07 November 2022: Database Analysis
Mechanism of Progesterone in Treatment of Traumatic Brain Injury Based on Network Pharmacology and Molecular Docking Technology
Chen Zheng1ABCDEFG, Jian Gong1CEF, Lili Zang2CDEF, Daiying Song2BCDF, Xinyue Ran2CDF, Juncen Li2BCDE, Bo Jiang1DF, Jianli Xu1BC, Qihua Wu1ACDFG*DOI: 10.12659/MSM.937564
Med Sci Monit 2022; 28:e937564
Abstract
BACKGROUND: Previous studies have confirmed that progesterone has a protective effect on traumatic brain injury (TBI). In this paper, network pharmacology and molecular docking technology were used to further explore the potential mechanism of progesterone in the treatment of TBI.
MATERIAL AND METHODS: Based on network pharmacology, potential targets of progesterone for TBI were obtained. The network diagram of interactions between target proteins was established to screen the key targets of progesterone for TBI. The DAVID database was used to analyze its biological function and enrichment pathway, and to explore and determine the biological pathway of progesterone in treating TBI. Molecular docking technology was used to simulate the interaction between progesterone and key target proteins.
RESULTS: Progesterone can treat TBI by anti-inflammatory action, repairing damaged cell membranes, stabilizing the structure of the blood-brain barrier, alleviating brain edema, reducing neuronal apoptosis, and improving neurological function. The molecular mechanism involves the PI3K/Akt signaling pathway, MAPK signaling pathway, and Ras signaling pathway.
CONCLUSIONS: Progesterone is a potential clinical treatment for TBI. Exploring the potential targets and pathways of TBI therapy through network pharmacology can provide a direction for subsequent research.
Keywords: Progesterone, Brain Injuries, Traumatic, Pharmacology, Molecular Docking Simulation, Humans, Phosphatidylinositol 3-Kinases, Network Pharmacology, Drugs, Chinese Herbal, Technology
Background
Traumatic brain injury (TBI) is head injury and brain dysfunction caused by external blows or head rotation [1]. At present, TBI is mostly caused by traffic collisions, falls, and violence, and is one of the main causes of disability and death among people of all ages, which greatly threatens human health and life [2]. More than 50 million people experience TBI globally every year [3]. The injury mechanism of TBI is complex, and the course of TBI can be divided into 2 stages: primary injury and secondary injury. Primary injury is usually irreversible and can directly lead to the death of nerve cells. Secondary injury is a reversible process secondary to primary injury [4], which is mainly caused by the secretion of inflammatory mediators such as cytokines, chemokines, cytotoxic proteases, and oxygen free radicals. It involves pathophysiological processes such as secondary brain injury, cell death, tissue injury, neurodegeneration, mitochondrial dysfunction, inflammatory reaction, and coagulation dysfunction [5,6], which is the main cause of death and disability in patients with TBI in clinical practice. At present, clinical treatment is mainly focused on prevention of secondary craniocerebral injury, and neuroprotection is strengthened in the whole course of treatment [7]. Treatment methods include mild hypothermia therapy, decompressive craniectomy (DC), traumatic cerebrovascular injury treatment, and drug therapy [4]. Preclinical drug studies have shown that progesterone, glucocorticoids, erythropoietin, citicoline, and recombinant human IL-1 receptor antagonists all have potential effects on reducing TBI secondary craniocerebral injury [8–10]. Among them, the therapeutic effect of progesterone on TBI has attracted great attention from the medical community [11].
Progesterone, a neuroactive steroid containing 21 carbon atoms, is secreted by the corpus luteum of the ovary and is essential during pregnancy. Ovarian granulosa cells and placenta secrete progesterone, and there are many progesterone receptors in the endometrial epithelium, stroma, and muscle smooth muscle nuclei. In the central nervous system, astrocytes, oligodendrocytes, and microglia also have the function of secreting progesterone, and progesterone receptors are widely distributed in various parts of the brain [12]. Animal experimental studies have shown that progesterone can reduce nerve cell death, inhibit apoptosis, alleviate brain edema, reduce hypoxic-ischemic brain injury, reduce inflammation, and promote neurological function recovery by regulating nerve cell autophagy [13]. Clinical reports have also shown that progesterone therapy has obvious curative effects in patients with acute severe TBI after admission. After progesterone treatment, TBI patients’ brain injury is alleviated, neurological function recovery is accelerated, and prognosis is significantly improved [12,14,15].
Although progesterone has such great advantages in the treatment of TBI, in high-quality, large-sample, and multicenter clinical studies, progesterone is not considered to effectively improve the prognosis of TBI patients [16], and the protective mechanism of progesterone on TBI still needs to be further studied. Therefore, the present study used network pharmacology and molecular docking technology to explore the specific mechanism of progesterone in the treatment of TBI at the molecular level, so as to provide a reliable theoretical basis for further basic and clinical research.
Material and Methods
TARGET PREDICTION OF PROGESTERONE:
The PubChem (
TBI TARGET SCREENING:
TBI targets were collected using the GeneCards (
CONSTRUCTION OF INTERSECTION TARGET PROTEIN–PROTEIN INTERACTION (PPI) NETWORK:
To clarify the interaction between progesterone and TBI intersection targets, the venny2.1.0 website (
ENRICHMENT ANALYSIS OF FUNCTIONS AND PATHWAYS:
Using the DAVID 6.8 (
CONSTRUCTION OF PROGESTERONE TARGET-PATHWAY NETWORK DIAGRAM:
The intersection targets and signaling pathways of progesterone and TBI were imported into Cytoscape3.8.2 network mapping software to construct a progesterone-target-pathway network. Among them, progesterone, targets, and signaling pathways are represented by nodes in the network, and the relationships between nodes are represented by edges.
MOLECULAR DOCKING VERIFICATION OF KEY TARGETS OF PROGESTERONE TREATMENT FOR TBI:
Molecular docking technology was used to simulate the interaction between progesterone molecules and the target and to predict the binding mode and binding capacity. We downloaded the crystal structures of key target proteins in the PPI network from the RCSB database (
Results
PROGESTERONE-RELATED TARGETS:
The pharmacophore model and molecular features of progesterone were obtained through the PharmMapper database (Figure 1), as well as the corresponding target protein, and the UniProt database was used to convert the obtained target protein into gene names. After eliminating the target gene information without corresponding genes, the corresponding 183 possible targets were screened (Table 1).
TBI-RELATED TARGETS:
From the GeneCards database, 1687 TBI targets were obtained with the keyword “traumatic brain injury”. The target with Score >median was set as the potential target of TBI. The maximum value of Score was 73.65, the minimum value was 0.25, and the median was 6.65. Therefore, the target with Score ≥6.65 was set as the target of the TBI potential target. After excluding the data, 844 targets were obtained.
INTERSECTION TARGET PPI NETWORK:
The screened progesterone targets and TBI targets were intersected, and Venn diagrams were drawn through the venny 2.1.0 website to obtain 48 progesterone-TBI common targets (Figure 2A). We submitted the target to the STRING platform, selected the species as “Homo sapiens”, set the clustering method as “kmeans clustering”, and obtained the PPI network interaction diagram of the intersection targets (Figure 2B). The MCODE plug-in in the CytoScape 3.8.2 software was used to perform cluster analysis on the interaction relationship, and 2 functional modules were obtained. The sub-network with the highest score value is shown (Figure 2C). The larger the node, the more important the node is. Using the CytoHubba plug-in, the top 10 key genes were obtained according to the MCC (Maximal Clique Centrality) analysis method (Figure 2D), followed by albumin (ALB), caspase 3 (CASP3), heat shock protein 90α family A class member 1 (HSP90AA1), epidermal growth factor receptor (EGFR), annexin A5 (ANXA5), kinase insertion domain receptor (KDR), mitogen-activated protein kinase 14 (MAPK14), peroxidase proliferator-activated receptor gamma (PPARG), mitogen-activated protein kinase 1 (MAPK1), and interleukin 2 (IL2).
ENRICHMENT ANALYSIS OF TARGET FUNCTION AND PATHWAY:
The common target information of progesterone and TBI was imported into the DAVID database for GO and KEGG enrichment analysis. We obtained 197 GO annotation entries, including 120 biological processes (BP), 22 cell compositions (CC), and 53 molecular functions (MF). Arranged in ascending order of P value, the top 10 GO items were selected to draw a histogram (Figure 3A). Bioprocess enrichment results indicated steroid hormone-mediated signaling, proteolysis, transcription initiation from RNA polymerase II promoters, positive regulation of nitric oxide biosynthesis, brake stress response, collagen catabolism, cellular biological processes such as ECM breakdown, positive regulation of RNA polymerase II promoter transcription, negative regulation of cholesterol storage, and exogenous apoptotic signaling pathways lacking ligands all play important roles in the treatment of TBI with progesterone. Most of the core targets are located in the extracellular domain, extracellular space, extracellular exosomes, mitochondria, protein complexes, receptor complexes, endolysosomal lumen, pits, membrane rafts, and cytoplasm. In the KEGG pathway enrichment analysis, 52 signaling pathways were screened and ranked in ascending order of P value. The top 20 items were selected to draw a bubble chart (Figure 3B). The results showed that progesterone can act on proteoglycans and estrogens in cancer via hormone signaling pathway, cancer pathway, insulin resistance, PPAR signaling pathway, prolactin signaling pathway, PI3K-Akt signaling pathway, FoxO signaling pathway, prostate cancer, GnRH signaling pathway, Ras signaling pathway, tumor necrosis factor signaling pathway, vascular endothelial growth factor signaling pathway, NOD-like receptor signaling pathway, etc. ErbB signaling pathway, HIF-1 signaling pathway, Toll-like receptor signaling pathway, serotonergic synapse, neurotrophin signaling pathway, sphingolipid signaling pathway, and arginine and proline metabolism. In addition, the results also showed that the target of progesterone is also involved in other diseases, such as hepatitis C, non-alcoholic fatty liver, whooping cough, influenza A, Alzheimer’s disease, toxoplasmosis, tuberculosis, focal adhesions, Shiga mycosis, melanoma, salmonella infection, bladder cancer, and pancreatic cancer. This suggests the potential advantages of progesterone in the treatment of these diseases.
DRUG-TARGET-PATHWAY NETWORK:
Based on progesterone, intersection target genes, GO enrichment biological process, and KEGG enrichment pathway, the CytoScape 3.8.2 network mapping software was used to construct a drug-target-pathway network (Figure 4).
ANALYSIS OF MOLECULAR DOCKING RESULTS:
Molecular docking verification was performed between progesterone and the top 10 key targets: ALB, CASP3, HSP90AA1, EGFR, ANXA5, KDR, MAPK14, PPARG, MAPK1, and IL2. The results showed that the binding energy of progesterone to key target proteins was less than −5 Kal/mol (Table 2), and could bind to the amino acids of EGFR (thr854, cys797) (Figure 5A), MAPK1 (met149, asn194) (Figure 5B), and ANXA5 (asp332, cys238) (Figure 5C). Residues interacted with each other, showing good binding force, which suggested that progesterone had high biological affinity with TBI-related targets and had good activity.
Discussion
The molecular docking results showed that the biological affinity of progesterone and key target proteins was high, and the binding energy between them was less than −5 Kal/mol. EGFR, MAPK1, and ANXA5 had the strongest binding energy with progesterone, reaching −9.4 Kal/mol, −9.2 Kal/mol, and −9.2 Kal/mol, respectively. EGFR, a member of the HER family, is a receptor tyrosine transmembrane glycoprotein [17]. Activation of EGFR can reduce the loss of BBB cell connexins (such as junction adhesion molecule-1 and sealing protein-5) in brain injury, stabilize BBB structure, reduce permeability, and reduce brain tissue edema [18]. Extracellular signal-regulated kinase (ERK), a member of the MAPK family, can be encoded by the MAPK1 gene, follows the 3-level enzymatic cascade of MAPKs, and is activated through the Ras/Raf/MEK/ERK1/2 classical pathway [19]. Activation of the ERK/MAPK pathway can cause neuronal injury and apoptosis, resulting in brain tissue damage [20,21]. The use of PD98059, a specific inhibitor of the ERK/MAPK pathway, to disinhibit the ERK/MAPK pathway can significantly reduce brain edema and neurological damage caused in the rat TBI model [22]. Annexins are caleidogenic phospholipid-binding proteins, which can participate in various physiological activities such as anticoagulation, anti-inflammation, signal transduction, and cell proliferation [23]. AnnexinA5 (AnxA5), a member of the AnxA5 family, is the most widely distributed in the body and can inhibit the polarization of Ml macrophages through the NF-κB signaling pathway to achieve an anti-inflammatory effect [24]. In the presence of Ca2+, it can bind to the edge of the damaged cell membrane and repair the damaged cell membrane [25]. These results suggest that progesterone may play a role in the treatment of TBI by acting on core targets such as EGFR, MAPK1, and ANXA5, and then play a role in cell membrane repair, anti-inflammatory, and brain edema reduction.
The GO enrichment results showed that the progesterone treatment target is the core of TBI, mostly located in the extracellular space, the extracellular foreign body, the cell membrane, cytoplasm, mitochondria, and protein complex area, participating in the steroid hormone-mediated signaling pathway, protein hydrolysis, RNA polymerase II promoter transcription start, and nitric oxide of positive regulation of biosynthesis of biological process. It regulates steroid hormone receptor activity, RNA polymerase II transcription factor activity, receptor signaling protein tyrosine kinase activity, MAP kinase, and other related protein kinase activity. Further analysis by KEGG showed that the core targets of progesterone treatment of TBI can participate in the negative regulation and signal transduction of apoptosis, and were significantly enriched in PI3K/Akt signaling pathway, Ras signaling pathway, and MAPK signaling pathway. This suggests that the treatment of TBI with progesterone has the characteristics of multi-target and multi-pathway, and it can exert different physiological effects through various signaling pathways to treat TBI.
MAPK signaling pathway is one of the key signaling pathways obtained by this KEGG analysis. It activates downstream transcription factors and acts as a signal transduction module through stepwise phosphorylation of the 3-stage enzymatic cascade (MAP3K/MAP2K/MAPK) [26]. JNK (c-Jun amino-terminal kinase) belongs to the MAPK family [27]. The JNK signaling pathway can be activated by MAPK enzymatic cascade reaction and participate in cell apoptosis, metabolism, movement, DNA repair damage, and other activities induced by extracellular stimulation [28]. The JNK signaling pathway is closely related to nerve cell apoptosis and nerve function recovery after TBI. After brain injury, JNK3 expression is downregulated, which can promote the recovery of nerve function after brain injury [29]. Luo et al [30] also showed that inhibiting activation of the JNK signaling pathway can significantly reduce the degree of brain edema after TBI in rats, inhibit the apoptosis of nerve cells, and improve neurological function after injury. The mechanism may be to reduce the expression of TNFα, IL-1α, IL-1β, and IL-6 by reducing the secondary inflammatory response after brain injury. P38 MAPK is a differentiated and conserved serine/threonine protein kinase, which is closely related to the pathophysiological process of neural tissues [31]. The P38 MAPK signaling pathway can also be activated by the enzymatic cascade of MAPK, which participates in the defense and protection of various brain diseases and becomes the pathogenic vector of various encephalopathies through oxidative stress, activating inflammatory factors, and triggering inflammatory reactions [31]. In the acute stage of ischemic brain injury, the P38 MAPK signaling pathway can induce neurotoxicity and promote inflammatory response [32], while in the subacute stage, this pathway inhibits the inflammatory response by a neuroprotective anti-apoptotic effect [33–35]. In addition, the P38 MAPK pathway can change the expression of pro-apoptosis-related protein (caspase-9), apoptosis regulator (Bcl-2/Bcl-2-related X protein and caspase-3, and induce neuronal apoptosis [36,37]. It can also enhance the activities of cytosolic phospholipase A2 and aquaporin 4, thereby increasing blood-brain barrier permeability and promoting brain tissue edema [38,39]. The Ras signaling pathway is a classical pathway of the MAPK signaling pathway, which plays a corresponding physiological role through the Ras/Raf/MEK/ERK1/2 pathway [19]. Inhibition of the ERK/MAPK pathway can significantly reduce neuronal injury and apoptosis, and improve brain edema and neurological damage [22]. In addition, studies have shown that the combination of EGFR with epidermal growth factor (EGF), double-regulated protein (AR), and other ligands can also activate the Ras signaling pathway [40]. The EGFR/ERK signaling pathway protects against ischemic stroke, and its activation can inhibit the inflammatory response in injured brain tissue [41], promote secretion of osteopontin, enhance the expression of IL-20, and reduce release of excitatory amino acids, thus protecting brain tissue [42]. These results suggest that progesterone can inhibit secondary TBI brain injury, reduce neuronal apoptosis, and improve neurological function by acting on the MAPK signaling pathway and Ras signaling pathway.
The PI3K/Akt signaling pathway is another key signaling pathway obtained by KEGG analysis. Core target genes such as GSK3B, HSP90AA1, NOS3, INSR, KDR, MAPK1, EGFR, IL2, and FGFR1 were enriched in this signaling pathway. Studies have shown that the EGFR/PI3K/Akt pathway plays an important role in brain injury, cerebral infarction, epilepsy, and other neurological diseases. It can participate in cell apoptosis and survival, cell growth and proliferation, angiogenesis, and other cellular activities, and can inhibit cell apoptosis through a variety of ways [43–45]. The specific pathways are as follows: (1) it can phosphorylate downstream Bad (bcl-2-related death protein), and then depolymerize the anti-apoptotic factor bcl-2 or bcl-xl from the apoptotic complex to play an anti-apoptotic role; (2) it can inactivate the pro-apoptotic protein Bax by phosphorylation and inhibit cell apoptosis; (3) it can deactivate downstream casspase-9 by inhibiting the phosphorylation of the caspase pathway, and then block the activation of downstream molecules, such as CasSPase-3, CasSPase-8, and CasSPase-10, thereby inhibiting its pro-apoptotic effect; (4) it can inhibit Forkhead family transcription factors (eg, Forkhead receptor and FKHR) and prevent them from regulating the transcription of apoptosis-related genes, thus inhibiting cell apoptosis; (5) it can phosphorylate glycogen synthase kinase-3β (GSK-3β) expressed in the nervous system and inactivate it, so as to reduce cell apoptosis, inhibit cell hypertrophy, promote vascular regeneration, and play an anti-apoptotic role; and (6) it can activate NF-κB, promote cell proliferation and inhibit cell apoptosis. It is suggested that progesterone can act on the EGFR/PI3K/Akt signaling pathway to stabilize the structure of the blood-brain barrier, reduce brain edema, and protect nerve tissue.
Conclusions
To sum up, based on network pharmacology and molecular docking technology, this study analyzed the action targets, biological processes, and related pathways of progesterone, showing that progesterone has multi-target and multi-channel interaction characteristics in treating TBI. The key target may treat TBI by anti-inflammation, repairing damaged cell membrane, stabilizing blood-brain barrier structure, alleviating brain edema, reducing neuron apoptosis, and improving nerve function. The molecular mechanism involves the PI3K/Akt signal pathway, MAPK signal pathway, and Ras signal pathway. These results suggest that progesterone has potential as a clinical drug for the treatment of TBI, but due to the limitations of network pharmacology research methods, further experimental verification is still needed in the later stage.
Figures
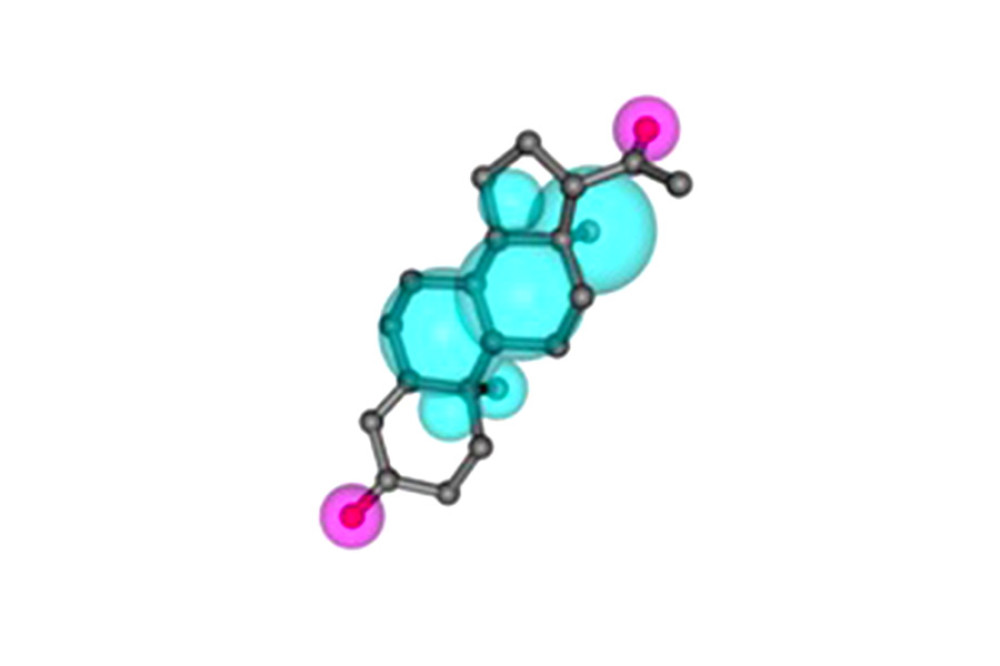 Figure 1. Pharmacophore model and molecular characteristics of progesterone (obtained from the PharmMapper online database).
Figure 1. Pharmacophore model and molecular characteristics of progesterone (obtained from the PharmMapper online database). 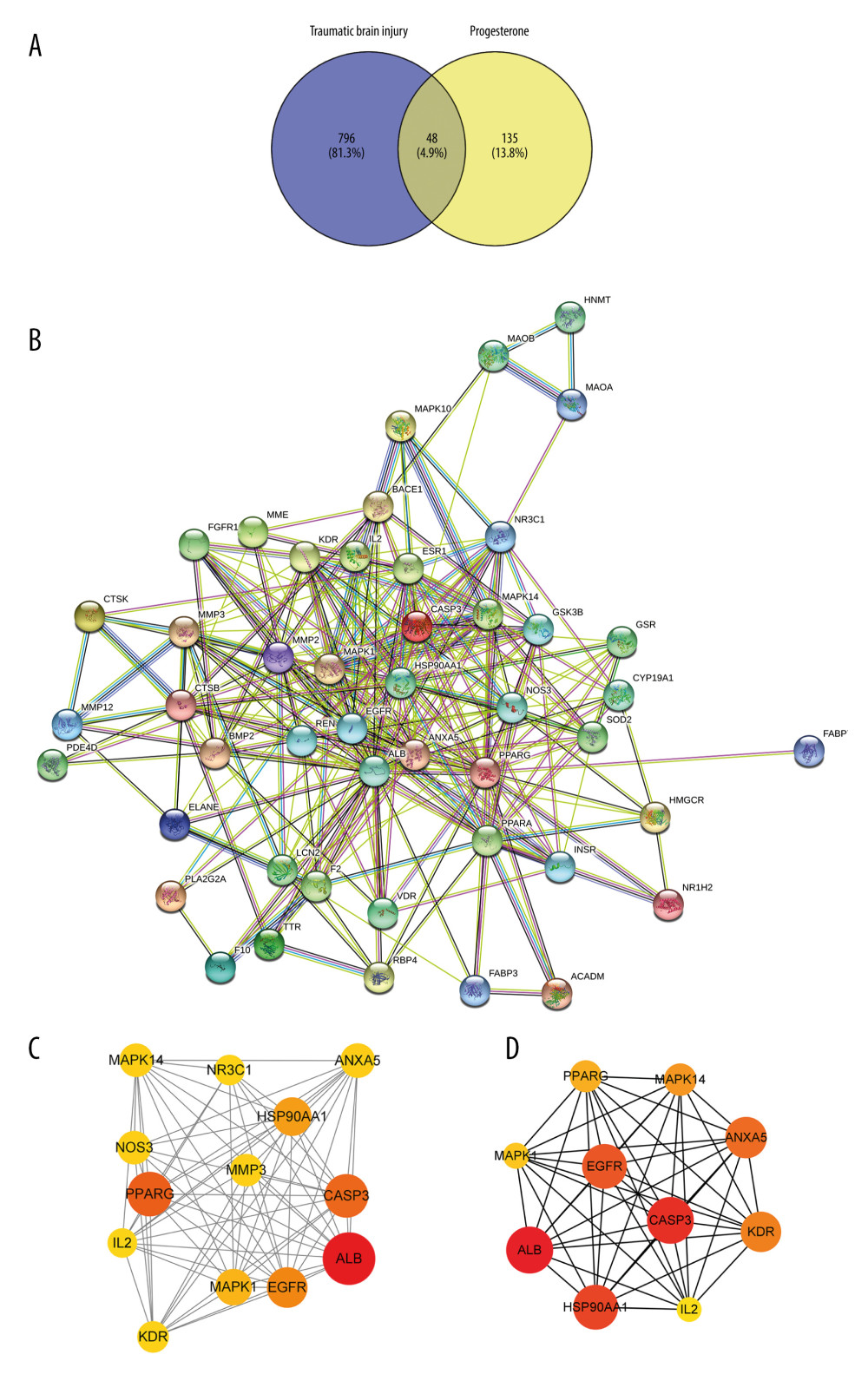 Figure 2. (A) The intersection target of progesterone target and traumatic brain injury target (created using Venny2.1.0 website). (B) PPI network of the intersection target of progesterone and traumatic brain injury (created using the STRING online database). (C) The main sub-networks (functional modules) in the target network, according to the figure. The node degree value sets the size of the node. The larger the node, the redder the color, indicating that the node is more important. Set the thickness of the edge according to the binding score. The thicker the edge, the stronger the PPI relationship (created using CytoScape 3.8.2 Software Mapping). (D) In the intersection target, the top 10 key target genes are calculated according to ECC. The larger the node, the redder the color, and the higher the ranking (created using CytoScape 3.8.2 Software Mapping).
Figure 2. (A) The intersection target of progesterone target and traumatic brain injury target (created using Venny2.1.0 website). (B) PPI network of the intersection target of progesterone and traumatic brain injury (created using the STRING online database). (C) The main sub-networks (functional modules) in the target network, according to the figure. The node degree value sets the size of the node. The larger the node, the redder the color, indicating that the node is more important. Set the thickness of the edge according to the binding score. The thicker the edge, the stronger the PPI relationship (created using CytoScape 3.8.2 Software Mapping). (D) In the intersection target, the top 10 key target genes are calculated according to ECC. The larger the node, the redder the color, and the higher the ranking (created using CytoScape 3.8.2 Software Mapping). 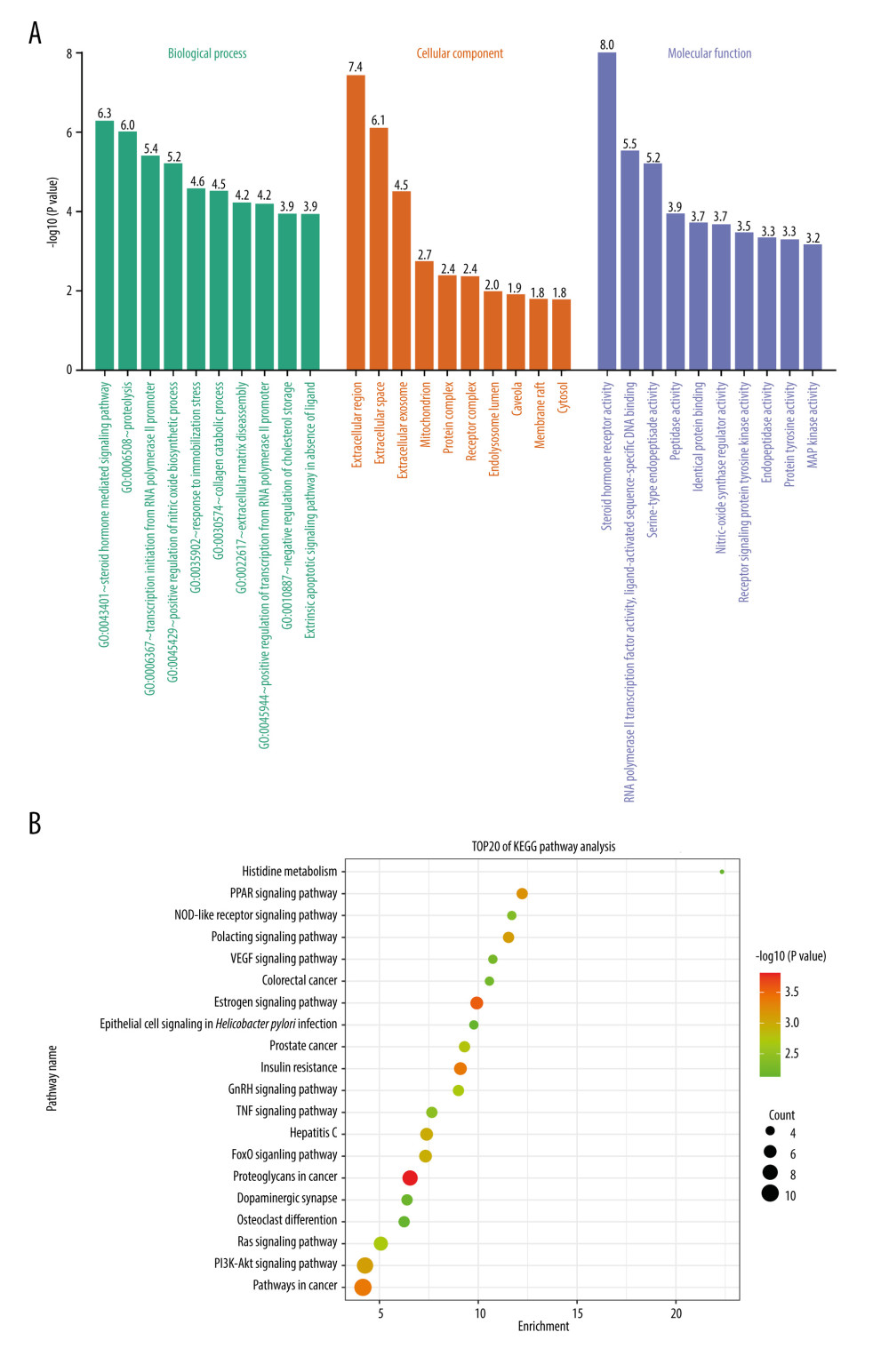 Figure 3. (A) GO enrichment analysis (created using online visualization tools, http://www.bioinformatics.com.cn/).(B) KEGG pathway enrichment (top 20) (created using online visualization tools, http://www.bioinformatics.com.cn/).
Figure 3. (A) GO enrichment analysis (created using online visualization tools, http://www.bioinformatics.com.cn/).(B) KEGG pathway enrichment (top 20) (created using online visualization tools, http://www.bioinformatics.com.cn/). 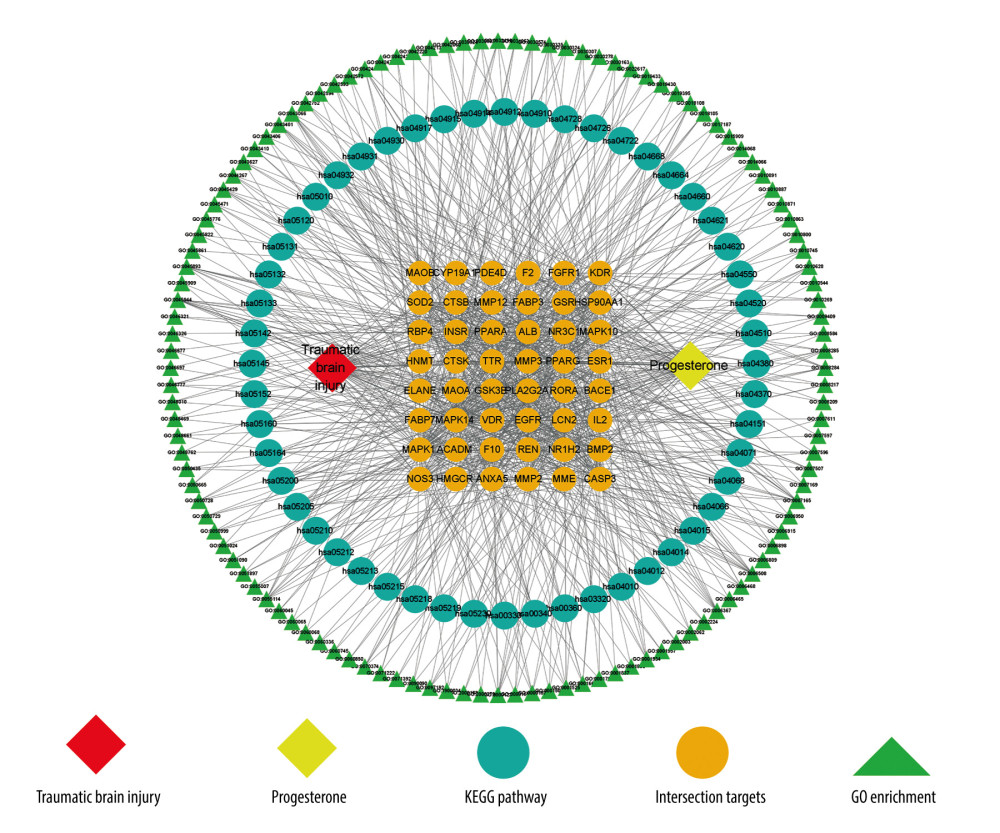 Figure 4. Drug-target-pathway network of progesterone in the treatment of traumatic brain injury (created using CytoScape 3.8.2 Software Mapping).
Figure 4. Drug-target-pathway network of progesterone in the treatment of traumatic brain injury (created using CytoScape 3.8.2 Software Mapping). 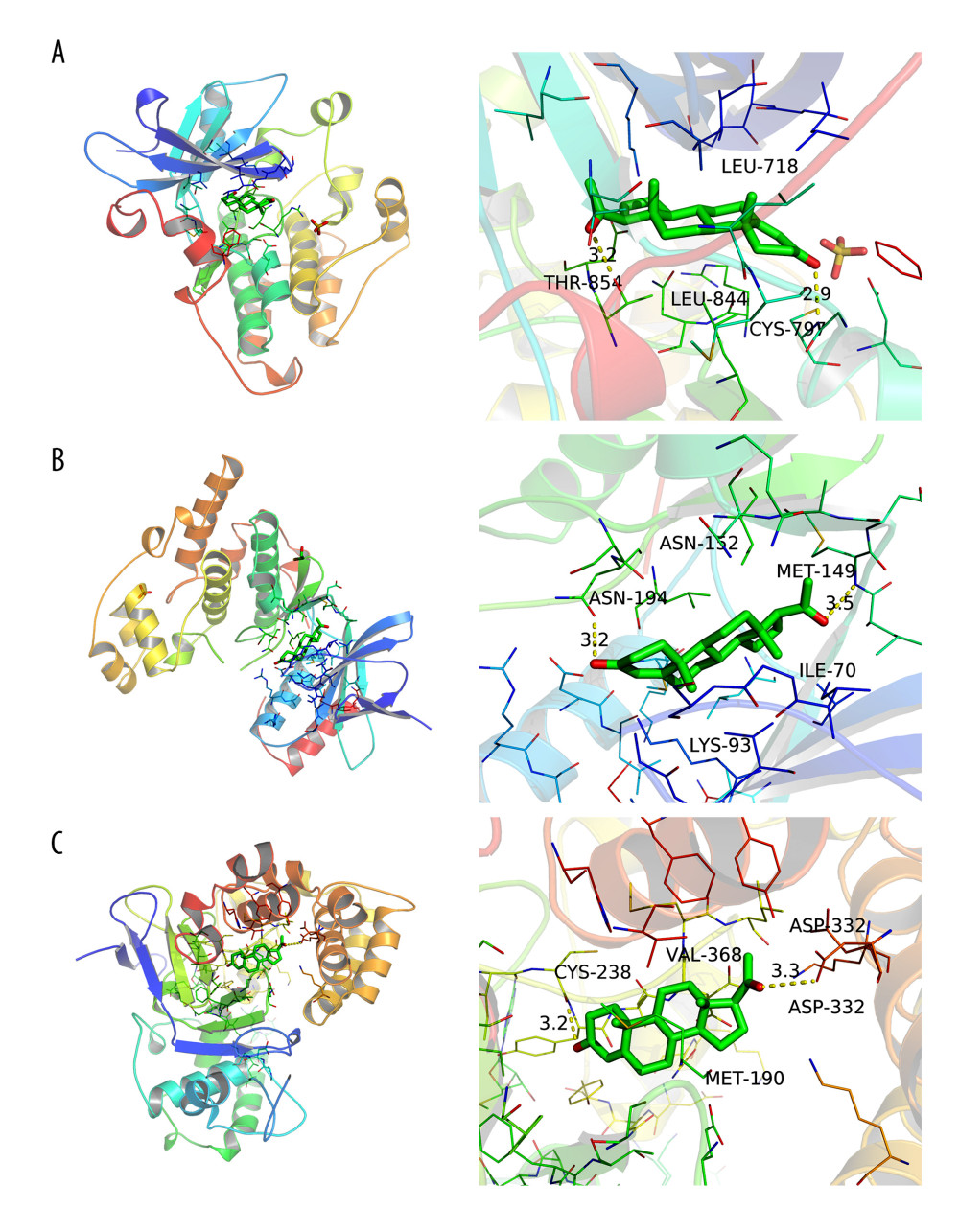 Figure 5. (A) Simulation of molecular docking between progesterone and EGFR (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). (B) Simulation of molecular docking between progesterone and MAPK1 (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). (C) Simulation of molecular docking between progesterone and ANXA5 (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software).
Figure 5. (A) Simulation of molecular docking between progesterone and EGFR (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). (B) Simulation of molecular docking between progesterone and MAPK1 (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). (C) Simulation of molecular docking between progesterone and ANXA5 (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). References
1. You CY, Fu YQ, Xu F, Research progress on the mechanism and prognosis of coagulation dysfunction after traumatic brain injury: Chongqing Medical, 2021; 50(18); 3210-14
2. Li PF, Li CR, Zhang P, Predictive value of blood lactate level and lactate clearance rate on survival and neurological outcome of patients with craniocerebral trauma: Journal of Medical Postgraduates, 2019; 32(10); 1049-54
3. Maas AIR, Menon DK, Adelson PD, Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research: Lancet Neurol, 2017; 16(12); 987-1048
4. Luan YY, Yao YM, New progress in research of traumatic brain injury: International Journal of Surgery, 2021; 48(01); 27-31
5. Jia YB, Wang GY, Kang EM, The role of microglia in neuroinflammatory environment of traumatic brain injury and its significance to nerve regeneration and repair: Chinese Journal of Neuromedicine, 2021; 20(7); 733-37
6. Huang YN, Yang LY, Nigel HG, Neuroprotective effects of pifithrin-α against traumatic brain injury in the striatum through suppression of neuroinflammation, oxidative stress, autophagy, and apoptosis: Sci Rep, 2018; 8(1); 2368
7. Xia ZF, Wu GS, Clinical research progress of traumatic brain injury: Academic Journal of Second Military Medical University, 2021; 42(02); 117-21
8. Puffer RC, Yue JK, Mesley M, Recovery trajectories and long-term outcomes in traumatic brain injury: A secondary analysis of the phase 3 citicoline brain injury treatment clinical trial: World Neurosurg, 2019; 125; e909-e915
9. Lee J, Cho YS, Choi KS, Efficacy and safety of erythropoietin in patients with traumatic brain injury: A systematic review and meta-analysis: Am J Emerg Med, 2019; 37(6); 1101-7
10. Helmy A, Guilfoyle MR, Carpenter KL, Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: A phase II randomized control trial: J Cereb Blood Flow Metab, 2014; 34(5); 845-51
11. Chen XC, Ren XJ, Gao WW, Yue SY, Experimental study on progesterone regulating inflammatory response and prognosis after traumatic brain injury: Lingnan Modern Clinical Surgery, 2020; 20(1); 84-88 +92
12. Jiang TW, Hang CH, Research progress of progesterone application in traumatic brain injury: Chinese Journal of Experimental Surgery, 2016; 33(3); 851-54
13. Wei SP, Ren SH, Ma SB, Study on nerve function repair of progesterone in traumatic brain injury: Hebei Medicine, 2019; 41(16); 2504-7
14. Jin MH, Wu LJ, Sun MF, Changes of blood hormones and prognosis analysis after hyperbaric oxygen combined with progesterone for moderate and severe traumatic brain injury: China Hospital Statistics, 2017; 24(5); 329-331+335
15. Guo LJ, Jing SY, Han FL, Zhang YP, Clinical significance of early dynamic changes of serum progesterone in patients with craniocerebral trauma: Guide of China Medicine, 2014(36); 151-52
16. Brett ES, Andrew IM, Raj KN, A clinical trial of progesterone for severe traumatic brain injury: N Engl J Med, 2014; 371(26); 2467-76
17. Wee P, Wang ZX, Epidermal growth factor receptor cell proliferation signaling pathways: Cancers (Basels), 2017; 9(5); 52
18. Xiang JY, Bandura JF, Zhang P, EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration: Nat Commun, 2017; 8(1); 15125
19. Wish PP, Shang YZ, Research progress of MAPK signaling pathway mediating apoptosis: Journal of Chengde Medical College, 2021; 38(3); 243-46
20. Zhang Q, Ao T, Xiao SY, Research progress of epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway in ischemic cerebrovascular disease: Chinese Medicine Guides, 2018; 15(9); 36-40
21. Qiao TT, Yu W, Cui YH, Research progress of ERK signaling pathway in Alzheimer’s disease: Medical Review, 2022; 28(3); 422-27
22. Zhang X, Chen B, Li M, Research progress of signal pathways involved in cerebral ischemia-reperfusion injury: Zhejiang Medical Science, 2022; 44(5); 557-62
23. Qiu HJ, Wu ZM, Wang XJ, Study on the relationship between Annexins and malignant tumors: Journal of Oncology, 2014; 20(2); 156-61
24. Annexin Xu F, A5 regulates macrophage polarization to improve inflammation [D]: Nanjing University, 2017
25. Wang XJ, Dai YR, Zhao YN, AnnexinA5 might suppress the phenotype of human gastric cancer cells via ERK pathway: Front Oncol, 2021; 11; 665105
26. Tikkanen R, David NP, Mitogen-activated protein kinases: Functions in signal transduction and human diseases: International Journal of Molecular Sciences, 2019; 20(19); 4844
27. Liu TT, Zhang SP, Qin XY, Pan RY, Research progress of MAPK signal transduction pathway and nerve injury: Chin J Public Health, 2016; 32(2); 248-54
28. Zhang KK, Wu MF, Xie DH, Relationship between mitogen-activated protein kinase signaling pathway and energy metabolism: World Journal of Chinese Medicine, 2022; 17(12); 1778-1782+1787
29. CC Xu: NK inhibitor SP600125 attenuates ischemic reperfusion brain injury in rats by down-regulating the expression of MMP-9 [D], 2019, Kunming University of Science and Technology
30. Luo LQ, Chen XR, Li YS, Role of JNK signaling pathway in inflammatory response after traumatic brain injury in rats: Chin J Neuromed, 2014; 13(12); 1233-38
31. Zhang KM, Cui YL, Ge LD, Research progress of P38 MAPK signaling pathway on ischemic stroke: Medical Review, 2021; 27(9); 1691-95
32. Alam S, Liu Q, Liu S, Up-regulated cathepsin C induces macrophage M1 polarization through FAK-triggered p38 MAPK/NF-κB pathway: Exp Cell Res, 2019; 382(2); 111472
33. Cheng CY, Ho TY, Hsiang CY, Angelica sinensis exerts angiogenic and anti-apoptotic effects against cerebral ischemia-reperfusion injury by activating p38MAPK/HIF-1/VEGF-A Signaling in Rats: Am J Chin Med, 2017; 45(8); 1683-708
34. Cheng CY, Tang NY, Kao ST, Hsieh CL, Ferulic acid administered at various time points protects against cerebral infarction by activating p38 MAPK/p90RSK/CREB/Bcl-2 anti-apoptotic signaling in the subacute phase of cerebral ischemia-reperfusion injury in rats: PLoS One, 2017; 11(5); e0155748
35. Yang SI, Yuan YJ, Jiao S, Calcitonin gene-related peptide protects rats from cerebral ischemia/reperfusion injury via a mechanism of action in the MAPK pathway: Biomed Rep, 2016; 4(6); 699-703
36. Li W, Li DY, Zhao SM, Rutin attenuates isoflurane-induced neuroapoptosis via modulating JNK and p38 MAPK pathways in the hippocampi of neonatal rats: Exp Ther Med, 2017; 13(5 Pt A); 2056-64
37. Su DY, Ma J, Zhang ZB, Protective effects of UCF-101 on cerebral ischemia-reperfusion (CIR) is depended on the MAPK/p38/ERK signaling pathway: Cell Mol Neurobiol, 2016; 36(6); 907-14
38. Sun J, Nan GX, The mitogen-activated protein kinase (MAPK) signaling pathway as a discovery target in stroke: J Mol Neurosci, 2016; 59(1); 90-98
39. Xie J, Zhang ZB, Mitogen-activated protein kinase signaling pathway and neuronal apoptosis after cerebral ischemia: International Journal of Cerebrovascular Diseases, 2015(6); 464-68
40. Song H, Bian ZG, Yang J, Expression of p2ras and epidermal growth factor receptor protein in laryngeal squamous cell carcinoma: Anatomy Research, 2020; 42(4); 314-17
41. Liu BH, Liu M, Liu HB, Gu Y, Dynamic changes and significance of ERK-MAPK signaling pathway in rats with traumatic brain injury: Chinese Journal of Gerontology, 2017; 37(17); 4212-14
42. LL Li: Study on the effect of silybin on neuronal apoptosis and EGFR/ERK signaling pathway in mice with cerebral ischemia [D], 2016, Hebei Medical University
43. Zhou DR: Study of Xuesaitong on LINGO-1, EGFR, PI3K/AKT pathway, PTEN and GSK-3β in MCAO rats and OGD/R damaged SH-SY5Y cells [D], 2021, Beijing University of Traditional Chinese Medicine
44. Wu S: Study on the action mechanism of Xuesaitong on Lingo-1, EGFR/PI3K/Akt pathway and BDNF in MCAO rats and SH-SY5Y cells damaged by OGD/R [D], 2020, Beijing University of Traditional Chinese Medicine
45. Chen B, Guo YT, Wang YL, miR-1231 inhibits the proliferation of meningioma cells by targeting EGFR/PI3K/AKT pathway: Hebei Medical Science, 2020; 26(8); 1233-37
Figures
 Figure 1. Pharmacophore model and molecular characteristics of progesterone (obtained from the PharmMapper online database).
Figure 1. Pharmacophore model and molecular characteristics of progesterone (obtained from the PharmMapper online database). Figure 2. (A) The intersection target of progesterone target and traumatic brain injury target (created using Venny2.1.0 website). (B) PPI network of the intersection target of progesterone and traumatic brain injury (created using the STRING online database). (C) The main sub-networks (functional modules) in the target network, according to the figure. The node degree value sets the size of the node. The larger the node, the redder the color, indicating that the node is more important. Set the thickness of the edge according to the binding score. The thicker the edge, the stronger the PPI relationship (created using CytoScape 3.8.2 Software Mapping). (D) In the intersection target, the top 10 key target genes are calculated according to ECC. The larger the node, the redder the color, and the higher the ranking (created using CytoScape 3.8.2 Software Mapping).
Figure 2. (A) The intersection target of progesterone target and traumatic brain injury target (created using Venny2.1.0 website). (B) PPI network of the intersection target of progesterone and traumatic brain injury (created using the STRING online database). (C) The main sub-networks (functional modules) in the target network, according to the figure. The node degree value sets the size of the node. The larger the node, the redder the color, indicating that the node is more important. Set the thickness of the edge according to the binding score. The thicker the edge, the stronger the PPI relationship (created using CytoScape 3.8.2 Software Mapping). (D) In the intersection target, the top 10 key target genes are calculated according to ECC. The larger the node, the redder the color, and the higher the ranking (created using CytoScape 3.8.2 Software Mapping). Figure 3. (A) GO enrichment analysis (created using online visualization tools, http://www.bioinformatics.com.cn/).(B) KEGG pathway enrichment (top 20) (created using online visualization tools, http://www.bioinformatics.com.cn/).
Figure 3. (A) GO enrichment analysis (created using online visualization tools, http://www.bioinformatics.com.cn/).(B) KEGG pathway enrichment (top 20) (created using online visualization tools, http://www.bioinformatics.com.cn/). Figure 4. Drug-target-pathway network of progesterone in the treatment of traumatic brain injury (created using CytoScape 3.8.2 Software Mapping).
Figure 4. Drug-target-pathway network of progesterone in the treatment of traumatic brain injury (created using CytoScape 3.8.2 Software Mapping). Figure 5. (A) Simulation of molecular docking between progesterone and EGFR (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). (B) Simulation of molecular docking between progesterone and MAPK1 (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). (C) Simulation of molecular docking between progesterone and ANXA5 (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software).
Figure 5. (A) Simulation of molecular docking between progesterone and EGFR (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). (B) Simulation of molecular docking between progesterone and MAPK1 (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). (C) Simulation of molecular docking between progesterone and ANXA5 (created using PyMOL2.4 Software and AutoDock Tools1.5.6 Software). Tables
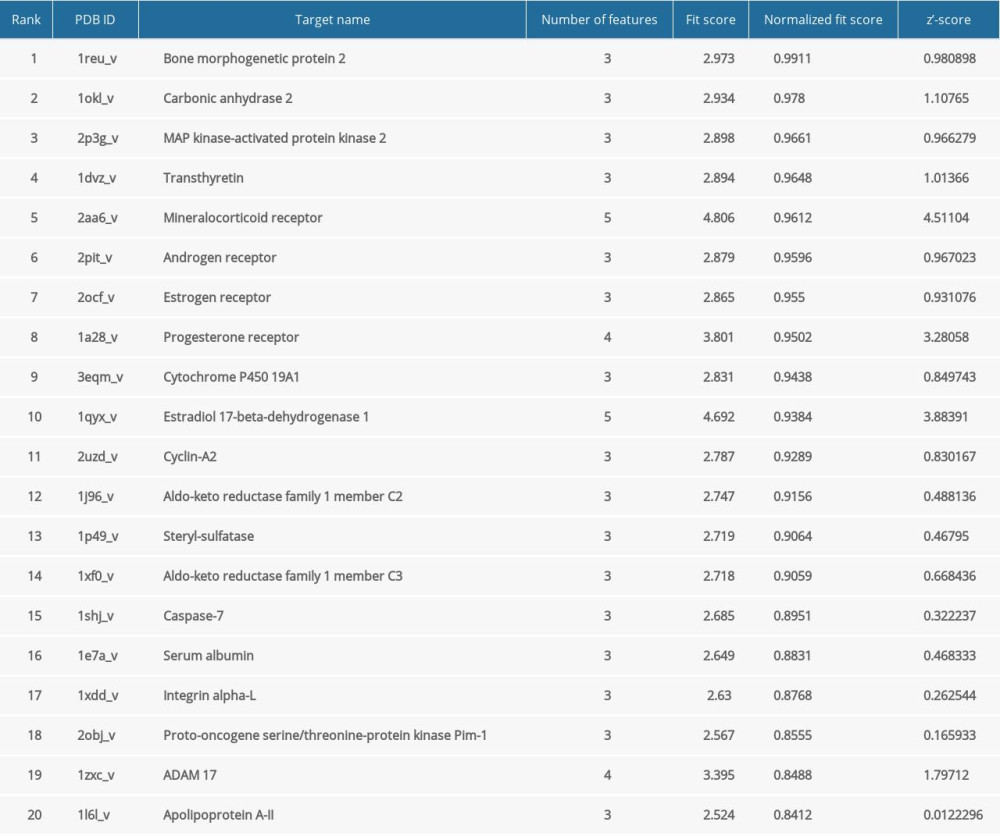 Table 1. Top 20 progesterone-related targets in descending order of standardized fit scores.
Table 1. Top 20 progesterone-related targets in descending order of standardized fit scores.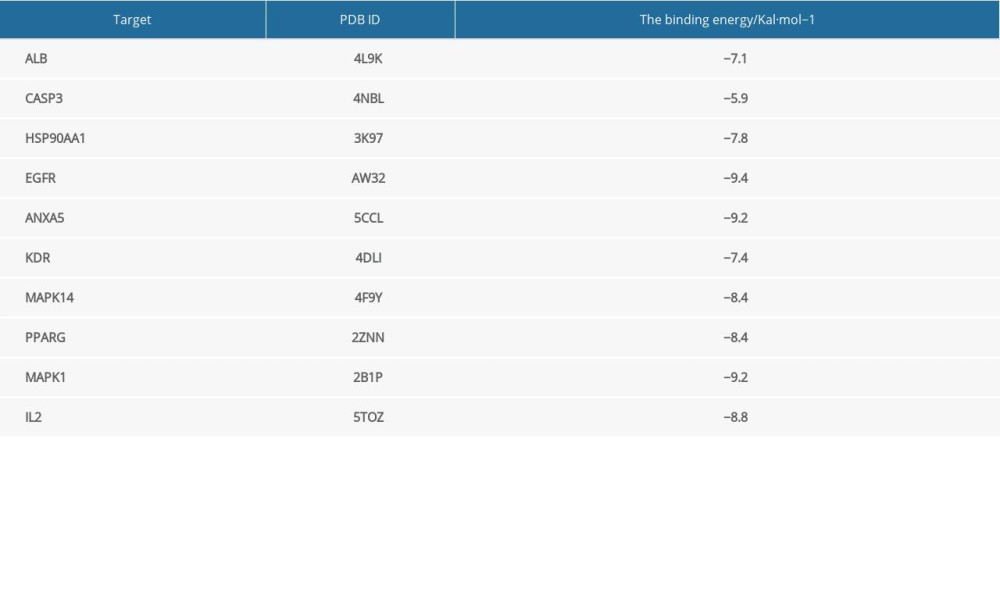 Table 2. Binding energies of progesterone and key targets.
Table 2. Binding energies of progesterone and key targets. Table 1. Top 20 progesterone-related targets in descending order of standardized fit scores.
Table 1. Top 20 progesterone-related targets in descending order of standardized fit scores. Table 2. Binding energies of progesterone and key targets.
Table 2. Binding energies of progesterone and key targets. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








