19 April 2023: Lab/In Vitro Research
Effects of the Dibenzofuran, Usnic Acid, on Inhibition of Ocular Biofilm Formation Due to Coagulase-Negative Staphylococci
Sertaç Argun KıvançDOI: 10.12659/MSM.940266
Med Sci Monit 2023; 29:e940266
Abstract
BACKGROUND: Coagulase-negative staphylococci (CoNS) are gram-positive, aerobic, commensal bacteria found on the skin and mucus membranes, including the conjunctiva. Usnic acid (UA) is a dibenzofuran derivative isolated from lichens. This study aimed to investigate the effects of usnic acid on inhibition of ocular biofilm formation due to CoNS.
MATERIAL AND METHODS: Nine Staphylococcus epidermidis isolates, 5 Staphylococcus hominis isolates, 2 Staphylococcus saprophyticus isolates, and 1 Staphylococcus capitis and Staphylococcus lentus isolates were taken as test bacteria. They were inoculated into brain heart infusion broth and incubated for 24 hours at 35°C and activated. Antibiotic susceptibility was investigated by Kirby-Bauer disc diffusion method. Biofilm production was determined using the microtiter plate method and optical densitometry was measured at 570 nm using an automated microplate reader. Anti-biofilm activity of UA was determined by microtitration method and biofilm removal percentage was calculated.
RESULTS: All tested bacteria were found as high biofilm-producer strains; they were generally resistant to methicillin, but susceptible to vancomycin. UA inhibited the biofilm formation of S. epidermidis isolates, ranging from 5.7% to 81.5%. It inhibited the biofilm formation of S. saprophyticus and S. lentus by 73.3% and 74.3%, respectively. There was no effect of UA on mature biofilms of S. epidermidis 17.7H, S. epidermidis 15.41, S. hominis 9.3, S. hominis 17.2H, S. saprophyticus, and S. lentus.
CONCLUSIONS: It was determined that UA exerted anti-biofilm activity on some CoNS isolated from the ocular surface. Anti-biofilm activity was found to be higher even in strains that did not show antibacterial activity.
Keywords: usnic acid, Biofilms, Eye, Eye Infections, Bacterial, Coagulase, Humans, Staphylococcal Infections, Anti-Bacterial Agents, Dibenzofurans, Microbial Sensitivity Tests
Background
Coagulase-negative staphylococci (CoNS) are a heterogeneous group, and the historical definition of this group is based on diagnostic procedures used to distinguish between
To the best of our knowledge, investigation of UA in the field of ophthalmology is very limited. This study aimed to investigate the effects of UA on inhibition of ocular biofilm formation due to CoNS.
Material and Methods
TESTED BACTERIA AND DETERMINATION OF ANTIBIOTIC SUSCEPTIBILITY:
The tested bacteria were Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus lentus, Staphylococcus saprophyticus, and Staphylococcus capitis. Bacteria isolated and stocked from previous studies regarding ocular surface were obtained from Eskisehir Technical University Microbiology Laboratory. After the bacteria were removed from the stock, they were inoculated into brain heart infusion (BHI) broth and incubated for 24 hours at 35°C and activated, then inoculated into BHI agar and incubated at 35°C for 24 hours. They were used in the tests after examining the colony characteristics and then checking their purity microscopically by gram staining. Antibiotic resistance status of test bacteria was investigated by Kirby-Bauer disc diffusion method using Mueller-Hinton agar (MHA). Tests were applied in line with the recommendation of the Clinical and Laboratory Standards Institute (CLSI) [31]. Penicillin G (P10), gentamicin (CN 10), kanamycin (K30), erythromycin (E15), tetracycline (TE30), and methicillin (ME5) antibiotic discs were used in susceptibility tests. Minimum inhibitory concentration (MIC) values of vancomycin were determined by microdilution broth method according to CLSI standards. MIC values were interpreted as ≤4: sensitive or ≥32: resistant.
DETERMINATION OF BIOFILM FORMATION:
Biofilm production was determined using the microtiter plate method. CoNS strains were inoculated with 10 ml of tryptic soy broth (TSB) with 0.25% glucose and incubated at 37°C for 24 hours. The cultures were then diluted 1: 40 with TSB with 0.25% glucose. We transferred 200 μl of the diluted cultures into the wells of 96-well polystyrene microtiter plates. Plates were incubated at 37°C for 24 hours. After incubation, the plates were washed 3 times with sterile phosphate-buffered solution (PBS, pH 7.2). After the plates dried, the wells were treated with 200 μl of 96% methanol for 5 minutes and washed again with PBS. Then, 200 μl of 2% crystal violet was transferred to the wells and kept at room temperature for 5 minutes. Excess dye was washed off by placing the plate under running tap water and the plates were air-dried. We added 160 ml of 33% (v/v) glacial acetic acid to the wells. Optical densitometry (OD) was measured at 570 nm using an automated microplate reader. Medium without bacteria was used as a negative control [32]. The study was done in pairs in parallel. The strains were classified as non-adherent (0), weakly (+), moderately (++), or strongly (+++) adherent, referring to the ODs of bacterial films.
DETERMINATION OF MINIMUM INHIBITORY AND MINIMUM BACTERICIDAL CONCENTRATION OF UA:
The minimum inhibition concentration of UA for the test bacteria was determined by the microdilution method [31]. Double-layer serial dilutions of UA in Muller-Hilton broth (MHB) were prepared, then we transferred 100 μl of different dilutions to the wells, and 100 μl of the overnight culture adjusted to 0.5 McFarland was added to each well. Plates were incubated at 37°C for 24 hours. After incubation, the lowest concentration without growth was determined as the MIC value. The plates were inoculated on BHI agar from all concentrations without growth, starting from the lowest concentration at which no bacterial growth was observed. After incubation at 37°C for 18–24 hours, we checked for growth, and the lowest concentration without growth was recorded as the minimum bactericidal concentration (MBC). Experiments were performed 3 times. Growth was controlled with tetrazolium chloride (TCC).
INVESTIGATION THE EFFECT OF UA ON BIOFILM FORMATION:
Anti-biofilm activity of UA was determined by microtitration method in multi-well flat bottom polystyrene plates. To determine the effect of UA on biofilm formation, 100 μl of TSB prepared with UA as much as its MIC value was transferred to each well. We transferred 100 μl of the test bacteria culture prepared in TSB containing 0.25% glucose, diluted 1/40 from the 18-hour culture of test bacteria developed in TSB containing 0.25% glucose. TSB was used as control. The amount of biofilm was determined after the plates were incubated for 24 hours at 37°C. To determine the effect of UA on the mature biofilm, a 1/40 diluted bacterial culture was prepared from the overnight culture prepared in TSB containing 0.25% glucose. We poured 200 μl of this bacterial culture into the wells and incubated at 37°C for 24 hours. At the end of the incubation period, 100 μl was removed from the wells and transferred from the solution containing 100 μl UA (as MIC). Plates were incubated at 37°C for 24 hours. After incubation, the biofilm was determined. All tests were done 3 times [33,34].
DATA ANALYSIS:
Descriptive statistical analyses were performed. Biofilm inhibition percentage was calculated and given according to an equation used in a previous study [35]:
Results
BIOFILM FORMATION PROPERTIES AND ANTIBIOTIC SUSCEPTIBILITY OF TESTED BACTERIA:
Nine S. epidermidis isolates, 5 S. hominis isolates, 2 S. saprophyticus isolates, and 1 S. capitis and S. lentus isolates were taken as test bacteria. All tested bacteria formed high biofilm on polystyrene (Table 1). The susceptibility of CoNS isolates to antibiotics was different. Antibiotic susceptibility properties of the tested bacteria are given in Table 1. While S. epidermidis isolates were resistant to penicillin and erythromycin, they were determined to be susceptible to gentamicin. All isolates were resistant to penicillin except S. epidermidis17.7H and S. lentus. All test isolates were susceptible to tetracycline except for S. epidermidis 4.11 and S. saprophyticus 2. Test isolates were generally resistant to methicillin but susceptible to vancomycin.
ANTIBACTERIAL EFFECTS OF UA:
The antibacterial effect of UA varied according to the isolates. While 62.5 μg/ml inhibited 5 S. epidermidis isolates, it was not effective on 4 S. epidermidis isolates (Table 1). It was not found to be effective on most of S. hominis, S. capitis, and S. saprophyticus. The MIC value for S. lentus was found to be 31.2 μg/mL. The MIC and MBC values of the tested CoNS strains are shown in Table 1.
ANTI-BIOFILM EFFECTS OF UA:
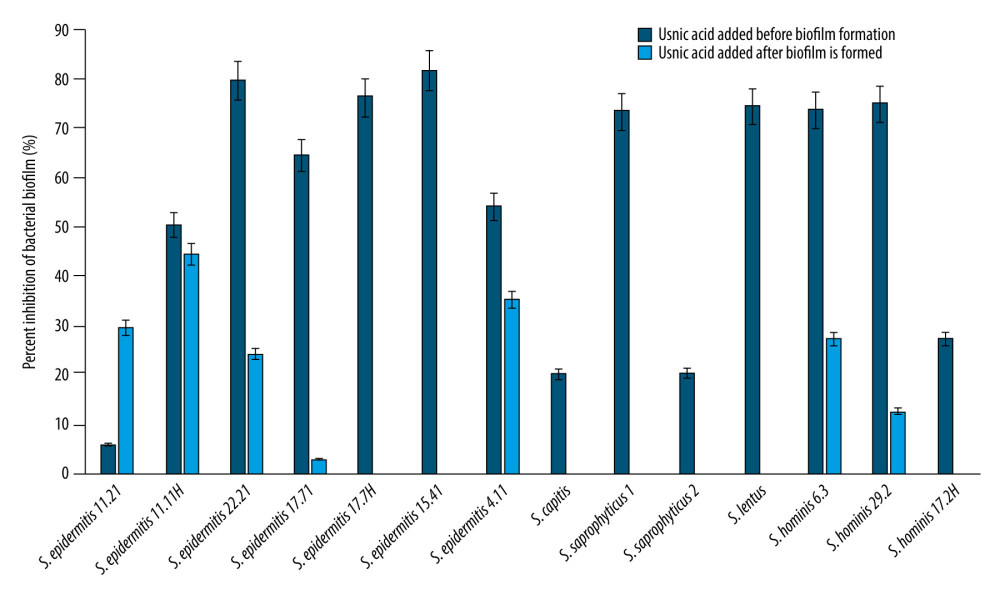
Anti-biofilm effects of UA were evaluated against biofilm formation of tested strains before and after adding into the media. Biofilm inhibition percentage was between 5.7% and 81.5% when UA was tested against
After the biofilm was formed, the inhibition effect of UA decreased in all strains (Figure 1, S. epidermidis 15.81, S. epidermidis17.11, S. hominis 17.2, and S. hominis 9.3 were excluded since they were not inhibited). UA could not inhibit biofilm against 11 out of 18 strains after biofilm formed. UA inhibited the formed biofilm of S. epidermidis 17.71 by 2.9%, S. epidermidis 22.21 by 24.2%, and S. epidermidis 11.21 by 29.5%, while it inhibited the biofilm of S. epidermidis 4.11 by 35.1% S. epidermidis 11.11H by 44.4%. No effect on mature biofilms were observed for S. epidermidis 17.7H, S. epidermidis 15.41, S. hominis 9.3, S. hominis 17.2H, S. saprophyticus, and S. lentus. Figure 1 shows the inhibitory effect of UA on coagulase-negative staphylococcal isolates before biofilm formation and after biofilm formation. None of the isolates were stimulated for biofilm formation by UA.
Discussion
Usnic acid has been shown to have antitumor, antiviral, and antimicrobial activities [36], and its antibacterial properties against many susceptible and multi-drug-resistant bacterial strains have been proven [37,38]. In the present study, the antimicrobial activity of UA varied according to the tested bacteria. Usnic acid did not show antibacterial activity against
There are few studies on the antibacterial and anti-biofilm effect of UA on CoNS, and to best of our knowledge, the effects of UA on ocular surface microbiota have not been studied previously. It was determined that while UA was antibacterial on some CoNS, it was not effective on some strains, and anti-biofilm activity was found to be higher. Although UA did not show antibacterial activity in some strains, it was shown to have anti-biofilm activity. However, liver toxicity and contact allergy have been reported in some studies [54], which greatly reduces its potential as an anti-biofilm agent. There are studies showing that it is beneficial as a controlled-release drug to prevent local toxicity [51,53,55]. It has been demonstrated that ocular microorganisms, including
Our study has some limitations. First, this was an in vitro study, and in vivo studies are needed to assess the effects of UA on eyes and on infected ocular surface. To avoid the toxic effects of UA, studies should be performed with controlled-release UA products. Another limitation is that scanning electron microscopy and confocal laser microscopy were not used to visualize the biofilm formation.
Conclusions
In this study, it was determined that UA was effective at different rates in terms of antibacterial and anti-biofilm effects against coagulase-negative staphylococci obtained from the ocular surface. However, anti-biofilm effects were found to be limited after mature biofilm was formed in vivo in medium. Further studies should focus on inhibition of the formed biofilm. Considering that drugs are used as topical drops in the field of eye diseases, and with the development of slow-release drugs with technological developments, topical use of UA may be possible without systemic and local toxicities. Our findings suggest that UA needs to be investigated in further studies for its antibacterial and anti-biofilm effects on the ocular surface. We think that UA, which our study shows has an effect on ocular surface bacteria, may be a molecule that can be used for antibacterial and anti-biofilm purposes. We believe that the changes in antibiotic resistance in recent years necessitate the search for such alternative molecules. Also, UA should be studied both in vivo and in vitro against other ocular pathogens such as
References
1. Becker K, Heilmann C, Peters G, Coagulase-negative staphylococci: Clin Microbiol Rev, 2014; 27(4); 870-926
2. Ozkan J, Willcox MD, The ocular microbiome: molecular characterisation of a unique and low microbial environment: Curr Eye Res, 2019; 44(7); 685-94
3. Aragona P, Baudouin C, Benitez Del Castillo JM, The ocular microbiome and microbiota and their effects on ocular surface pathophysiology and disorders: Surv Ophthalmol, 2021; 66(6); 907-25
4. Zhou Y, Holland MJ, Makalo P, The conjunctival microbiome in health and trachomatous disease: A case control study: Genome Med, 2014; 6(11); 99
5. Doan T, Akileswaran L, Andersen D, Paucibacterial microbiome and resident DNA virome of the healthy conjunctiva: Invest Ophthalmol Vis Sci, 2016; 57(13); 5116-26
6. Wen X, Miao L, Deng Y, The influence of age and sex on ocular surface microbiota in healthy adults: Invest Ophthalmol Vis Sci, 2017; 58(14); 6030-37
7. Graham JE, Moore JE, Jiru X, Ocular pathogen or commensal: A PCR-based study of surface bacterial flora in normal and dry eyes: Invest Ophthalmol Vis Sci, 2007; 48(12); 5616-23
8. Kıvanç SA, Akova B, Kıvanç M: Experimed, 2022; 12(3); 103-7
9. Zegans ME, Shanks RMQ, O’Toole G, Bacterial biofilms and ocular infections: Ocul Surf, 2005; 3; 73-80
10. Taban M, Behrens A, Newcomb RL, Acute endophthalmitis following cataract surgery: A systematic review of the literature: Arch Ophthalmol, 2005; 123; 613-20
11. West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD, The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001: Ophthalmology, 2005; 112; 1388-94
12. Parsa K, Schaudinn C, Gorur A, Demonstration of bacterial biofilms in culture-negative silicone stent and jones tube: Ophthalmic Plast Reconstr Surg, 2010; 26; 426-30
13. Samimi DB, Bielory BP, Miller D, Johnson TE, Microbiologic trends and biofilm growth on explanted periorbital biomaterials: A 30-year review: Ophthalmic Plast Reconstr Surg, 2013; 29; 376-81
14. Bispo PJ, Haas W, Gilmore MS, Biofilms in infections of the eye: Pathogens, 2015; 4; 111-36
15. Gonçalves NL, Borges VM, de Arruda JAA: Photodiagnosis Photodyn Ther, 2020; 32; 102042
16. Ciric AD, Petrovic JD, Glamoclija JM, Natural products as biofilm formation antagonists and regulators of quorum sensing functions: A comprehensive review update and future trends: South Afr J Botany, 2019; 120; 65-80
17. Sasirekha B, Megha DM, Chandra MS, Soujanya R, Study on effect of different plant extracts on microbial biofilms: Asian J Biotechnol, 2015; 7; 1-12
18. Lu L, Hu W, Tian Z, Developing natural products as potential anti-biofilm agents: Chin Med, 2019; 14; 11
19. Akova B, Kıvanç SA, Kıvanç M: Eur Rev Med Pharmacol Sci, 2021; 25; 7799-805
20. Sharma D, Misba L, Khan AU, Antibiotics versus biofilm: An emerging battleground in microbial communities: Antimicrob Resist Infect Control, 2019; 8; 76
21. Mishra R, Panda AK, De Mandal S, Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens: Front Microbiol, 2020; 11; 566325
22. Sousa M, Gomes IB, Simões LC, The action of phytochemicals in the control of pathogenic biofilms: Antibiofilm strategies Springer Series on Biofilms, 2022; 11; 371-98, Cham, Springer
23. Huneck S, Yoshimura I: Identification of lichen substances, 1996, Berlin, Heidelberg, Springer
24. Vartia KO, Antibiotics in lichens: The lichens, 1973; 547-61, New York, Academic Press
25. Okuyama E, Umeyama K, Yamazaki M: Planta Med, 1995; 61; 113-15
26. Ingolfsdottir K, Usnic acid: Phytochemistry, 2002; 61; 729-36
27. Cocchietto M, Skert N, Nimis PL, Sava G, A review on usnic acid, an interesting natural compound: Naturwissenschaften, 2002; 89; 137-46
28. Araújo AA, de Melo MG, Rabelo TK, Review of the biological properties and toxicity of usnic acid: Nat Prod Res, 2015; 29; 2167-80
29. Elo H, Matikainen J, Pelttari E: Naturwissenschaften, 2007; 94; 465-68
30. Francolini I, Norris P, Piozzi A, Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces: Antimicrob Agents Chemother, 2004; 48(11); 4360-65
31. Clinical and Laboratory Standards Institute, (CLSI) guidelines – performance standards for antimicrobial susceptibility testing: CLSI Document M100, 2013 ()m10033_samplepages-1.pdf
32. Stepanovic S, Vukovic D, Dakic I, A modified microtiter-plate test for quantification of staphylococcal biofilm formation: J Microbiol Methods, 2000; 40(2); 175-79
33. Pompilio A, Pomponio S, Di Vincenzo V: Future Microbiol, 2013; 8; 281-92
34. Pompilio A, Riviello A, Crocetta V: Future Microbiol, 2016; 11; 1315-38
35. Kıvanç SA, Akova Budak B, Kıvanç M, Göz Yüzeyinden Elde Edilen Bakterilerin Oluşturduğu Biyofilme Karşı Probiyotik Bakterilerin Hücresiz Filtratlarının Antibiyofilm Etkilerinin Araştırılması: Süleyman Demirel Üniversitesi Sağlık Bilimleri Dergisi, 2022; 13(3); 432-40
36. Molnár K, Farkas E, Current results on biological activities of lichen secondary metabolites: A review: Z Naturforsch C J Biosci, 2010; 65; 157-73
37. Selbmann L, Zucconi L, Ruisi S, Culturable bacteria associated with Antarctic lichens: Affiliation and psychrotolerance: Polar Biology, 2010; 33; 71-83
38. Bate PNN, Orock AE, Nyongbela KD, In vitro activity against multi-drug resistant bacteria and cytotoxicity of lichens collected from Mount Cameroon: Journal of King Saud University-Science, 2020; 32(1); 614-19
39. Lauterwein M, Oethinger M, Belsner K, In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (−)-usnic acid against aerobic and anaerobic microorganisms: Antimicrob Agents Chemother, 1995; 39; 2541-43
40. Shibata S, Ukita T, Tamura T, Miura Y, Relation between chemical constitutions and antibacterial effects of usnic acid and its derivatives: Jpn J Med, 1948; 1; 152-55
41. Yilmaz M, Türk AO, Tay T, Kivanç M, The antimicrobial activity of extracts of the lichen Cladonia foliacea and its (−)-usnic acid, atranorin, and fumarprotocetraric acid constituents: Z Naturforsch C J Biosci, 2004; 59; 249-54
42. Tay T, Türk AO, Yilmaz M: Z Naturforsch C J Biosci, 2004; 59; 384-88
43. Tozatti MG, Ferreira DS, Flauzino LG: Nat Prod Commun, 2016; 11(4); 493-96
44. Victor K, Boris L, Athina G, Design, synthesis and antimicrobial activity of usnic acid derivatives: Medchemcomm, 2018; 9; 870-82
45. Sinha S, Gupta VK, Kumar P, Usnic acid modifies MRSA drug resistance through down-regulation of proteins involved in peptidoglycan and fatty acid biosynthesis: FEBS Open Bio, 2019; 9; 2025-40
46. Maciąg-Dorszyńska M, Węgrzyn G, Guzow-Krzemińska B, Antibacterial activity of lichen secondary metabolite usnic acid is primarily caused by inhibition of RNA and DNA synthesis: FEMS Microbiol Lett, 2014; 353; 57-62
47. Gupta VK, Verma S, Gupta S: Eur J Clin Microbiol Infect Dis, 2012; 31; 3375-83
48. Lauinger IL, Vivas L, Perozzo R, Potential of lichen secondary metabolites against Plasmodium liver stage parasites with FAS-II as the potential target: J Nat Prod, 2013; 76; 1064-70
49. Antonenko YN, Khailova LS, Rokitskaya TI, Mechanism of action of an old antibiotic revisited: Role of calcium ions in protonophoric activity of usnic acid: Biochim Biophys Acta Bioenerg, 2019; 1860(4); 310-16
50. Shcherbakova A, Strömstedt AA, Göransson U: World J Microbiol Biotechnol, 2021; 37; 129
51. Chandika P, Khan F, Heo SY, Enhanced wound-healing capability with inherent antimicrobial activities of usnic acid incorporated poly(ɛ-caprolactone)/decellularized extracellular matrix nanofibrous scaffold: Biomater Adv, 2022; 140; 213046
52. Sun F, Qu F, Ling Y, Biofilm-associated infections: Antibiotic resistance and novel therapeutic strategies: Future Microbiol, 2013; 8; 877-86
53. Pandit S, Rahimi S, Derouiche A, Sustained release of usnic acid from graphene coatings ensures long term antibiofilm protection: Sci Rep, 2021; 11; 9956
54. Guo L, Shi Q, Fang JL: J Environ Sci Health C Environ Carcinog Ecotoxicol Rev, 2008; 26; 317-38
55. da Silva FR, Silva RO, de Castro Oliveira HM, Gelatin-based membrane containing usnic acid-loaded liposomes: A new treatment strategy for corneal healing: Biomed Pharmacother, 2020; 130; 110391
56. Bharathi MJ, Ramakrishnan R, Shivakumar C, Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in south India: Indian J Ophthalmol, 2010; 58; 497-507
57. Kim SJ, Toma HS, Ophthalmic antibiotics and antimicrobial resistance a randomized, controlled study of patients undergoing intravitreal injections: Ophthalmology, 2011; 118; 1358-63
58. Shimizu Y, Toshida H, Honda R, Prevalence of drug resistance and culture-positive rate among microorganisms isolated from patients with ocular infections over a 4-year period: Clin Ophthalmol, 2013; 7; 695-702
59. Barry P, Seal DV, Gettinby G, ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study: J Cataract Refract Surg, 2006; 32; 407-10
60. Seal D, Reischl U, Behr A, Laboratory diagnosis of endophthalmitis: Comparison of microbiology and molecular methods in the European Society of Cataract & Refractive Surgeons multicenter study and susceptibility testing: J Cataract Refract Surg, 2008; 34; 1439-50
61. Cocchietto M, Skert N, Nimis PL, Sava G, A review on usnic acid, an interesting natural compound: Naturwissenschaften, 2002; 89(4); 137-46
Tables
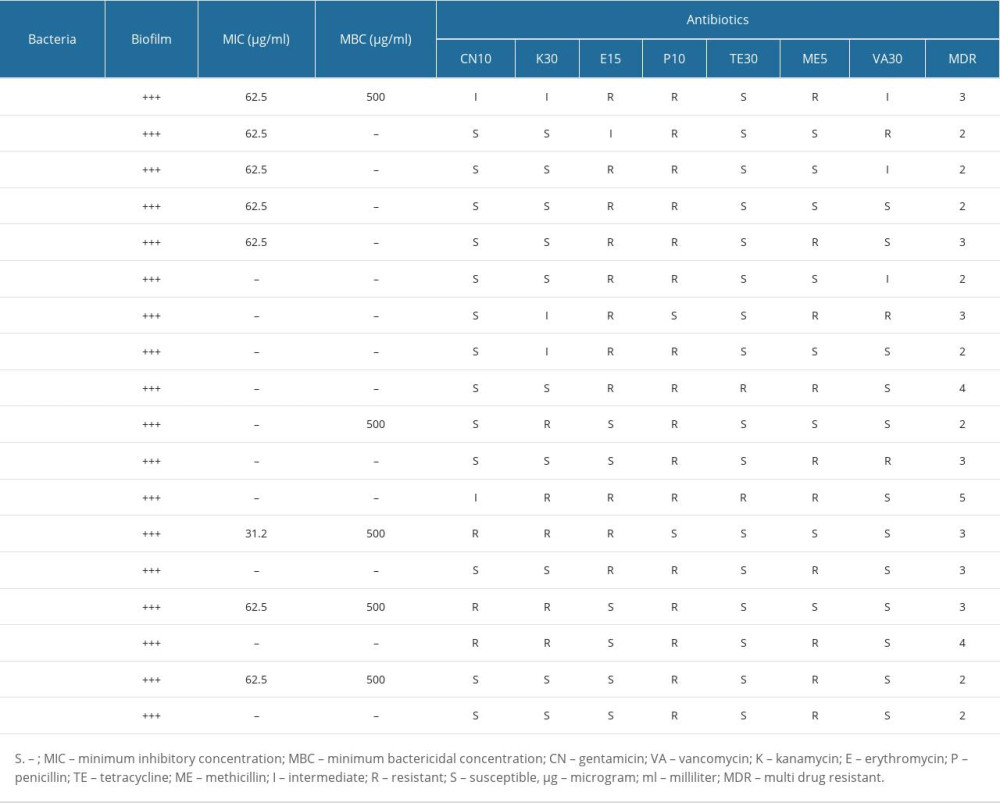 Table 1. The antibiotic susceptibilities of coagulase-negative staphylococci that formed biofilm and minimum inhibitory concentration and minimum bactericidal concentration values of usnic acid.
Table 1. The antibiotic susceptibilities of coagulase-negative staphylococci that formed biofilm and minimum inhibitory concentration and minimum bactericidal concentration values of usnic acid. Table 1. The antibiotic susceptibilities of coagulase-negative staphylococci that formed biofilm and minimum inhibitory concentration and minimum bactericidal concentration values of usnic acid.
Table 1. The antibiotic susceptibilities of coagulase-negative staphylococci that formed biofilm and minimum inhibitory concentration and minimum bactericidal concentration values of usnic acid. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952