31 August 2022: Clinical Research
Chemotherapy-Induced Peripheral Neuropathy (CIPN) in Patients Receiving 4–6 Cycles of Platinum-Based and Taxane-Based Chemotherapy: A Prospective, Single-Center Study from Kosovo
Blerim Myftiu12ABCDEFG*, Zylfije Hundozi12ACDF, Faton Sermaxhaj3ABD, Afrim Blyta1ACDF, Nexhmedin Shala12CD, Fisnik Jashari12C, Hasime Qorraj Bytyqi4CDF, Ekrem Hyseni3BC, Ilir Kurtishi3BCDOI: 10.12659/MSM.937856
Med Sci Monit 2022; 28:e937856
Abstract
BACKGROUND: Chemotherapy-induced peripheral neuropathy (CIPN) is most commonly associated with platinum-based drugs, taxanes, and vinca alkaloids. This prospective study from a single center in Kosovo aimed to evaluate CIPN in 120 patients receiving 4-6 cycles of platinum-based and taxane-based chemotherapy.
MATERIAL AND METHODS: One hundred twenty patients underwent neurological examination and nerve conduction studies (NCS) before chemotherapy, and after 4 to 6 cycles of treatment. Sixty patients were treated with platinum-based chemotherapy, 30 were treated with taxane-based chemotherapy, and 22 patients received a combination of platinum- and taxane-based chemotherapy. The most commonly used platinum-based compounds were oxaliplatin and carboplatin, whereas the most commonly used taxane medications were paclitaxel and docetaxel. Presence of neuropathy was confirmed with neurological examination of electrophysiological criteria applicable for polyneuropathies. Total Neuropathy Score (TNSr) was used to combine clinical and electrophysiological values.
RESULTS: Around 90% of patients self-reported neuropathic symptoms, and in 60% of them polyneuropathy was present in NCS. All sensory and motor nerves had significantly lower amplitudes (P<0.01). Platinum-based agents caused more pronounced decrease in ulnar nerve compound motor action potential (CMAP) (P<0.05); when used solely or in combination with taxanes, they caused significant decrease in tibial nerve CMAP (P<0.01). TNSr did not reach statistical significance between groups; only clinical muscle strength showed pronounced weakness in the combined protocol (P<0.05).
CONCLUSIONS: These findings support previous studies and show that CIPN, including sensory and motor symptoms, is commonly associated with chemotherapy. Platinum-based chemotherapy agents were more commonly associated with ulnar and tibial nerve damage in this study population.
Keywords: Ulnar Nerve, Tibial Nerve, Polyneuropathies, Chemotherapy, Adjuvant, Antineoplastic Agents, Bridged-Ring Compounds, Humans, Kosovo, Peripheral Nervous System Diseases, Platinum, Platinum Compounds, Prospective Studies, Taxoids
Background
Chemotherapeutic or cytostatic drugs are chemical agents used for treating solid cancers and hematological neoplastic diseases. While the aim is to fight rapidly differentiating malignant cells, chemotherapy drugs are not selective toward the targets. These medications also damage normal cells and tissues, especially those which differentiate quickly, like those in the gastrointestinal, skin, and hematopoietic systems [1].
Among the many kinds of damage that chemotherapy can cause, of utmost importance for neurology is peripheral nervous system damage, leading to chemotherapy-induced peripheral neuropathy (CIPN). The terminal parts of axons and support cells in the peripheral nervous system are sensitive to these drugs. Damage to those structures is caused through a range of pathophysiological mechanisms [2].
Epidemiological registers show that CIPN is present in 30–55% of patients treated with chemotherapy [3,4]. This figure covers a wide range of conditions because not all chemotherapy drugs cause CIPN: some of them are known to cause severe damage, while others are not known to cause any harm. In most of the patients with polyneuropathy, the severity can last and progress even months after chemotherapy ends, a phenomenon described as the “coasting effect” [5]. The drugs most linked to CIPN are platinum-based compounds (eg, cisplatin, carboplatin, oxaliplatin) [6], antimitotic agents like taxanes (eg, paclitaxel, docetaxel) [7–9], vinca alkaloids (eg, vincristine, vinblastine, vinorelbine, vinflunine) [10], protease inhibitors (eg, bortezomib) [11], thalidomide [12], alkylating agents (eg, cyclophosphamide, procarbazine) [13], and antimetabolites (eg, methotrexate, cytosine arabinoside, gemcitabine, 5-fluorouracil, capecitabine).
The severity of CIPN correlates with the cumulative dose of administered medication. In the absence of clear therapeutic options, limiting the treatment time and dose to the minimal effective level is presently the most beneficial method for prophylaxis of this complication [14]. The recently updated American Society of Clinical Oncology (ASCO) guidelines have not found additional studies supporting the use of any preventative approach for neuropathy [15]. Beside the medication type and its cumulative dose, other factors that stem from medication and affect the manifestation of polyneuropathy are infusion speed and frequency, administration method, and treatment protocol. Patient factors relevant to CIPN include pre-existing polyneuropathy, concomitant diseases, and surgery [16].
The diagnosis of CIPN can be established by clinical history and by development of symptoms and signs attributable to neuropathy. Neurologic electrodiagnostic tests such as nerve conduction studies (NCS) and electromyography (EMG) are used to evaluate the peripheral nervous system, but in the case of CIPN they often lack the capacity to detect changes in otherwise clinically present neuropathy [15,17]. This derives from the limitation of NCS being able to detect changes only in thick and myelinated fibers [17]. Overall, CIPN is clinically characterized by the appearance of tactile symptoms, initially in distal parts of the extremities, progressing to more proximal parts. Motor symptoms often follow the sensory ones, are less pronounced, and rarely cause functional difficulties [18]. Distal axonopathy is the most frequent form of CIPN. It reflects damage to axonal function and transport that leads to retrograde Wallerian type of degeneration of the terminal parts of sensory axons [19].
There are some general toxicity scales in use, such as the WHO scale, the National Cancer Institute Common Toxicity Criteria 3.0, and the Eastern Cooperative Oncology Group (ECOG) scale [20]. There are also some more specific scales in the field of neurology that are more suitable for assessing the clinical and electrophysiological features of peripheral nerve involvement due to chemotherapy treatment. Of these, the Total Neuropathy Score (TNS) is easy to use and provides more specific neuropathy information [21]. A reduced version was used in the present study.
This prospective study from a single center in Kosovo aimed to evaluate CIPN in 120 patients receiving 4–6 cycles of platinum-based and taxane-based chemotherapy.
Material and Methods
CASES:
Clinical and electrophysiological evaluations prior initiation of chemotherapy, and after 4–6 cycles of chemotherapy treatment, were done in 120 subjects (63 females and 57 males). All of the patients had been diagnosed with cancer. The cancer type was irrelevant for this study. All pre-chemotherapy measurements had to be within normal physiologic ranges; patients with previously diagnosed polyneuropathy or other conditions known to cause polyneuropathy were excluded from the study.
CLINICAL EVALUATION:
All patients were asked to self-report sensory, motor, and autonomous symptoms. They were recorded within the TNSr grading. Patients underwent identical neurological examination before and after the chemotherapy treatment, with emphasis on clinical evaluation of muscle strength, superficial sensitivity, deep sensitivity, deep-tendon reflexes, and autonomous symptoms.
Muscle strength evaluation consisted of grading the force of the respective key muscles in the upper and lower extremities through evaluation of their full range of movement. Patients were first asked to perform physiological movements of a muscle to its optimal position and hold it against gravity for 5 s. Later, the examiner applied optimal counterforce against the muscle and evaluated the resistance. Muscles evaluated were deltoid, biceps, triceps, hand extensor and flexor, hand finger flexor and abductor muscles, iliopsoas, quadriceps femoris, anterior tibial, and gastrocnemius muscles. The muscle strength was graded by using the Oxford scale (0 for no movement up to 5 for full range of movement, resistant against examiner counterforce) [22].
In regard to superficial sensitivity, patients were examined for sensitivity to touch, pain, and two-point discrimination modalities. Decrease of touch and pain sensation and/or inability to discriminate qualitative stimulation between 2 points were considered to be pathologic [23]. Deep sensitivity was evaluated using a standardized tuning vibration fork (128 Hz). Examiner activated it by hitting the palm, and the stem was placed in the distal interphalangeal joint of the index finger and great toe. Time passed from the activation of the tuning fork and its placement until the cessation of vibration feeling by the patient was recorded and compared to the examiner’s perception. Earlier cessation of feeling by the patient was considered pathologic [24].
Deep-tendon reflexes (DTR) were measured based on conventional grading from 0, which indicates no response, to 4, which indicates a repeating reflex, usually called clonus [25].
ELECTROPHYSIOLOGICAL METHODS:
All patients underwent an identical protocol of electrophysiologic study, which consisted of nerve conduction studies for certain sensory and motor nerves in the right upper and lower extremities. For the purposes of NCS, the MEB-9400K Neuropack S1 EMG/EP Measuring System of Nihon Kohden was used. The temperature of the procedure room was kept under 24°C and the temperature of the patients’ extremities was 35°C. Their extremities were heated if necessary.
Surface electrodes were used for NCSs. The stimulating electrode was placed above the nerve to be recorded; and the recording electrode was placed above the respective nerve (for sensory nerves) or muscle (for motor nerves). Right median, ulnar, and sural nerves were examined for sensory nerve conduction studies. Median and ulnar responses were recorded orthodromically, whereas sural response was recorded antidromically. Parameters recorded were distal latency, amplitude of the sensory nerve action potential (SNAP), and the nerve conduction velocity.
For motor nerve conduction evaluation, right median, ulnar, peroneal, and tibial nerves were selected. The peak latency, the amplitude of compound muscle action potential (CMAP), and the nerve conduction velocity were determined. Decreased sensory nerve action potential (SNAP) and/or compound motor action potential (CMAP) amplitudes and decreased nerve conduction velocities were considered to be diagnostic for presence of neuropathy.
The clinical and electrophysiological observations were combined and scaled into the Total Neuropathy Score (TNS). This scale occurs in 2 variants: the reduced score (TNSr), which combines the electrophysiological and clinical observations, and a clinical score (TNSc), which uses clinical observations only [21]. We used the reduced Total Neuropathy Score because of its documented correlation with polyneuropathic changes [5,21]. The reduced version of the TNS consists of information on 9 parameters: sensory symptoms, motor symptoms, autonomic symptoms, pin sensitivity, vibration sensitivity, strength, DTR, sural nerve SNAP, and peroneal nerve CMAP. Based on the findings, each category is graded as 1 to 4 points, with the total score ranging from 0 (absence of any sign of neuropathy) to 36 (full neuropathy).
STATISTICAL ANALYSIS:
Descriptive statistics – mean, standard deviation, minimum, and maximum – were calculated for each of the examined parameters. The means were compared parametrically (
Results
Patients were selected from the Oncology Clinic of the University Clinical Center of Kosovo (UCCK), and their clinical and electrophysiological evaluation was performed in the Neurology Clinic of UCCK. Examined patients were almost equally divided by sex (63 females to 57 males), and their mean age was 59.4±11 years. They had been diagnosed with various cancers and were expecting to begin chemotherapy treatment (Table 1). Breast, gastric, colon, and lung cancers were the most frequent diagnoses.
Patients were treated with 15 different chemotherapy protocols (Table 2). Approximately 93% (112 of 120) of them had either platinum-based compounds or taxanes, or both of them, at the core of the regimen. Oxaliplatin and carboplatin were the most frequently used platinum-based agents, whereas the most often used taxane medications were paclitaxel and docetaxel. Platinum-based and/or taxane agents were usually combined with alkylating agents (eg, cyclophosphamide), antimetabolites (eg, capecitabine, 5-fluorouracil) and doxorubicin, in different combinations.
Since almost all protocols included platinum-based and/or taxane agents, and because these are mostly associated with CIPN, we continued with the comparison of clinical and electrophysiological findings between patients treated with platinum-based medications only, taxanes only, or both (Table 3).
Almost 90% of patients self-reported sensory symptoms. In almost two-thirds of them, these symptoms were localized in the fingers, hands, and feet, predominantly in the lower extremities. A few patients reported minor motor and/or autonomous dysfunction. Objective physical sensory tactile dysfunction was shown in 75% of patients, with changes reported mostly in the hands and feet, and rarely extending to more proximal parts. Diminished vibration sensitivity was detected at a similar frequency and distribution with tactile dysfunction. Muscle strength was generally well preserved.
In initial (pre-chemotherapy) assessment, sensory and motor responses were within normal limits in 120 examined patients. After 4–6 chemotherapy cycle treatments, the sensory sural responses were not obtained in 64 patients, the ulnar responses were not obtained in 19, while sural responses were not obtained in 23. The SNAPs of all sensory nerves were significantly lower (
The CMAPs and velocities of the median, tibial, peroneal (all
Treatment with platinum-based compounds had a more pronounced effect in decreasing ulnar nerve CMAP (
The mean TNSr after chemotherapy was 11.2 (8.5 for no platinum/taxanes regimen, 12.1 for platinum-based, 9.6 for taxane-based, and 11.6 for platinum+taxanes). TNSr did not show any significant difference among groups. Only muscle strength was significantly decreased in patients treated with the combination of platinum- and taxane-based compounds (
Discussion
Chemotherapy drugs damage peripheral nerve fibers to varying degrees, thus leading to polyneuropathy. Almost all patients were treated with platinum- and/or taxane-based compounds. These agents are mostly linked to peripheral nerve damage and thus a high degree of self-reported sensory symptoms, and objective clinical findings supported neuropathy in our cohort.
Terminal parts of axons and the supportive cells of the peripheral nervous system are prone to damage by these drugs through various pathophysiological mechanisms [2]. Two-thirds of the cohort reported moderate to severe sensory symptoms in distal parts of the extremities, associated with diminished deep-tendon reflexes and vibration sensitivity. If mild symptoms are included, then almost 90% of patients described sensory symptoms. In most of these cases, symptoms were reported to have manifested after the third or fourth treatment cycle. Even though almost 90% of patients self-reported sensory symptoms, an objective decrease in sensitivity was found in 75% of them. Other major studies have reported similar sensory system involvement due to chemotherapy exposure [3,26–29]. Muscle strength was generally preserved, with a very few cases of mild decrease in strength, which were more pronounced in the groups treated with platinum compounds only or in combination with taxanes [30].
We were unable to obtain sensory sural response from 64 patients, ulnar response from 19, and median response from 23. The SNAPs for all sensory nerves were lower in patients after chemotherapy. These findings indicated axonal degeneration in the sensory fibers of these nerves, and corroborated previously reported cases of platinum and taxane exposure [9,14,30]. There was no significant difference in sensory nerve involvement between exposures to different types of chemotherapy drugs.
The CMAPs and velocities of the tibial, median, peroneal, and ulnar nerves were lower and slower, respectively. Slowing seems to be a consequence of axonal degeneration rather than primary demyelinating neuropathy. Despite decreased CMAPs, the absence of significant prolongation in distal latencies also suggested primarily axonal involvement of the peripheral nerves, corroborating the predominantly axonal involvement reported in previous studies [30–33]. Treatment with platinum-based compounds resulted in a greater decrease in motor ulnar and tibial nerve CMAPs in comparison to taxane-based chemotherapy regimens; this finding has not been reported by other studies involving treatment with both options in their cohort [26,28,30].
Reported symptoms and detected signs were mainly sensory which, in the case of platinum compounds, is suggested to derive from the accumulation of the toxin in dorsal root ganglia (DRG) and by initiating axonal hyperexcitability and repetitive discharges via changes in the voltage-gated sodium channels [29,34–37]. These agents are believed to have a strong affinity for the DNA of these neurons [38] which, while important for antineoplastic purposes, is apoptotic for the neurons [14]. Most motor neurons have larger diameters and are highly myelinated; motor nerve involvement is usually present when sensory involvement is more pronounced [19].
Susceptibility to damage is associated with length, diameter, and myelination of nerve fibers. Since no significant difference was found in length and myelination between median and ulnar nerves, and tibial and peroneal nerves, respectively, these 2 factors do not appear to explain the more pronounced ulnar and tibial nerve involvement in patients treated with platinum-based compounds. Based on ultrasonographic measurements of the cross-sectional areas and maximal thicknesses of peripheral nerves, ulnar and tibial nerves have larger mean cross-sectional area than median and peroneal nerves, respectively [39–41]. A larger cross-sectional area is associated with to increased susceptibility to nerve damage, as shown in previous studies. However, this finding cannot fully explain our observations, since those investigations were conducted for peripheral nerve entrapment cases, where the underlying polyneuropathy mechanism is different. There is thus a need to conduct ultrasound measurements of the cross-sectional area of nerves in the setting of CIPN.
Since most of our patients treated with platinum-based compounds were treated with oxaliplatin, some specific genomic changes can be used as an indicator of their specific nerve predilection. Results of a prospective and multicenter study have demonstrated that nucleotide polymorphism of voltage-gated sodium channels gene (SCN4A-rs2302237) is a predictive factor for the severity of acute polyneuropathy. Also, the presence of other single-nucleotide polymorphisms makes it possible to predict the severity of polyneuropathy in patients treated with oxaliplatin [42,43]. There is still a need to link specific genotype to particular clinical symptoms or signs and to specific electrophysiologic findings.
One limitation of this study was that the number of participants was small. Involving more patients, and systematic clinical and electrophysiological evaluation of patients to be treated with chemotherapy, would have given more comprehensive data about peripheral nervous system involvement. Using quantitative sensory testing could have been an additional tool in evaluation of small fiber involvement in CIPN.
Conclusions
The findings supported previous studies and showed that CIPN, including sensory and motor symptoms, was commonly associated with chemotherapy. Platinum-based chemotherapy agents were more commonly associated with ulnar and tibial nerve damage in this study population.
Tables
Table 1. Type of diagnosed cancer and distribution by number, age, and sex.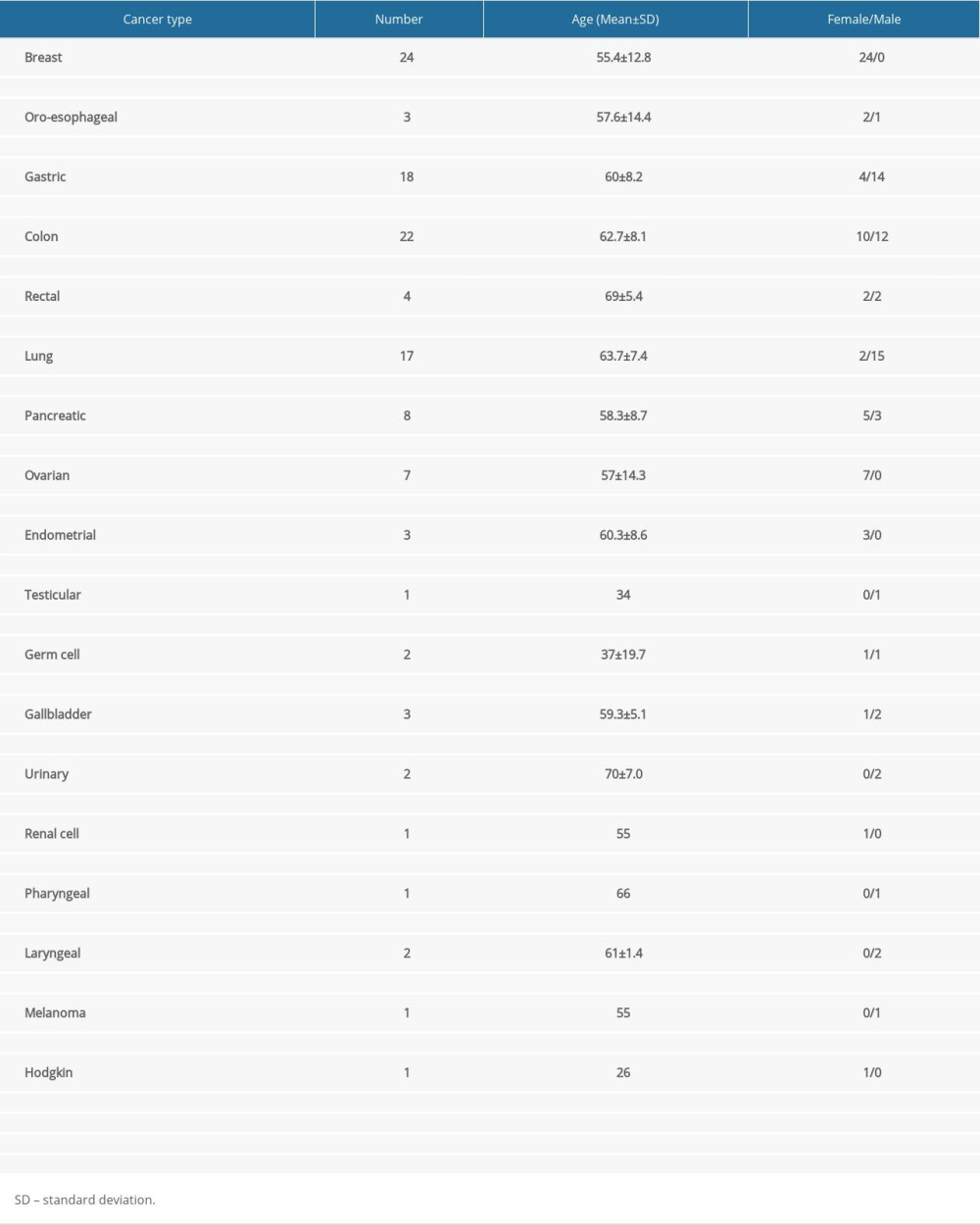 Table 2. Chemotherapy protocols.
Table 2. Chemotherapy protocols.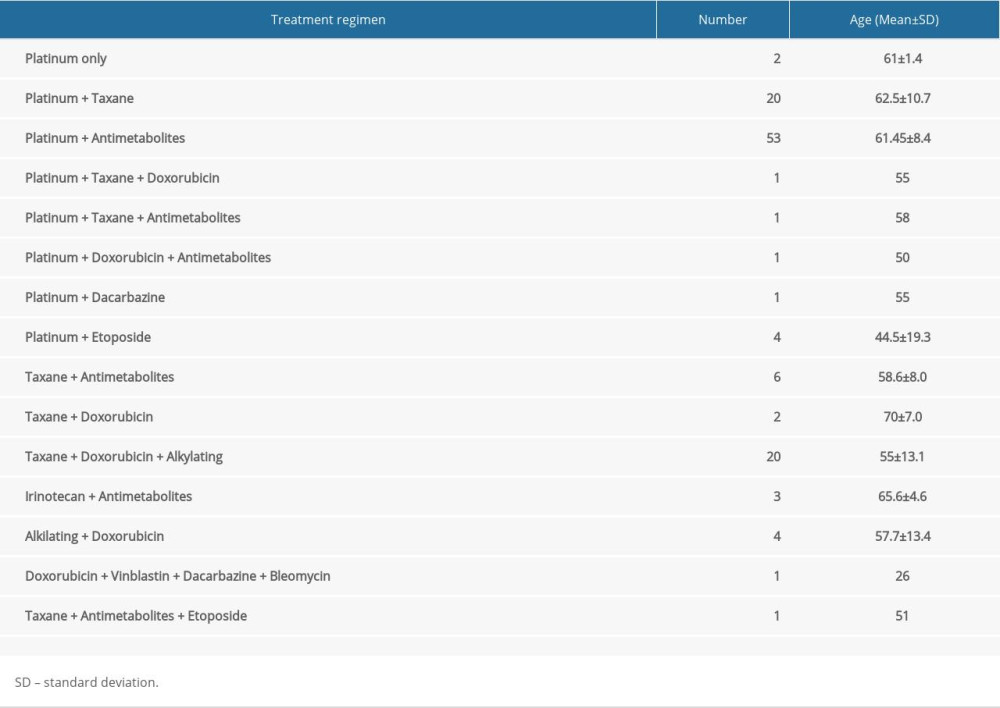 Table 3. Chemotherapy protocols based on platinum- and taxane-based compounds.
Table 3. Chemotherapy protocols based on platinum- and taxane-based compounds.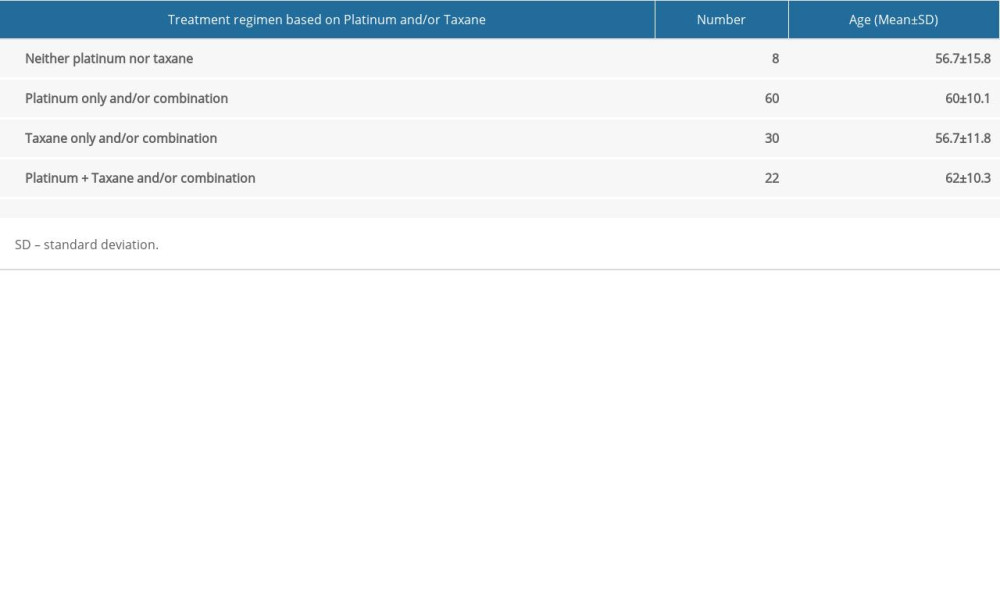 Table 4. Sensory nerve conduction studies.
Table 4. Sensory nerve conduction studies.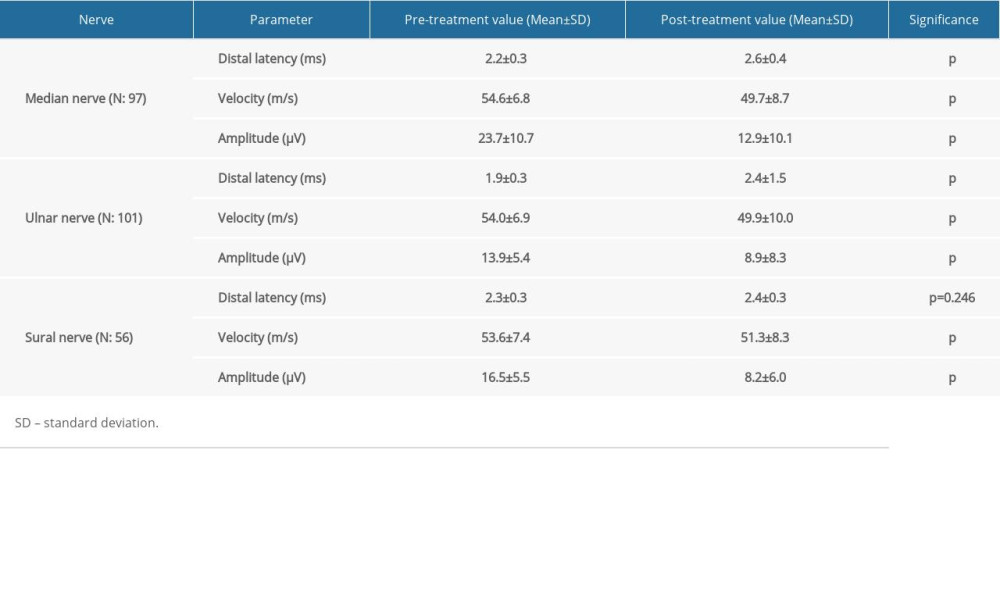 Table 5. Motor nerve conduction studies.
Table 5. Motor nerve conduction studies.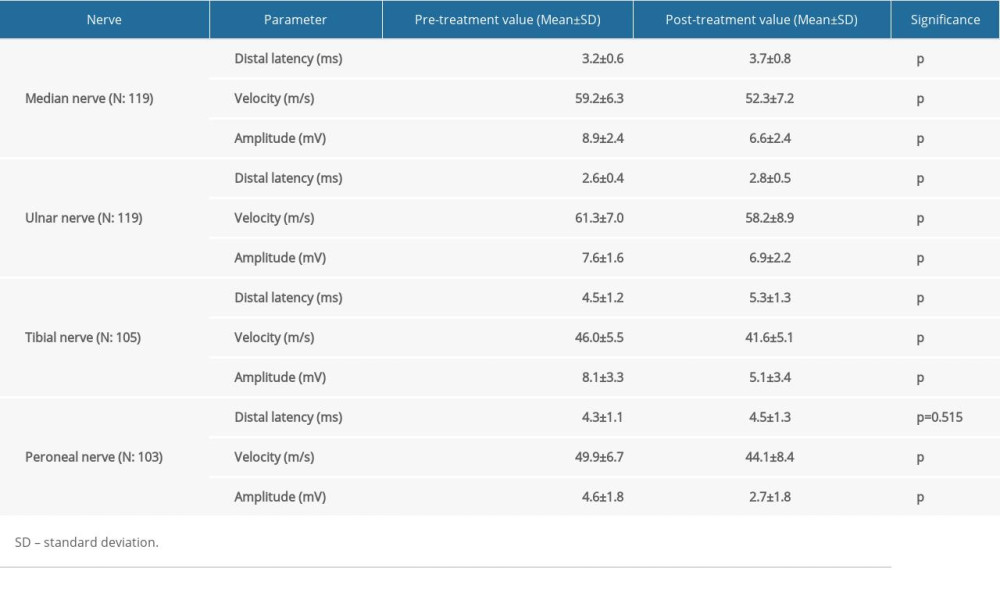 Table 6. Electrophysiological values comparison between platinum-only, taxane-only, and combination groups.
Table 6. Electrophysiological values comparison between platinum-only, taxane-only, and combination groups.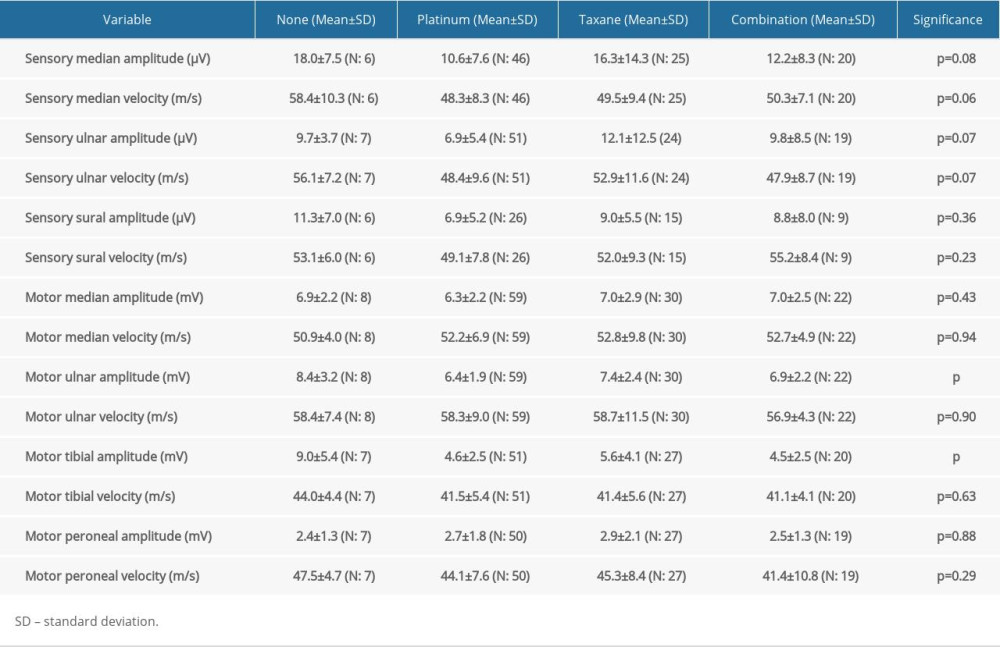 Table 7. Clinical comparison between platinum-only, taxane-only, and combination groups.
Table 7. Clinical comparison between platinum-only, taxane-only, and combination groups.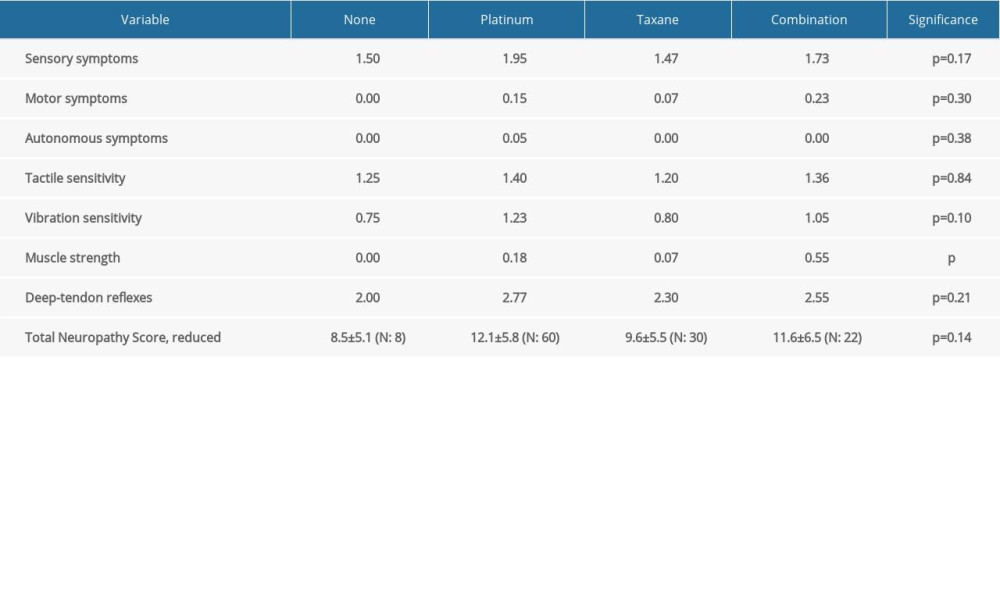
References
1. Tobias JS, Hochhauser D: Cancer and its management, 2014, London, Wiley-Blackwell
2. Windebank AJ, Grisold W, Chemotherapy-induced neuropathy: J Peripher Nerv Syst, 2008; 13(1); 27-46
3. Zedan Ahmed H, Vilholm Ole J, Chemotherapy-induced polyneuropathy: Major agents and assessment by questionnaires: Basic Clin Pharmacol Toxicol, 2014; 115; 193-200
4. Izycki D, Niezgoda A, Kazmierczak M, Nowak-Markwitz E, Chemotherapy-induced peripheral neuropathy – epidemiology and pathogenesis: Ginekol Pol, 2016; 87; 293-99
5. Burgess J, Ferdousi M, Gosal D, Chemotherapy-induced peripheral neuropathy: epidemiology, pathomechanisms and treatment: Oncol Ther, 2021; 9; 385-450
6. Cavaletti G, Nicolini G, Marmiroli P, Neurotoxic effects of antineoplastic drugs: The lesson of pre-clinical studies: Front Biosci, 2008; 13; 3506-24
7. Quasthoff S, Hartung HP, Chemotherapy-induced peripheral neuropathy: J Neurol, 2002; 249; 9-17
8. Balayssac D, Ferrier J, Descoeur J, Chemotherapy-induced peripheral neuropathies: From clinical relevance to preclinical evidence: Expert Opin Drug Saf, 2011; 10; 407-17
9. Hilkens PH, Verweij J, Vecht CJ, Clinical characteristics of severe peripheral neuropaty induced by docetaxel (Taxotere): Ann Oncol, 1997; 8; 187-90
10. Paulson JC, McClure WO, Inhibition of axoplasmic transport by colchicine, podophyllotoxin, and vinblastine: An effect on microtubules: Ann NY Acad Sci, 1975; 253; 517-27
11. Argyriou AA, Iconomou G, Kalofonos HP, Bortezomib-induced peripheral neuropathy in multiple myeloma: A comprehensive review of the literature: Blood, 2008; 112; 1593-99
12. Cavaletti G, Beronio A, Reni L, Thalidomide sensory neurotoxicity: A clinical and neurophysiologic study: Neurology, 2004; 62; 2291-93
13. Patel SR, Vadhan-Raj S, Papadopolous N, High-dose ifosfamide in bone and soft tissue sarcomas: Results of phase II and pilot studies – dose-response and schedule dependence: J Clin Oncol, 1997; 15; 2378-84
14. Brzezinski K, Chemotherapy-induced polyneuropathy. Part I. Pathophysiology: Contemp Oncol (Pozn), 2012; 16(1); 72-78
15. Loprinzi CL, Lacchetti C, Bleeker J, Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO Guideline Update: J Clin Oncol, 2020; 38(28); 3325-48
16. Chaudhry V, Chaudhry M, Crawford TO, Toxic neuropathy in patients with pre-existing neuropathy: Neurology, 2003; 60; 337-40
17. Kandula T, Farrar MA, Kiernan MC, Neurophysiological and clinical outcomes in chemotherapy-induced neuropathy in cancer: Clin Neurophysiol, 2017; 128(7); 1166-75
18. Ruddy KJ, Pachman D, Qin R, A comparison of the natural history of oxaliplatin- and paclitaxel-induced neuropathy. (NCCTG N08C1, N08CB/Alliance): J Clin Oncol, 2015; 33(Suppl 15); 9564
19. Hausheer FH, Schilsky RL, Bain S, Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy: Semin Oncol, 2006; 33; 15-49
20. Cavaletti G, Frigeni B, Lanzani F, Chemotherapy-induced peripheral neurotoxicity assessment: A critical revision of the currently available tools: Eur J Cancer, 2010; 46(3); 479-94
21. Cavaletti G, Jann S, Pace A, Multi-center assessment of the Total Neuropathy Score for chemotherapy-induced peripheral neurotoxicity: J Peripheral Nervous System, 2006; 11; 135-41
22. Clarkson HM: Musculoskeletal assessment: joint range of motion and manual muscle strength, 2000, Philadelphia, Lippincott Williams & Wilkins
23. Bigley GK, Sensation: Clinical methods: The history, physical, and laboratory examinations, 1990; Chapter 67, Boston, Butterworths
24. Prabhakar AT, Suresh T, Kurian DS, Timed vibration sense and joint position sense testing in the diagnosis of distal sensory polyneuropathy: J Neurosci Rural Pract, 2019; 10(2); 273-77
25. Walker HK, Deep-Tendon Reflexes: Clinical methods: The history, physical, and laboratory examinations, 1990; Chapter 72, Boston, Butterworths
26. Kroigard T, Schroder HD, Qvotrup C, Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies: Eur J Neurol, 2014; 21(4); 623-29
27. Beijers A, Mols F, Dercksen W, Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy: J Community Support Oncol, 2014; 12(11); 401-6
28. Staff NP, Grisold A, Grisold W, Windebank AJ, Chemotherapy-induced peripheral neuropathy: A current review: Ann Neurol, 2017; 81(6); 772-81
29. Park SB, Lin CS, Krishnan AV, Dose effects of oxaliplatin on persistent and transient Na+ conductances and the development of neurotoxicity: PLoS One, 2011; 6; e18469
30. Argyriou AA, Polychronopoulos P, Koutras A, Clinical and electrophysiological features of peripheral neuropathy induced by administration of cisplatin plus paclitaxel-based chemotherapy: Eur J Cancer Care (Engl), 2007; 16; 231-37
31. Velasco R, Bruna J, Briani C, Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients: J Neurol Neurosurg Psychiatry, 2014; 85(4); 392-98
32. McHugh JC, Tryfonopoulos D, Fennelly D, Electroclinical biomarkers of early peripheral neurotoxicity from oxaliplatin: Eur J Cancer Care (Engl), 2012; 21(6); 782-89
33. Chen X, Stubblefield MD, Custodio CM, Electrophysiological features of taxane-induced polyneuropathy in patients with breast cancer: J Clin Neurophysiol, 2013; 30(2); 199-203
34. Ocean AJ, Vahdat LT, Chemotherapy-induced peripheral neuropathy: Pathogenesis and emerging therapies: Support Care Cancer, 2004; 12; 619-25
35. Krishnan AV, Goldstein D, Friedlander M, Kiernan MC, Oxaliplatin-induced neurotoxicity and the development of neuropathy: Muscle Nerve, 2005; 32; 51-60
36. Park SB, Lin CS-Y, Krishnan AV, Utilizing natural activity to dissect the pathophysiology of acute oxaliplatin-induced neuropathy: Exp Neurol, 2011; 227; 120-27
37. Pasetto LM, D’Andrea MR, Rossi E, Monfardini S, Oxaliplatin-related neurotoxicity: How and why?: Crit Rev Oncol Hematol, 2006; 59; 159-68
38. Ta LE, Espeset L, Podratz J, Windebank AJ, Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding: Neurotoxicology, 2006; 27; 992-1002
39. Kunwarpal S, Kamlesh G, Sukhdeep K, High resolution ultrasonography of the tibial nerve in diabetic peripheral neuropathy: J Ultrason, 2017; 17(71); 246-52
40. Ribak S, Fonseca JR, Tietzmann A, The anatomy and morphology of the superficial peroneal nerve: J Reconstr Microsurg, 2016; 32(4); 271-75
41. Bedewi MA, Yousef AMM, Abd-Elghany , Estimation of ultrasound reference values for the ulnar nerve fascicular number and cross-sectional area in young males. A cross-sectional study: Medicine (Baltimore), 2017; 96(10); e6204
42. Custodio A, Moreno-Rubio J, Aparicio J, Pharmacogenetic predictors of severe peripheral neuropathy in colon cancer patients treated with oxaliplatin-based adjuvant chemotherapy: A GEMCAD group study: Ann Oncol, 2014; 25; 398-403
43. Argyriou AA, Cavaletti G, Antonacopoulou A, Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: Results from a prospective multicenter study: Cancer, 2013; 119; 3570-77
Tables
 Table 1. Type of diagnosed cancer and distribution by number, age, and sex.
Table 1. Type of diagnosed cancer and distribution by number, age, and sex. Table 2. Chemotherapy protocols.
Table 2. Chemotherapy protocols. Table 3. Chemotherapy protocols based on platinum- and taxane-based compounds.
Table 3. Chemotherapy protocols based on platinum- and taxane-based compounds. Table 4. Sensory nerve conduction studies.
Table 4. Sensory nerve conduction studies. Table 5. Motor nerve conduction studies.
Table 5. Motor nerve conduction studies. Table 6. Electrophysiological values comparison between platinum-only, taxane-only, and combination groups.
Table 6. Electrophysiological values comparison between platinum-only, taxane-only, and combination groups. Table 7. Clinical comparison between platinum-only, taxane-only, and combination groups.
Table 7. Clinical comparison between platinum-only, taxane-only, and combination groups. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








