30 August 2022: Clinical Research
Postoperative Outcomes Following a Modified Method of Surgical Division of the Splenic Pedicle in 719 Patients During Splenectomy for Portal Hypertension: A 12-Year, Retrospective, Single-Center Study
Long Huang1ABDEFG*, Qingsheng Yu1DG, Hui Peng1BCF, Zhou Zhen2CDFDOI: 10.12659/MSM.937763
Med Sci Monit 2022; 28:e937763
Abstract
BACKGROUND: Portal hypertension associated with liver cirrhosis can be treated by splenectomy. During splenectomy, the gastrosplenic and the splenorenal ligaments that form the hilar splenic pedicle can be surgically divided by several approaches, with the aim to reduce portal vein thrombosis (PVT) and postoperative pancreatic fistula (PPF). This 12-year retrospective study from a single center aimed to evaluate postoperative outcomes following use of a modified method of surgical division of the splenic pedicle (MSDSP) in 719 patients who underwent splenectomy for portal hypertension (PH).
MATERIAL AND METHODS: From January 2010 to December 2021, 719 consecutive cirrhotic patients with PH and splenomegaly underwent splenectomy in our department. According to different methods of surgical division of the splenic pedicle, patients were divided into a Control Group (n=349) and a Study Group (n=370). The characteristics of the patients, perioperative indicators, postoperative complications (PVT, PPF and abdominal hemorrhage) and follow-up data were compared between the 2 groups. Propensity score matching was conducted to adjust for differences in preoperative characteristics at a 1: 1 ratio, resulting in 260 patients in each group.
RESULTS: After PSM was conducted, intraoperative blood loss, PVT, PPF, and hospital stay were decreased significantly in the matched Study Group (all P<0.01). Both groups showed similar results concerning recurrent esophagogastric variceal bleeding and overall survival during the follow-up period.
CONCLUSIONS: Our MSDSP help to reduce postoperative complications and shorten hospital stay.
Keywords: Fibrosis, Follow-Up Studies, Hypertension, Portal, Pancreatic Fistula, Splenectomy, Venous Thrombosis, Esophageal and Gastric Varices, Gastrointestinal Hemorrhage, Humans, Liver Cirrhosis, Portal Vein, Postoperative Complications
Background
As the final result of various clinical chronic liver injuries, liver cirrhosis can lead to some critical complications, including portal hypertension (PH), esophagogastric variceal bleeding (EGVB), splenomegaly, and hypersplenism [1,2]. For cirrhotic patients, splenomegaly and hypersplenism can result in a series of abnormal blood indicators, such as leukopenia, erythropenia, and thrombocytopenia [3,4].
Splenectomy is the optimal choice for patients with cirrhosis and hypersplenism [5], and splenectomy combined with paraesophagogastric devascularization is an effective treatment for cirrhotic patients with EGVB and PH [6,7], but postoperative complications such as postoperative pancreatic fistula (PPF), portal vein thrombosis (PVT), and abdominal hemorrhage remain crucial challenges for smooth postoperative recovery [8]. PVT, PPF, and abdominal hemorrhage are all severe complications after splenectomy [9]. These complications can easily occur in splenectomy due to insufficient preoperative preparation and careless surgical procedure [10,11]. The gastrosplenic and the splenorenal ligaments that form the hilar splenic pedicle can be surgically divided by several approaches, including primary and secondary splenic pedicle dissection [12], employing the LigaSure vessel sealing system, Harmonic Scalpel, EndoGIA [13], clip ligation [14], and conventional ligation [15], with the aim to reduce postoperative complications and promote smooth recovery. However, there are few studies on predictive factors of PVT, PPF, and abdominal hemorrhage after splenectomy regarding surgical techniques [16–18]. To avoid postoperative complications, the technical optimization of crucial steps for splenectomy may be a better way. Careful separation and dissection of the splenic pedicle vessels is a crucial step during splenectomy, which is closely related to the occurrence of postoperative complications [19,20]. A safe and effective splenic pedicle division strategy should effectively avoid injury to the pancreatic tail and splenic vascular intima, and reduce the occurrence of PVT and PPF [21].
Few studies have focused on the technical optimization of splenic pedicle division to reduce the surgical risk of splenectomy and avoid serious postoperative complications [22]. To solve these issues during the perioperative period, our team developed a series of technical optimizations and an innovative approach for splenectomy and proposed a modified method of surgical division of the splenic pedicle (MSDSP).
In the process of long-term clinical practice, we have gradually optimized and standardized a series of technical optimization and innovations (TOI) for splenectomy to ensure safety of the operation and smooth postoperative recovery. As the crucial step of splenectomy, MSDSP dissects the secondary splenic pedicle vessels, and even the tertiary splenic pedicle vessels, if possible, which are close to the proximal end of the spleen and away from the pancreatic tail, so the new method can effectively prevent injury to the pancreatic tail and reduce the occurrence of PPF when compared with the conventional method of surgical division of the splenic pedicle (CSDSP). Since the MSDSP strategy is performed away from the primary splenic pedicle vessel, it can reduce the traction of the splenic vein and the damage to vascular endothelium, so as to reduce the risk of PVT.
Therefore, this 12-year retrospective study from a single center aimed to evaluate postoperative outcomes following use of a MSDSP in 719 patients who underwent splenectomy for portal hypertension.
Material and Methods
Patient Selection and Study Design
PATIENT SELECTION AND STUDY DESIGN:
From January 2010 to December 2021, 719 consecutive patients with cirrhosis and PH underwent splenectomy at the First Hospital Affiliated to Anhui University of Traditional Chinese Medicine (Figure 1).
All included patients met the following inclusion criteria: (1) diagnosis of cirrhosis and PH with any etiology; (2) secondary splenomegaly and hypersplenism related to cirrhosis. Hypersplenism was defined as a leukocyte count <3500/μl and a platelet count <7.5×104/μl [23]; and (3) patients who underwent splenectomy.
The exclusion criteria were: (1) liver cirrhosis without hypersplenism and splenomegaly; (2) patients with severe coagulation dysfunction; (3) liver or any other malignancy; (4) refused to participate in the study; and (5) early postoperative transfer. All surgical procedures were conducted as non-emergent operations.
The clinical characteristics of included patients, including age, sex, etiology of cirrhosis, Child-Pugh classification, spleen size, and diameter of the portal vein system, were carefully evaluated. The preoperative blood test results, including white blood cell (WBC), red blood cell (RBC), hemoglobin (HGB), platelet (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), total bilirubin (TBIL), prothrombin time (PT), PT-INR, and D-dimer, were also collected in detail. Data on intraoperative outcomes (intraoperative blood loss and duration of surgery) and postoperative complications (PVT, PPF, and abdominal hemorrhage) were collected and analyzed in detail. The follow-up data, including recurrent esophagogastric variceal bleeding (REGVB) and overall survival (OS), were also reviewed retrospectively. The clinical characteristics of included patients, preoperative blood examinations, intraoperative outcomes, postoperative complications, and follow-up data were collected and analyzed carefully from a standardized database. Color Doppler ultrasound (ACUSON S2000, Siemens, Shanghai, China) detection was performed for preoperative evaluation and postoperative monitoring by an experienced examiner with a broadband convex array probe (3 to 5 MHz). The color Doppler ultrasound detection was performed to measure the diameter of the portal and splenic vein, the spleen size, and the occurrence of PVT. All of the operations were performed by 6 surgeons in a surgical team.
According to different methods of surgical division of the splenic pedicle, patients who underwent splenectomy with CSDSP were assigned to the Control Group (n=349), and those who underwent splenectomy with MSDSP were assigned to the Study Group (n=370).
CSDSP:
After the ligaments around the spleen were separated and dissected, the splenic pedicle was completely explored. The primary splenic pedicle was dissected, ligated, and sutured after dissociation.
MSDSP: After the bifurcation of the splenic pedicle was determined clinically, the serosa tissue in front of the secondary splenic pedicle space was separated and dissected, then the secondary splenic pedicle or even the tertiary splenic pedicle space could be clearly displayed. The dissection should be performed as close as possible to the splenic parenchyma to keep away from the pancreatic tail and stomach. Every secondary splenic pedicle vessel or even tertiary splenic pedicle vessel was dissected and ligated in direct vision. During the operation, vascular forceps were placed in the upper splenic pedicle and lower splenic pedicle through the secondary splenic pedicle space or the tertiary splenic pedicle space, if possible, and then the splenic tissue was removed, ligated, and sutured (Figure 2).
All included patients underwent splenectomy. Surgery was performed in the supine position, and a paramedian incision was made on the left upper abdomen. The separation and ligation of the splenic artery was the first main procedure. Then, the splenic pedicle was treated with CSDSP or MSDSP. A drainage tube was routinely placed in the splenic fossa.
THREE PERIODS OF SURGICAL OPTIMIZATION AND INNOVATION:
According to the development periods of surgical technology and innovation, the included patients were divided into 3 groups: the early-period group (n=288, from January 2010 to December 2013), the intermediate-period group (n=241, from January 2014 to December 2017), and the late-period group (n=190, from January 2018 to December 2011) (Figure 3).
Through the accumulation of long-term clinical experience, our team has gradually carried out a series of TOI in splenectomy to reduce postoperative complications and promote the smooth rehabilitation of patients to a greater extent. The perioperative management was gradually optimized and standardized over time, as follows: (1) The optimized and standardized management in the perioperative period contains effective preoperative preparation, careful intraoperative procedure, and accurate postoperative monitoring. (2) Effective preoperative preparation: Etiological treatment is key for the treatment of cirrhosis whenever feasible [24,25]. D-penicillamine is the first treatment in patients with cirrhosis due to Wilson’s disease, and antiviral therapy is also the key treatment for hepatitis cirrhosis. Anti-inflammation drugs, including glycyrrhizic acid preparation, polyene phosphatidyl choline, silymarin, ademetionine, and reduced glutathione, are basic liver-protecting treatments. Liver-protecting treatment and regulation of blood coagulation and nutrition were routinely performed in patients ranked as Child C, and human albumin and Vit-K1 were common therapeutic schedules. Fresh plasma and prothrombin complex were used for patients with severe coagulation dysfunction. Thrombopoietin (TPO) receptor agonists were usually given to patients when preoperative platelets were <30 g/L [26]. Preoperative splenic artery embolism was an effective preoperative preparation method for patients with huge splenomegaly or severe coagulation dysfunction. (3) Careful intraoperative procedure: Splenic artery ligation is necessary for splenectomy. The intraoperative management of the splenic pedicle was performed by MSDSP. The application of an autologous blood transfusion technique was performed when necessary [27]. (4) Accurate postoperative monitoring: Monitoring of clinical symptoms and vital signs was performed in all postoperative patients. Generally, drainage volume and amylase were monitored daily by drainage tubes in all patients after the operation to prevent postoperative abdominal hemorrhage and PPF. On admission and every 7 days thereafter, all included splenectomy patients underwent routine ultrasound for monitoring PVT. Liver function and coagulation indexes were monitored every 3 days after the operation. These methods of technical optimization and innovation for splenectomy were all used during the perioperative period.
In the early period, based on long-term clinical practice, we proposed use of MSDSP to reduce postoperative complications. Although we proposed and applied the MSDSP in clinical practice, we preferred to use the CSDSP in most cases to ensure the safety of the operation in light of the lack of surgical separation technique of splenic pedicle vessels in the early period. With the improvement of the surgical separation technique of splenic pedicle vessels, we applied the MSDSP for splenectomy in most included patients unless the splenic pedicle space was small or the splenic pedicle was bleeding in the intermediate period. Due to the maturity of the surgical separation technique of splenic pedicle vessels, we used the MSDSP for splenectomy in almost all included patients in the late period, with a lower incidence of complications.
POSTOPERATIVE COMPLICATIONS:
The data on postoperative complications after splenectomy were routinely collected and assessed during hospitalization, including PVT, postoperative abdominal hemorrhage, abdominal infection, PPF, liver failure, severe ascites, and encephalopathy. PPF was defined as follows: (1) postoperative drainage time was more than 3 days; (2) amylase in drainage fluid was higher than 3 times the upper limit of normal value; (3) biochemical leakage was not recorded [28]. Clinical details regarding the operation, including the operation time, intraoperative blood loss volume, and postoperative hospital stay, were also recorded.
FOLLOW-UP:
Routine follow-up examinations, which were performed by telephone or at the outpatient department, were conducted in all included patients who underwent splenectomy. The deadline for the follow-up examination was December 25, 2021. Overall survival (OS) and the cumulative hazard of recurrent esophagogastric variceal bleeding (REGVB) were the 2 main primary endpoints. The definition of OS was the time from surgery to death for any reason. REGVB was defined as the time from surgery to the first EGVB. Patients who were still alive at the last follow-up date were considered for censored observations.
STATISTICAL ANALYSIS:
All statistical analyses were performed using SPSS Version 24.0 software (Chicago, IL, USA). Comparisons among the 3 period groups were performed using one-way ANOVA (analysis of variance) or the Kruskal-Wallis test, as appropriate. Continuous variables with a normal distribution were reported as mean±standard deviation (χ±SD); those with a non-normal distribution were reported as median (Q1, Q3). Comparison between the 2 groups was carried out using the
PSM was conducted to adjust for the confounding between the 2 groups. Propensity scores were estimated by logistic regression model using the following data as covariates: age, sex, etiology of cirrhosis, Child-Pugh classification, spleen size, diameter of the portal vein system, variceal hemorrhage history, operation, the splenic pedicle division method, operation period, and preoperative blood examinations [29]. Propensity score matching was carried out according to the 1: 1 nearest-neighbor caliper matching method, and the caliper value was set to 0.2.
Results
COMPARISON AMONG DIFFERENT OPERATION PERIODS:
Of the 740 patients who met the inclusion criteria, 21 were excluded: 6 patients with ITP and 2 patients with hereditary spherocytosis underwent splenectomy without hypersplenism and splenomegaly, 4 developed severe coagulation dysfunction, 3 had liver malignancy, and 4 had early postoperative transfers. Finally, 719 patients who met the inclusion criteria were enrolled in the study.
Among 719 patients, 405 were men and 314 were women. There were 288 patients in the early-period group, 241 patients in the intermediate-period group, and 190 patients in the late-period group, respectively.
No significant differences were found regarding sex, etiology of cirrhosis, Child-Pugh classification, RBC, HGB, ALB, PT, PT-INR, variceal hemorrhage history, PPF, and operation time among the 3 groups (all
COMPARISON BETWEEN THE 2 GROUPS BY PSM ANALYSIS:
Comparing with the clinical data of included patients with or without MSDSP, the indicators of age, sex, etiology of cirrhosis, Child-Pugh classification, and length of the spleen revealed no significant differences between the 2 groups (all P>0.05). No significant differences were found regarding WBC, RBC, HGB, PLT, TBIL, ALT, AST, ALB, PT, PT-INR, and D-dimer between the 2 groups (all P>0.05) (Table 1). In terms of preoperative indicators, the thickness of the spleen (69.0±16.8 mm versus 72.3±16.4 mm, P=0.01), the portal vein diameter (PVD) (1.5±0.2 cm versus 1.4±0.2 cm, P<0.01), the splenic vein diameter (1.3±0.5cm versus 1.2±0.3 cm, P<0.01) and operation period (185/101/63 versus 103/140/127, P<0.01) showed significant differences between the 2 groups. Compared with the Control Group, hospital stay (13.8±3.1 days versus 13.0±2.2 days), PVT (148/349 versus 81/370), as well as PPF (40/349 versus 13/340) were significantly lower in the Study Group (all P<0.01). However, the operation time (196.2±34.6 min versus 172.4±35.4 min) in the Study Group was significantly longer when compared with the Control Group (P<0.01).
PSM was conducted at a 1: 1 ratio, resulting in 260 patients in each group. After PSM, the thickness of spleen, PVD, splenic vein diameter, and operation period in the matched Study Group were comparable to those in the matched Control Group (all P>0.05). In terms of intra- and post-operative outcomes, although postoperative abdominal hemorrhage was similar between the 2 groups (8/260 versus 5/260, P=0.40), intraoperative blood loss (200.8±103.7 mL versus 316.0±212.2 mL), hospital stay (12.8±1.9 days versus 14.0±3.4 days), PVT (55/260 versus 124/260), and PPF (10/260 versus 34/260) were significantly lower in the matched Study Group when compared with the matched Control Group (all P<0.01). However, the operation time (199.4±34.7 min versus 173.1±36.3 min, P<0.01) was significantly longer in the matched Study Group (Table 2).
FOLLOW-UP:
The follow-up rate was 89.4% (312/349) in the Control Group and 86.8% (321/370) in the Study Group. The median follow-up time was 77.5 (46/112) months in the Control Group and 67 (36/104) months in the Study Group (Figure 4). The 1-, 3-, 5-, and 12-year REGVB rates were 1.6%, 2.2%, 2.9% and 3.8% in the Control Group, versus 1.2%, 2.2%, 3.1%, and 3.7% in the Study Group, respectively. There were no significant differences in the follow-up data between the 2 groups with log-rank test (P=0.66). The 1-, 3-, 5-, and 12-year OS rates were 99.7%, 99%, 98.7%, and 98.7% in the Control Group, versus 100%, 99.7%, 99.4%, and 99.1% in the Study Group, and the results showed no significant differences with log-rank test (P=0.63) (Table 3).
Four patients died of liver failure during follow-up in the Control Group. Two patients died of liver failure and 1 patient developed refractory ascites and died of a spontaneous ascitic fluid infection in the Study Group. After endoscopy treatment and conservative drug treatment, the 24 patients with REGVB all recovered smoothly.
Discussion
In the past 12 years, we have carried out a series of TOI in splenectomy and proposed the use of MSDSP to reduce postoperative complications and promote the smooth rehabilitation of patients to a greater extent. In our present study, patients who underwent splenectomy with MSDSP showed a significant reduction in intraoperative blood loss, PVT, PPF, and hospital stay compared to those without MSDSP(
At present, there are few published studies on technical innovation of the splenic pedicle division method to reduce the surgical risk of splenectomy and avoid postoperative complications. Previous studies regarding secondary splenic pedicle division mostly focused on the normal size spleen, such as ITP [30], or surgical instruments [12–14], such as Endo GIA and LigaSure. Although previous studies also concluded that the secondary splenic pedicle dissection could decrease the incidence of pancreatic fistula, these studies mainly focused on complications after the splenic pedicle division with different surgical instruments. The previous literature paid little attention to the impact of surgical separation and division technology on postoperative complications and the follow-up data, and our study focused on the effect of splenic pedicle division technology on postoperative complications and the follow-up data. Therefore, we performed this comprehensive study to evaluate the safety and effectiveness of our MSDSP in splenectomy, especially for patients with splenomegaly, and PSM was conducted to adjust for differences in spleen size, PVD, splenic vein diameter, and operation period between the 2 groups.
Spleen size is a major factor in selecting the splenic pedicle division method [31–34]. In the light of our experience, selecting a splenic pedicle division strategy mainly depends on the size of the spleen and the pedicle space in the early period. In our study, spleen thickness in the Study Group revealed significant differences, which indicated that spleen size may be one of the factors to consider when performing the splenic pedicle division method. However, with the progress of surgical technique and the accumulation of surgical experience, spleen size is no longer a limiting factor for surgical procedures [35,36]. The size of the secondary splenic pedicle space and the degree of adhesion around the spleen were also influencing factors. Preoperative splenic artery embolization could be a useful method of autologous blood transfusion and diminish the splenic volume preoperatively. The preoperative splenic artery embolization was reported to be an effective preoperative preparation method for resection of huge splenomegaly with shorter duration of surgery and decreased intraoperative blood loss [37,38].
MSDSP helped to reduce the incidence of PPF. In terms of our MSDSP, the separation and dissection of the splenic pedicle, which was performed as close as possible to the splenic parenchyma, was more elaborate, complicated, and difficult than the CSDSP. The splenic pedicle consists of the splenic artery, vein, lymph, and nerve tissues, and the gap between the upper and lower splenic lobes vessel is the secondary splenic pedicle space. After the space is detected during the operation, the operation is carried out to avoid the pancreatic tail, thus reducing pancreatic tail injury and the incidence of postoperative complications [39]. To avoid the risk of pancreatic injury in splenectomy, the following points of the MSDSP need to be seriously considered: (1) ensuring excellent and direct visualization of the tail of the pancreas; (2) accurate dissection and ligation of the splenic pedicle; and (3) separation plane as close as possible to the splenic parenchyma. The MSDSP fit the above points exactly, thus revealing a lower incidence of PPF. In this study, the incidence of PPF was 3.5% (10/287) in the matched Study Group versus 11.9% (34/287) in the matched Control Group. The difference was statistically significant (
Because of PH and coagulation disorder in cirrhotic patients, postoperative hemorrhage is more prone to occur and is hard to stop [40]. To ensure the safety of splenectomy, an optimal splenic vessel division method was the crucial step, thus we used the MSDSP to deal with the splenic pedicle, especially for the ligaments and small vessels around the spleen. Using the MSDSP strategy, every secondary or even tertiary branch of splenic pedicle vessels was dissected and ligated in direct vision; therefore, intraoperative abdominal hemorrhage was adequately managed. In this study, the mean blood loss was (200.8±103.7) ml in the matched Study Group versus (316.0±212.2) ml in the matched Control Group. The difference was statistically significant (
PVT is a critical complication in cirrhotic patients after splenectomy, which can give rise to liver dysfunction and liver failure [41]. Although many causes can lead to PVT, including large splenic vein diameter, wider diameters of the portal vein, and hemorheological abnormalities, different methods of splenic pedicle division are also one of the causes of PVT [42–44]. The splenic pedicle vessels in the Study Group, which were dissected as close as possible to the splenic parenchyma, minimized the vascular intimal injury of the main splenic vein to the greatest extent, thus reducing the occurrence of PVT. In our study, the incidence of PVT was 21.2% (55/260) in the matched Study Group versus 47.7% (124/260) in the matched Control Group, and the difference was statistically significant (
Postoperative complications have a vital influence on the smooth recovery of patients. Therefore, knowing how to ensure the safety of the operation and avoid complications is crucial for a surgical team. In our experience, good cooperation and surgical experience of a surgical team are particularly important for a smooth and successful operation [47].
In the past 12 years, through accumulated long-term clinical experience, our team has carried out a series of TOI in splenectomy to reduce postoperative complications and promote the smooth rehabilitation of patients to a greater extent. With the continuous progress of our surgical team in use of surgical technology, the rate of utilization of the MSDSP method (66.8%, 127/190) was higher in the late period. Intraoperative blood loss, postoperative hemorrhage, and hospital stay in the late period showed significant differences when compared to those in the intermediate period (
Postoperative EGVB and mortality are 2 main important indicators in the follow-up data [49]. In our study, the 2 methods of splenic pedicle division revealed similar results regarding the incidence of postoperative EGVB. Endoscopic treatment and drug conservative treatment are 2 main treatments for postoperative EGVB [50–52]. In the present study, the included patients who underwent splenectomy had a satisfactory OS rate, and the 2 methods of splenic pedicle division had similar results, which suggested the splenic pedicle division method does not affect OS.
There are some limitations to this study. This retrospective study was a single-institution investigation, and a few discharged patients were lost to follow-up. Clinical data, which were retrospectively collected, contained a few confounding variables that may have affected the results. To further evaluate the efficacy of the MSDSP, a prospective, randomized, multicenter trial is needed.
Conclusions
In conclusion, our MSDSP helps to reduce postoperative complications including PVT and PPF, decrease intraoperative blood loss and operation time, promote smooth recovery, and shorten hospital stay.
Figures
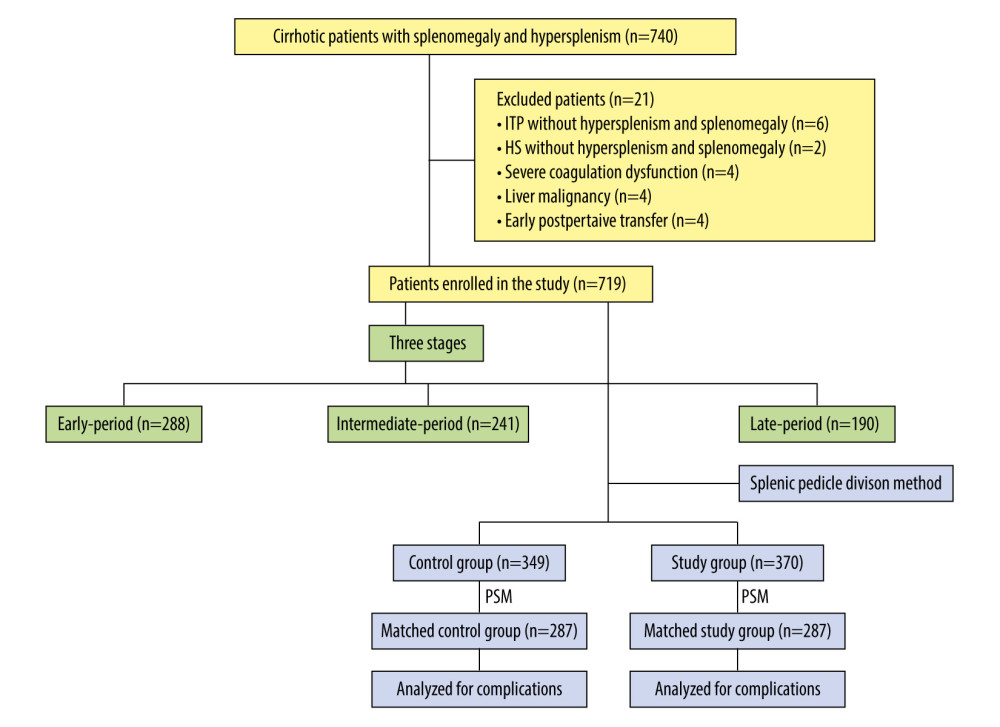 Figure 1. Classification and flow diagram of the study population. ITP – idiopathic thrombocytopenic purpura; HS – hereditary spherocytosis; PSM – propensity score matching.
Figure 1. Classification and flow diagram of the study population. ITP – idiopathic thrombocytopenic purpura; HS – hereditary spherocytosis; PSM – propensity score matching. 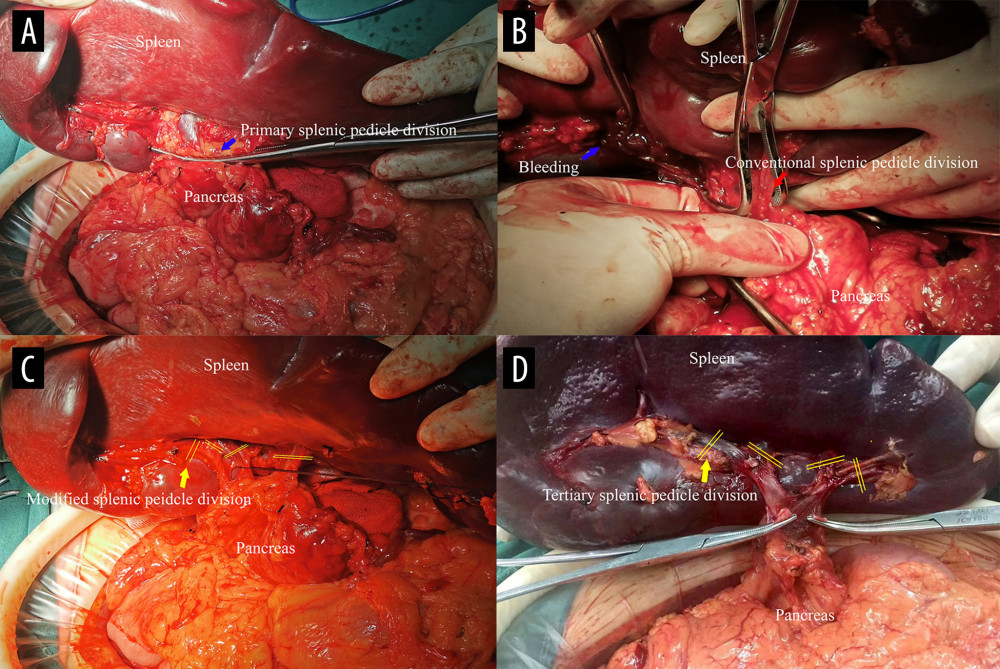 Figure 2. Comparison of the 2 splenic pedicle division methods. (A) Conventional splenic pedicle division method; (B) Emergency treatment of splenic pedicle hemorrhage by conventional splenic pedicle division method; (C) Modified splenic pedicle division method; (D) Tertiary splenic pedicle vessels division.
Figure 2. Comparison of the 2 splenic pedicle division methods. (A) Conventional splenic pedicle division method; (B) Emergency treatment of splenic pedicle hemorrhage by conventional splenic pedicle division method; (C) Modified splenic pedicle division method; (D) Tertiary splenic pedicle vessels division. 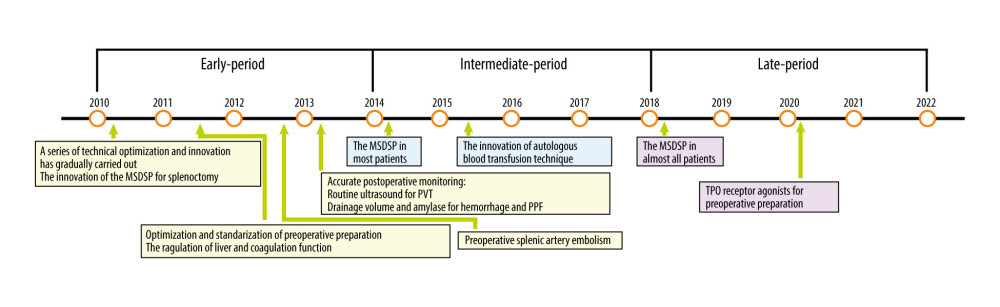 Figure 3. The development of technical optimization and innovation for splenectomy. MSDSP – modified method of surgical division of the splenic pedicle; CSDSP – conventional method of surgical division of the splenic pedicle; PVT – portal vein thrombosis; PPF – postoperative pancreatic fistula; TPO – thrombopoietin.
Figure 3. The development of technical optimization and innovation for splenectomy. MSDSP – modified method of surgical division of the splenic pedicle; CSDSP – conventional method of surgical division of the splenic pedicle; PVT – portal vein thrombosis; PPF – postoperative pancreatic fistula; TPO – thrombopoietin. 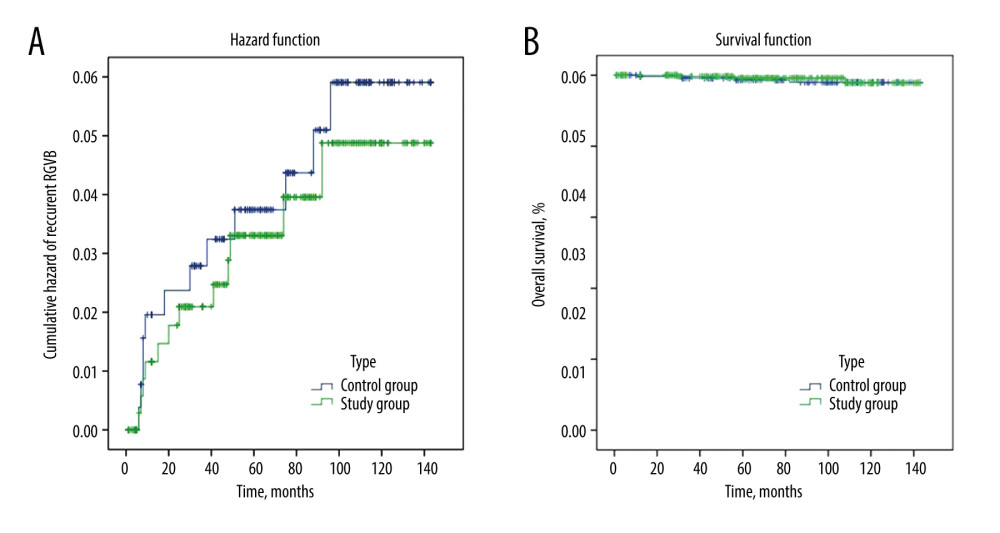 Figure 4. The follow-up data comparing the 2 groups. (A) Cumulative hazard of recurrent EGVB; (B) Overall survival. EGVB – esophagogastric variceal bleeding.
Figure 4. The follow-up data comparing the 2 groups. (A) Cumulative hazard of recurrent EGVB; (B) Overall survival. EGVB – esophagogastric variceal bleeding. References
1. Peck-Radosavljevic M, Thrombocytopenia in chronic liver disease: Liver Int, 2017; 37; 778-93
2. Chikamori F, Inoue A, Okamoto H, Relationships between types of esophagogastric varices and systemic hemodynamics in patients with liver cirrhosis: Hepatogastroenterology, 2011; 58; 909-15
3. Yongxiang W, Zongfang L, Guowei L, Effects of splenomegaly and splenic macrophage activity in hypersplenism due to cirrhosis: Am J Med, 2002; 113; 428-31
4. Orlando R, Lirussi F, Basso SM, Lumachi F, Splenomegaly as risk factor of liver cirrhosis. A retrospective cohort study of 2,525 patients who underwent laparoscopy: In Vivo, 2011; 25; 1009-12
5. Ushitora Y, Tashiro H, Takahashi S, Splenectomy in chronic hepatic disorders: portal vein thrombosis and improvement of liver function: Dig Surg, 2011; 28; 9-14
6. Habermalz B, Sauerland S, Decker G, Laparoscopic splenectomy: The clinical practice guidelines of the European Association for Endoscopic Surgery (EAES): Surg Endosc, 2008; 22; 821-48
7. Xu XY, Ding HG, Li WG, Chinese guidelines on the management of liver cirrhosis (abbreviated version): World J Gastroenterol, 2020; 26; 7088-103
8. Nomura Y, Kage M, Ogata T, Influence of splenectomy in patients with liver cirrhosis and hypersplenism: Hepatol Res, 2014; 44; E100-9
9. Du Z, Dong J, Zhang J, Incidence and risk factors associated with a high comprehensive complication index score after splenectomy in cirrhotic patients with hypersplenism: J Surg Res, 2018; 222; 69-74
10. Denninger MH, Chaït Y, Casadevall N, Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors: Hepatology, 2000; 31; 587-91
11. Tsutsumi N, Tomikawa M, Akahoshi T, Pancreatic fistula after laparoscopic splenectomy in patients with hypersplenism due to liver cirrhosis: Effect of fibrin glue and polyglycolic acid felt on prophylaxis of postoperative complications: Am J Surg, 2016; 212; 882-88
12. Yan Q, Zhu J, Zhan X, Primary versus secondary splenic pedicle dissection in laparoscopic splenectomy for splenic diseases: J Am Coll Surg, 2013; 216; 266-71
13. Romano F, Gelmini R, Caprotti R, Laparoscopic splenectomy: Ligasure versus EndoGIA: a comparative study: J Laparoendosc Adv Surg Tech A, 2007; 17; 763-67
14. Shabahang H, Maddah G, Tavassoli A, Laparoscopic splenectomy: Ligasure or clip ligation?: Surg Laparosc Endosc Percutan Tech, 2012; 22; 136-38
15. Amirkazem VS, Malihe K, Randomized clinical trial of ligasure™ versus conventional splenectomy for injured spleen in blunt abdominal trauma: Int J Surg, 2017; 38; 48-51
16. Zhang X, Wang Y, Yu M, Effective prevention for portal venous system thrombosis after splenectomy: A meta-analysis: J Laparoendosc Adv Surg Tech A, 2017; 27; 247-52
17. Aziret M, Koyun B, Karaman K, Intraoperative hemorrhage and increased spleen volume are risk factors for conversion to open surgery in patients undergoing elective robotic and laparoscopic splenectomy: Turk J Surg, 2020; 36; 72-81
18. Mehdorn AS, Schwieters AK, Mardin WA, Pancreatic fistula and biochemical leak after splenectomy: Incidence and risk factors-a retrospective single-center analysis: Langenbecks Arch Surg, 2022 [Online ahead of print]
19. Vecchio R, Marchese S, Swehli E, Intagliata E, Splenic hilum management during laparoscopic splenectomy: J Laparoendosc Adv Surg Tech A, 2011; 21; 717-20
20. Feldman LS, Laparoscopic splenectomy: Standardized approach: World J Surg, 2011; 35; 1487-95
21. Liu YB, Kong Y, Wang XA, Role of dissection of secondary branches of splenic pedicle in portal hypertension cases undergoing splenectomy: Chin Med J (Engl), 2008; 121; 2250-53
22. Wysocki M, Radkowiak D, Zychowicz A, Prediction of technical difficulties in laparoscopic splenectomy and analysis of risk factors for postoperative complications in 468 cases: J Clin Med, 2018; 7; 547
23. Ikegami T, Soejima Y, Taketomi A, Hypersplenism after living donor liver transplantation: Hepatogastroenterology, 2009; 56; 778-82
24. Guidelines of prevention and treatment for alcoholic liver disease: A 2018 update: Zhonghua Gan Zang Bing Za Zhi, 2018; 26; 188-94 [in Chinese]
25. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update: Zhonghua Gan Zang Bing Za Zhi, 2018; 26; 195-203 [in Chinese]
26. Tomiyama Y, Guest editorial: Pathophysiology and management of thrombocytopenia: Possible clinical application of TPO receptor agonists: Int J Hematol, 2013; 98; 8-9
27. Jiang GQ, Bai DS, Chen P, Modified laparoscopic splenectomy and azygoportal disconnection combined with cell salvage is feasible and might reduce the need for blood transfusion: World J Gastroenterol, 2014; 20; 18420-26
28. Bassi C, Marchegiani G, Dervenis C, The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After: Surgery, 2017; 161; 584-91
29. Austin PC, Some methods of propensity-score matching had superior performance to others: Results of an empirical investigation and Monte Carlo simulations: Biom J, 2009; 51; 171-84
30. Cai XJ, Shen B, Yu H, Laparoscopic splenectomy by secondary pedicle division strategy: A highly cost-effective method: Chin Med J (Engl), 2008; 121; 105-7
31. Wang D, Chen X, Lv L, Laparoscopic splenectomy and devascularization for massive splenomegaly in portal hypertensive patients: A retrospective study of a single surgical team’s experience with 6-year follow-up data: Ann Transl Med, 2022; 10; 207
32. Koshenkov VP, Németh ZH, Carter MS, Laparoscopic splenectomy: Outcome and efficacy for massive and supramassive spleens: Am J Surg, 2012; 203; 517-22
33. Zhou J, Wu Z, Cai Y, The feasibility and safety of laparoscopic splenectomy for massive splenomegaly: A comparative study: J Surg Res, 2011; 171; e55-60
34. Smith L, Luna G, Merg AR, Laparoscopic splenectomy for treatment of splenomegaly: Am J Surg, 2004; 187; 618-20
35. Tsamalaidze L, Stauffer JA, Permenter SL, Asbun HJ, Laparoscopic Splenectomy for massive splenomegaly: Does size matter?: J Laparoendosc Adv Surg Tech A, 2017; 27; 1009-14
36. Casaccia M, Sormani MP, Palombo D, Laparoscopic splenectomy versus open splenectomy in massive and giant spleens: Should we update the 2008 EAES guidelines?: Surg Laparosc Endosc Percutan Tech, 2019; 29; 178-81
37. Wu Z, Zhou J, Pankaj P, Peng B, Comparative treatment and literature review for laparoscopic splenectomy alone versus preoperative splenic artery embolization splenectomy: Surg Endosc, 2012; 26; 2758-66
38. Reso A, Brar MS, Church N, Outcome of laparoscopic splenectomy with preoperative splenic artery embolization for massive splenomegaly: Surg Endosc, 2010; 24; 2008-12
39. Chand B, Walsh RM, Ponsky J, Brody F, Pancreatic complications following laparoscopic splenectomy: Surg Endosc, 2001; 15; 1273-76
40. Kercher KW, Novitsky YW, Czerniach DR, Litwin DE, Staple line bleeding following laparoscopic splenectomy: Intraoperative prevention and postoperative management with splenic artery embolization: Surg Laparosc Endosc Percutan Tech, 2003; 13; 353-56
41. Jiang GQ, Bai DS, Chen P, Predictors of portal vein system thrombosis after laparoscopic splenectomy and azygoportal disconnection: A retrospective cohort study of 75 consecutive patients with 3-months follow-up: Int J Surg, 2016; 30; 143-49
42. Kinjo N, Kawanaka H, Akahoshi T, Risk factors for portal venous thrombosis after splenectomy in patients with cirrhosis and portal hypertension: Br J Surg, 2010; 97; 910-16
43. Ruiz-Tovar J, Priego P, Portal vein thrombosis after splenic and pancreatic surgery: Adv Exp Med Biol, 2017; 906; 241-51
44. Kuroki T, Kitasato A, Tokunaga T, Predictors of portal and splenic vein thrombosis after laparoscopic splenectomy: A retrospective analysis of a single-center experience: Surg Today, 2018; 48; 804-9
45. Kwon J, Koh Y, Yu SJ, Yoon JH, Low-molecular-weight heparin treatment for portal vein thrombosis in liver cirrhosis: Efficacy and the risk of hemorrhagic complications: Thromb Res, 2018; 163; 71-76
46. Tripodi A, Primignani M, Braham S, Coagulation parameters in patients with cirrhosis and portal vein thrombosis treated sequentially with low molecular weight heparin and vitamin K antagonists: Dig Liver Dis, 2016; 48; 1208-13
47. Wu Z, Zhou J, Cai YQ, The learning curve for laparoscopic splenectomy for massive splenomegaly: A single surgeon’s experience: Chin Med J (Engl), 2013; 126; 2103-8
48. Bai DS, Qian JJ, Chen P, Modified laparoscopic and open splenectomy and azygoportal disconnection for portal hypertension: Surg Endosc, 2014; 28; 257-64
49. Selzner M, Tuttle-Newhall JE, Dahm F, Current indication of a modified Sugiura procedure in the management of variceal bleeding: J Am Coll Surg, 2001; 193; 166-73
50. Rakotomalala JA, Razafindrazoto CI, Randriamifidy NH, Splenectomy combined with endoscopic variceal ligation (EVL) versus EVL alone for secondary prophylaxis of variceal bleeding in hepatosplenic schistosomiasis: A retrospective case-control study: Hepat Med, 2022; 14; 79-85
51. Al-Khazraji A, Curry MP, The current knowledge about the therapeutic use of endoscopic sclerotherapy and endoscopic tissue adhesives in variceal bleeding: Expert Rev Gastroenterol Hepatol, 2019; 13; 893-97
52. Ba DS, Zhang C, Jin SJ, Laparoscopic splenectomy and azygoportal disconnection combining with pre- and postoperative endoscopic intervention – a sandwich-style sequential therapy for portal hypertensive bleeding: A retrospective cohort study: Turk J Gastroenterol, 2018; 29; 669-75
Figures
 Figure 1. Classification and flow diagram of the study population. ITP – idiopathic thrombocytopenic purpura; HS – hereditary spherocytosis; PSM – propensity score matching.
Figure 1. Classification and flow diagram of the study population. ITP – idiopathic thrombocytopenic purpura; HS – hereditary spherocytosis; PSM – propensity score matching. Figure 2. Comparison of the 2 splenic pedicle division methods. (A) Conventional splenic pedicle division method; (B) Emergency treatment of splenic pedicle hemorrhage by conventional splenic pedicle division method; (C) Modified splenic pedicle division method; (D) Tertiary splenic pedicle vessels division.
Figure 2. Comparison of the 2 splenic pedicle division methods. (A) Conventional splenic pedicle division method; (B) Emergency treatment of splenic pedicle hemorrhage by conventional splenic pedicle division method; (C) Modified splenic pedicle division method; (D) Tertiary splenic pedicle vessels division. Figure 3. The development of technical optimization and innovation for splenectomy. MSDSP – modified method of surgical division of the splenic pedicle; CSDSP – conventional method of surgical division of the splenic pedicle; PVT – portal vein thrombosis; PPF – postoperative pancreatic fistula; TPO – thrombopoietin.
Figure 3. The development of technical optimization and innovation for splenectomy. MSDSP – modified method of surgical division of the splenic pedicle; CSDSP – conventional method of surgical division of the splenic pedicle; PVT – portal vein thrombosis; PPF – postoperative pancreatic fistula; TPO – thrombopoietin. Figure 4. The follow-up data comparing the 2 groups. (A) Cumulative hazard of recurrent EGVB; (B) Overall survival. EGVB – esophagogastric variceal bleeding.
Figure 4. The follow-up data comparing the 2 groups. (A) Cumulative hazard of recurrent EGVB; (B) Overall survival. EGVB – esophagogastric variceal bleeding. Tables
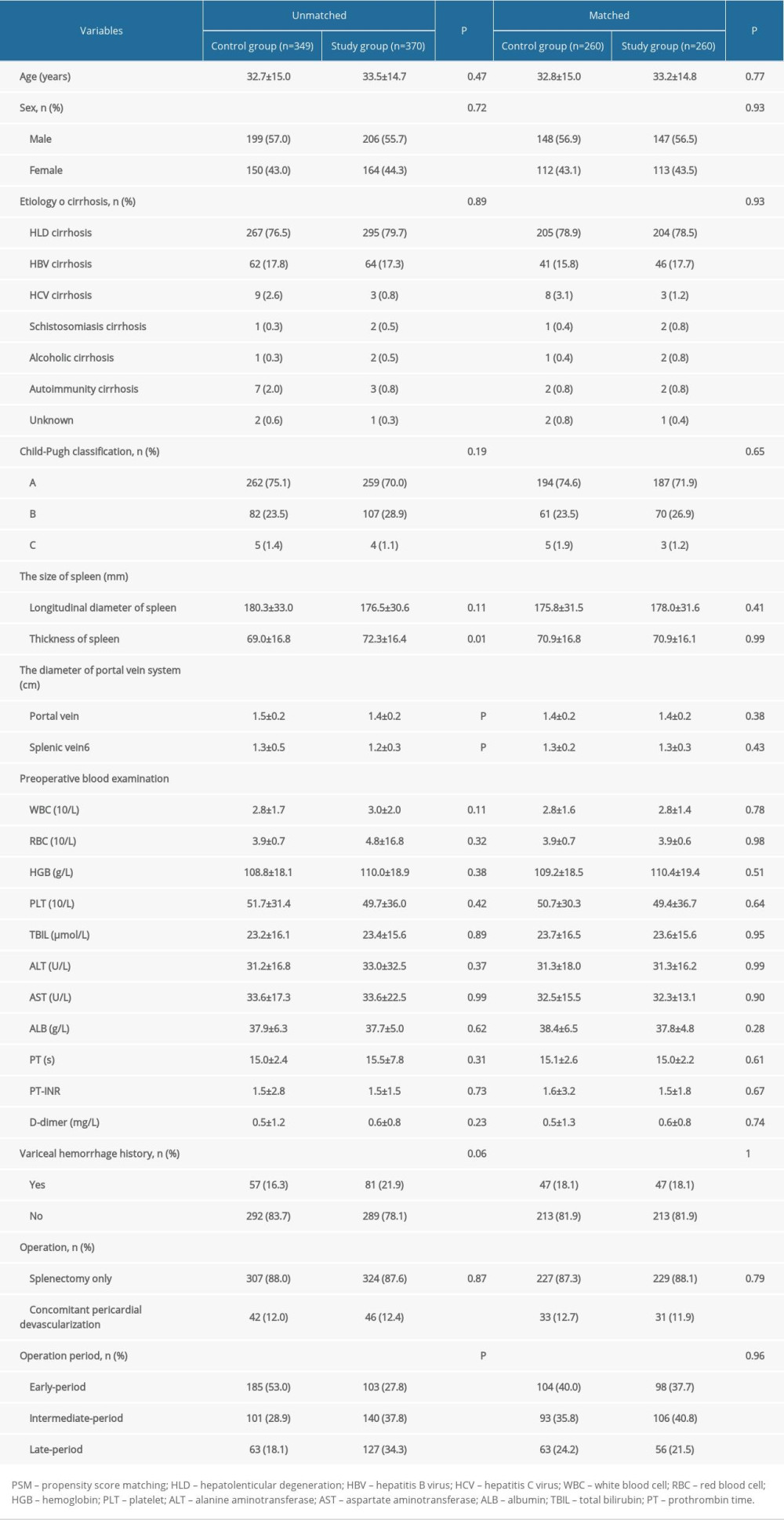 Table 1. Comparison of the backgrounds of included patients between the 2 groups by PSM analysis.
Table 1. Comparison of the backgrounds of included patients between the 2 groups by PSM analysis.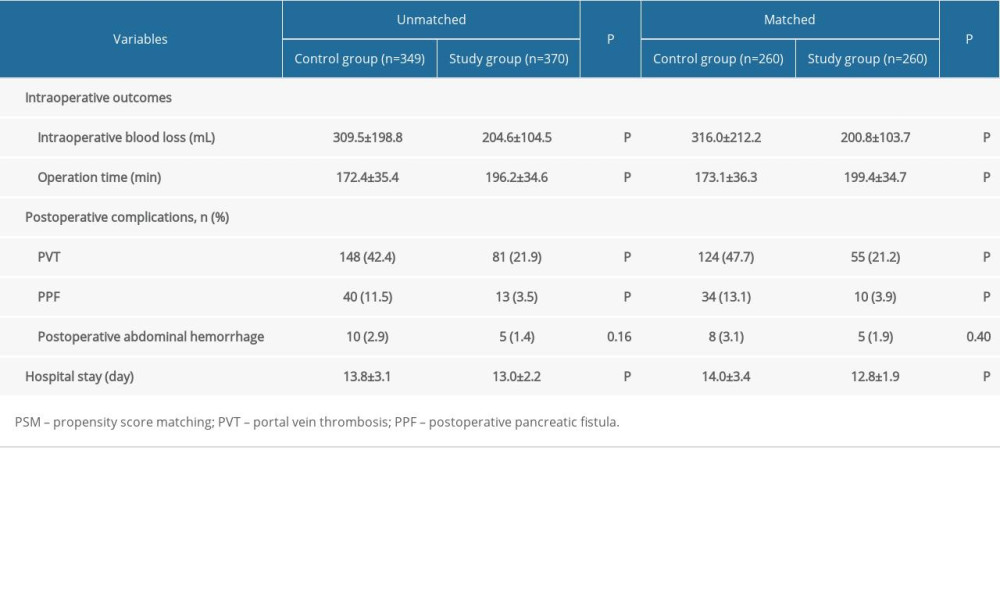 Table 2. Comparison of the outcomes of included patients between the 2 groups by PSM analysis.
Table 2. Comparison of the outcomes of included patients between the 2 groups by PSM analysis.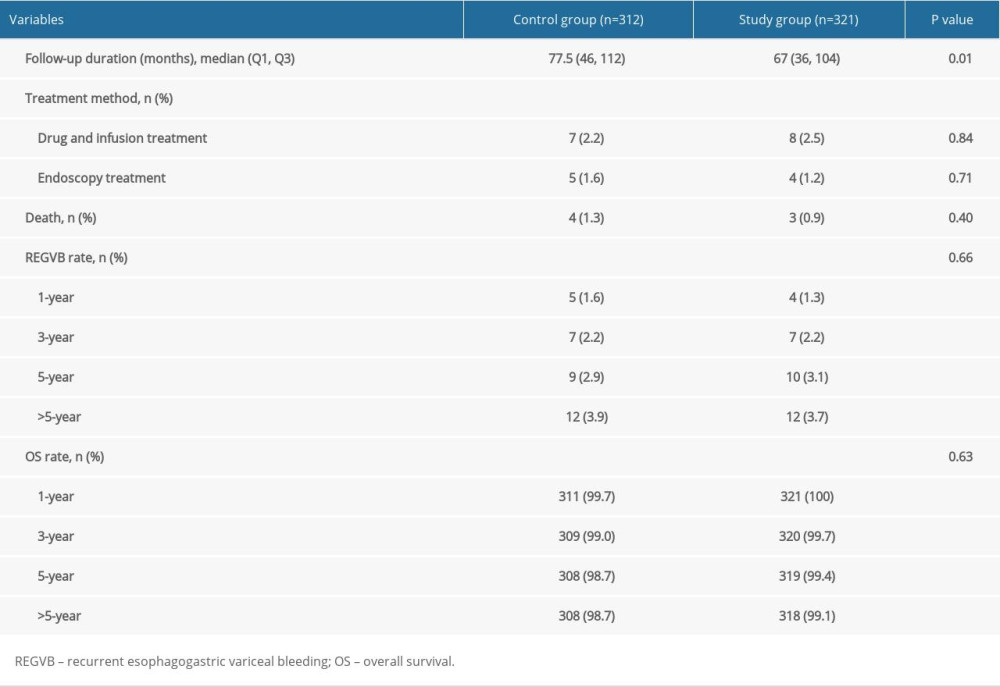 Table 3. Follow-up data of the 2 groups.
Table 3. Follow-up data of the 2 groups. Table 1. Comparison of the backgrounds of included patients between the 2 groups by PSM analysis.
Table 1. Comparison of the backgrounds of included patients between the 2 groups by PSM analysis. Table 2. Comparison of the outcomes of included patients between the 2 groups by PSM analysis.
Table 2. Comparison of the outcomes of included patients between the 2 groups by PSM analysis. Table 3. Follow-up data of the 2 groups.
Table 3. Follow-up data of the 2 groups. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








