05 December 2022: Clinical Research
Application of Indocyanine Green Fluorescence Imaging Combined with Laparoscopic Ultrasound in Laparoscopic Microwave Ablation of Liver Cancer
Yu Hu1BC, Wenting Guo1CD, Jinliang Ma2D, Jihai Yu2B, Wenbin Liu2DE, Chuanhai Zhang2C, Weidong Jia1AE*, Yongsheng Ge1EGDOI: 10.12659/MSM.937832
Med Sci Monit 2022; 28:e937832
Abstract
BACKGROUND: The aim of this study was to assess the effect of indocyanine green (ICG) fluorescence imaging combined with laparoscopic ultrasound in laparoscopic microwave ablation of liver cancer.
MATERIAL AND METHODS: This study retrospectively analyzed 61 patients who underwent laparoscopic microwave ablation of liver cancer, including laparoscopic microwave ablation with and without ICG fluoroscopy.
RESULTS: The operative times, ablation times, postoperative hospital stay, postoperative complication rate, hospitalization cost, postoperative liver function changes, and postoperative overall survival were similar between the 2 groups, but there was a statistically significant difference in recurrence-free survival (P<0.05). A total of 5 lesions were found in the fluorescence laparoscopy group that were not found by preoperative imaging, while no new lesions were found in the ordinary laparoscopy group. Fluorescence laparoscopy has obvious advantages over ordinary laparoscopy in finding small lesions that were not found before surgery. In terms of complete ablation rate, 3 patients in the ordinary laparoscopy group and 1 patient in the fluorescence laparoscopy group were judged to be incompletely ablated and were ablated again at 1 month after the operation.
CONCLUSIONS: For small hepatocellular carcinoma with severe liver cirrhosis and located on the liver surface, fluorescence laparoscopy can better reveal the location and boundary of the tumor, and fluorescence laparoscopy can detect tiny lesions that cannot be detected by preoperative imaging. The combination of fluorescence laparoscopy and microwave ablation has a good effect on the treatment of small hepatocellular carcinoma located on the surface of the liver that is difficult to distinguish.
Keywords: Indocyanine Green, Laparoscopy, Liver Neoplasms, Microwaves, Humans, Carcinoma, Hepatocellular, optical imaging
Background
Primary liver cancer is one of the most common cancers in China [1], ranking fourth in incidence and third in mortality rate [2]. Surgical resection remains the preferred treatment for liver cancer [3], but some patients are not candidates for hepatectomy due to cirrhosis, hepatic insufficiency, or poor general health. Local ablation, including microwave ablation, is a widely used minimally invasive treatment for small liver cancers, especially in patients with severe cirrhosis. Laparoscopic microwave ablation of liver cancers is a safer and more effective treatment for liver cancers in some anatomic sites [4], such as beneath the liver capsule, and lesions protruding beyond the liver capsule, in the diaphragmatic dome, or close to the gallbladder and gastrointestinal tract, and adjacent to great vessels and the hepatic portal [5,6]. Accurately locating tiny tumor tissue under laparoscopic direct vision has become a key factor to improve the success rate of laparoscopic microwave ablation. Indocyanine green (ICG) fluorescence imaging during laparoscopy has emerged as an auxiliary technique in recent years [7]. ICG injected into the human bloodstream binds to proteins and emits near-infrared light with a wavelength of 840 nm when excited by light at 750–810 nm. The emitted near-infrared light can penetrate connective tissues at a 5–10 mm thickness [8–10]. ICG can be used to indicate tumor position and delineate tumor boundaries based on this principle [11,12]. From 1 April 2019 to 31 December 2021, our department used the intraoperative navigation of ICG fluorescence imaging laparoscopy, combined with laparoscopic ultrasound, to perform fluorescence laparoscopic microwave ablation of liver cancer in 29 patients with small liver cancer. Microwave ablation of hepatocellular carcinoma was performed in 32 patients with small hepatocellular carcinoma. The data and results of the 2 groups of patients are reported as follows.
Material and Methods
CLINICAL DATA:
We recruited 61 patients who underwent laparoscopic microwave ablation to treat liver cancers at the First Affiliated Hospital of the University of Science and Technology of China from 1 April 2019 to 31 December 2021 with a total of 67 tumors in 61 patients. The research was approved by the Ethics Committee of Anhui Provincial Hospital, He Fei, China (No. 2021-S(H)-017). All patients signed an informed consent form conforming to medical ethics requirements before surgery. Inclusion criteria were: (1) pathologically confirmed or clinically diagnosed with primary liver cancer using 2 or more imaging modalities (liver MRI, upper-abdomen contrast-enhanced CT, ultrasonography, and PET-CT) combined with tumor markers (AFP, DCP, and human prothrombin complex); (2) single or multiple liver cancers with a diameter ≤3 cm and totaling <3; (3) no evidence of vascular invasion, bile duct invasion, or extrahepatic metastases; and (4) assessed as class A or B according to the Child-Pugh classification system. The exclusion criteria were: (1) single or multiple liver cancers with a diameter >3 cm and totaling >3; (2) combined with extrahepatic metastases; (3) class C liver function according to the Child-Pugh classification system; and (4) severe underlying diseases, such as heart and renal failure. There were 35 males and 26 females with an average age of 58.91 years. Among the 61 patients, 54 and 7 had class A and B liver function, respectively, according to the Child-Pugh classification system. According to the 2020 China liver cancer (CNLC) staging system, 55 and 6 patients were classified as CNLC stage Ia and Ib, respectively. All patients were thoroughly evaluated before surgery to exclude surgical contraindications. All tumors located inside the liver parenchyma and positive iodine allergy test were in the general laparoscopy group.
EQUIPMENT AND INSTRUMENTS:
We used a high-resolution fluorescence laparoscope manufactured by Guangdong Optomedic Technologies, Inc. (Guangdong, China). The microwave ablation system was the ECO-100C cold cycle microwave knife (Nanjing Yigao Microwave System Engineering Co., Ltd., Nanjing, China). We used an ECO-100AI8 disposable microwave ablation needle (2.0×150.0 mm).
SURGICAL METHOD:
The patients were placed in supine position after endotracheal anesthesia was established. The right shoulder was slightly elevated in some patients who had cancers in the right posterior lobe of the liver. A pneumoperitoneum was established, with the pressure maintained at 10–14 mmHg and the head elevated. The laparoscope was inserted through a subumbilical incision to inspect the peritoneal cavity, the tumor position, number of tumors, and whether there was a need to dissociate the liver from the peri-adherent tissues. If necessary, 2 to 4 incisions were made in the following positions: below the xiphoid process, right midclavicular line, below the costal margin at the anterior axillary line, and below the costal margin at the left midclavicular line. The specific positions of the additional incisions were adjusted according to the patients’ body size, tumor position, and intraoperative manipulations performed. The ablation fully covered and exceeded the liver tumor by 0.5–1 cm. For larger tumors, overlapping ablation was performed.
TIMING AND DOSE OF ICG INJECTION:
All patients underwent iodine allergy testing before surgery. An ICG injection was contraindicated in patients with positive iodine allergy testing. Otherwise, ICG was injected into a peripheral vein 2–3 days before surgery at a dose of 0.3–0.5 mg/kg.
POSTOPERATIVE FOLLOW-UP:
The patients were re-examined radiographically (by contrast-enhanced MR, or contrast-enhanced CT, or CEUS of the liver) 1 month after surgery, and the ablation effect was assessed by tumor-related indicators (eg, AFP, DCP). Contrast-enhanced ultrasonography indicates that there is no contrast agent filling inside the tumor, and contrast-enhanced CT or MR indicates that the tumor is not enhanced, indicating that the ablation is complete. If there is still tumor residue, re-ablation can be performed until the tumor is completely inactivated. The patient was followed up every 2–3 months in the outpatient clinic thereafter. Color Doppler ultrasound or CT showed new lesions around the ablated tumor, in the liver, or in other parts, which was defined as recurrence.
STATISTICAL METHODS:
Statistical analyses were performed using SPSS26.0 software. Quantitative data are expressed as χ̄±s, and the intergroup comparison was conducted using an independent-samples
Results
The 2 groups of patients were comparable with respect to general preoperative information inpatient observation index, and postoperative complete ablation rate (
Discussion
Local ablation and surgical resection had similar efficacy for a single lesion ≤5 cm or 2–3 lesions with a maximum diameter ≤3 cm not combined with vascular, bile duct, and adjacent organ invasion or distant metastases, and class A and B liver function. These patients usually achieved a radical cure by local ablation [13]. Local ablation has the advantages of simplicity and effectiveness, less trauma, better safety and reliability, and wide indications, and has been widely used in the treatment of small hepatocellular carcinoma. Microwave ablation uses heat produced by high-speed rotational friction of polar molecules and collision movement of polar ions. This process usually creates considerable heating in the tissues. Protein denaturation can be induced in tumor cells when the tissue temperature is >60°C, resulting in irreversible necrosis. The tumor cells release heat shock proteins, which activate the immune response to inhibit tumor spread [14,15].
Microwave ablation can be delivered via 3 pathways: percutaneous, laparotomy, and laparoscopic. B-mode-guided, CT-guided, and guided microwave ablation of liver cancers have the benefits of convenience and minimal invasiveness [4]. This ablation procedure has been widely used clinically, but it requires high-level physician skill in puncture techniques under the guidance of images such as B-ultrasound, CT, or MRI. In addition, intense pain may be induced because the liver capsule is irritated during the microwave ablation. Body position changes and respiratory movement can lead to inaccurate puncture or displacement of the ablation needle, thus resulting in incomplete ablation, as shown in Figure 3. Using B-mode-, CT- and MRI-guided percutaneous microwave ablation, the clinician is operating blindly. The ablation procedure is more likely to cause bleeding, tumor rupture, and incidental injury to the surrounding organs and tissues if the liver cancers are protruding outside the liver capsule, located in the diaphragmatic dome or near the gallbladder and the gastrointestinal tract, or near the great vessels and hepatic portal [16]. Lesions close to the portal vein are even more difficult to manage and are a potential risk factor for incomplete ablation [17]. Laparoscopic microwave ablation is a better treatment for liver cancers in these positions [18–20].
Liver cancer patients undergoing microwave ablation usually have severe cirrhosis and cirrhotic liver nodules of varying sizes on the liver surface [21,22]. Laparoscopic ultrasound cannot demonstrate the liver surface or accurately detect tumor position in these patients. The cancers are difficult to differentiate from cirrhotic liver nodules. Laparoscopic ultrasound with ICG fluorescence navigation can visualize the tumors, as shown in Figure 4. In patients undergoing conventional laparoscopy combined with laparoscopic ultrasonography, the tumor cannot be found and located at all, but the tumor can be clearly located under ICG fluorescence laparoscopy.
The advantage of ICG fluorescence laparoscopy over conventional laparoscopy is the ability to detect micrometastases and satellite lesions during surgery [23,24]. Intraoperative detection and management of micrometastases other than primary lesions are very important for patient prognosis. Preoperative imaging examinations, including enhanced CT, MRI, and contrast-enhanced ultrasonography, cannot easily identify small liver cancer lesions, and small lesions are easily confused with cirrhotic nodules in imaging and cannot be located during surgery [25]. Intraoperative ultrasound has a low sensitivity for small hepatocellular carcinoma lesions, is difficult to distinguish from normal cirrhotic nodules, and has extremely high requirements for the surgeon [26]. ICG fluorescence imaging has high sensitivity for small liver cancer. It can detect and locate liver cancer lesions in real time during surgery, and can also detect small lesions not found by preoperative imaging examination, thereby reducing the residual risk of tumor and improving the prognosis of patients. The comparison of the 2 groups of patients in the present study shows that the ability of ICG fluorescence laparoscopy to detect small lesions that cannot be detected by preoperative imaging is significantly better than that of conventional laparoscopy, so the patients in the ICG fluorescence laparoscopy group had better recurrence-free survival. There was no significant difference in the overall survival of the 2 groups of patients, which may be related to the shorter follow-up time and the small number of cases. Multicenter studies with larger samples and longer follow-up are needed.
It is noteworthy that some cirrhotic liver nodules, hepatic cysts, and hyperplastic bile ducts are also capable of ICG uptake, thus leading to false-positive results [27]. Differentiation can be made by surgeons based on their experience and by combining laparoscopic ultrasound findings with preoperative imaging.
We identified 4 major benefits of laparoscopic microwave ablation of liver cancers with ICG fluorescence navigation through the comparative analysis of 29 cases of fluorescence laparoscopic microwave ablation of liver cancer and 32 cases of conventional laparoscopic microwave ablation of liver cancer undergoing this procedure in our department: (1) This procedure has high safety, and all manipulations are done under direct vision. In addition to dissociating the liver and the perihepatic adhesion, safe spaces can be created by artificial ascites or separation with wet gauze for cancers in special positions, such as lesions close to segments VII and VIII of the right liver and lesions close to the gallbladder and the gastrointestinal tract. In this way, undesired damage caused by needle puncture and burn injury caused by heat conduction can be avoided. (2) The procedure has high reliability and can be repeated under direct vision. The procedure also allows for puncture from different directions, thereby preventing incomplete ablation as much as possible. (3) The procedure achieves a more precise tumor localization by combining fluorescence navigation with laparoscopic ultrasound. The tumor position and the relationship between the tumor and the surrounding blood vessels and organs can be more accurately determined. A clearer delineation of tumor boundaries is conducive to manipulations during the ablation. Liver cancers can be difficult to differentiate from the cirrhotic liver nodules in some patients when combined with severe cirrhosis. This situation may still exist, even under laparoscopic ultrasound. ICG staining offers a good solution to this problem. (4) For detection of small lesions, ICG staining can reveal minimal lesions that are otherwise not visible using preoperative imaging modalities [28].
However, the application of fluorescence laparoscopy also has certain limitations. For tumors located inside the liver parenchyma, fluorescence cannot be localized and imaged. In addition, fluorescence cannot monitor the ablation effect in real time, and cannot judge whether the ablation is complete. In addition, in this study, tumors located deep in the liver parenchyma were more difficult to ablate than tumors located on the surface of the liver. The advantages of fluorescence laparoscopy in microwave ablation of liver cancer require further research with large samples, multicenter evaluation, and longer follow-up.
Conclusions
To conclude, ICG fluorescence navigation during laparoscopic microwave ablation of liver cancers achieved good efficacy. This procedure has advantages compared with conventional laparoscopic microwave ablation. The complete ablation rate was even higher for those patients with severe cirrhosis and small liver cancers on the liver surface. In addition, the new ablation procedure more accurately detected tumor position and differentiated between cancers and cirrhotic liver nodules, especially in patients with severe cirrhosis. More importantly, fluorescence laparoscopy can effectively detect small lesions that cannot be displayed by preoperative imaging. Therefore, laparoscopic microwave ablation with ICG fluorescence navigation has high clinical utility for liver cancers and is highly recommended.
Figures
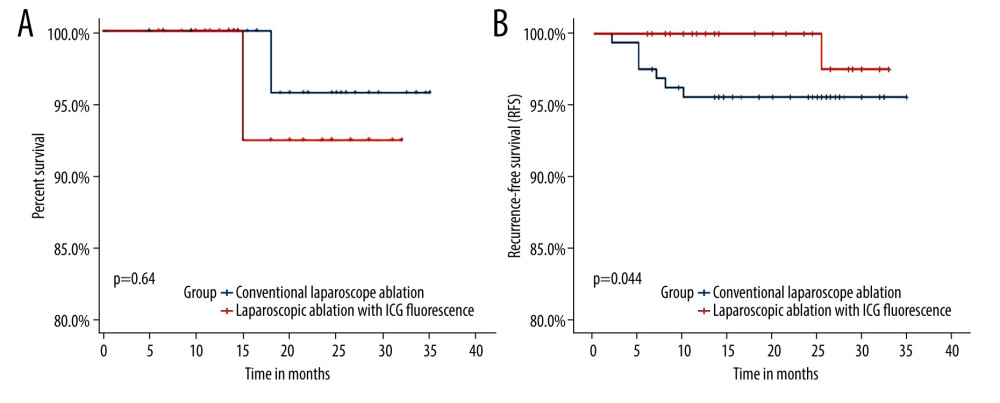 Figure 1. (A) Overall survival. (B) Recurrence-free survival.
Figure 1. (A) Overall survival. (B) Recurrence-free survival. 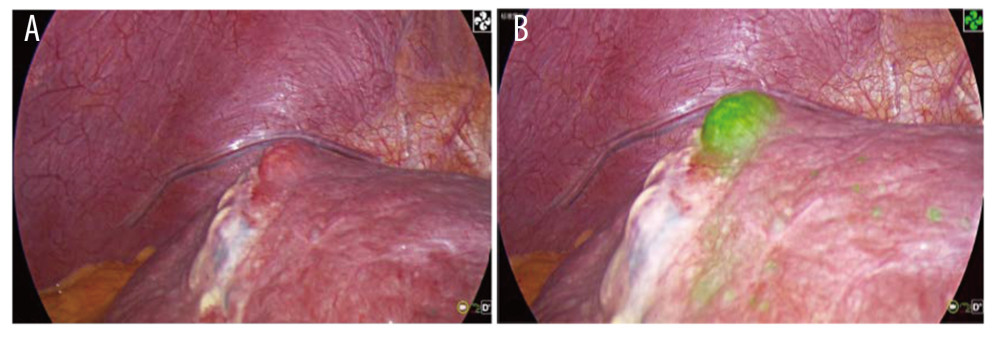 Figure 2. (A) Tumor residues after ultrasound-guided percutaneous microwave ablation. (B) Fluorescence imaging of tumor residues after ultrasound-guided percutaneous microwave ablation.
Figure 2. (A) Tumor residues after ultrasound-guided percutaneous microwave ablation. (B) Fluorescence imaging of tumor residues after ultrasound-guided percutaneous microwave ablation. 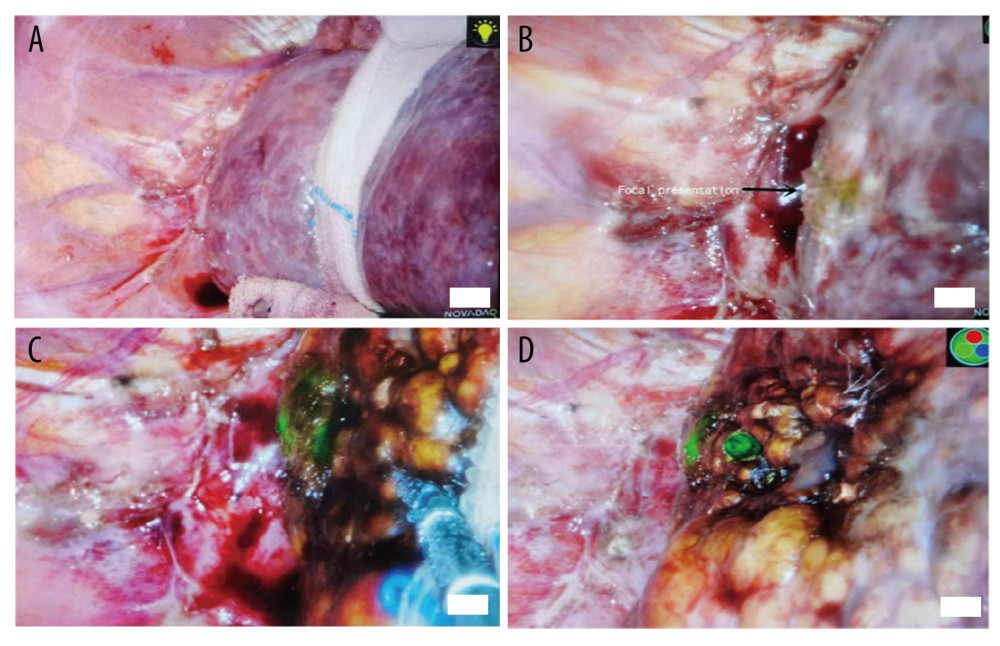 Figure 3. (A) No lesions can be found in conventional laparoscopic mode, and no lesions can be found by laparoscopic ultrasonography. (B) Fluorescence imaging of the lesion. (C) During lesion ablation. (D) After lesion ablation.
Figure 3. (A) No lesions can be found in conventional laparoscopic mode, and no lesions can be found by laparoscopic ultrasonography. (B) Fluorescence imaging of the lesion. (C) During lesion ablation. (D) After lesion ablation. 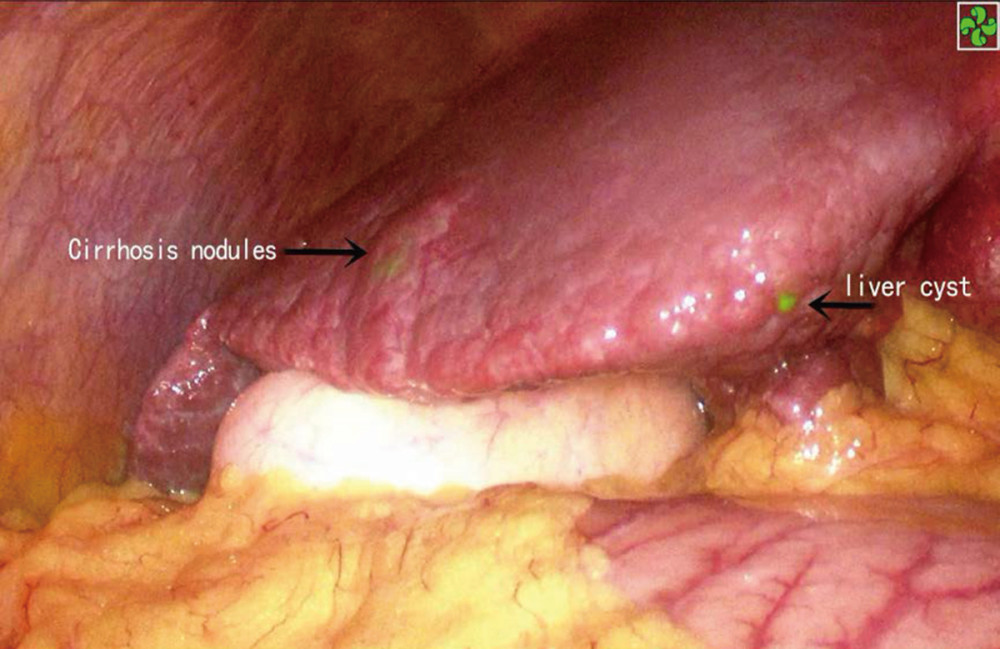 Figure 4. ICG staining of a benign liver lesion.
Figure 4. ICG staining of a benign liver lesion. References
1. Chen W, Zheng R, Baade PD, Cancer statistics in China, 2015: Cancer J Clin, 2016; 66; 115-32
2. Mogahed MM, Zytoon AA, Eysa B, Outcome of laparoscopic assisted percutaneous microwave ablation for exophytic versus non-exophytic hepatocellular carcinoma: J Gastrointest Cancer, 2021; 52; 892-98
3. Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D, Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification: Adv Cancer Res, 2021; 149; 1-61
4. akamoto T, Improvement and development in anatomical hepatectomy for hepatocellular carcinoma: Hepatobiliary Surg Nutr, 2021; 10; 545-47
5. Vogl TJ, Nour-Eldin NA, Hammerstingl RM, Microwave ablation (MWA): Basics, technique and results in primary and metastatic liver neoplasms – review article: Rofo, 2017; 189; 1055-66
6. Della Corte A, Ratti F, Monfardini L, Marra P, Comparison between percutaneous and laparoscopic microwave ablation of hepatocellular carcinoma: Int J Hyperthermia, 2020; 37; 542-48
7. Mediouni M, DRS , Madry H, Cucchiarini M, Rai B, A review of translational medicine. The future paradigm: How can we connect the orthopedic dots better?: Curr Med Res Opin, 2018; 34; 1217-29
8. Hwang S, Ha TY, Song GW, Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume: J Gastrointest Surg, 2015; 19; 1305-14
9. Han HW, Shi N, Zou YP, Zhang YP, Functional anatomical hepatectomy guided by indocyanine green fluorescence imaging in patients with localized cholestasis: Report of four cases: World J Gastrointest Surg, 2021; 13; 323-29
10. Ishizawa T, Saiura A, Kokudo N, Clinical application of indocyanine green-fluorescence imaging during hepatectomy: Hepatobiliary Surg Nutr, 2016; 5; 322-28
11. Ishizawa T, Fukushima N, Shibahara J, Real-time identification of liver cancers by using indocyanine green fluorescent imaging: Cancer, 2009; 115; 2491-504
12. Yokoyama N, Otani T, Hashidate H, Maeda C, Real-time detection of hepatic micrometastases from pancreatic cancer by intraoperative fluorescence imaging: Preliminary results of a prospective study: Cancer, 2012; 118; 2813-19
13. Ng KKC, Chok KSH, Chan ACY, Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma: Br J Surg, 2017; 104; 1775-84
14. Liu W, Zheng Y, He W, Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: A propensity score analysis: Aliment Pharmacol Ther, 2018; 48; 671-81
15. Ge N, Huang J, Shi Z, Safety and efficacy of microwave ablation for periductal hepatocellular carcinoma with intraductal cooling of the central bile ducts through a percutaneous transhepatic cholangial drainage tube: J Interv Med, 2019; 2; 84-90
16. Chen QY, Xie JW, Zhong Q, Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: A randomized clinical trial: JAMA Surg, 2020; 155; 300-11
17. Chen J, Peng K, Hu D, Tumor location influences oncologic outcomes of hepatocellular carcinoma patients undergoing radiofrequency ablation: Cancers (Basel), 2018; 10; 378
18. Kawaguchi Y, Nagai M, Nomura Y, Kokudo N, Tanaka N, Usefulness of indocyanine green-fluorescence imaging during laparoscopic hepatectomy to visualize subcapsular hard-to-identify hepatic malignancy: J Surg Oncol, 2015; 112; 514-16
19. Terasawa M, Ishizawa T, Mise Y, Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy: Surg Endosc, 2017; 31; 5111-18
20. Nishino H, Hatano E, Seo S, Real-time navigation for liver surgery using projection mapping with indocyanine green fluorescence: Development of the novel medical imaging projection system: Ann Surg, 2018; 267; 1134-40
21. Vietti Violi N, Duran R, Guiu B, Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: A randomised controlled phase 2 trial: Lancet Gastroenterol Hepatol, 2018; 3; 317-25
22. Cheng X, Huang J, Li WFAnalysis of the effect of microwave ablation in the treatment of small liver cancer: Zhonghua Gan Zang Bing Za Zhi, 2021; 29; 1059-62 [in Chinese]
23. Zhou SC, Tian YT, Wang XW, Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer: World J Gastroenterol, 2019; 25; 4502-11
24. Jianxi W, Xiongfeng Z, Zehao Z, Indocyanine green fluorescence-guided laparoscopic hepatectomy versus conventional laparoscopic hepatectomy for hepatocellular carcinoma: A single-center propensity score matching study: Front Oncol, 2022; 12; 930065
25. Zhong X, Tang H, Lu B, Differentiation of small hepatocellular carcinoma from dysplastic nodules in cirrhotic liver: Texture analysis based on MRI improved performance in comparison over gadoxetic acid-enhanced MR and diffusion-weighted imaging: Front Oncol, 2019; 9; 1382
26. Abd Alkhalik Basha M, Abd El Aziz El Sammak D, El Sammak AA, Diagnostic efficacy of the Liver Imaging-Reporting and Data System (LI-RADS) with CT imaging in categorising small nodules (10–20 mm) detected in the cirrhotic liver at screening ultrasound: Clin Radiol, 2017; 72; 901e1-e11
27. Ishizawa T, Masuda K, Urano Y, Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma: Ann Surg Oncol, 2014; 21; 440-48
28. Santambrogio R, Bruno S, Kluger MD, Laparoscopic ablation therapies or hepatic resection in cirrhotic patients with small hepatocellular carcinoma: Dig Liver Dis, 2016; 48; 189-96
Figures
 Figure 1. (A) Overall survival. (B) Recurrence-free survival.
Figure 1. (A) Overall survival. (B) Recurrence-free survival. Figure 2. (A) Tumor residues after ultrasound-guided percutaneous microwave ablation. (B) Fluorescence imaging of tumor residues after ultrasound-guided percutaneous microwave ablation.
Figure 2. (A) Tumor residues after ultrasound-guided percutaneous microwave ablation. (B) Fluorescence imaging of tumor residues after ultrasound-guided percutaneous microwave ablation. Figure 3. (A) No lesions can be found in conventional laparoscopic mode, and no lesions can be found by laparoscopic ultrasonography. (B) Fluorescence imaging of the lesion. (C) During lesion ablation. (D) After lesion ablation.
Figure 3. (A) No lesions can be found in conventional laparoscopic mode, and no lesions can be found by laparoscopic ultrasonography. (B) Fluorescence imaging of the lesion. (C) During lesion ablation. (D) After lesion ablation. Figure 4. ICG staining of a benign liver lesion.
Figure 4. ICG staining of a benign liver lesion. Tables
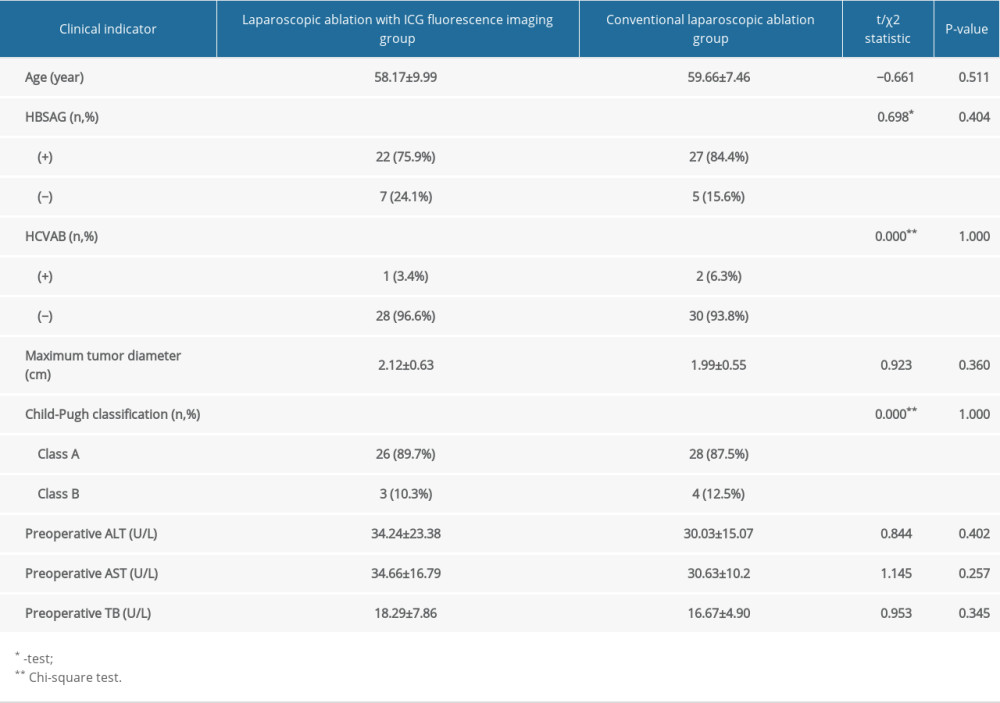 Table 1. Comparison of preoperative clinical indicators between laparoscopic ablation with and without ICG fluorescence imaging.
Table 1. Comparison of preoperative clinical indicators between laparoscopic ablation with and without ICG fluorescence imaging.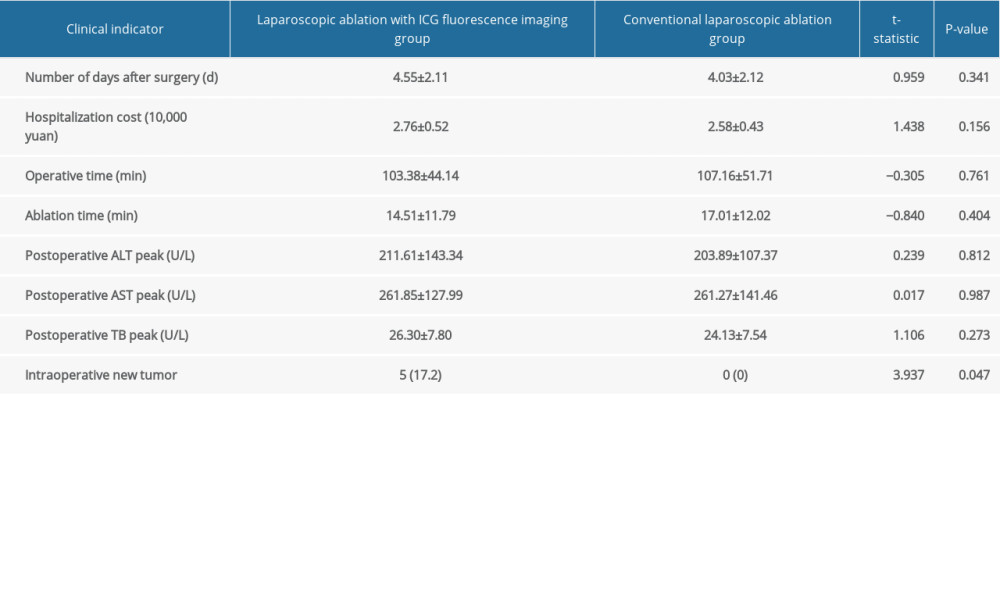 Table 2. Comparison of intraoperative and postoperative clinical indicators between the laparoscopic ablation with and without ICG fluorescence imaging.
Table 2. Comparison of intraoperative and postoperative clinical indicators between the laparoscopic ablation with and without ICG fluorescence imaging. Table 1. Comparison of preoperative clinical indicators between laparoscopic ablation with and without ICG fluorescence imaging.
Table 1. Comparison of preoperative clinical indicators between laparoscopic ablation with and without ICG fluorescence imaging. Table 2. Comparison of intraoperative and postoperative clinical indicators between the laparoscopic ablation with and without ICG fluorescence imaging.
Table 2. Comparison of intraoperative and postoperative clinical indicators between the laparoscopic ablation with and without ICG fluorescence imaging. In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








