15 February 2023: Clinical Research
Outcomes Following Carotid Endarterectomy and Carotid Artery Stenting in Patients with Carotid Artery Stenosis: A Retrospective Study from a Single Center in South Korea
Myung Ji Kim1BCDE, Sung-Kon Ha1AFG*DOI: 10.12659/MSM.939223
Med Sci Monit 2023; 29:e939223
Abstract
BACKGROUND: Previous randomized controlled trials and meta-analyses comparing carotid endarterectomy (CEA) and carotid artery stenting (CAS) included a large number of patients, but the diagnosis, treatment selection, and performance were heterogeneous. This retrospective study from a single center in South Korea aimed to evaluate outcomes following CEA and CAS in patients with carotid artery stenosis.
MATERIAL AND METHODS: A retrospective analysis was performed using the data of patients who underwent carotid revascularization between September 2016 and June 2021 at a single institution. The primary outcomes were stroke, myocardial infarction (MI), and death during the periprocedural period.
RESULTS: We enrolled a total of 61 (44 symptomatic and 17 asymptomatic) patients who underwent CEA or CAS. Among them, 36 (59%) underwent CEA and 25 (41%) underwent CAS. Statistically significant differences were found between the groups in degree of carotid stenosis (CEA: 87.0±9.1, CAS: 80.5±9.3, P=0.007). All patients with confirmed plaque ulceration before carotid revascularization underwent CEA. Two (3.3%) periprocedural strokes occurred, 1 in each group, on the ipsilateral side. There were no significant differences between CEA and CAS in the event-free survival rate for stroke during the follow-up (log-rank test=0.806).
CONCLUSIONS: Favorable outcomes in terms of periprocedural stroke were observed. We found no significant difference between the 2 carotid revascularization techniques in the incidence of periprocedural stroke in symptomatic and asymptomatic patients. To confirm our findings, further studies involving a larger number of patients and continuous follow-up are necessary.
Keywords: carotid stenosis, Endarterectomy, Carotid, Stents, Stroke, Ischemic Attack, Transient, Humans, Treatment Outcome, Carotid Arteries, Risk Factors
Background
The prevalence of stroke in adults with carotid artery stenosis ranges from 10% to 20% [1,2]. Stroke following carotid revascularization is a serious consequence that has a significant impact on treatment options [3]. Many randomized controlled trials (RCTs) [4–9] and meta-analyses [3,10–13] comparing carotid endarterectomy (CEA) and carotid artery stenting (CAS) have been conducted. Although the results of each study vary somewhat, CEA has been suggested as a more effective and safer treatment for the prevention of stroke in patients with symptomatic or asymptomatic CS compared with CAS [14–17]. A recent meta-analysis revealed that the increased risk of CAS is mostly due to an increase in periprocedural stroke in patients aged >70 years [18]. However, CAS offers a number of advantages: it does not require general anesthesia, it is less invasive, and it requires a shorter hospital stay after the procedure [13]. Previous studies have suggested that the outcomes of CAS in patients with symptomatic or asymptomatic CS were noninferior to CEA in terms of periprocedural death, stroke, and myocardial infarction (MI) [5,15,19,20]. Existing RCTs and meta-analyses included many patients, but the diagnosis, treatment selection, and performance were heterogeneous. Therefore, this retrospective study from a single center in South Korea aimed to evaluate outcomes following carotid endarterectomy or carotid artery stenting in patients with carotid artery stenosis.
Material and Methods
PATIENTS:
A retrospective analysis was performed on all patients who underwent carotid revascularization between September 2016 and June 2021 at a single institution. Clinical follow-up included neurologic examination, carotid artery ultrasonography, and radiologic assessment. All patients underwent cerebral angiography prior to CEA or CAS, and postoperative computed tomography (CT) scans and diffusion-weighted magnetic resonance imaging (MRI). Patients with a follow-up period of less than 6 months after CEA or CAS were excluded. Patients with a transient ischemic attack (TIA) or stroke within 180 days, and with angiography-confirmed ≥50% carotid stenosis, according to the method described by the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [21] before carotid revascularization were deemed symptomatic. Patients with no recent (in the last 6 months) neurological events and carotid stenosis ≥60% on angiography were considered asymptomatic [20]. Patients who previously had a disabling stroke or persistent AF were not eligible for the present study. This study received ethics approval from the Institutional Review Board of Korea University Ansan Hospital (IRB number: 2021AS0374), and all procedures were performed in accordance with the Declaration of Helsinki. The requirement to obtain informed consent was waived, as this was a retrospectively designed study.
CEA AND CAS:
Diagnostic angiography, treatment selection, and treatment for each patient were performed by a single neurosurgeon. The decision to perform CEA or CAS was based on patient characteristics, surgical anatomy, vessel anatomy, and plaque morphology. The procedures for CEA and CAS have been previously described [22]. CAS was performed under local anesthesia using transfemoral arterial access. An embolic protection device (Emboshield NAV6 [ev3, Plymouth, Minnesota, USA]; RX Accunet [Abbott, Santa Clara, California, USA]) was carefully placed at the distal end of the stenosis. The stenosis was predilated with a balloon of appropriate size (Sterling [Boston Scientific, Marlborough, Massachusetts, USA], and the self-expanding stent (AccuLink [Abbott, Santa Clara, California, USA]). Because CAS was not performed in an emergent manner, patients received dual antiplatelets, aspirin (100 mg), and clopidogrel (75 mg daily). All patients who underwent CAS were recommended to continue dual antiplatelet therapy. CEA was performed under general anesthesia. For CEA, patients received 100 mg of aspirin daily, and those who took dual antiplatelet therapy stopped clopidogrel 5–7 days before CEA. After the Hemovac was removed, the patient continued dual antiplatelet therapy. If contralateral carotid stenosis was detected among CEA candidates, balloon occlusion tests (BOT) were performed to confirm whether patients would tolerate the procedure. An arterial shunt was used selectively for high-risk patients who could not tolerate BOT.
OUTCOMES:
The primary outcomes were stroke, MI, and death during the periprocedural period. The periprocedural period was defined as the 30 days after CEA or CAS, as described elsewhere [20]. We also evaluated asymptomatic diffusion-weighted image (DWI) hyperintense lesions, TIA within 30 days, ipsilateral intracerebral hemorrhage, cerebral hyperperfusion syndrome, cranial deficit, ipsilateral stroke or TIA after 30 days, restenosis, and mobile thrombus during the follow-up period.
STATISTICAL ANALYSIS:
Descriptive statistics are presented as the mean and standard deviation for continuous variables and as frequencies and percentages for categorical variables. The chi-squared test, Fisher’s exact test, and Mann-Whitney U test were used to evaluate differences between the CEA and CAS groups. Kaplan-Meier survival analysis was used to investigate the association between treatment modality and the occurrence of stroke following carotid revascularization after stratification using the log-rank test. Statistical significance was indicated by a
Results
PATIENTS:
A total of 61 patients (50 men and 11 women) who underwent CEA or CAS were enrolled in the present study. Among them, 36 (59%) underwent CEA and 25 (41%) underwent CAS. Of the 44 symptomatic patients (72.1%), 35 (79.5%) experienced infarction and 9 (20.5%) experienced TIA within 180 days prior to carotid revascularization. Seventeen (27.9%) patients had asymptomatic carotid stenosis. The mean degree of carotid stenosis was 84.3±9.6. Severe contralateral carotid stenosis (70–100%) was observed in 13 patients (21.3%). Patient baseline demographics and characteristics of carotid stenosis are presented in Table 1.
COMPARISON OF BASELINE CHARACTERISTICS BETWEEN CEA AND CAS GROUP:
There was no significant difference in age between the 2 groups (CEA, 70.3±8.1 vs CAS 68.9±10.6, P=0.582) (Table 1). None of the patient-specific characteristics, including medical comorbidities, differed significantly between the 2 groups. Statistically significant differences were found between the 2 groups with respect to degree of carotid stenosis (CEA, 87.0±9.1 vs CAS, 80.5±9.3, P=0.007). All patients with confirmed plaque ulceration before carotid revascularization underwent CEA (CEA, 100% vs CAS 0%, P<0.0001).
COMPARISON OF OUTCOME BETWEEN CEA AND CAS GROUP:
The treatment outcomes for carotid revascularization are described in Table 2. No periprocedural death or MI was reported in the present study. Two (3.3%) periprocedural strokes occurred, both on the ipsilateral side. One (2.8%) was a disabling stroke that occurred immediately after surgery in the CEA group and the other (4.0%) was a non-disabling stroke that occurred 6 days after the intervention in the CAS group. Both patients with periprocedural stroke were in the symptomatic group, and periprocedural stroke did not occur in the asymptomatic group. There was no significant difference in the rates of periprocedural stroke, MI, or death between the 2 groups. No ipsilateral intracerebral hemorrhage following carotid revascularization was reported. One patient (2.8%) in the CEA group reported severe headache and deterioration of consciousness without any additional ischemic or hemorrhagic lesions, which were diagnosed as cerebral hyperperfusion syndrome. Cranial nerve deficit occurred in 1 patient (2.8%) in the CEA group. One patient (4.0%) in the CAS group, who experienced TIA after 30 days, was diagnosed with restenosis, and CEA with bovine angioplasty was performed. A mobile thrombus was found in 1 patient (2.8%) in the CEA group, and there were no other complications while antiplatelet therapy was continued. Kaplan-Meier curves that evaluated CEA and CAS in relation to stroke are shown in Figure 1. There were no significant differences between CEA and CAS in the event-free survival rate for stroke following carotid revascularization (P=0.806).
CASE 1: A patient was admitted to the emergency room with concerns of sudden loss of sensation in the left hand and blurred vision. No underlying diseases were identified at the time of admission. The MRI revealed acute right posterior cerebral artery (PCA) territory infarction (Figure 2A). A week later, angiography revealed chronic occlusion of the right intracerebral artery (ICA), and the left ICA flow was considered to supply to the right anterior cerebral artery, middle cerebral artery (MCA), and PCA through the anterior communicating artery (Figure 2B). The degree of left carotid stenosis was 80% on angiography (Figure 2C). Right PCA territory infarction might have resulted from decreased blood flow due to left carotid stenosis. The patient continued to take dual antiplatelet and lipid-lowering drugs. Two weeks later, the patient underwent CAS with a 9×50 mm stent instead of CEA owing to contralateral ICA occlusion (Figure 2D). The patient did not have any periprocedural complications or stroke during the follow-up period.
CASE 2: A patient presented with left MCA territory scattered infarction and 50% carotid stenosis. The best medical treatment was recommended. Four months later, recurrent left MCA territory infarction occurred (Figure 3A), and the carotid stenosis was slightly increased to 55% on angiography (Figure 3B). A neurosurgeon recommended CEA and continuation of aspirin until the surgery. There was a sign of neurologic deterioration following CEA; therefore, MR angiography (MRA) was performed and left common carotid artery occlusion was identified (Figure 3C). Emergency surgery was immediately performed to remove a massive thrombus. Postoperative MRA showed non-visualization from the left proximal ICA to the distal ICA (Figure 3D). We investigated why an immediate complication occurred, and we suspected that aspirin was not administered to prepare the patient for an endobronchial ultrasound bronchoscopy to diagnose lung cancer.
Discussion
We retrospectively reviewed the outcomes of carotid revascularization performed by a single neurosurgeon at a single institute. More favorable outcomes in terms of periprocedural stroke were observed in the present study compared with those in previous studies (Table 3). The periprocedural stroke rates were 3.3% overall and 2.8% and 4.0% for CEA and CAS, respectively. There were no significant differences between the 2 groups. No periprocedural stroke occurred following carotid revascularization in the asymptomatic patients. According to the American Heart Association, the combined risk of stroke and death from CEA should not exceed 3% for asymptomatic patients and 6% for symptomatic patients. The overall periprocedural risk of carotid revascularization should be less than 3% or 6% for asymptomatic and symptomatic carotid stenosis, respectively, according to the European guidelines for choosing CAS or CEA [23]. Our study results were consistent with the recommendations of these guidelines. Previous randomized controlled trials (RCTs) and meta-analyses [3,10–12,24] reported that CAS had a significantly higher risk of periprocedural stroke than CEA. The International Carotid Stenting Study (ICSS) [9] and endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) [6] suggested that CEA should be the treatment of choice owing to significantly higher periprocedural complication rates (stroke/deaths) in CAS. However, the Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) [7,8] did not reveal inferiority of CAS compared to CEA regarding periprocedural complication rate (stroke/death/MI); this was also shown in 10-year follow-up data. The Stent-Protected Angioplasty versus Carotid Endarterectomy study (SPACE) [4] demonstrated that the rate of recurrent ipsilateral ischemic strokes was not significantly different between the 2 groups until 2-year follow-up. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy study (SAPPHIRE) [5] concluded that CAS with the use of an embolic protection device is not inferior to CEA among “high-risk” patients with severe carotid stenosis and coexisting conditions for 3 years. In the Asymptomatic Carotid Trial (ACT-1) [19] comparing CEA and CAS in asymptomatic patients, in terms of periprocedural death/stroke/MI or ipsilateral stroke within 1 year, CAS was shown to be noninferior to CEA. A meta-analysis reported that stenting for symptomatic carotid stenosis carries a higher risk of periprocedural stroke or death, especially in patients over the age of 70. Although carotid stenting is just as effective as endarterectomy in preventing recurrent stroke, endarterectomy is still preferred when combining procedural safety and long-term efficacy. The risk of periprocedural stroke or death with stenting compared to CEA may be slightly higher in people with asymptomatic carotid stenosis [13]. Despite the small sample size of the current study, periprocedural stroke did not differ significantly between the CEA and CAS groups or between the symptomatic and asymptomatic groups. There is still a need for further RCTs regarding asymptomatic stenosis.
Systemic review of guidelines reported 86% of guidelines recommended CEA for 50–99% average-CEA-risk asymptomatic carotid stenosis and 61% recommended CAS; 47% of guidelines recommended CAS for high-CEA-risk asymptomatic carotid stenosis; 94% of guidelines 94% of guidelines recommended CEA for patients with 50–99% average-CEA-risk symptomatic carotid stenosis and 58% recommended CAS; and 82% of guideline recommended CAS for high-CEA-risk symptomatic carotid stenosis [25]. The European Stroke Organization guideline recommends CEA in patients with 60–99% asymptomatic carotid stenosis and with 70–99% symptomatic stenosis, and suggests CEA with 50–69% symptomatic stenosis. CAS might be considered in patients less than 70 years old with symptomatic 50–99% carotid stenosis [26]. The Society for Vascular Surgery recommends CEA as the first-line treatment for symptomatic low-risk surgical patients with stenosis of 50% to 99% and asymptomatic patients with stenosis of 70% to 99% [27].
As CAS techniques and materials have advanced, favorable outcomes in CAS have been reported recently, with careful consideration of patient, plaque, and vessel characteristics. Age has been suggested as an important factor to consider when selecting CEA or CAS. Meta-analyses of major trials revealed that increasing age (≥70 years) is associated with an increase in periprocedural stroke with CAS relative to CEA [18,28–31]. A recent study suggested that older patients should be treated with CEA [18]. In the present study, there was no significant difference in age between the 2 groups (CEA, 70.3±8.1 vs CAS 68.9±10.6,
We believe that the favorable outcomes in the present study are attributable to the proper allocation of patients for the right procedure, despite the small number of patients enrolled in the study. A hybrid vascular neurosurgeon can assess which treatment is safer and more feasible during diagnostic angiography and perform the selected treatment. Medical comorbidities, vascular anatomy, and carotid plaque morphology are factors that determine the decision between CEA and CAS. In the current study, statistically significant differences were found between the 2 groups with respect to the degree of carotid stenosis (CEA, 87.0±9.1 vs CAS, 80.5±9.3;
CEA was selected in cases of ulcerative plaque (Figure 4A), severe diffuse calcification (Figure 4B), aortic arch type II or III (Figure 4C), and acute angle of entry (Figure 4D). CEA is known to have a higher morbidity rate in patients with contralateral occlusive internal carotid artery stenosis [21,34,35]. Patients with severe medical comorbidities and challenging surgical anatomy, resulting in difficult cervical dissection, might be deemed high-risk for CEA. CEA is difficult to perform surgically if lesions are too high (above C2) or too low (below the clavicle). The difficulty in visualizing the endpoint of the endarterectomy, as well as the higher incidence of cranial nerve injury, may be attributed to the high carotid exposure [36].
On the other hand, plaque morphology and unfavorable vessel anatomy, including the complexity of the aortic arch and vessel tortuosity, can also influence the feasibility and safety of CAS [37,38]. A carotid lesion with more than 90° bends within a short distance of the target lesion makes CAS more challenging, and the insertion of a distal embolic protection device might be disturbed by distal ICA tortuosity [36,39]. CAS was selected in cases of stenosis distant from the carotid bifurcation (Figure 5A), long segment (Figure 5B), and contralateral intracerebral artery (ICA) occlusion or severe stenosis (Figure 5C). Patients who are regarded as too high-risk for surgery, or who have surgically inaccessible lesions, have been offered CAS [3,23,40]. In asymptomatic patients, CAS is preferred because it is less invasive and does not require general anesthesia. When vulnerable plaques or heavily calcified, extensive pre-occlusive lesions were identified [41], CEA was selected, even if a patient preferred CAS. A previous study identified that periprocedural stroke risk was higher in patients with lipid-rich plaques treated with CAS [42]. During the implantation of the wire or stent over the carotid lesion, unstable plaques increase the risk of embolization. As technology advances, using flow-reversal embolic protection instead of distal filter protection could lessen the risk of embolization [36,43].
The major limitation of the current study is that it was a retrospective review of procedures performed at a single institution with a small number of patients. The comparison of the CEA and CAS groups was limited by selection bias, which may have a critical role in patient selection and the decision to perform procedures. However, all patients enrolled in the present study were allocated and operated upon by a single surgeon, and we believe that this minimizes the selection bias resulting from procedures performed by multiple surgeons. Further investigation with a larger number of patients and continuous follow-up are needed to confirm our findings.
Conclusions
Favorable outcomes in terms of periprocedural stroke were observed in the present study. The 2 carotid revascularization techniques did not result in a difference in the incidence of periprocedural stroke in both symptomatic and asymptomatic patients. Safety and effectiveness can be improved with both CEA and CAS if plaque morphology and vessel anatomy, rather than age, are fully considered during treatment selection. To confirm our findings, further investigations involving a larger number of patients and continuous follow-up are necessary.
Figures
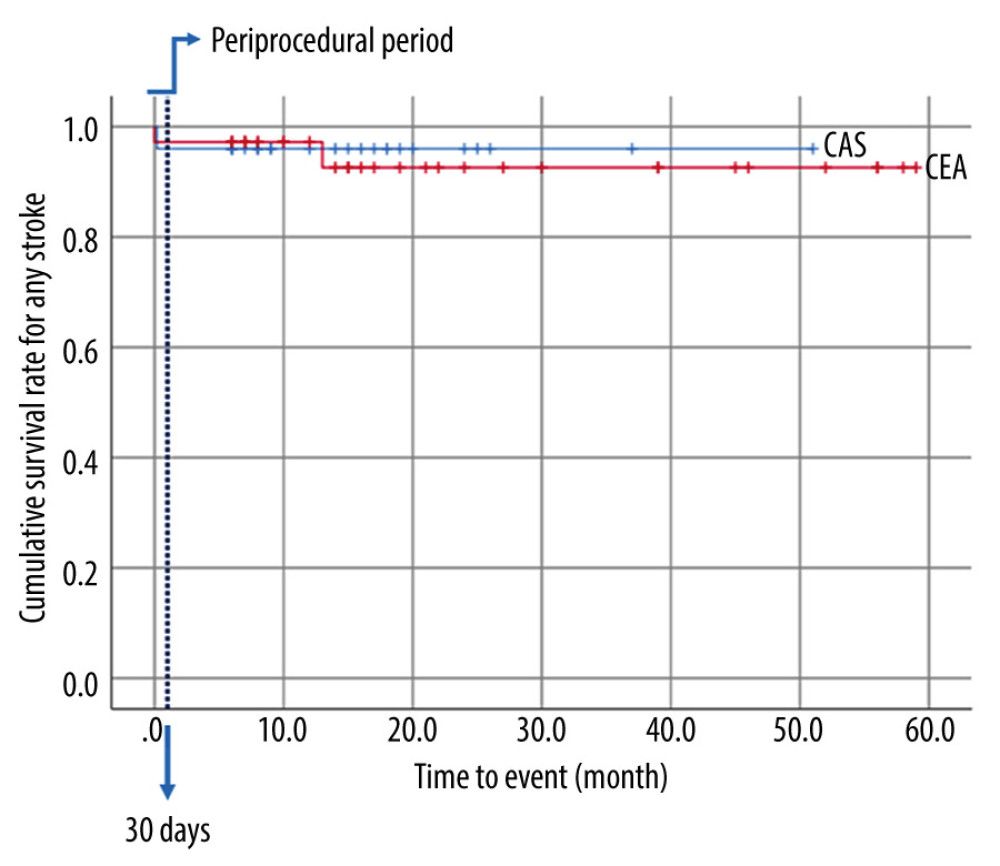 Figure 1. Kaplan-Meier event-free survival rate for strokeThe blue line represents “Periprocedural period” within 30 days after the procedure.
Figure 1. Kaplan-Meier event-free survival rate for strokeThe blue line represents “Periprocedural period” within 30 days after the procedure. 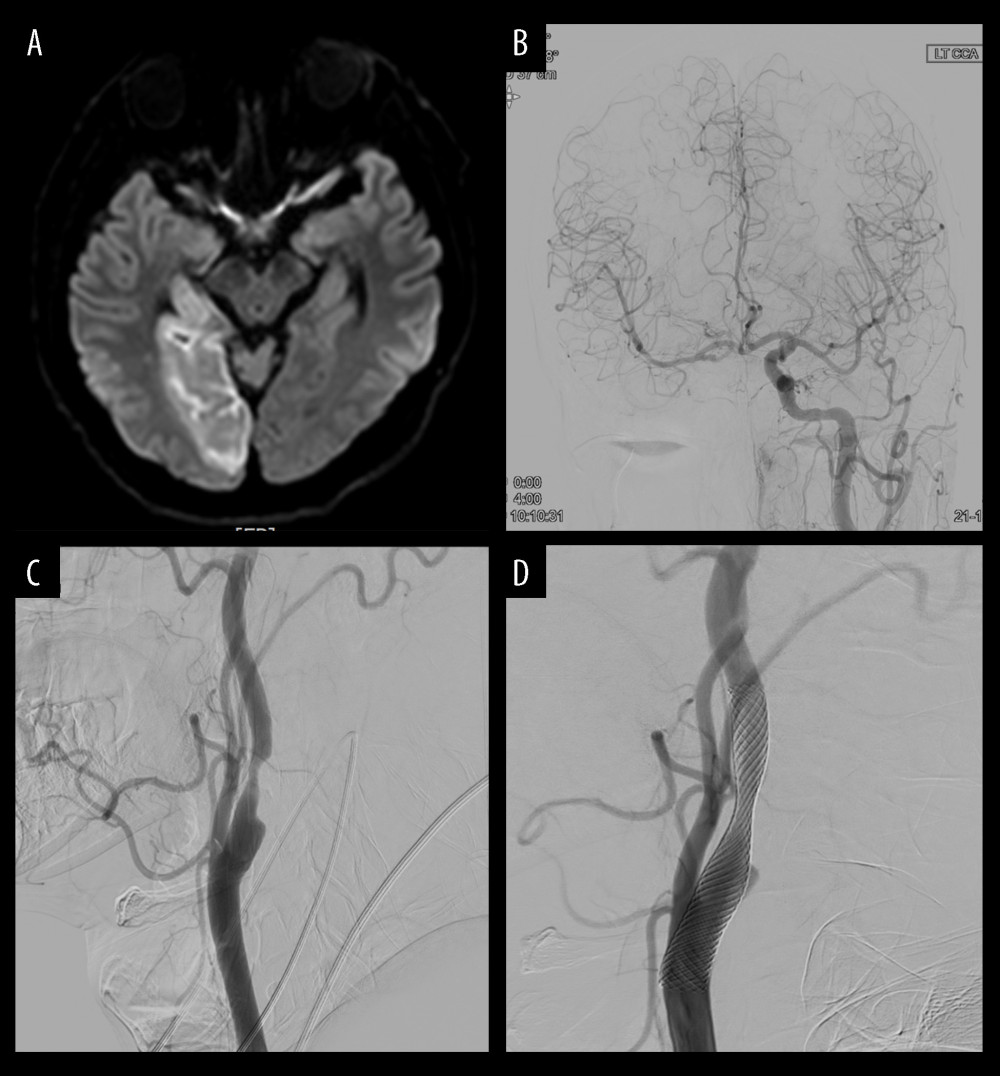 Figure 2. Illustration of case 1(A) Magnetic resonance imaging reveals an acute right posterior cerebral artery (PCA) territory infarction. (B) Angiography reveals chronic occlusion of the right intracerebral artery (ICA), and the left ICA flow was considered to supply to the right anterior cerebral artery, middle cerebral artery, and PCA through the anterior communicating artery. (C) Degree of left carotid stenosis is 80% on angiography. (D) Carotid artery stenting (9×50 mm) is performed for left carotid stenosis.
Figure 2. Illustration of case 1(A) Magnetic resonance imaging reveals an acute right posterior cerebral artery (PCA) territory infarction. (B) Angiography reveals chronic occlusion of the right intracerebral artery (ICA), and the left ICA flow was considered to supply to the right anterior cerebral artery, middle cerebral artery, and PCA through the anterior communicating artery. (C) Degree of left carotid stenosis is 80% on angiography. (D) Carotid artery stenting (9×50 mm) is performed for left carotid stenosis. 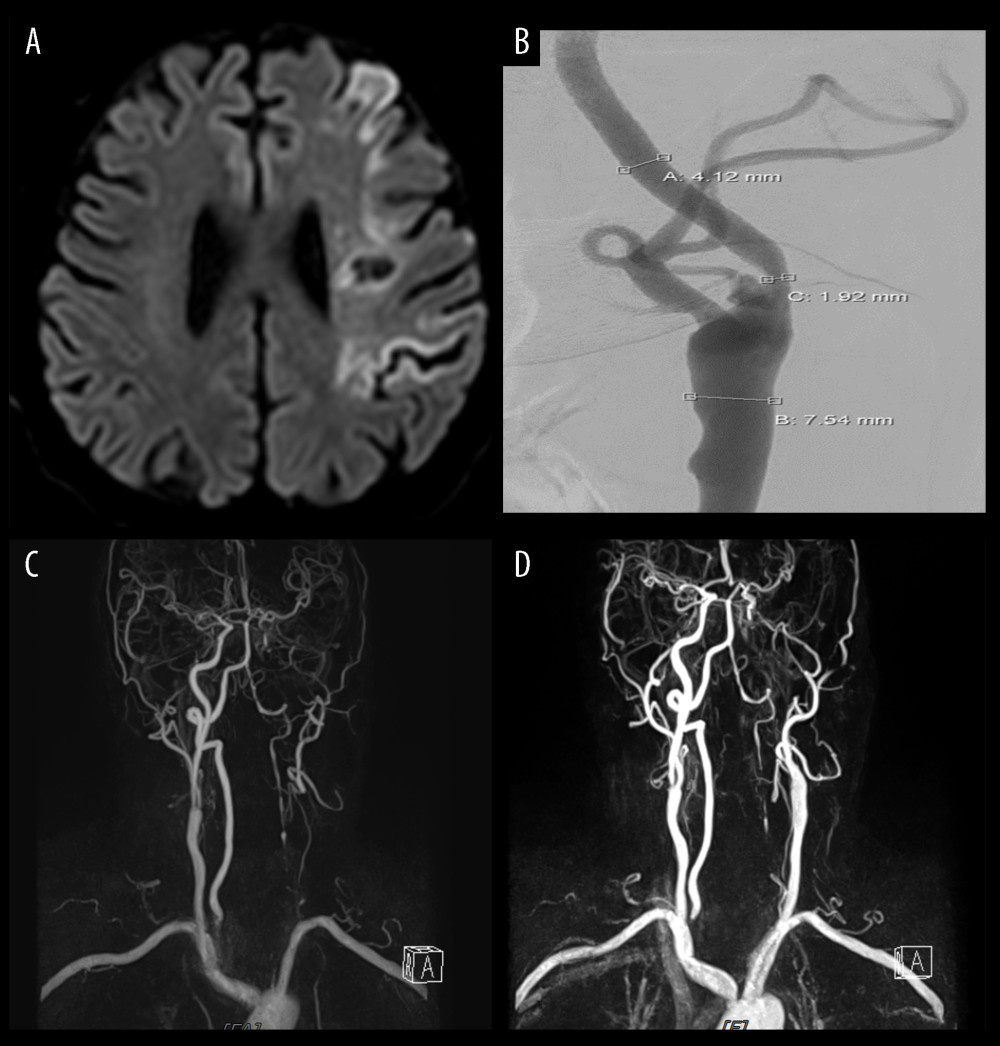 Figure 3. Illustration of case 2(A) Magnetic resonance image reveals recurrent left middle cerebral artery territory infarction. (B) Degree of left carotid stenosis is 55% on angiography, which has slightly increased from 50%. (C) Magnetic resonance angiography shows left common carotid artery occlusion following carotid endarterectomy. (D) Magnetic resonance angiography shows left intracerebral artery occlusion following emergent thrombus removal.
Figure 3. Illustration of case 2(A) Magnetic resonance image reveals recurrent left middle cerebral artery territory infarction. (B) Degree of left carotid stenosis is 55% on angiography, which has slightly increased from 50%. (C) Magnetic resonance angiography shows left common carotid artery occlusion following carotid endarterectomy. (D) Magnetic resonance angiography shows left intracerebral artery occlusion following emergent thrombus removal. 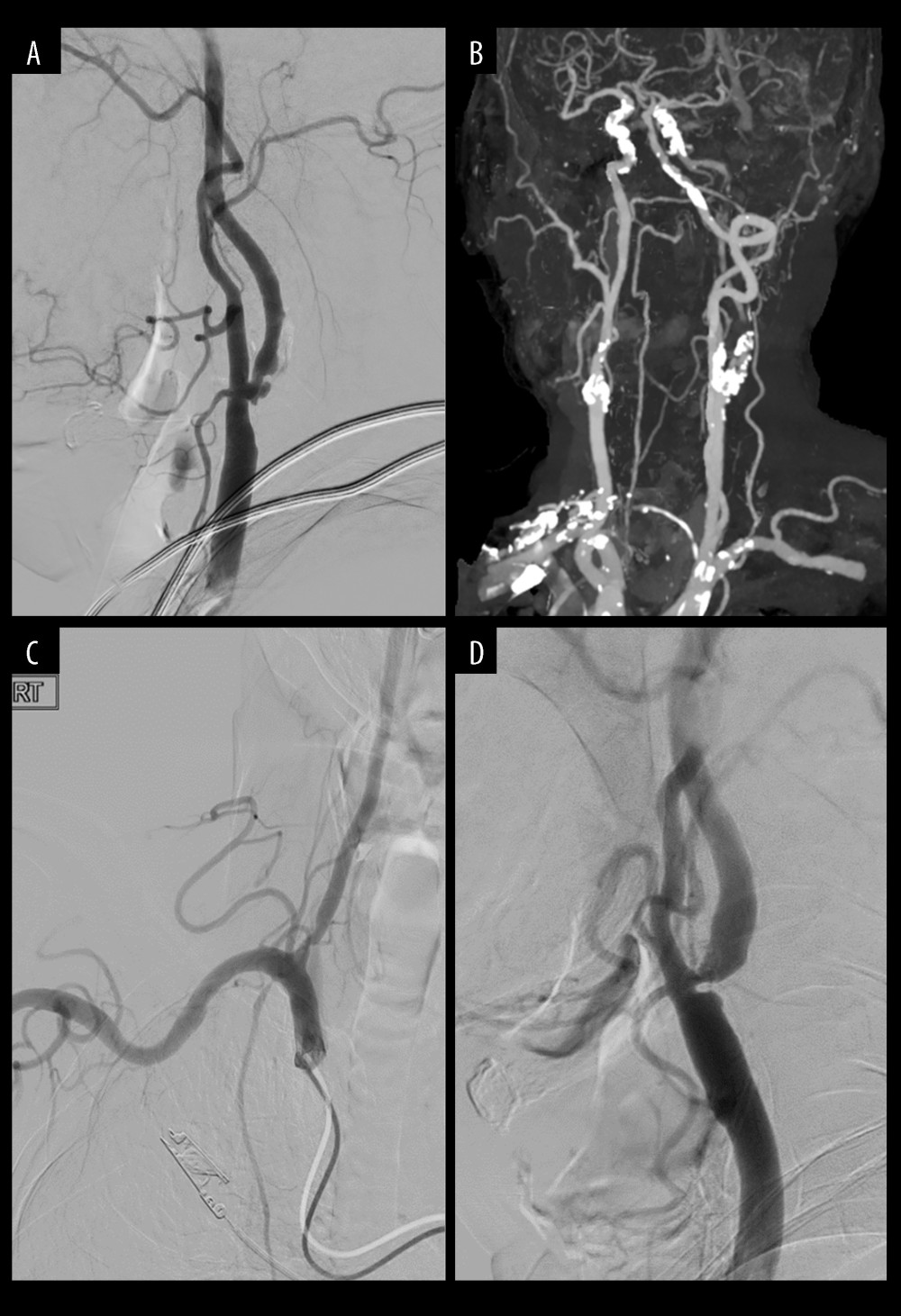 Figure 4. Cases of selecting carotid endarterectomy(A) Plaque ulcerative change. (B) Heavily, extensive calcified plaque. (C) Aortic arch type II (or III). (D) Acute entry of angle.
Figure 4. Cases of selecting carotid endarterectomy(A) Plaque ulcerative change. (B) Heavily, extensive calcified plaque. (C) Aortic arch type II (or III). (D) Acute entry of angle. 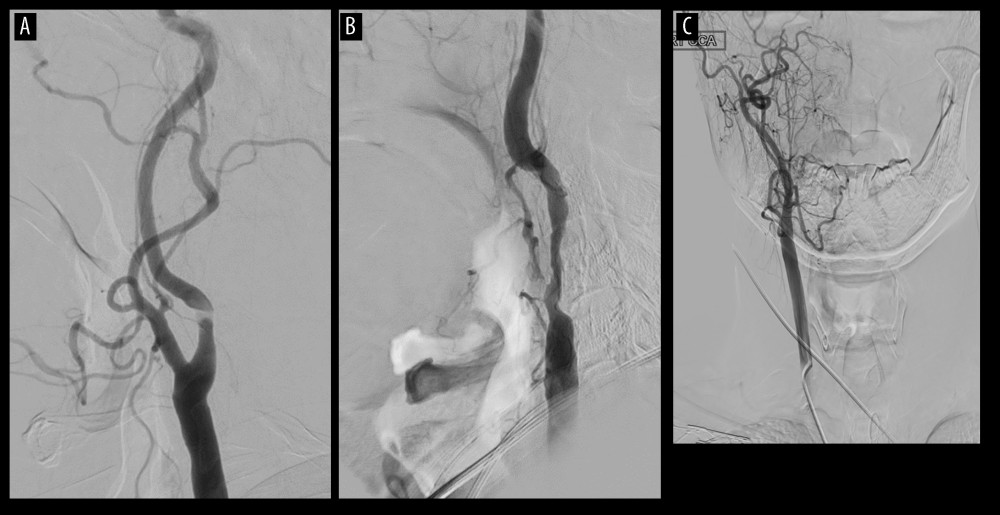 Figure 5. Cases of selecting carotid artery stenting(A) Stenosis at high position. (B) Long segment of stenosis. (C) Contralateral intracerebral artery occlusion (or severe stenosis).
Figure 5. Cases of selecting carotid artery stenting(A) Stenosis at high position. (B) Long segment of stenosis. (C) Contralateral intracerebral artery occlusion (or severe stenosis). References
1. Silver FL, Mackey A, Clark WM, Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST): Stroke, 2011; 42(3); 675-80
2. Lovett JK, Coull AJ, Rothwell PM, Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies: Neurology, 2004; 62(4); 569-73
3. Xin W, Yang S, Li Q, Yang X, Endarterectomy versus stenting for the prevention of periprocedural stroke or death in patients with symptomatic or asymptomatic carotid stenosis: A meta-analysis of 10 randomized trials: Ann Transl Med, 2021; 9(3); 256
4. Eckstein HH, Ringleb P, Allenberg JR, Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial: Lancet Neurol, 2008; 7(10); 893-902
5. Yadav JS, Wholey MH, Kuntz RE, Protected carotid-artery stenting versus endarterectomy in high-risk patients: N Engl J Med, 2004; 351(15); 1493-501
6. Mas JL, Chatellier G, Beyssen B, Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis: N Engl J Med, 2006; 355(16); 1660-71
7. Mantese VA, Timaran CH, Chiu D, The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): Stenting versus carotid endarterectomy for carotid disease: Stroke, 2010; 41(10 Suppl); S31-34
8. Otite FO, Khandelwal P, Malik AM, Chaturvedi S, National patterns of carotid revascularization before and after the Carotid Revascularization Endarterectomy vs Stenting Trial (CREST): JAMA Neurol, 2018; 75(1); 51-57
9. Bonati LH, Dobson J, Featherstone RL, Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: The International Carotid Stenting Study (ICSS) randomised trial: Lancet, 2015; 385(9967); 529-38
10. Lokuge K, de Waard DD, Halliday A, Meta-analysis of the procedural risks of carotid endarterectomy and carotid artery stenting over time: Br J Surg, 2018; 105(1); 26-36
11. Kakkos SK, Kakisis I, Tsolakis IA, Geroulakos G, Endarterectomy achieves lower stroke and death rates compared with stenting in patients with asymptomatic carotid stenosis: J Vasc Surg, 2017; 66(2); 607-17
12. Poorthuis MHF, Brand EC, Halliday A, A systematic review and meta-analysis of complication rates after carotid procedures performed by different specialties: J Vasc Surg, 2020; 72(1); 335-43.e17
13. Müller MD, Lyrer P, Brown MM, Bonati LH, Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis: Cochrane Database Syst Rev, 2020; 2(2); CD000515
14. Gurm HS, Yadav JS, Fayad P, Long-term results of carotid stenting versus endarterectomy in high-risk patients: N Engl J Med, 2008; 358(15); 1572-79
15. Brott TG, Howard G, Roubin GS, Long-term results of stenting versus endarterectomy for carotid-artery stenosis: N Engl J Med, 2016; 374(11); 1021-31
16. Ederle J, Bonati LH, Dobson J, Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): Long-term follow-up of a randomised trial: Lancet Neurol, 2009; 8(10); 898-907
17. Ederle J, Dobson J, Featherstone RL, Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): An interim analysis of a randomised controlled trial: Lancet, 2010; 375(9719); 985-97
18. Müller MD, Lyrer PA, Brown MM, Bonati LH, Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis: Stroke, 2021; 52(1); e3-e5
19. Rosenfield K, Matsumura JS, Chaturvedi S, Randomized trial of stent versus surgery for asymptomatic carotid stenosis: N Engl J Med, 2016; 374(11); 1011-20
20. Brott TG, Hobson RW, Howard G, Stenting versus endarterectomy for treatment of carotid-artery stenosis: N Engl J Med, 2010; 363(1); 11-23
21. North American Symptomatic Carotid Endarterectomy Trial, Methods, patient characteristics, and progress: Stroke, 1991; 22(6); 711-20
22. Sheffet AJ, Roubin G, Howard G, Design of the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST): Int J Stroke, 2010; 5(1); 40-46
23. Sastry RA, Pertsch NJ, Sagaityte E, Early outcomes after carotid endarterectomy and carotid artery stenting: A propensity-matched cohort analysis: Neurosurgery, 2021; 89(4); 653-63
24. Cui L, Han Y, Zhang S, Safety of stenting and endarterectomy for asymptomatic carotid artery stenosis: A meta-analysis of randomised controlled trials: Eur J Vasc Endovasc Surg, 2018; 55(5); 614-24
25. Abbott AL, Paraskevas KI, Kakkos SK, Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis: Stroke, 2015; 46(11); 3288-301
26. Bonati LH, Kakkos S, Berkefeld J, European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis: Eur Stroke J, 2021; 6(2); I-XLVII
27. AbuRahma AF, Avgerinos ED, Chang RW, Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease: J Vasc Surg, 2022; 75(1S); 4S-22S
28. Howard G, Roubin GS, Jansen O, Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: A meta-analysis of pooled patient data from four randomised trials: Lancet, 2016; 387(10025); 1305-11
29. Bonati LH, Dobson J, Algra A, Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: A preplanned meta-analysis of individual patient data: Lancet, 2010; 376(9746); 1062-73
30. Texakalidis P, Chaitidis N, Giannopoulos S, Carotid revascularization in older adults: A systematic review and meta-analysis: World Neurosurg, 2019; 126; 656-63.e1
31. Naylor AR, Endarterectomy versus stenting for stroke prevention: Stroke Vasc Neurol, 2018; 3(2); 101-6
32. Lee J, You JH, Oh SH, Outcomes of stenting versus endarterectomy for symptomatic extracranial carotid stenosis: A retrospective multicenter study in Korea: Ann Vasc Surg, 2019; 54; 185-92e1
33. Chiam PTL, Roubin GS, Iyer SS, Carotid artery stenting in elderly patients: Importance of case selection: Catheter Cardiovasc Interv, 2008; 72(3); 318-24
34. Perkins WJ, Lanzino G, Brott TG, Carotid stenting vs endarterectomy: New results in perspective: Mayo Clin Proc, 2010; 85(12); 1101-8
35. Rothwell PM, Eliasziw M, Gutnikov SA, Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery: Lancet, 2004; 363(9413); 915-24
36. Ricotta JJ, Aburahma A, Ascher E, Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: J Vasc Surg, 2011; 54(3); e1-31
37. Pasqui E, de Donato G, Alba G, Early and long-term outcomes of carotid stenting and carotid endarterectomy in women: Front Surg, 2021; 8; 646204
38. Noiphithak R, Liengudom A, Recent update on carotid endarterectomy versus carotid artery stenting: Cerebrovasc Dis, 2017; 43(1–2); 68-75
39. Setacci C, Chisci E, Setacci F, Siena carotid artery stenting score: A risk modelling study for individual patients: Stroke, 2010; 41(6); 1259-65
40. Mathur A, Roubin GS, Iyer SS, Predictors of stroke complicating carotid artery stenting: Circulation, 1998; 97(13); 1239-45
41. Lindsay AC, Biasiolli L, Lee JM, Plaque features associated with increased cerebral infarction after minor stroke and TIA: A prospective, case-control, 3-T carotid artery MR imaging study: JACC Cardiovasc Imaging, 2012; 5(4); 388-96
42. Biasi GM, Froio A, Diethrich EB, Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study: Circulation, 2004; 110(6); 756-62
43. Schnaudigel S, Gröschel K, Pilgram SM, Kastrup A, New brain lesions after carotid stenting versus carotid endarterectomy: A systematic review of the literature: Stroke, 2008; 39(6); 1911-19
44. Rizwan M, Aridi HD, Dang T, Long-term outcomes of carotid endarterectomy and carotid artery stenting when performed by a single vascular surgeon: Vasc Endovascular Surg, 2019; 53(3); 216-23
Figures
 Figure 1. Kaplan-Meier event-free survival rate for strokeThe blue line represents “Periprocedural period” within 30 days after the procedure.
Figure 1. Kaplan-Meier event-free survival rate for strokeThe blue line represents “Periprocedural period” within 30 days after the procedure. Figure 2. Illustration of case 1(A) Magnetic resonance imaging reveals an acute right posterior cerebral artery (PCA) territory infarction. (B) Angiography reveals chronic occlusion of the right intracerebral artery (ICA), and the left ICA flow was considered to supply to the right anterior cerebral artery, middle cerebral artery, and PCA through the anterior communicating artery. (C) Degree of left carotid stenosis is 80% on angiography. (D) Carotid artery stenting (9×50 mm) is performed for left carotid stenosis.
Figure 2. Illustration of case 1(A) Magnetic resonance imaging reveals an acute right posterior cerebral artery (PCA) territory infarction. (B) Angiography reveals chronic occlusion of the right intracerebral artery (ICA), and the left ICA flow was considered to supply to the right anterior cerebral artery, middle cerebral artery, and PCA through the anterior communicating artery. (C) Degree of left carotid stenosis is 80% on angiography. (D) Carotid artery stenting (9×50 mm) is performed for left carotid stenosis. Figure 3. Illustration of case 2(A) Magnetic resonance image reveals recurrent left middle cerebral artery territory infarction. (B) Degree of left carotid stenosis is 55% on angiography, which has slightly increased from 50%. (C) Magnetic resonance angiography shows left common carotid artery occlusion following carotid endarterectomy. (D) Magnetic resonance angiography shows left intracerebral artery occlusion following emergent thrombus removal.
Figure 3. Illustration of case 2(A) Magnetic resonance image reveals recurrent left middle cerebral artery territory infarction. (B) Degree of left carotid stenosis is 55% on angiography, which has slightly increased from 50%. (C) Magnetic resonance angiography shows left common carotid artery occlusion following carotid endarterectomy. (D) Magnetic resonance angiography shows left intracerebral artery occlusion following emergent thrombus removal. Figure 4. Cases of selecting carotid endarterectomy(A) Plaque ulcerative change. (B) Heavily, extensive calcified plaque. (C) Aortic arch type II (or III). (D) Acute entry of angle.
Figure 4. Cases of selecting carotid endarterectomy(A) Plaque ulcerative change. (B) Heavily, extensive calcified plaque. (C) Aortic arch type II (or III). (D) Acute entry of angle. Figure 5. Cases of selecting carotid artery stenting(A) Stenosis at high position. (B) Long segment of stenosis. (C) Contralateral intracerebral artery occlusion (or severe stenosis).
Figure 5. Cases of selecting carotid artery stenting(A) Stenosis at high position. (B) Long segment of stenosis. (C) Contralateral intracerebral artery occlusion (or severe stenosis). Tables
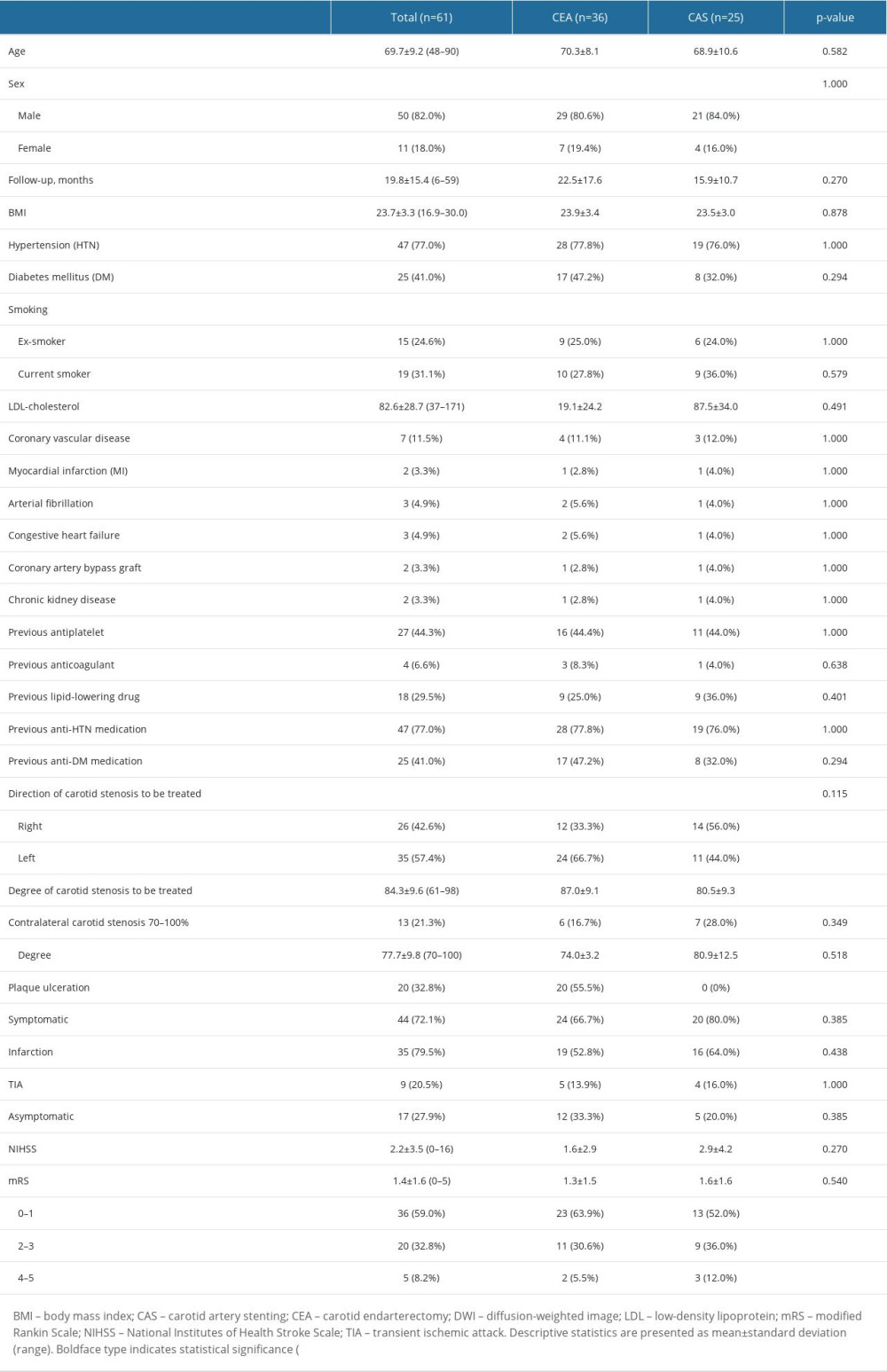 Table 1. Comparison of baseline demographics and characteristics of carotid stenosis between CEA and CAS groups.
Table 1. Comparison of baseline demographics and characteristics of carotid stenosis between CEA and CAS groups.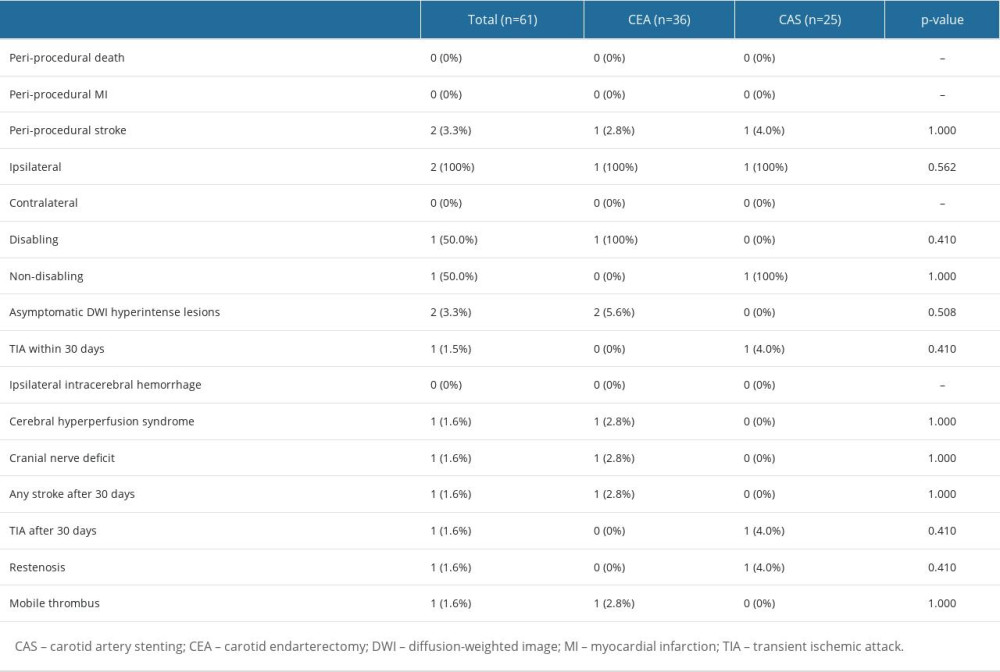 Table 2. Comparison of treatment outcomes between CEA and CAS groups.
Table 2. Comparison of treatment outcomes between CEA and CAS groups.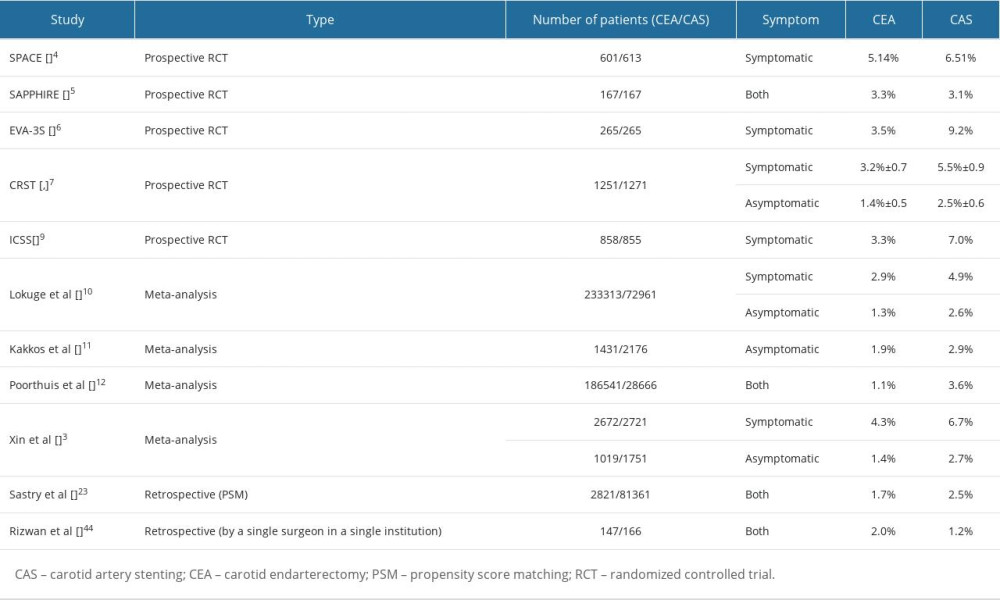 Table 3. Risk of periprocedural stroke in previous studies.
Table 3. Risk of periprocedural stroke in previous studies. Table 1. Comparison of baseline demographics and characteristics of carotid stenosis between CEA and CAS groups.
Table 1. Comparison of baseline demographics and characteristics of carotid stenosis between CEA and CAS groups. Table 2. Comparison of treatment outcomes between CEA and CAS groups.
Table 2. Comparison of treatment outcomes between CEA and CAS groups. Table 3. Risk of periprocedural stroke in previous studies.
Table 3. Risk of periprocedural stroke in previous studies. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








