07 September 2022: Clinical Research
Measurement of Serum Levels of 5 Amino Acids and Dimethylamine Using Liquid Chromatography-Tandem Mass Spectrometry in Patients without Septic Associated Acute Kidney Injury and with Septic Associated Acute Kidney Injury Requiring Continuous Renal Replacement Therapy
Patrycja Leśnik1ABCDEFG*, Lidia Łysenko2ADEG, Mariusz G. FleszarDOI: 10.12659/MSM.937784
Med Sci Monit 2022; 28:e937784
Abstract
BACKGROUND: Acute kidney injury (AKI) is one of the most common organ failures. An early diagnosis of AKI using specific biomarkers is essential for effective treatment. This study determined the serum concentrations of selected amino acids and amines using targeted liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) in patients with AKI during sepsis and septic shock treated in the Intensive Care Unit (ICU).
MATERIAL AND METHODS: A sample of 41 patients was divided into 2 groups: (1) patients with sepsis and septic shock along required continuous renal replacement therapy (CRRT) due to AKI (n=13), and (2) patients with sepsis and septic shock but without AKI (n=28). LC-MS/MS was used to measure a serum concentration of 6 amino acids and amines: arginine, ornithine, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), dimethylamine (DMA), and citrulline.
RESULTS: There was a statistically significantly higher median DMA level in AKI patients compared to those without AKI (8.1 vs 5.2 umol/L; P=0.022). The results for the remaining molecules showed no significant differences (P>0.05). Patients with DMA ≥14.95 umol/L (n=5; 100%) and treated with CRRT presented DMA level below the cut-off point (n=7; 20%). Subjects with creatinine levels ≥1.19 mg/dL (n=11; 28%) and treated with CRRT presented creatinine levels below the cut-off point (n=1; 3%).
CONCLUSIONS: In patients with sepsis, increased serum levels of DMA were significantly associated with AKI requiring CRRT. It remains unclear whether increased DMA concentrations are secondary to sepsis-induced AKI or are a cause.
Keywords: Acute Kidney Injury, Dimethylamine, Intensive Care Units, renal insufficiency, Sepsis, Amines, Amino Acids, Chromatography, Liquid, Continuous Renal Replacement Therapy, Creatinine, Dimethylamines, Humans, Renal Replacement Therapy, Shock, Septic, tandem mass spectrometry
Background
Acute kidney injury (AKI) is one of the most common organ failures in patients hospitalized in intensive care units (ICUs) [1]. The incidence varies from 5% to 50% depending on the literature and is associated with a mortality rate of up to 50% [2–4]. Additionally, 20–50% of patients who survive later develop chronic kidney disease (CKD), and about 5% develop end-stage renal disease [5–9]. Renal tubular ischemia can be caused by sepsis, cardiogenic shock, low cardiac output, drug toxicity, surgery, and hypovolemia [10].
An early diagnosis of AKI is essential for effective treatment. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, the 2 basic diagnostic criteria in the diagnosis of AKI are the dynamics of the increase in serum creatinine concentration and urine output [11,12]. CRRT is the method of choice for AKI treatment in patients treated in the ICU. Due to the high hemodynamic instability of patients, it has an advantage over IHD. The main indications for starting CRRT are life-threating electrolyte, fluid, and acid-based changes [13].
In kidney injuries, when the glomerular filtration rate (GFR) decreases, the half-life of creatinine increases from 4 to 24–72 h, meaning that change in creatinine level is delayed by up to 24–36 h [14,15]. New biomarkers, such as neutrophil-gelatinase associated lipocalin (NGAL), cystatin C (CC), kidney injury molecule-1 (KIM-1), interleukin-18 (IL18), liver-type fatty acid-binding protein (L-FABP), N-acetyl-β-D-glucosaminidase (NAG), α-glutathione transferase (alpha-GST), metalloproteinase tissue inhibitor type 2 (TIMP-2), and insulin-like growth factor binding protein 7 (IGFBP-7), might be more beneficial for the early detection of acute kidney injuries [16–18].
In the diagnosis of AKI in the course of sepsis and septic shock, metabolic and cytokine factors associated with mortality and organ dysfunction, such as IL-6, IL-10, IL8, IL9, TNFβ, and DMA, may also be useful [19]. A clinically relevant biomarker should have high sensitivity and specificity, and the ability to predict clinical outcomes through the use of a rapid, low-cost measurement technique [20].
Dimethylamine (DMA) is an amine circulating in human blood that is excreted in the urine. The occurrence of DMA in human urine was described in 1935 and 1938 [21,22]. A major precursor for endogenous DMA is asymmetric dimethylarginine (ADMA), an endogenous amino acid and inhibitor of nitric oxide (NO) synthesis. ADMA is hydrolyzed to DMA and l-citrulline by dimethylarginine dimethylaminohydrolase (DDAH). The concentration of circulating DMA in healthy young women (n=18) was determined to be 1.43±0.23 μM in serum, 1.73±0.17 μM in lithium heparin plasma, and 9.84±1.43 μM in EDTA plasma. Serum is recommended as the most appropriate matrix for measuring the DMA level in human blood [23].
In adults, about 90% of daily produced ADMA is estimated to be hydrolyzed to DMA, with the remaining 10% being excreted unchanged in the urine [24]. DMA circulates in the blood in the lower μM range and is excreted in the urine in the upper μM range. The diurnal variation of DMA excretion is fairly constant, and drugs such as the diuretic acetazolamide do not affect its urinary excretion rate in healthy humans [25].
The mass spectrometry (MS) technique is based on measurement of the charge-to-mass ratio (m/z) of ions of the tested substances present in the gas phase. Mass spectrometry is a highly specific and sensitive technique; coupled with an appropriate separation technique (liquid chromatography, gas chromatography, capillary electrophoresis), it allows for characterization of many groups of compounds and their quantitative analysis at very low levels, even in such complex biological matrices as serum, plasma, urine, tissues, and cell lines. MS technique is considered the criterion standard in qualitative and quantitative analysis. Due to its potential, it is often used in metabolomics studies, both targeted and untargeted. The method used in the present study is efficient and sensitive, and it allows for simultaneous analysis of L-arginine, L-citrulline, L-ornithine, ADMA, SDMA, and DMA in human serum using benzoyl chloride as a derivatization reagent, analyzed by reversed-phase chromatography, with full separation of ADMA and SDMA in a relatively short time. In contrast to methods using classical detectors, the method using mass spectrometry is highly specific, which generates fewer false high results [26].
Therefore, this study aimed to investigate serum levels of 5 amino acids – , arginine, ornithine, ADMA, SDMA, L-citrulline – and the amine, DMA, measured using LC-MS/MS in 41 patients with and without AKI admitted to the ICU of a single center who required CRRT.
Material and Methods
ETHICS:
The study protocol was approved by the Bioethics Committee of the Wrocław Medical University, Poland (approval no. KB-166/2018). Confidentiality and privacy were observed for all personal, laboratory, and clinical data. The study was carried out in accordance with the guidelines of the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all the participants prior to the study.
DESIGN AND SETTINGS:
This single-center retrospective observational study was conducted at the Department of Anaesthesiology and Intensive Therapy, 4th Military Clinical Hospital, Poland. The standards of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) were followed, and the relevant checklist was used for enrollment and allocation of participants [27].
PARTICIPANTS:
The study group consisted of 41 patients treated in an ICU. This study used a prospective blood sampling and retrospective data analysis of a cohort of patients admitted to the Department of Anesthesiology and Intensive Therapy at Wrocław Medical University, Poland. Patients with sepsis and septic shock within the first 24 h of admittance were enrolled. The inclusion criteria were sepsis or septic shock according to the Sepsis-3 criteria, age >18, while the exclusion criteria disqualified patients with severe immunosuppression (cancer, chronic steroid therapy, chemotherapy) or patients aged <18 years old. All septic patients were treated according to the Surviving Sepsis Campaign guidelines. The detailed characteristics of the patients are presented in Table 1.
After their admission to the ICU, 2 groups of patients were distinguished. One group included patients with AKI in the course of sepsis and septic shock, which required the use of CRRT; the second group included patients with sepsis and septic shock without AKI and CRRT. The criteria were established based on the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [28]. AKI was defined as an increase in creatinine serum (SCr) by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h, an increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days, or a urine volume of less than 0.5 mL/kg/h for 6 h. If there was no improvement in urine output or a decrease in creatinine, renal replacement therapy was started in patients with corrected volemia and a MAP >65 mmHg or with life-threatening metabolic and electrolyte disorders. All CRRT patients were treated in CVVHDF mode with citrate anticoagulation. The applied ultrafiltrate dose varied in the range of 20–25 mL/kg/bw.
LABORATORY AND CLINICAL PARAMETERS:
The patients’ demographic, clinical, and biochemical data at the time of ICU admission were recorded. The Acute Physiology and Chronic Health Evaluation (APACHE) II [29] and Sequential Organ Failure Assessment (SOFA) [30] scores were evaluated at the initial time of ICU admission. The SOFA scores were also recorded on days 1, 3, 5, and 7. Data were collected for the following parameters: age, sex, total fluid input and output, serum creatinine, urea, procalcitonin, CRP, Hb, blood lactate, arterial and venous blood gas analysis, parameters of coagulation system and liver, serum electrolytes, and arginine, ornithine, ADMA, SDMA, DMA, and citrulline. Additionally, patients were assessed with respect to their need for CRRT [31].
SERUM SAMPLE COLLECTION:
Blood samples were collected into serum separator tubes in the morning of the first, third, fifth and seventh days. Blood samples were allowed to clot at room temperature (22–24°C for 15 min and then centrifuged at 720 g for 10 min. For amino acid analysis, the serum was divided into aliquots and stored at −80°C [32].
PREPARATION OF CALIBRATION STANDARDS AND URINE AND SERUM SAMPLES – LC-MS SAMPLE ANALYSIS:
Analysis of Arg/NO pathway metabolites was performed using a Xevo G2 Q-TOF MS mass spectrometer coupled with the Acquity UPLC system (Waters, Milford, MA, USA). Calibration standards and plasma samples were conducted in the same manner according to the method described by Fleszar et al [26]. Briefly, 100 μl aliquots was pipetted into 2.0 mL polypropylene tubes with 10 μl of internal standard solution [IS D6-DMA (50 μM); D7-ADMA (20 μM); D7-Arg (100 μM)] and 50 μl of borate buffer. Derivatization was performed by adding the 10% benzoyl chloride (BC)l in acetonitrile and 400 μl of acetonitrile as precipitation solution. After centrifuging, 100 μl of the supernatant was transferred into autosampler glass vials with 400 μl of water for the LC-MS analysis. Samples were separated on a Waters HSS T3 column (1.8 μm, 1.0×50 mm), using the following gradient: 0.0 min – 5% B hold for 1 min, 3.5 min – 14% B, 5.0 min – 60% B, 5.5 min – 90% B hold for 1.1 min, 6.60 min – 5% B, where eluent A was water with 0.1% formic acid (FA) and eluent B was methanol with 0.1% FA. The temperature of the column was 60°C and total flow rate was 0.220 mL/min.
The mass spectrometer was operated in full scan mode from 100 to 650 m/z with leucine–enkephalin as lock mass. The spray voltage, source temperature, and the desolvation temperature were set at 0.5 kV, 120°C, and 400°C, respectively. For quantitative analysis, extracted ion chromatograms (EIC) of compounds were used. Data were acquired and quantified using MassLynx software (Waters) [33].
PREPARATION OF CALIBRATION STANDARDS AND URINE AND SERUM SAMPLES – CHEMICALS AND REAGENTS:
The Arg/NO pathway metabolites were of analytical standard: hydrochloride salts of unlabeled dimethylamine (DMA), hexadeutero- dimethylamine (D6-DMA, declared as 99 atom% 2H), L-arginine, SDMA, ADMA, L-citrulline, L-ornithine monohydrochloride, labeled L-ornithine hydrochloride (3,3,4,4,5,5-D6-ornithine), benzoyl chloride (BCl) and sodium tetraborate were procured from Sigma-Aldrich (Poznan, Poland). Isotope labeled L-arginine: HCl (D7-arginine, 98%) and asymmetric dimethylarginine (2,3,3,4,4,5,5-D7-ADMA, 98%) were obtained from Cambridge Isotope Laboratories (Tewksbury, MA, USA). Methanol, acetonitrile (ACN), water, and formic acid (FA) were acquired from Merck Millipore (Warsaw, Poland), and leucine–enkephalin was obtained from Waters (Warsaw, Poland) [34,35].
SAMPLE SIZE:
Sample size analysis was performed using Statistica 13 (TIBCO Software, Inc.). Based on the available preliminary results carried out in our center (pilot incidence, n=9), we assessed how the results differ between the 2 groups (group A – without AKI and without CRRT, n=5; group B – with AKI and CRRT, n=4). In the sample size estimation analysis, the means, and standard deviations of the amino acid level scores in both groups were used. The estimated sample size is based on a two-sample
STATISTICAL ANALYSIS:
Statistical analysis was performed using the Statistica 13 program (TIBCO, Inc., USA). For measurable variables, the medians, the lower quartile, and the upper quartile were calculated. All investigated quantitative variables were checked with the Shapiro-Wilk test to establish the type of distribution. The results were compared using the Mann-Whitney U test. The comparison of qualitative variables was performed using the chi-square test. The receiver operating characteristic (ROC) curves for selected variables were presented along with the determined optimal cut-off points (Youden’s method) and the relationship between sensitivity and specificity are shown. Moreover, to assess the reliability of diagnostic tests, sensitivity and specificity were determined. Additionally, the positive (PPV) and negative (NPV) predictive value was indicated, as well as the likelihood ratio of positive (LR+) and negative (LR−) results. An attempt was made to evaluate the relationship of the results of selected parameters with AKI and for CRRT (logistic regression for each variable separately – one-way model of predictors included in the analysis). Odds ratios and confidence intervals were determined.
Results
AKI BIOMARKERS AND PROCALCITONIN LEVEL:
There was a statistically significantly higher median serum level of procalcitonin obtained in patients with AKI who underwent CRRT (Me=54.7 ng/mL) compared to those without AKI and CRRT (Me=−2.9 ng/mL) (
It was found that statistically significant higher levels of urine output were obtained by patients who did not undergo CRRT (Me=1675.0 mL/day) compared to those who received CRRT (Me=100.0 mL/day) (P<0.001). In addition, there was a statistically significant difference in blood bicarbonate (HCO3−) results: the median in patients without CRRT was 21.9 mmol/L and was higher than in those with CRRT (Me=17.5 mmol/L) (P=0.029). A statistically significant difference in lactate levels was also shown, with a higher median for the group of patients undergoing CRRT (Me=5.0) compared to the patients not undergoing CRRT (Me=1.7) (P=0.012). Table 2 compares the results of selected blood tests in patients with and without AKI and CRRT.
SELECTED AMINO ACIDS AND DMA:
Table 3 compares the concentrations of amino acids (arginine, ornithine, ADMA, SDMA, and citrulline) and DMA in the serum (expressed as μmol/L) between the septic group with and without AKI.
There was a statistically significantly higher median level of DMA obtained from the AKI patients (Me=8.1 umol/L) compared to the patients without AKI (Me=5.2 umol/L) (P=0.022). The results for the remaining molecules, as well as for the values of selected amino acids and DMA in patients with sepsis and septic shock, revealed no statistically significant differences (P>0.05) (Table 4).
AUC/ROC ANALYSIS OF THE CONCENTRATION OF SELECTED AMINO ACIDS, AMIN DMA, AND SERUM CREATININE RELATIVE TO THE INCIDENCE OF AKI IN SEPSIS AND SEPTIC SHOCK:
The AUC analysis of the selected amino acids, arginine, ornithine, ADMA, SDMA, citrulline, and DMA, as predictors of AKI, is presented in Table 5.
Only DMA was selected for ROC analysis as its concentrations were shown to be significantly different between septic patients with and without AKI and CRRT (Table 5, Figure 1). The ROC curves of DMA and creatinine were plotted to identify a cut-off value that could differentiate patients with and without AKI and CRRT (Figures 1, 2). For DMA and creatinine, a measure of relationship, such as sensitivity, specificity, PPV, NPV, ACC, LR+, and LR−, is shown. Table 6 summarizes the AUC results for the variables DMA and creatinine.
Table 7 shows the number of people who obtained a result above or below the established cut-off point for DMA and creatinine, according to whether they were treated with CRRT. In subjects with DMA greater than or equal to 14.95 umol/L, 100% (n=5) were treated with CRRT, and in the group of subjects with a DMA score below the cut-off point, 20% were treated with CRTT (n=7). It was also observed that in subjects with creatinine levels greater than or equal to 1.19 mg/dL, 28% of the subjects (n=11) were treated with CRRT, and in the group of subjects with creatinine scores below the cut-off point, only 3% were so treated (n=1). The table also includes the results of sensitivity, specificity, PPV, NPV, AVV, and LR−/+. Additionally, a positive, statistically significant correlation between creatinine and DMA was observed (Figure 3).
SURVIVAL ANALYSIS:
A comparison of APACHE II and SOFA scores in the groups of patients with and without AKI is presented in Table 8. Patients with AKI (Me=25.0 points) had statistically significantly higher APACHE II scores than patients without AKI (Me=21.0 points) (P=0.038). In addition, a statistically significant difference in SOFA scores (P<0.001) was demonstrated; patients with AKI (Me=14.0 points vs Me=9.0 points) had higher median SOFA scores.
Discussion
In our study, the usefulness of determining the value of DMA as a marker of AKI in the course of sepsis and septic shock and the need for CRRT was demonstrated. As a biomarker of septic AKI, a very high sensitivity (100%) and a specificity of 42% were demonstrated for and above a 14.95 μmol/L of DMA serum level. Due to the renal metabolism of dimethylamine and its excretion by urine, it seems to be useful in the diagnosis of kidney damage.
The kidney plays an important role in the metabolism of amino acids and the control of plasma concentrations. Abnormal plasma and muscle amino acid profiles in chronic renal failure first indicates malnutrition, which can be partially corrected by supplementation. Investigations in normal kidneys have shown that glutamine uptake maintains acid-base homeostasis, removes glycine and citrulline, and releases serine and arginine into the circulation. These metabolic processes are impaired in chronic renal failure. Uremia affects most tissues and causes malnutrition, while acidosis activates the catabolism of amino acids and proteins in muscle.
The kidney converts citrulline to arginine. Loss of this function likely contributes to the increasing ratio of citrulline to arginine as the GFR declines below 50 mL/min/1.73 m2 [36]. Similarly, reduced renal production of serine from glycine probably underlies the rise in the plasma glycine-to-serine ratio.
In particular, DMA, which circulates in human blood and is excreted in the urine, is a useful biomarker of kidney injury. The major precursor for endogenous DMA is ADMA, an endogenous inhibitor of NO synthesis, which is hydrolyzed to DMA and l-citrulline by DAH. In adults, about 90% of daily produced ADMA is estimated to be hydrolyzed to DMA, with the remaining 10% being excreted unchanged in the urine. DMA circulates in the blood in the lower mM range and is excreted in the urine in the upper mM range. The variation of DMA excretion in the urine is fairly constant, and drugs, such as the diuretic acetazolamide, do not affect its urinary excretion rate in healthy humans [25]. ADMA is an endogenous inhibitor of nitric oxide synthase (NOS) [37]. Protein-bound arginine is methylated [38,39] by endothelial cells to form ADMA. Elevated ADMA concentrations are observed during renal failure and have been shown to contribute to cardiovascular mortality in those patients [40,41]. ADMA is converted to arginine and dimethylamine by the enzyme N-G,N-G-dimethylarginine dimethylaminohydrolase in the kidney or in the liver [42–44]. Approximately 4.5% of ADMA is excreted in the urine [45], and the remainder is metabolized to arginine, arginine-derived amino acids, and by-products.
Sepsis is defined as a life-threating organ dysfunction caused by the dysregulation of the host’s response to an infection [39]. Due to abnormal tissue perfusion, over 47% of patients with sepsis and septic shock develop an AKI [46]. Patients with sepsis-associated AKIs have a 62% risk of death compared to 32% in patients without associated renal injury. For both sets of patients, diagnosis using sensitive and specific biomarkers of kidney damage can significantly affect the speed of diagnosis and the prevention of sepsis-associated AKIs. Biomarkers can also help in monitoring kidney function during sepsis treatment, for which drugs and fluids with nephrotoxic potential are often used.
The basic markers of renal failure used today are serum creatinine and the measurement of daily urine output. In the case of creatinine, there is no clear position on the determination of the baseline value in the absence of primary concentration measurements. In addition, changes in creatinine concentration are delayed due to renal reserves and the kinetics of creatinine itself. Since serum creatinine determination remains the standard in defining AKIs, the positive correlation of serum creatinine with DMA confirms the usefulness of DMA determination in the diagnosis of AKIs. At a DMA serum concentration of >14.95 umol/L, the high sensitivity of DMA determination (100%) in patients treated with CRRT was observed. Rodrigues et al [19] described the usefulness of monitoring DMA concentration in urine as a biomarker of kidney function.
Urine output depends mainly on the patient’s volemic state before admission to the hospital. Urine output is the 1 of 2 KDIGO criteria of AKI diagnosis, but urine output is dependent upon many factors, including fluid administration, volemic status, and drug administration. Decrease of urine output does not necessarily indicate a decrease of glomerular filtration rate, but can be physiological renal adaptation to the body volume or electrolytes homeostasis. In the case of hypovolemia, urine output may be inadequately low.
A highly specific and sensitive biomarker to assess kidney damage is still being sought. In the course of sepsis and septic shock, the pathomechanism of kidney damage results from hypoperfusion and ischemia, as well as inflammation and apoptosis [47]. In addition, during sepsis there are immunological disturbances and macrovascular and microvascular organ dysfunctions. In the course of sepsis, there is an increase in the expression of proinflammatory cytokines and the activation of leukocytes, leading to capillary plugging and micro clots. As a result, reactive oxygen species and the induction of NOS production occurs, which can damage the endothelium and glycocalyx [24]. All of this changes during sepsis and septic shock, resulting in structural and functional changes in the kidneys.
In our study, an attempt was made to assess the usefulness of amino acids and DMA, synthesized in the human body, as biomarkers of kidney damage. Only DMA seems to be an independent biomarker of kidney damage caused by sepsis and septic shock.
Our study has some potential limitations to be discussed. It was a single-center study and the number of patients enrolled was relatively small. The method of measurement of amino acids and DMA, as we explained before, is sensitive and highly specific; however, like any quantitative method, it has specific inaccuracies regarding quantification and detection, as well as limitations related to the amount of sample necessary to use, confirmed by validation, which was carried out in accordance with the Food and Drug Administration (FDA) guidelines. Method is characterized by the limits of detection (1.7 μM L-arginine, 0.03 μM ADMA, 0.02 μM SDMA, 0.36 μM l-citrulline, 0.06 μM DMA) and quantification (3.2 μM L-arginine, 0.08 μM ADMA, 0.05 μM SDMA, 1.08 μM l-citrulline, 0.19 μM DMA). The volume of the sample, depending on the concentration of the tested substances in analyzed matrix, ranges from 50 μL to 100 μL [26].
Conclusions
In patients with sepsis, increased serum levels of DMA were significantly associated with AKI requiring CRRT. It remains unclear whether increased DMA concentrations are secondary to sepsis-induced AKI, or are a cause. Serum DMA might be useful as a specific and sensitive biomarker of renal failure in patients with sepsis and septic shock.
Figures
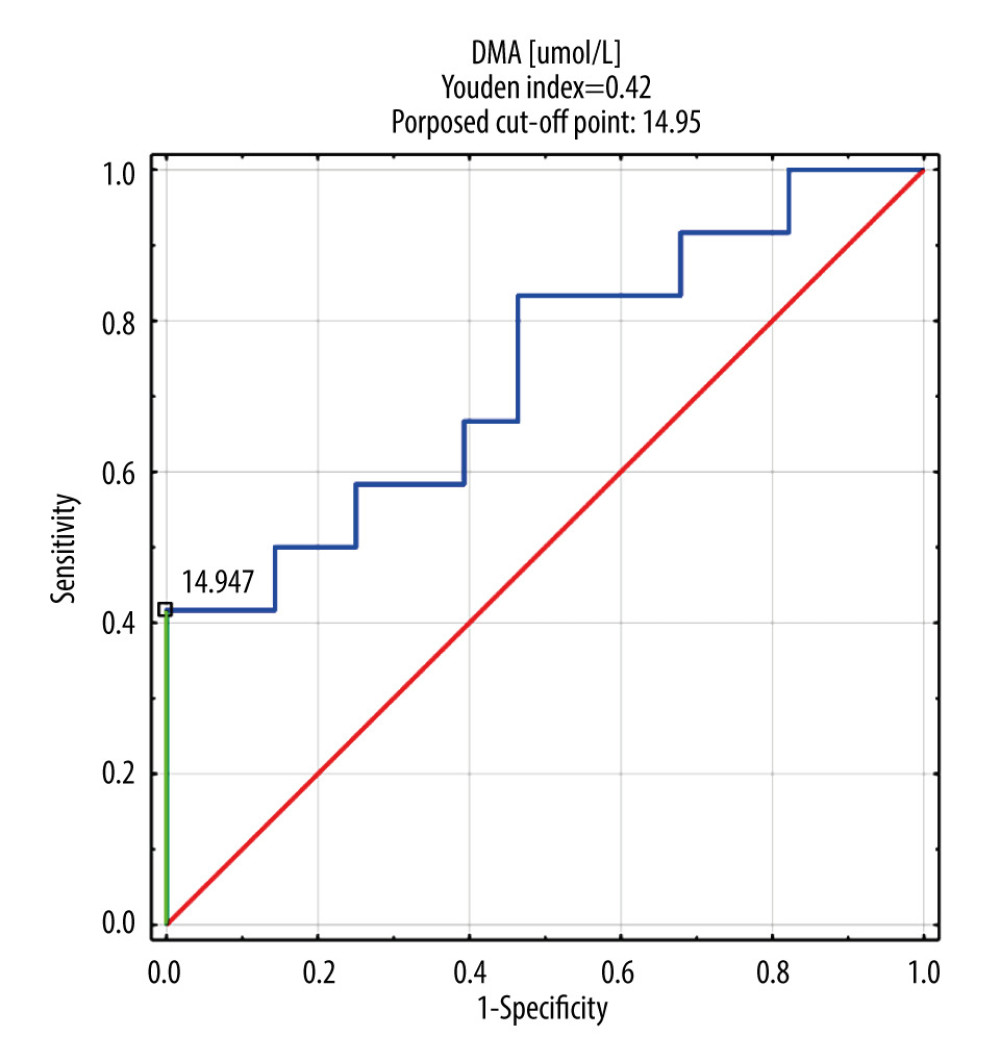 Figure 1. DMA ROC curve in AKI and CRRT patient group. DMA – dimethylamine; ROC – receiver operating characteristic; AKI – acute kidney injury; CRRT – continuous renal replacement therapy.
Figure 1. DMA ROC curve in AKI and CRRT patient group. DMA – dimethylamine; ROC – receiver operating characteristic; AKI – acute kidney injury; CRRT – continuous renal replacement therapy. 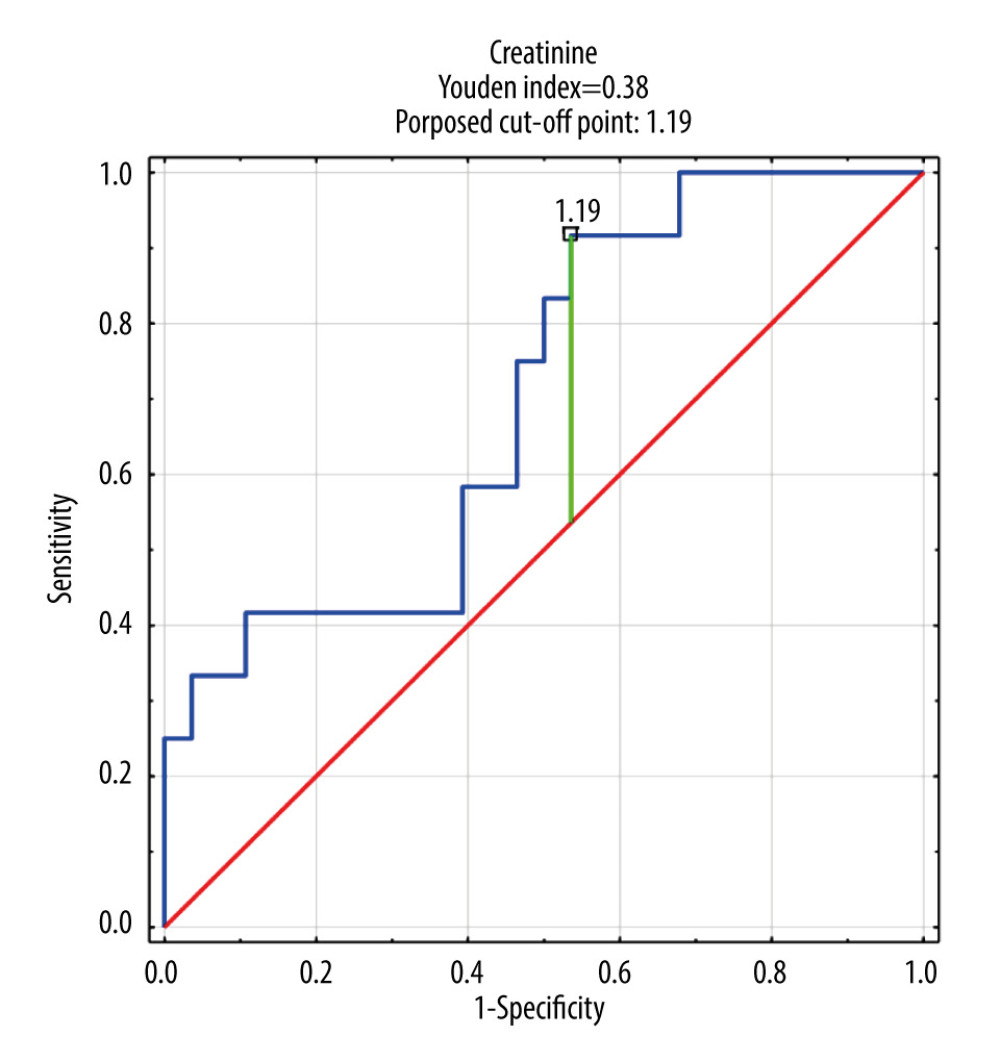 Figure 2. Creatinine ROC curve in AKI and CRRT patient group. ROC – receiver operating characteristic; AKI – acute kidney injury; CRRT – continuous renal replacement therapy.
Figure 2. Creatinine ROC curve in AKI and CRRT patient group. ROC – receiver operating characteristic; AKI – acute kidney injury; CRRT – continuous renal replacement therapy. 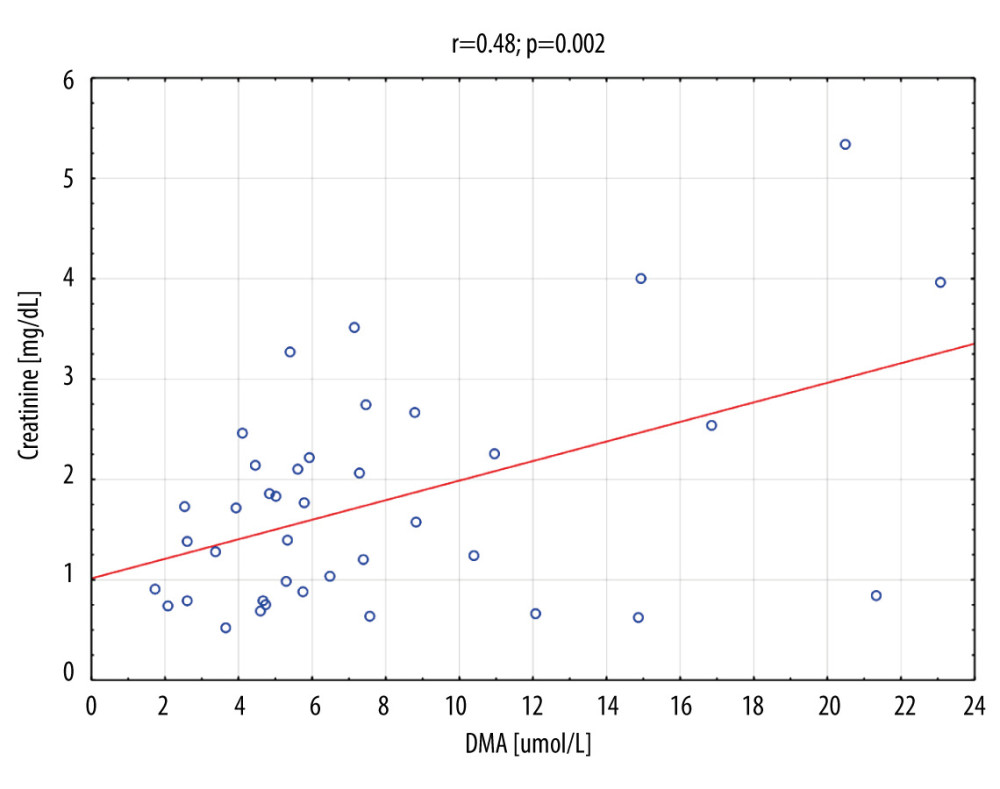 Figure 3. Creatinine vs DMA correlation for the whole group (n=41). DMA – dimethylamine.
Figure 3. Creatinine vs DMA correlation for the whole group (n=41). DMA – dimethylamine. Tables
Table 1. Study group characteristics.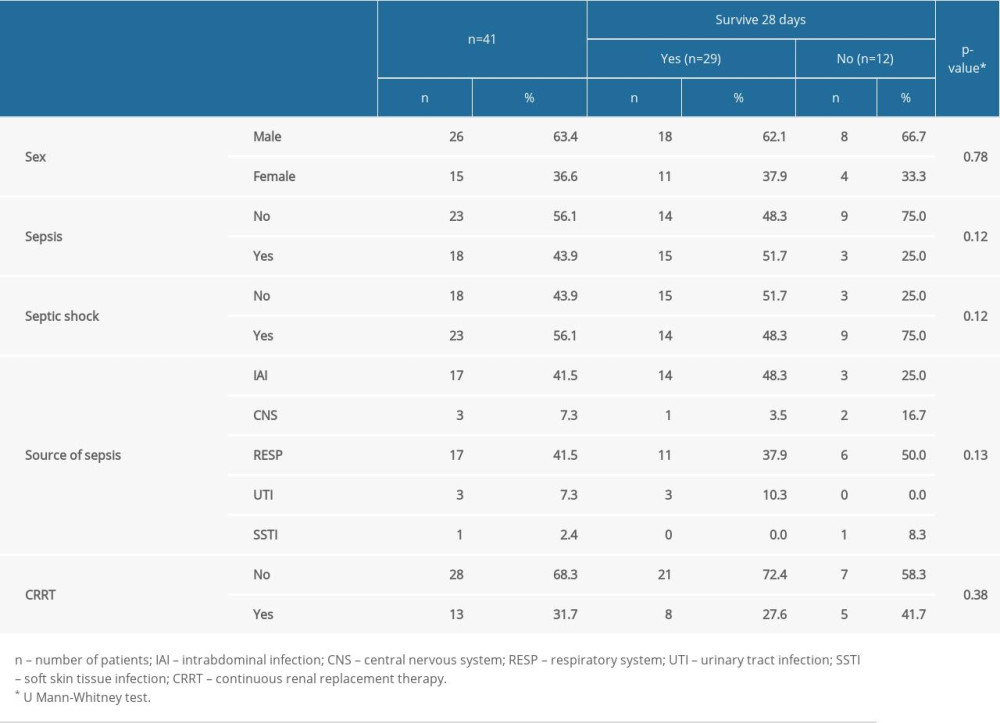 Table 2. Comparison of selected laboratory blood tests in patients with and without AKI and CRRT.
Table 2. Comparison of selected laboratory blood tests in patients with and without AKI and CRRT.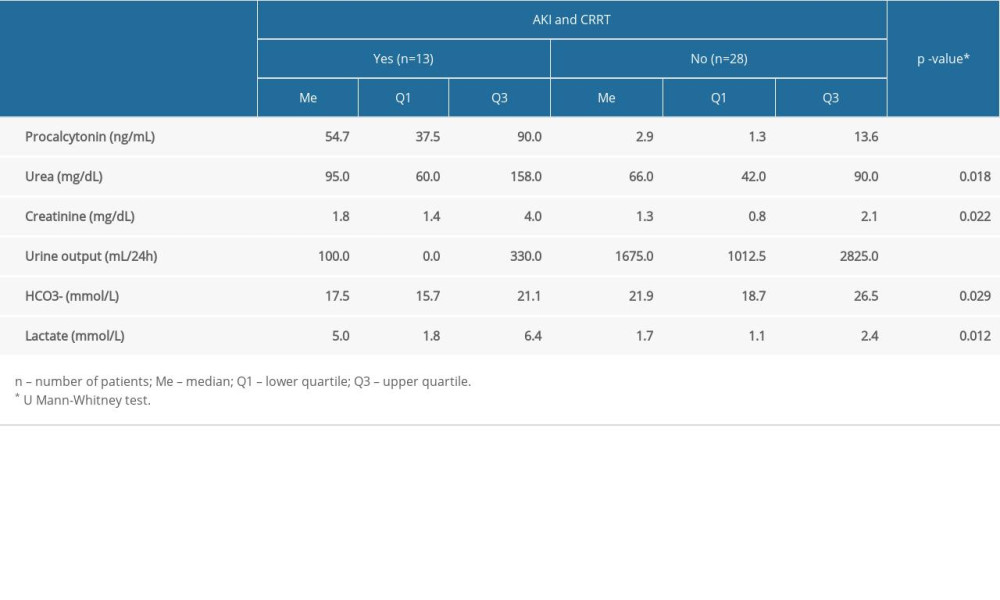 Table 3. Comparison of the level of amine DMA and selected amino acids in the group with and without AKI.
Table 3. Comparison of the level of amine DMA and selected amino acids in the group with and without AKI.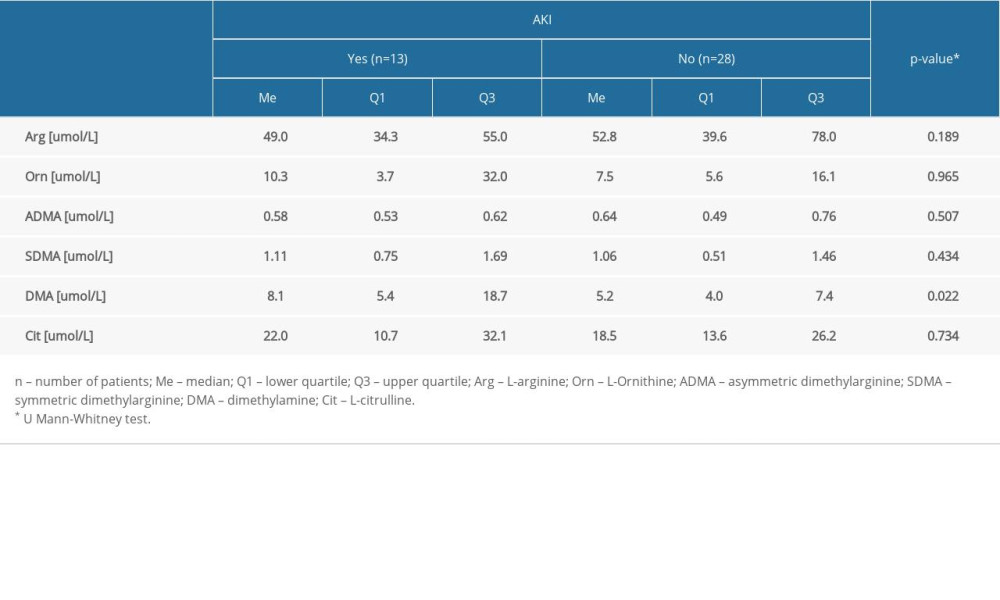 Table 4. Comparison of the level of amines and amino acids in the group with and without sepsis and septic shock.
Table 4. Comparison of the level of amines and amino acids in the group with and without sepsis and septic shock.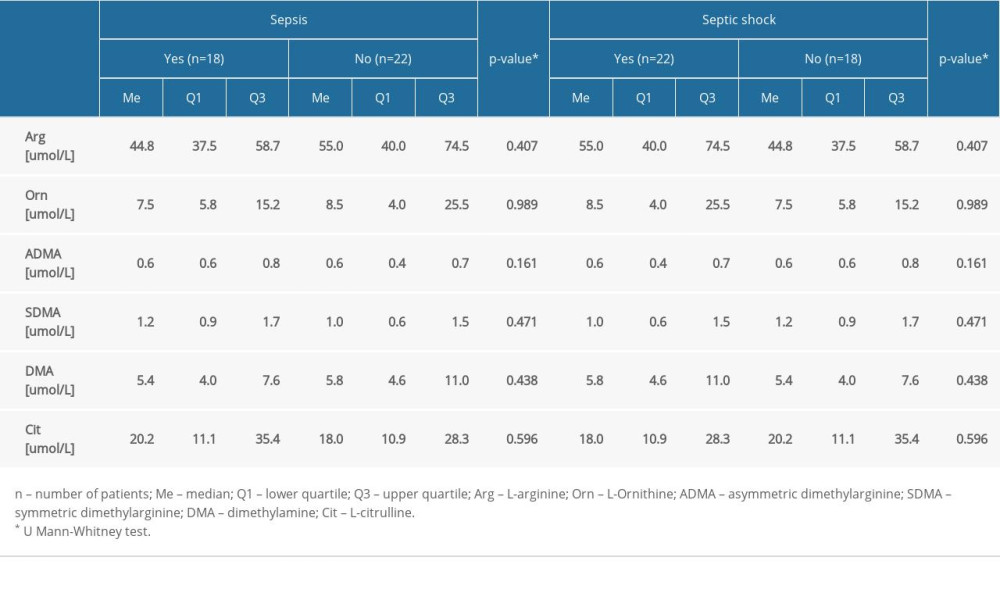 Table 5. The AUC of biomarkers for detection of AKI.
Table 5. The AUC of biomarkers for detection of AKI.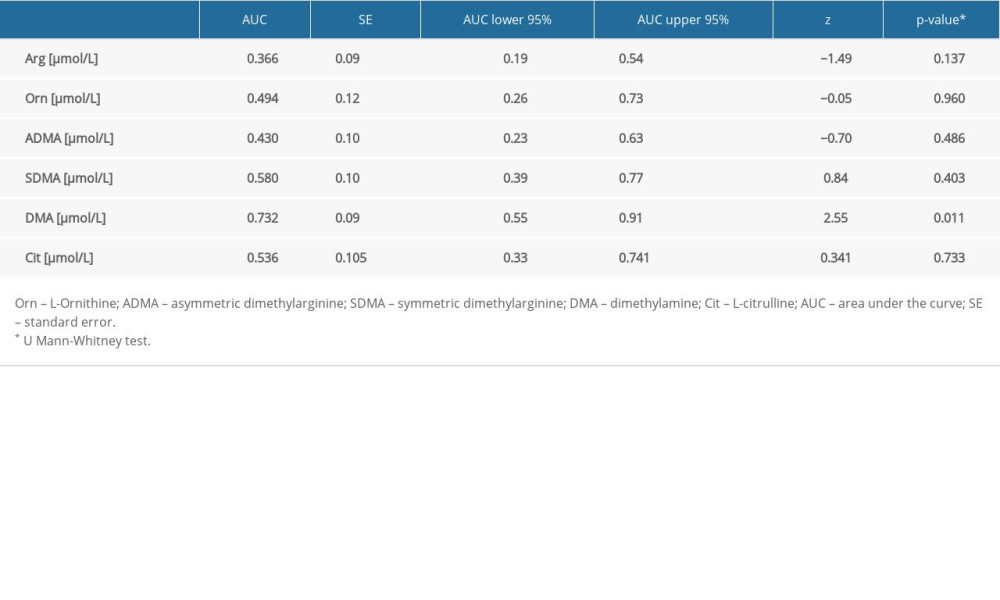 Table 6. The AUC of DMA biomarker and creatinine for detection of CRRT patients.
Table 6. The AUC of DMA biomarker and creatinine for detection of CRRT patients.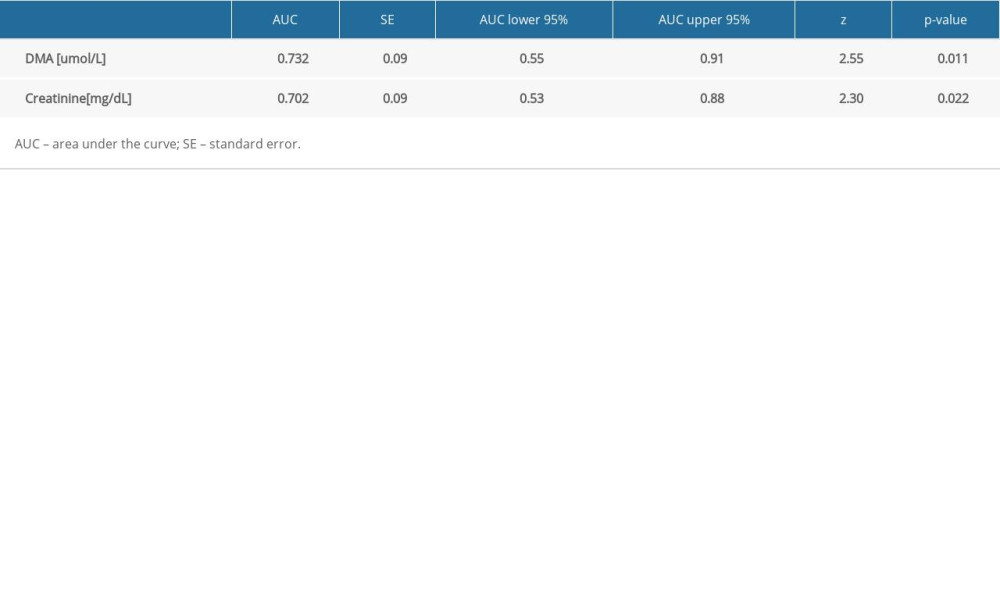 Table 7. The sensitivity, specificity, PPV, NPV, AVV, and LR of DMA and creatinine for detection of CRRT patients.
Table 7. The sensitivity, specificity, PPV, NPV, AVV, and LR of DMA and creatinine for detection of CRRT patients.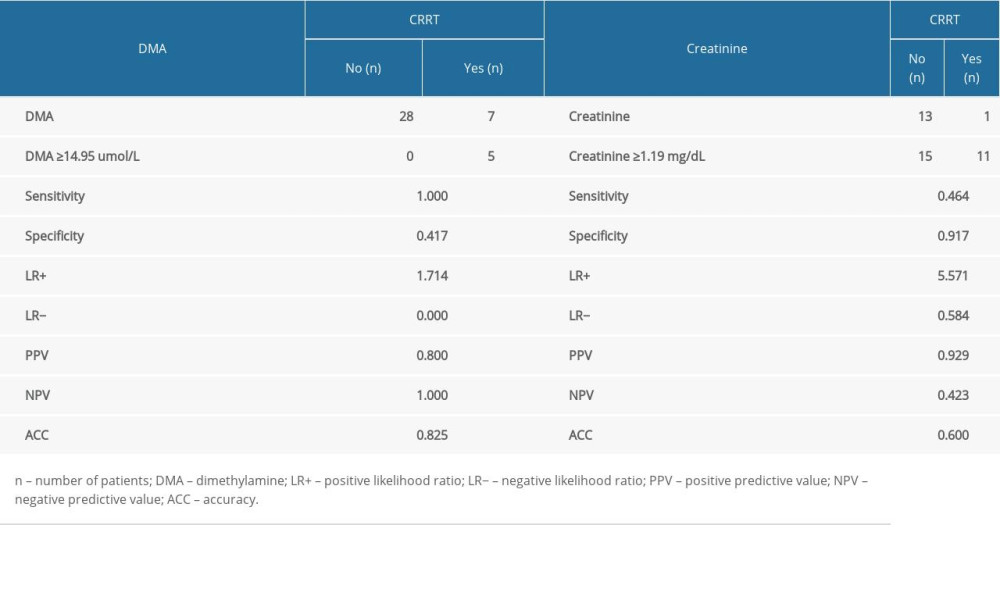 Table 8. Comparison of APACHE II and SOFA scale results against the occurrence of AKI.
Table 8. Comparison of APACHE II and SOFA scale results against the occurrence of AKI.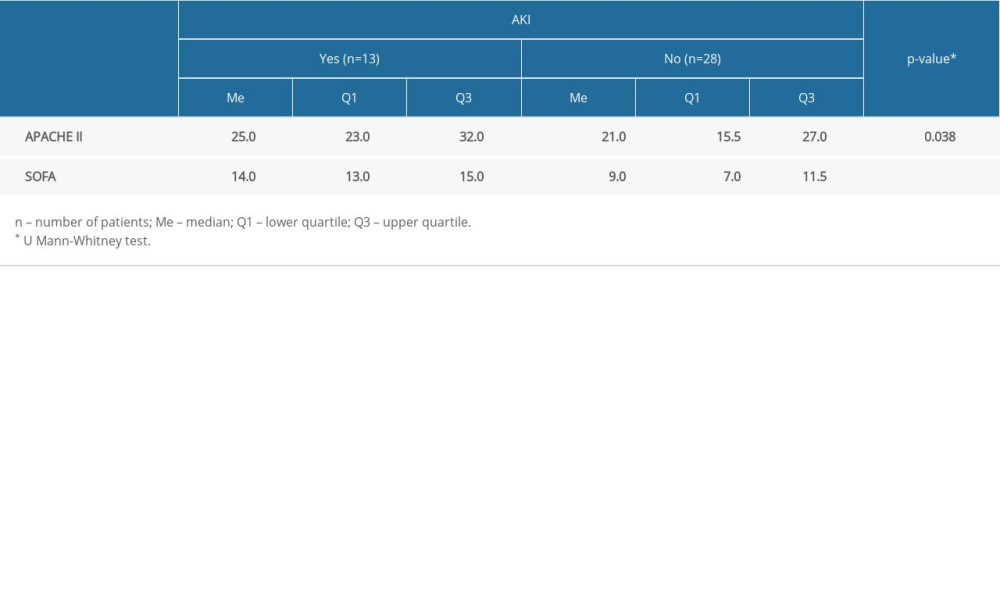
References
1. García AF, Manzano-Nunez R, Bayona JG, Acute kidney injury in severely injured patients admitted to the intensive care unit: Mil Med Res, 2020; 7; 47
2. Rodrigo E, Suberviola B, Santibáñez M, Association between recurrence of acute kidney injury and mortality in intensive care unit patients with severe sepsis: J Intensive Care, 2017; 5; 28
3. Samimagham HR, Kheirkhah S, Haghighi A, Najmi Z, Acute kidney injury in Intensive Care Unit: Incidence, risk factors and mortality rate: Saudi J Kidney Dis Transplant, 2011; 22; 464-70
4. Case J, Khan S, Khalid R, Khan A, Epidemiology of acute kidney injury in the Intensive Care Unit: Crit Care Res Pract, 2013; 2013; 479730
5. Herget-Rosenthal S, Metzger J, Proteomic biomarkers for the early detection of acute kidney injury: Prilozi, 2012; 33; 27-48
6. Aydoğdu M, Gürsel G, Sancak B, The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and Cystatin C in early diagnosis of septic acute kidney injury in critically ill patients: Dis Markers, 2013; 34; 237-46
7. Omerika L, Rasić S, Serdarević N, Importance of determination of urine neutrophile gelatinase associated lipocalin in early detection of acute kidney injury: Coll Antropol, 2014; 38; 161-66
8. Ghonemy TA, Amro GM, Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery: Saudi J Kidney Dis Transpl, 2014; 25; 582-88
9. Mason J, Olbricht C, Takabatake T, Thurau K, The early phase of experimental acute renal failure. I. Intratubular pressure and obstruction: Pflugers Arch, 1977; 370; 155-63
10. Hanif MO, Bali A, Ramphul K: Acute renal tubular necrosis, 2022, StatPearls, StatPearls Publishing http://www.ncbi.nlm.nih.gov/books/NBK507815/
11. Kellum JA, Diagnostic criteria for acute kidney injury: Present and future: Crit Care Clin, 2015; 31; 621-32
12. , Section 2: AKI definition: Kidney Int Suppl 2011, 2012; 2(1); 19-36
13. , Section 5: Dialysis Interventions for Treatment of AKI: Kidney Int Suppl , 2011; 2012(2); 89-115
14. Ostermann M, Joannidis M, Acute kidney injury 2016: diagnosis and diagnostic workup: Crit Care, 2016; 20; 299
15. Parikh CR, Mansour SG, Perspective on clinical application of biomarkers in AKI: J Am Soc Nephrol, 2017; 28; 1677-85
16. Ricci Z, Villa G, Ronco C, Management of AKI: The role of biomarkers: Annual update in Intensive Care and Emergency Medicine 2015., 2015; 365-77, Springer International Publishing
17. Alge JL, Arthur JM, Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications: Clin J Am Soc Nephrol, 2015; 10; 147-55
18. Wang K, Xie S, Xiao K, Biomarkers of sepsis-induced acute kidney injury: Biomed Res Int, 2018; 2018; 6937947
19. Rodrigues FA, de P, Santos AD, da C, de Medeiros PHQS, Gingerol suppresses sepsis-induced acute kidney injury by modulating methylsulfonylmethane and dimethylamine production: Sci Rep, 2018; 8; 12154
20. Koyner JL, Zarbock A, Basu RK, Ronco C, The impact of biomarkers of acute kidney injury on individual patient care: Nephrol Dial Transplant, 2019; 35; 1295-305
21. Dowden HC, The determination of small amounts of dimethylamine in biological fluids: Biochem J, 1938; 32; 455-59
22. Löffler H, Die flüchtigen Amine des menschlichen Harnes: Hoppe-Seylers Z Für Physiol Chem, 1935; 232; 259-62 [in German]
23. Stichtenoth DO, Marhauer V, Tsikas D, Effects of specific COX-2-inhibition on renin release and renal and systemic prostanoid synthesis in healthy volunteers: Kidney Int, 2005; 68; 2197-207
24. Tsikas D, Urinary dimethylamine (DMA) and its precursor asymmetric dimethylarginine (ADMA) in clinical medicine, in the context of nitric oxide (NO) and beyond: J Clin Med, 2020; 9; 1843
25. Tsikas D, Thum T, Becker T, Accurate quantification of dimethylamine (DMA) in human urine by gas chromatography-mass spectrometry as pentafluorobenzamide derivative: evaluation of the relationship between DMA and its precursor asymmetric dimethylarginine (ADMA) in health and disease: J Chromatogr B Analyt Technol Biomed Life Sci, 2007; 851; 229-39
26. Fleszar MG, Wiśniewski J, Krzystek-Korpacka M, Quantitative analysis of l-arginine, dimethylated arginine derivatives, l-citrulline, and dimethylamine in human serum using liquid chromatography-mass spectrometric method: Chromatographia, 2018; 81; 911-21
27. von Elm E, Altman DG, Egger M, The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies: Int J Surg Lond Engl, 2014; 12; 1495-99
28. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group, KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease: Kidney Int, 2021; 99; S1-S87
29. Knaus WA, Draper EA, Wagner DP, Zimmerman JE, APACHE II: A severity of disease classification system: Crit Care Med, 1985; 13; 818-29
30. Vincent JL, de Mendonça A, Cantraine F, Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine: Crit Care Med, 1998; 26; 1793-800
31. Lobo VA, Renal replacement therapy in acute kidney injury: Which mode and when?: Indian J Crit Care Med Peer-Rev, 2020; 24; S102-106
32. Tuck MK, Chan DW, Chia D, Standard operating procedures for serum and plasma collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group: J Proteome Res, 2009; 8; 113-17
33. Vogeser M, Stone JA, A suggested standard for validation of LC-MS/MS based analytical series in diagnostic laboratories: Clin Mass Spectrom, 2020; 16; 25-32
34. Fleszar MG, Wiśniewski J, Zboch M, Targeted metabolomic analysis of nitric oxide/L-arginine pathway metabolites in dementia: Association with pathology, severity, and structural brain changes: Sci Rep, 2019; 9; 13764
35. Krzystek-Korpacka M, Fleszar MG, Bednarz-Misa I, Transcriptional and metabolomic analysis of L-arginine/nitric oxide pathway in inflammatory bowel disease and its association with local inflammatory and angiogenic response: Preliminary findings: Int J Mol Sci, 2020; 21; 1641
36. Himmelfarb J, Sayegh MH: Chronic kidney disease, dialysis, and transplantation: A Companion to Brenner and Rector’s The Kidney, 2010, Saunders
37. Vallance P, Leone A, Calver A, Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure: Lancet Lond Engl, 1992; 339; 572-75
38. Kakimoto Y, Akazawa S, Isolation and identification of N-G,N-G- and N-G,N′-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine: J Biol Chem, 1970; 245; 5751-58
39. Singer M, Deutschman CS, Seymour CW, The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3): JAMA, 2016; 315; 801-10
40. Zoccali C, Benedetto FA, Maas R, Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease: J Am Soc Nephrol, 2002; 13; 490-96
41. Böger RH, Zoccali C, ADMA: A novel risk factor that explains excess cardiovascular event rate in patients with end-stage renal disease: Atheroscler Suppl, 2003; 4; 23-28
42. MacAllister RJ, Rambausek MH, Concentration of dimethyl-L-arginine in the plasma of patients with end-stage renal failure: Nephrol Dial Transplant, 1996; 11; 2449-52
43. Ogawa T, Kimoto M, Sasaoka K, Occurrence of a new enzyme catalyzing the direct conversion of NG,NG-dimethyl-L-arginine to L-citrulline in rats: Biochem Biophys Res Commun, 1987; 148; 671-77
44. Ogawa T, Kimoto M, Sasaoka K, Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney: J Biol Chem, 1989; 264; 10205-9
45. Ogawa T, Kimoto M, Watanabe H, Sasaoka K, Metabolism of NG,NG-and NG,N′G-dimethylarginine in rats: Arch Biochem Biophys, 1987; 252; 526-37
46. Xu X, Nie S, Liu Z, Epidemiology and clinical correlates of AKI in Chinese hospitalized adults: Clin J Am Soc Nephrol, 2015; 10; 1510-18
47. Uchino S, Kellum JA, Bellomo R, Acute renal failure in critically ill patients: A multinational, multicenter study: JAMA, 2005; 294; 813-18
Figures
 Figure 1. DMA ROC curve in AKI and CRRT patient group. DMA – dimethylamine; ROC – receiver operating characteristic; AKI – acute kidney injury; CRRT – continuous renal replacement therapy.
Figure 1. DMA ROC curve in AKI and CRRT patient group. DMA – dimethylamine; ROC – receiver operating characteristic; AKI – acute kidney injury; CRRT – continuous renal replacement therapy. Figure 2. Creatinine ROC curve in AKI and CRRT patient group. ROC – receiver operating characteristic; AKI – acute kidney injury; CRRT – continuous renal replacement therapy.
Figure 2. Creatinine ROC curve in AKI and CRRT patient group. ROC – receiver operating characteristic; AKI – acute kidney injury; CRRT – continuous renal replacement therapy. Figure 3. Creatinine vs DMA correlation for the whole group (n=41). DMA – dimethylamine.
Figure 3. Creatinine vs DMA correlation for the whole group (n=41). DMA – dimethylamine. Tables
 Table 1. Study group characteristics.
Table 1. Study group characteristics. Table 2. Comparison of selected laboratory blood tests in patients with and without AKI and CRRT.
Table 2. Comparison of selected laboratory blood tests in patients with and without AKI and CRRT. Table 3. Comparison of the level of amine DMA and selected amino acids in the group with and without AKI.
Table 3. Comparison of the level of amine DMA and selected amino acids in the group with and without AKI. Table 4. Comparison of the level of amines and amino acids in the group with and without sepsis and septic shock.
Table 4. Comparison of the level of amines and amino acids in the group with and without sepsis and septic shock. Table 5. The AUC of biomarkers for detection of AKI.
Table 5. The AUC of biomarkers for detection of AKI. Table 6. The AUC of DMA biomarker and creatinine for detection of CRRT patients.
Table 6. The AUC of DMA biomarker and creatinine for detection of CRRT patients. Table 7. The sensitivity, specificity, PPV, NPV, AVV, and LR of DMA and creatinine for detection of CRRT patients.
Table 7. The sensitivity, specificity, PPV, NPV, AVV, and LR of DMA and creatinine for detection of CRRT patients. Table 8. Comparison of APACHE II and SOFA scale results against the occurrence of AKI.
Table 8. Comparison of APACHE II and SOFA scale results against the occurrence of AKI. Table 1. Study group characteristics.
Table 1. Study group characteristics. Table 2. Comparison of selected laboratory blood tests in patients with and without AKI and CRRT.
Table 2. Comparison of selected laboratory blood tests in patients with and without AKI and CRRT. Table 3. Comparison of the level of amine DMA and selected amino acids in the group with and without AKI.
Table 3. Comparison of the level of amine DMA and selected amino acids in the group with and without AKI. Table 4. Comparison of the level of amines and amino acids in the group with and without sepsis and septic shock.
Table 4. Comparison of the level of amines and amino acids in the group with and without sepsis and septic shock. Table 5. The AUC of biomarkers for detection of AKI.
Table 5. The AUC of biomarkers for detection of AKI. Table 6. The AUC of DMA biomarker and creatinine for detection of CRRT patients.
Table 6. The AUC of DMA biomarker and creatinine for detection of CRRT patients. Table 7. The sensitivity, specificity, PPV, NPV, AVV, and LR of DMA and creatinine for detection of CRRT patients.
Table 7. The sensitivity, specificity, PPV, NPV, AVV, and LR of DMA and creatinine for detection of CRRT patients. Table 8. Comparison of APACHE II and SOFA scale results against the occurrence of AKI.
Table 8. Comparison of APACHE II and SOFA scale results against the occurrence of AKI. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








