16 March 2023: Clinical Research
Double C-Arm Digital Subtraction Angiography Guidance During Transjugular Intrahepatic Portosystemic Shunt Placement
Xiao Bai1ABCDEFG, Ping Ma2CD, Junjie TanDOI: 10.12659/MSM.938912
Med Sci Monit 2023; 29:e938912
Abstract
BACKGROUND: This study aimed to evaluate the safety and efficacy of portal vein puncture with a new guidance system using double C-arm digital subtraction angiography (DSA) during transjugular intrahepatic portosystemic shunt (TIPS) placement.
MATERIAL AND METHODS: The procedure details of TIPS placements performed on 39 patients in our center between January and December 2021 were retrospectively analyzed. The procedure was performed under double C-arm DSA guidance (study group) and C-arm DSA (control group) in 18 and 21 patients, respectively. We analyzed the procedure’s technical success, duration of the overall procedure, portal vein puncture, fluoroscopy, radiation exposure, complications, and mortality and morbidity rates 30 days after the procedure.
RESULTS: TIPS placement was performed successfully in all patients. The mean portal vein puncture time in the study group (9±5.7 min) was significantly shorter than in the control group (33±14.9 min, p=0.02). The complete mean dose area product of the procedure showed no significant differences (study group, 126±53 Gy/cm²; control group. 142±66 Gy/cm²; p=0.42). The intraprocedural complication rates were 0% and 19% in the study and control groups, respectively (p=0.04). The 30-day post-procedural mortality rate in the control group was 4.8% (1/21), with no deaths from technical complications.
CONCLUSIONS: Double C-arm DSA guidance is a safe and effective method to assist TIPS placement. This approach may result in shorter portal vein puncture time and lower intraprocedural complication rates.
Keywords: Angiography, Digital Subtraction, Hypertension, Portal, Portasystemic Shunt, Transjugular Intrahepatic, Humans, Portal Vein, Punctures, Treatment Outcome
Background
Viral hepatitis, chronic alcohol use, and nonalcoholic steatohepatitis are common causes of chronic liver disease (CLD) [1]. Liver cirrhosis leads to portal hypertension (PH) and increased blood flow resistance. It may also cause severe complications, with 50% of patients developing ascites and some progressing to refractory ascites. In addition, 30–40% and 60% of patients with compensated and decompensated CLDs, respectively, develop gastroesophageal varices that can cause life-threatening hemorrhages if the varicose veins rupture and bleed [2]. Hypertensive gastric disease can cause chronic anemia or acute bleeding, and hepatorenal syndrome can lead to renal failure. Hepatic encephalopathy (HE), secondary to the neurological activity of peptides shunting from the portal vein (PV) to the systemic circulation, can range from subtle neurocognitive changes to coma [3]. In patients with PH, the mortality rate due to bleeding varices is 20%, and refractory ascites have a 1-year mortality rate of 50% [4].
Transjugular intrahepatic portosystemic shunt (TIPS) was first described in 1969 [5] and is currently performed to manage PH-related complications, particularly in patients with variceal bleeding and refractory ascites [6]. TIPS placement can reduce PH symptoms in over 90% of patients [7]. The most challenging and time-consuming step of this procedure is the intrahepatic PV puncture, mainly because the PV and liver parenchyma are indistinguishable during fluoroscopy [8]. Even in experienced TIPS clinical research centers, only 25% of procedures require fewer than 5 punctures [9]. Several complications occurring during a TIPS placement are associated with this step, such as subcapsular or intrahepatic hematoma, intra-abdominal hemorrhage, and damage to the hepatic artery, bile duct, or gallbladder [10].
Several approaches have been tested to improve the targeting of the PV, including carbon dioxide (CO2) or iodinated contrast agent wedged hepatic portography [11], guidance with transabdominal ultrasound (US) [12], intravascular ultrasound (IVUS) [13], and magnetic resonance angiography [14]. More recently, several three-dimensional (3D) image-fusion guidance methods have been suggested, using cone-beam computed tomography (CBCT) during TIPS placement to reduce the procedure time and complication risks [15]. In our clinical practice, PV puncture under C-arm digital subtraction angiography (DSA) guidance is the current criterion standard method. To the best of our knowledge, real-time PV puncture guided by double C-arm DSA has not been described in previous reports; therefore, this is the first study to investigate the use of double C-arm DSA guidance for PV puncture.
This study aimed to evaluate the safety and efficacy of PV puncture with a new guidance system using double C-arm DSA during TIPS placement.
Material and Methods
PATIENT CHARACTERISTICS AND STUDY DESIGN:
The study protocols were approved by the Ethics Review Committee of our hospital. The requirement for informed consent for medical research was waived owing to the retrospective design of this study.
The clinical data of all patients who had undergone TIPS at our intervention center between January and December 2021 were collected from electronic hospital records. The only exclusion criterion for the procedure was hypersensitivity to contrast agents. Relative contraindications to liver puncture, such as coagulopathy (as indicated by the international normalized ratio), thrombocytopenia, and anemia, were corrected with blood product administration before the intervention. For patients with mild HE or pulmonary hypertension (PH), TIPS placement is performed after the symptoms of drug treatment are improved.
From January to June 2021, we performed TIPS placement in the C-arm DSA. After our center introduced double C-arm DSA in July 2021, we started all TIPS placement in double C-arm DSA guidance. Depending on the intervention method, we classified the procedures into the study (double C-arm DSA) and control (C-arm DSA) groups. Preprocedural contrast-enhanced computed tomography (CT) was performed in the most critical patients. Prior to the endovascular PV puncture in the study group, US-guided (Aplio 500, Toshiba Corporation) abdominal PV puncture was completed, and a marked pigtail catheter was placed in the main PV for reference.
TIPS PROCEDURE:
All TIPS procedures were performed by the same radiologist, who had 7 years of experience with this procedure. All patients underwent tracheal intubation under general anesthesia. The study group underwent the TIPS procedure on the double C-arm DSA table (Innova IGS 630, GE Healthcare; Figure 1) and the control group on the C-arm DSA table (Discovery IGS 740, GE Healthcare). Percutaneous access was obtained by puncturing the right internal jugular vein under US guidance to allow the placement of a Rösch-Uchida transjugular liver access set (Cook Medical). Following a US-guided puncture of the right jugular vein, a 10-Fr sheath was inserted over a 0.035-inch guidewire. At the interventional radiologist’s discretion, the right or middle HV was catheterized using a 5-Fr catheter and 0.035-inch guidewire. The stent is easier to maneuver after the PV is punctured through the right HV.
Based on the preoperative contrast-enhanced CT of the PV, the puncture route was planned for the control group, and the PV was blindly punctured through the middle or right HV under fluoroscopy. In the study group, using the right or middle HV as the puncture pathway, the PV image was located through the marked pigtail catheter placed in advance. Based on the PV shape, the front and side positions were assessed in detail, and the most appropriate puncture point was determined. Under angiographic guidance, the metal marker point of the first gold-marked catheter on the right side of the portal PV at the PV bifurcation was selected as the most suitable puncture point. Subsequently, the PV puncture was performed quickly and accurately under double C-arm DSA guidance (Figure 2). Once the PV branch access was confirmed via blood aspiration and iodinated contrast material injection, a portography was acquired, and the portal pressure was measured to determine the initial portosystemic pressure gradient. Subsequently, a hardened guidewire (Amplatz, Boston Scientific) was introduced and left in the superior mesenteric or splenic vein, marking the successful PV puncture. A 6-mm-diameter balloon was introduced via the guidewire and placed between the PV and HV. The puncture channel was expanded, and the balloon notch between the PV and HV was carefully monitored (if necessary, a marked pigtail catheter was introduced for the angiographic evaluation of the length of the puncture passage from the PV to the HV; the length of the stent graft can be accurately calculated according to the scale on the catheter). Moreover, a stent of the appropriate length was selected. Based on our experience, when the balloon was retrieved, the 10-Fr long sheath was moved into the main PV to prevent the dislocation of the guidewire and the need for an additional PV puncture. Then, an 8-mm-diameter Viatorr stent (Gore Medical) was deployed between the PV and HV to create the shunt. The stent was then dilated to an adequate size using an 8-mm-diameter balloon, and the portosystemic gradient was measured again. The number and size of stents used were recorded in detail. After TIPS placement, the pigtail catheter was removed from the liver area, and the puncture channel was embolized with a Gelfoam strip through the vascular sheath.
DATA ANALYSIS:
The original data recorded for the subsequent analysis included the success of TIPS placement, time of overall procedure and PV puncture, fluoroscopy time, total radiation dose (dose area product [DAP]) for the clinicians, portal system pressure gradient change, intraoperative contrast media usage, intraprocedural complications, and morbidity and mortality rates 30 days after the procedure. The image data information of all patients was stored in a Picture Archiving and Communication System and saved on a CD. The introduction of the guidewire through the HV into the main PV was labeled a successful puncture. The PV puncture time included the US-guided percutaneous transhepatic portal puncture and intrahepatic portal puncture time. The intrahepatic portal puncture time refers to the interval from the first attempt to the successful PV puncture. The TIPS placement was deemed successful when the subsequent PV gradient pressure was <8 mmHg. The overall procedure time refers to the interval from the first to the last recorded image. The radiation dose (DAP) was based on the image acquisition automatically recorded by the system, and intraprocedural complications included injuries to the hepatic artery and bile duct. The TIPS clinical success was defined by the patient’s survival time longer than 30 days after the procedure.
STATISTICAL ANALYSES:
Statistical analyses were performed using the Statistical Package for the Social Sciences software (version 25.0, IBM). The median was used for descriptive statistics, and the Wilcoxon signed-rank test was applied to assess the level of significance for non-normally distributed data. For categorical data, the chi-squared test was used. A p-value <0.05 was considered significant.
Results
PATIENT CHARACTERISTICS:
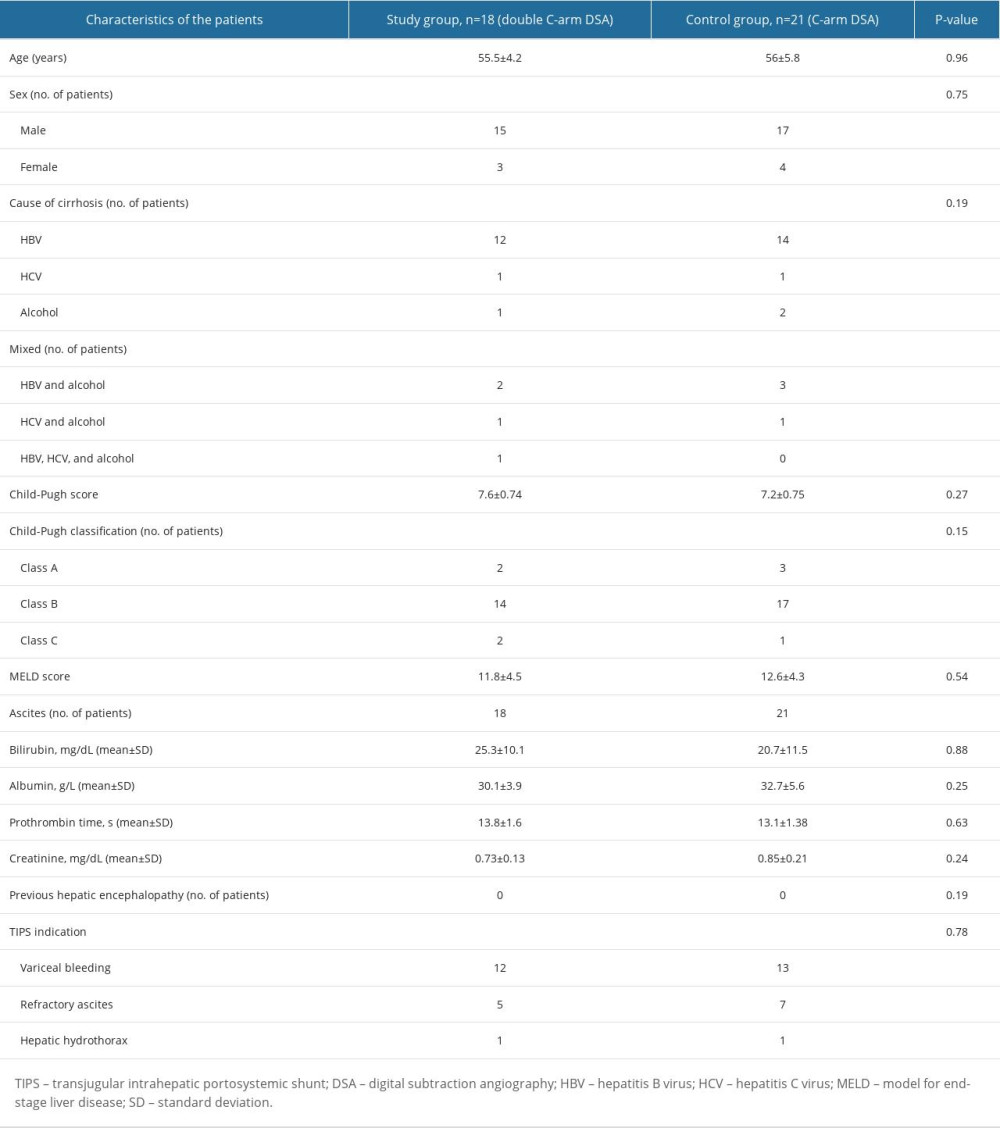
Based on the clinical information, the 39 patients included were divided into 2 groups according to the intervention method. The demographic and clinical characteristics of the 2 groups were not significantly different. The indications for TIPS placement were as follows: recurrent bleeding from esophageal varices despite medical and endoscopic treatments (study group, 12/18 [66.7%] patients; control group, 13/21 [61.9%] patients); refractory ascites (study group, 5/18 [27.8%]; control group, 7/21 [33.3%]), and hepatic hydrothorax (study group, 1/18 [5.6%]; control group, 1/21 [4.8%]). The patients’ demographics and clinical characteristics are shown in Table 1, including the primary cause of liver cirrhosis.
PROCEDURAL SUCCESS:
In total, TIPS placement was successful in all patients. Most TIPS shunts were created between the right HV and right PV (study group, 14/18 [77.8%]; control group, 16/21 [76.2%]) or the middle HV and right PV (study group, 4/18 [22.2%]; control group, 5/21 [23.8%]).
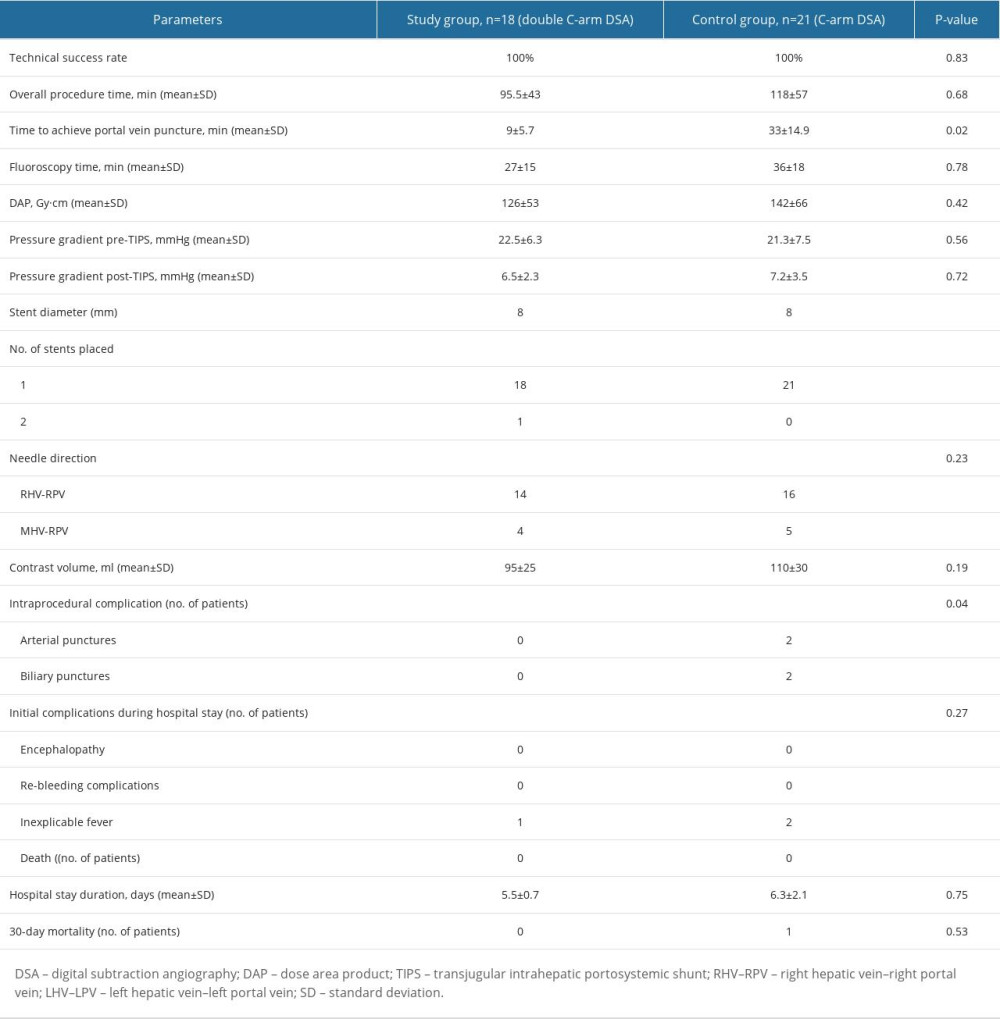
The overall procedure time was shorter in the study group (95.5±43 min) than in the control group (118±57 min); however, the difference in total operating time between the 2 groups was not statistically significant (p=0.68). The time to achieve the PV puncture was significantly shorter in the study group (9±5.7 min) than in the control group (33±14.9 min, p=0.02). The mean DAP of the complete procedure showed no significant differences between the 2 groups (study group, 126±53 Gy/cm2; control group, 142±66 Gy/cm2; p=0.42). The fluoroscopy time in the study group (27±15 min) was slightly shorter than in the control group (36±18 min, p=0.78). The portosystemic gradients in the study and control groups before TIPS placement were 22.5±6.3 mmHg and 21.3±7.5 mmHg, respectively, and 6.5±2.3 mmHg and 7.2±3.5 mmHg afterward, respectively. One patient in the study group had the stent positioned incorrectly; an extra stent graft was placed to prevent the occurrence of stent caps. The amount of contrast agent used was slightly smaller in the study group (95±25 mL) than in the control group (110±30 mL, p=0.19).
PROCEDURE SAFETY:
No patients in the study group had serious complications. In contrast, the intraprocedural complication rate was 19% (4/21) in the control group (p=0.04). Two arterial punctures (9.5%) occurred during the intraoperative PV puncture; the bleeding was successfully stopped after hepatic artery embolization in one patient, whereas no specific treatment was performed in the other patient. Two biliary punctures (9.5%) were noted on angiography during the puncture. Postoperatively, hepatic arteriography revealed no evident bleeding or biliary tract development. Among all patients, 1 and 2 in the study and control groups, respectively, developed an inexplicable fever after the procedure. Drug intervention was not provided, and the patients were discharged after rehydration. The 30-day post-procedure mortality rate was 4.8% (1/21) in the control group, with no mortalities from technical complications. These results are summarized in Table 2.
Discussion
The current study presents a new approach for PV puncture guidance in TIPS placement. During the procedure guided by double C-arm DSA, the time to achieve the PV puncture was significantly shorter in the study group than in the control group. Moreover, the intraprocedural complication rate was significantly lower in the study group than in the control group.
The most technically challenging step in TIPS procedure, and frequently the longest, is the puncture of the PV following HV access [16,17]. Double C-arm DSA has been widely used in clinical practice, especially in neurointerventional practice [18]. Because it has dual flat panel probes, which can provide both front and side images in real-time, we tried to place TIPS under its guidance for the first time. Compared with intravascular ultrasound or real-time 3D CT guidance, double C-arm DSA guidance can help achieve this without expensive post-processing software and complex 3D post-reconstruction. Simple operation and precise guidance help to promote TIPS placement. In particular, a less experienced or less practiced interventional radiologist might benefit from double C-arm DSA guidance in emergency settings. In a study with TIPS placement in 21 patients and a CBCT acquisition post-PV puncture, Georg et al [19] reported a longer mean overall procedural time (115±52 min) than we observed. We found that double C-arm DSA guidance has potential application in emergency TIPS because reducing the operation and anesthesia time can increase the survival rate of patients and reduce postoperative complications. In our study, most cases of TIPS placement were electively treated. A large case study of patients receiving emergency TIPS placement will be of interest in the future.
Although technology and algorithm changes are constantly being used to increase the accuracy of image matching, errors may occur in the positioning of the reconstructed PV image in relation to the actual intrahepatic PV after image matching [20,21]. This discrepancy might mislead the clinicians, resulting in an increased number of punctures and subsequent complications. In our study, the incidence rate of puncture complications was significantly lower in the study group than in the control group. As the marked pigtail catheter is located inside the PV, it can accurately display its shape; furthermore, its relative position will not change with the patient’s breathing or the mechanical pressure of the needle, thereby accurately guiding the PV puncture and reducing puncture complications. In contrast to US guidance, double C-arm DSA guidance uses a contrast agent to delineate the puncture path, reducing the operator’s dependence on image quality. In addition, double C-arm DSA guidance is particularly useful for PV puncture in patients with severe liver cirrhosis, small liver size, and a poor positional relationship between the HV and PV. In a further study, a group of such patients will be specially selected for TIPS placement, and a prospective controlled study will be developed, which may yield gratifying results.
In our study, we found an interesting problem. In comparison of the 2 groups, the time to achieve PV puncture was significantly shorter in the study group; however, there was no significant difference in the total operation time between the 2 groups. It may be that the PV Gelfoam embolization step was finally enhanced in the study group, resulting in no significant difference in the total operation time between the 2 groups. One patient in the study group had a large amount of ascites. After a successful TIPS operation, puncture and drainage were performed; angiography showed that the proximal end of the stent was located in the HV, and this displacement might have been caused by the change in liver position. An ordinary bare-metal stent was re-implanted to prevent stent cap occlusion. According to our interventional medical center’s experience, an 8-mm stent is sufficient to relieve the portal pressure, minimizing the occurrence of postoperative HE. Postoperative drug treatment is necessary to avoid intestinal obstruction, acidify the intestinal environment, adjust the intestinal flora, and help patients follow a low-protein diet.
Another important issue in TIPS placement is reducing the operator’s radiation dose [22,23]. Although the operators have appropriate radiation protection and their exposure is significantly lower than that of the patients, the cumulative dose of radiation exposure throughout their career is still relatively high [24]. Some clinical reports have indicated that doctors who perform fluoroscopy are at an increased risk of radiation-related cataracts [25]. Our study shows that compared with TIPS placement under fluoroscopy, double C-arm guidance reduces the DAP of the operator, although not significantly. In a prospective study, Luo et al [26] assessed TIPS placement and CBCT acquisition post-PV puncture in 20 patients, reporting a higher mean overall DAP (295.5±66.6 Gy/cm2) than that observed in the present study. However, the DAP comparison between different studies is unreliable because TIPS is performed with different angiography equipment that is not directly comparable.
This study has 2 major limitations. The first is that due to its retrospective design, the number of needles passed could not be evaluated directly. It is well known that puncture-related complications are directly related to the number of needle passes. The second limitation is the small sample size. Future prospective and large sample studies should be conducted to further evaluate duration, radiation exposure, and number of punctures needed to access the portal vein system using double C-arm DSA guidance.
Conclusions
In summary, the new double C-arm DSA guidance method for PV puncture in the TIPS procedure appears to be safe and effective. We observed a significantly shorter PV puncture time and fewer intraprocedural complications. However, further studies including more patients and specific analyses are required to quantify these benefits accurately.
Figures
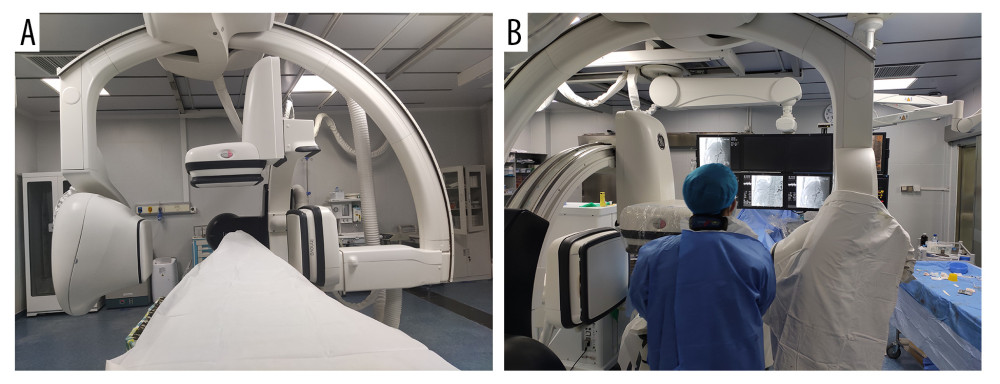 Figure 1. Operating the machine and doctor’s position. (A) The double C-arm digital subtraction angiography system. (B) Position of the operator and operation process. Image Software: Adobe Photoshop, CS6, Adobe Systems.
Figure 1. Operating the machine and doctor’s position. (A) The double C-arm digital subtraction angiography system. (B) Position of the operator and operation process. Image Software: Adobe Photoshop, CS6, Adobe Systems. 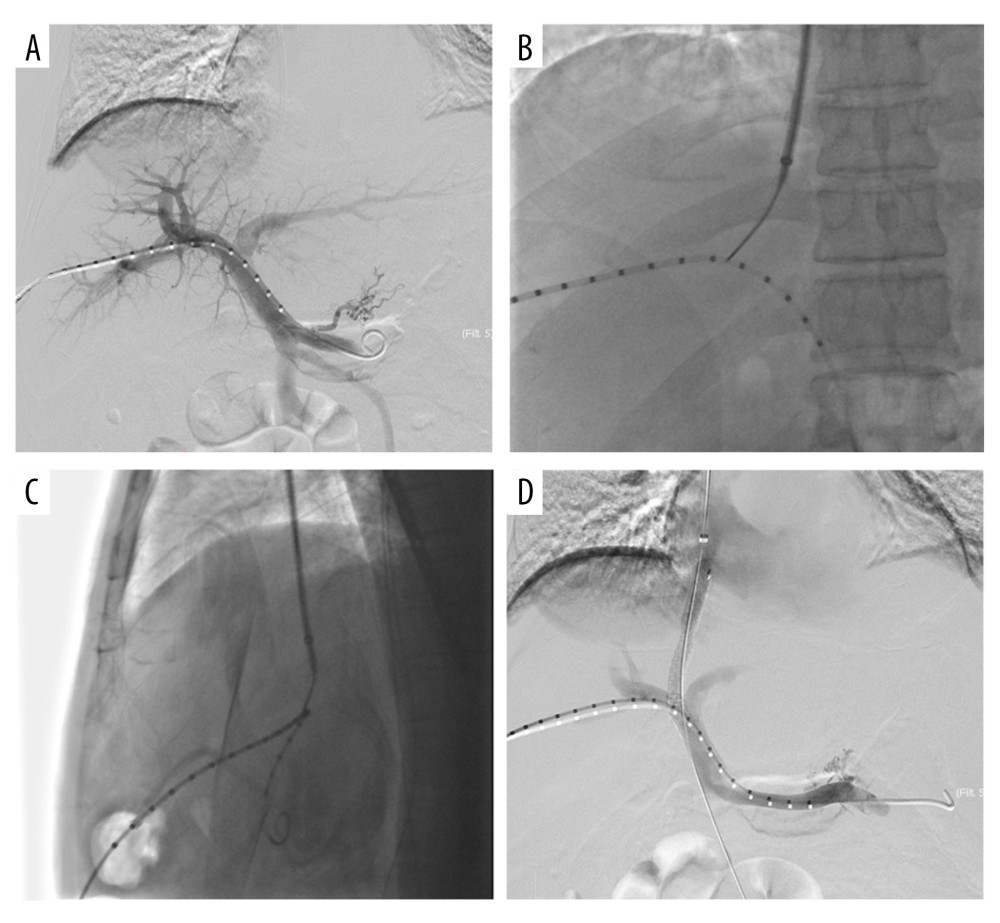 Figure 2. Double C-arm digital subtraction angiography (DSA) guidance for portal vein (PV) puncture. (A) Angiography via a marked pigtail catheter placed in the PV to assess the puncture site. (B, C) The front and side PV positions are compared in detail using double C-arm DSA, and the most appropriate puncture point is determined. (D) After transjugular intrahepatic portosystemic shunt placement, angiography reveals that the left and right branches of the PV and shunt channels are unobstructed. Image Software: Adobe Photoshop, CS6, Adobe Systems.
Figure 2. Double C-arm digital subtraction angiography (DSA) guidance for portal vein (PV) puncture. (A) Angiography via a marked pigtail catheter placed in the PV to assess the puncture site. (B, C) The front and side PV positions are compared in detail using double C-arm DSA, and the most appropriate puncture point is determined. (D) After transjugular intrahepatic portosystemic shunt placement, angiography reveals that the left and right branches of the PV and shunt channels are unobstructed. Image Software: Adobe Photoshop, CS6, Adobe Systems. References
1. Berzigotti A, Advances and challenges in cirrhosis and portal hypertension: BMC Med, 2017; 15; 200
2. Bosch J, Abraldes JG, Berzigotti A, Portal hypertension and gastrointestinal bleeding: Semin Liver Dis, 2008; 28; 3-25
3. Simonetto DA, Gines P, Kamath PS, Hepatorenal syndrome: Pathophysiology, diagnosis, and management: BMJ, 2020; 370; m2687
4. Turon F, Casu S, Hernández-Gea V, Variceal and other portal hypertension related bleeding: Best Pract Res Clin Gastroenterol, 2013; 27; 649-64
5. Rösch J, Hanafee WN, Snow H, Transjugular portal venography and radiologic portacaval shunt: an experimental study: Radiology, 1969; 92; 1112-14
6. Loffroy R, Favelier S, Pottecher P, Transjugular intrahepatic portosystemic shunt for acute variceal gastrointestinal bleeding: Indications, techniques and outcomes: Diagn Interv Imaging, 2015; 96; 745-55
7. Fidelman N, Kwan SW, LaBerge JM, The transjugular intrahepatic portosystemic shunt: an update: Am J Roentgenol, 2012; 199; 746-55
8. Ripamonti R, Ferral H, Alonzo M, Transjugular intrahepatic portosystemic shunt-related complications and practical solutions: Semin Intervent Radiol, 2006; 23; 165-76
9. Shah RP, Sze DY, Complications during transjugular intrahepatic portosystemic shunt creation: Tech Vasc Interv Radiol, 2016; 19; 61-73
10. Horhat A, Bureau C, Thabut D, Transjugular intrahepatic portosystemic shunt in patients with cirrhosis: Indications and posttransjugular intrahepatic portosystemic shunt complications in 2020: U Eur Gastroenterol J, 2021; 9; 203-8
11. Leger T, Petit A, Moustarhfir Y, Use of virtual target fluoroscopic display of three-dimensional CO2 wedged hepatic vein portography for TIPS placement: Cardiovasc Intervent Radiol, 2021; 44; 1817-22
12. David A, Liberge R, Meyer J, Ultrasonographic guidance for portal vein access during transjugular intrahepatic portosystemic shunt (TIPS) placement: Diagn Interv Imaging, 2019; 100; 445-53
13. Kao SD, Morshedi MM, Narsinh KH, Intravascular ultrasound in the creation of transhepatic portosystemic shunts reduces needle passes, radiation dose, and procedure time: A retrospective study of a single-institution experience: J Vasc Interv Radiol, 2016; 27; 1148-53
14. Kee ST, Ganguly A, Daniel BL, MR-guided transjugular intrahepatic portosystemic shunt creation with use of a hybrid radiography/MR system: J Vasc Interv Radiol, 2005; 16; 227-34
15. Rouabah K, Varoquaux A, Caporossi JM, Image fusion-guided portal vein puncture during transjugular intrahepatic portosystemic shunt placement: Diagn Interv Imaging, 2016; 97; 1095-102
16. Allaire M, Walter A, Sutter O, TIPS for management of portal-hypertension-related complications in patients with cirrhosis: Clin Res Hepatol Gastroenterol, 2020; 44; 249-63
17. Tacher V, Petit A, Derbel H, Three-dimensional image fusion guidance for transjugular intrahepatic portosystemic shunt placement: Cardiovasc Intervent Radiol, 2017; 40; 1732-39
18. Yu JF, Pung L, Minami H, Virtual 2D angiography from four-dimensional digital subtraction angiography (4D-DSA): A feasibility study: Interv Neuroradiol, 2021; 27; 307-13
19. Böning G, Lüdemann WM, Chapiro J, Clinical experience with real-time 3-D guidance based on C-arm-acquired cone-beam CT (CBCT) in transjugular intrahepatic portosystemic stent shunt (TIPSS) placement: Cardiovasc Intervent Radiol, 2018; 41; 1035-42
20. Meine TC, Dewald CLA, Becker LS, Transjugular intrahepatic portosystemic shunt placement: portal vein puncture guided by 3D/2D image registration of contrast-enhanced multi-detector computed tomography and fluoroscopy: Abdom Radiol (NY), 2020; 45; 3934-43
21. Morosetti D, Lenci I, Argirò R, Use of intravascular ultrasound to improve diagnosis and treatment of transjugular intrahepatic portosystemic shunt dysfunction in patients in the long-term follow-up: Euroasian J Hepatogastroenterol, 2022; 12; 50-56
22. Cam I, Gencturk M, Shrestha P, Ultrasound-guided portal vein access and percutaneous wire placement in the portal vein are associated with shorter procedure times and lower radiation doses during TIPS placement: Am J Roentgenol, 2021; 216; 1291-99
23. Miraglia R, Gerasia R, Maruzzelli L, Radiation doses to operators performing transjugular intrahepatic portosystemic shunt using a flat-panel detector-based system and ultrasound guidance for portal vein targeting: Eur Radiol, 2017; 27; 1783-86
24. Rajesh S, Philips CA, Betgeri SS, Transjugular intrahepatic portosystemic shunt (TIPS) placement at index portal hypertensive decompensation (anticipant TIPS) in cirrhosis and the role of early intervention in variceal bleeding and ascites: Indian J Gastroenterol, 2021; 40; 361-72
25. Hidajat N, Wust P, Kreuschner M, Radiation risks for the radiologist performing transjugular intrahepatic portosystemic shunt (TIPS): Br J Radiol, 2006; 79; 483-86
26. Luo X, Wang X, Zhao Y, Real-time 3D CT image guidance for transjugular intrahepatic portosystemic shunt creation using preoperative CT: A prospective feasibility study of 20 patients: Am J Roentgenol, 2017; 208; W11-16
Figures
 Figure 1. Operating the machine and doctor’s position. (A) The double C-arm digital subtraction angiography system. (B) Position of the operator and operation process. Image Software: Adobe Photoshop, CS6, Adobe Systems.
Figure 1. Operating the machine and doctor’s position. (A) The double C-arm digital subtraction angiography system. (B) Position of the operator and operation process. Image Software: Adobe Photoshop, CS6, Adobe Systems. Figure 2. Double C-arm digital subtraction angiography (DSA) guidance for portal vein (PV) puncture. (A) Angiography via a marked pigtail catheter placed in the PV to assess the puncture site. (B, C) The front and side PV positions are compared in detail using double C-arm DSA, and the most appropriate puncture point is determined. (D) After transjugular intrahepatic portosystemic shunt placement, angiography reveals that the left and right branches of the PV and shunt channels are unobstructed. Image Software: Adobe Photoshop, CS6, Adobe Systems.
Figure 2. Double C-arm digital subtraction angiography (DSA) guidance for portal vein (PV) puncture. (A) Angiography via a marked pigtail catheter placed in the PV to assess the puncture site. (B, C) The front and side PV positions are compared in detail using double C-arm DSA, and the most appropriate puncture point is determined. (D) After transjugular intrahepatic portosystemic shunt placement, angiography reveals that the left and right branches of the PV and shunt channels are unobstructed. Image Software: Adobe Photoshop, CS6, Adobe Systems. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952