05 April 2023: Clinical Research
Exploration of Perioperative Sleep Disturbance in 208 Patients Undergoing Non-Cardiac Surgery: Protocol for a Prospective Cohort Study
Jiaojiao Yang1BEF, Xue Han1B, Xiao Liu1C, Ying Cao1D, Kang Yu1D, Lei Liu2C, Huihui Miao1AEG*, Tianzuo Li1AEDOI: 10.12659/MSM.938832
Med Sci Monit 2023; 29:e938832
Abstract
BACKGROUND: Sleep disorder is a common complication for postoperative patients, which can impact their recovery and prognosis. In the perioperative period of non-cardiac surgery, multiple factors can be involved in abnormal sleep in patients, including changes in sleep quality and quantity. Thus, the purpose of this study is to explore the incidence of postoperative sleep disturbance and related influencing factors in 208 patients undergoing non-cardiac surgery.
MATERIAL AND METHODS: This is a single-center prospective cohort study including 208 eligible patients who will undergo non-cardiac surgery. All participants will implement the assessment and monitoring of perioperative sleep quality using the Pittsburgh Sleep Quality Index (PSQI) and a wearable electroencephalogram (EEG) sleep monitor on the night before surgery and on the first, third, and fifth nights after surgery (the first night is the day of surgery). Meanwhile, we will collect the patient’s basic information, past history, and surgery-related data from the hospital electronic medical record and will perform follow-up before and after surgery.
RESULTS: The primary outcome is the occurrence of sleep disturbance on the first, third, and fifth nights after surgery. The secondary outcomes are the factors related to sleep disturbance and changes in sleep structure on the first, third, and fifth nights after surgery.
CONCLUSIONS: This study will record the incidence of postoperative sleep disturbance, explore the risk factors of postoperative sleep disturbance, and clarify the change of postoperative sleep structure, which will provide ideas for clinicians to manage patients’ sleep disturbance during the perioperative period.
Keywords: Risk Factors, Sleep Wake Disorders, Postoperative Period, Humans, Prospective Studies, Sleep
Background
Sleep is one of the main basic physiological needs of human beings, and it has irreplaceable effects on maintaining a normal physical and psychological state [1]. Postoperative sleep disorder is a common clinical problem [2]. In a previous study, 42% of patients experienced a sleep disorder after orthopedic, vascular, and general surgery compared with before surgery [3]. Other studies showed about 25% of patients still lacked sleep for more than 15 days after surgery [4], and even at over 6 months of follow-up, the incidence of abnormal sleep was 10% to 61% [5]. Postoperative sleep disorder can bring many adverse effects for patients, such as depression, anxiety, acute and chronic pain, cardiovascular diseases, dementia, and cognitive decline [6,7]. Early postoperative sleep disorder is characterized by reduced total sleep time, decreased or absent rapid eye movement sleep (REM) and slow wave sleep, and frequent fragmented sleep [8].
At present, it is believed that multiple factors are involved in the occurrence and development of postoperative sleep disturbance, such as age, sex, pain, type of anesthesia, and magnitude of surgery [9]. Moreover, the degree and duration of postoperative sleep disturbance are related to the type of surgery [4]. For example, sleep disturbance is more severe after gastrectomy than after hernia repair [10]. Similarly, different anesthesia methods have different effects on postoperative sleep [11]; patients undergoing spinal and regional anesthesia seem to experience less bad sleep in the night after surgery than do those undergoing general anesthesia [12]. This may be because the stress response after regional or spinal anesthesia is less than that of general anesthesia and the need for anesthetics [13], especially opioids, are reduced. Although opioids have great advantages in pain relief, they can lead to changes in sleep architecture [14,15]. Taken together, monitoring and management of perioperative sleep quality are of great significance.
There are currently several tools for evaluating sleep quality. Subjective evaluation scales are widely used, such as the Pittsburgh Sleep Quality Index (PSQI), Athens Insomnia Scale, and Insomnia Severity Index [16,17]. Among them, the PSQI, which can assess patients’ sleep status in the past month, is widely used in clinical practice [18]. The PSQI takes into account both the time and efficiency of sleep. It is not to be used only to analyze the sleep quality of the general population, but more importantly, to assist in the diagnosis of sleep disturbance in clinical patients [17]. In addition, the PSQI has high sensitivity and reliability in identifying sleep disturbance [19,20]. Objective sleep assessment methods, such as polysomnography and actigraphy, are also increasingly used [21]. Polysomnography, as the criterion standard for diagnosing sleep disorders [22], conducts comprehensive and continuous monitoring of physiological activities during the night and determines various behavioral states in wakefulness and sleep, including stages of wakefulness, non-rapid eye movement sleep, and REM sleep [23]. However, due to its limitations, such as complicated operation, high cost, and the need to establish a standard sleep monitoring room [24], various novel portable sleep monitoring devices have been developed in recent years. Compared with polysomnography, the portable EEG sleep monitor is less intrusive and expensive, more ecological, and more practical for continuous data collection over days or weeks [25]. The wearable EEG sleep monitor developed by Beijing Easymonitor Technology Co, Ltd, Beijing, China, is a novel measurement reflecting the functional state of the brain based on integrated EEG wavelet analysis [26]. The principle of the wearable EEG sleep monitor is to use brain electrodes similar to bispectral index to collect EEG signals from the forehead (FPz), above the bilateral eyebrows (left Fp1, right Fp2), and the bilateral mastoid (left T5, right T6) [27]. Then, a cloud server calculates and automatically generates the sleep report, including sleep efficiency, sleep distribution and latency, and proportion of sleep stages (N1, N2, N3, N4, and REM). In summary, this study plans to use the PSQI and the wearable EEG sleep monitor to assess the perioperative sleep quality in patients undergoing non-cardiac surgery.
Hence, this study’s intended goals are (1) to obtain the incidence of postoperative sleep disturbance, (2) to explore the risk factors of postoperative sleep disturbance, and (3) to clarify the change of postoperative sleep structure.
Material and Methods
ETHICS STATEMENT:
This is a single-center prospective cohort study that has been granted approval by the Medical Ethics Committee of Beijing Shijitan Hospital, Capital Medical University, China (approval no. Sjtky11-1x-2022[19]). The study was registered at https://www.chictr.org.cn (registration no. ChiCTR2200056250) on February 2, 2022. All participants will sign written informed consent before entering the study.
STUDY DESIGN:
We plan to recruit 208 patients who meet the inclusion criteria, and all participants will have sleep quality assessed by the PSQI and the wearable EEG sleep monitor on the night before the surgery and on the first, third, and fifth nights after surgery (the first night is on the same day as the surgery). Meanwhile, we will collect patients’ demographic information, chronic diseases, and surgery-related data from the hospital electronic medical record and will follow up before and after surgery. The primary outcome is the occurrence of sleep disturbances on the first, third, and fifth nights after surgery.
INCLUSION AND EXCLUSION CRITERIA:
Patient eligibility criteria are a willingness to participate in the trial and ability to provided written informed consent.
The inclusion criteria are as follows: (1) age 18 years or older; (2) American Society of Anesthesiologists physical status class I to III; (3) operation time is 2 h or more; and (4) hospital stay is at least 5 days after surgery.
The exclusion criteria are as follows: (1) patients undergoing brain surgery and cardiac surgery; (2) history of mental and psychological disorders with use of sedatives, hypnotics, anti-psychotic drugs, or anti-depression drugs in the past 3 months, and hospitalization; (3) severe language, hearing, vision and cognitive dysfunction, which will prevent patients from cooperating with the questionnaire and sleep monitoring; (4) patients admitted to the Intensive Care Unit unexpectedly or who have significant postoperative complications needing investigations, interventions, or clinical care at night; (5) patients with poor-quality EEG data or significant artifacts on EEG monitoring; and (6) other types of sleep disturbances, such as restless leg syndrome or periodic limb movement.
RANDOMIZATION AND ENROLLMENT:
Due to the limitations of equipment and human resources, only up to 5 patients a day can be included in our study. Therefore, we will ensure the randomization of the research and consider the effect of different operating times on postoperative sleep quality [28,29]. Every day before surgery, we will first use the random number table to select 3 to 5 patients who are scheduled to undergo surgery in the daytime (8: 00 a.m.–12: 00 p.m.) as target research participants. Then, team members will visit these patients in the ward and invite them to join the study. Once these patients are interested in the research, their written informed consent will be obtained prior to enrollment in the trial. If any of the target participants obtained through the random number table method refuse to participate in the study, we will use the random number table again to extract study participants until 3 to 5 patients participate in the study every day.
INTERVENTION:
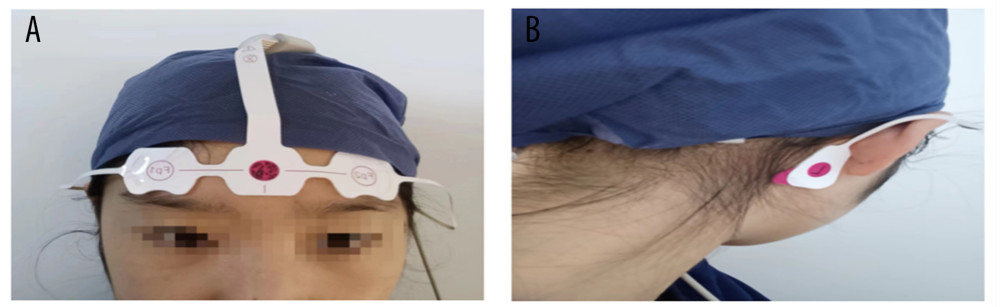
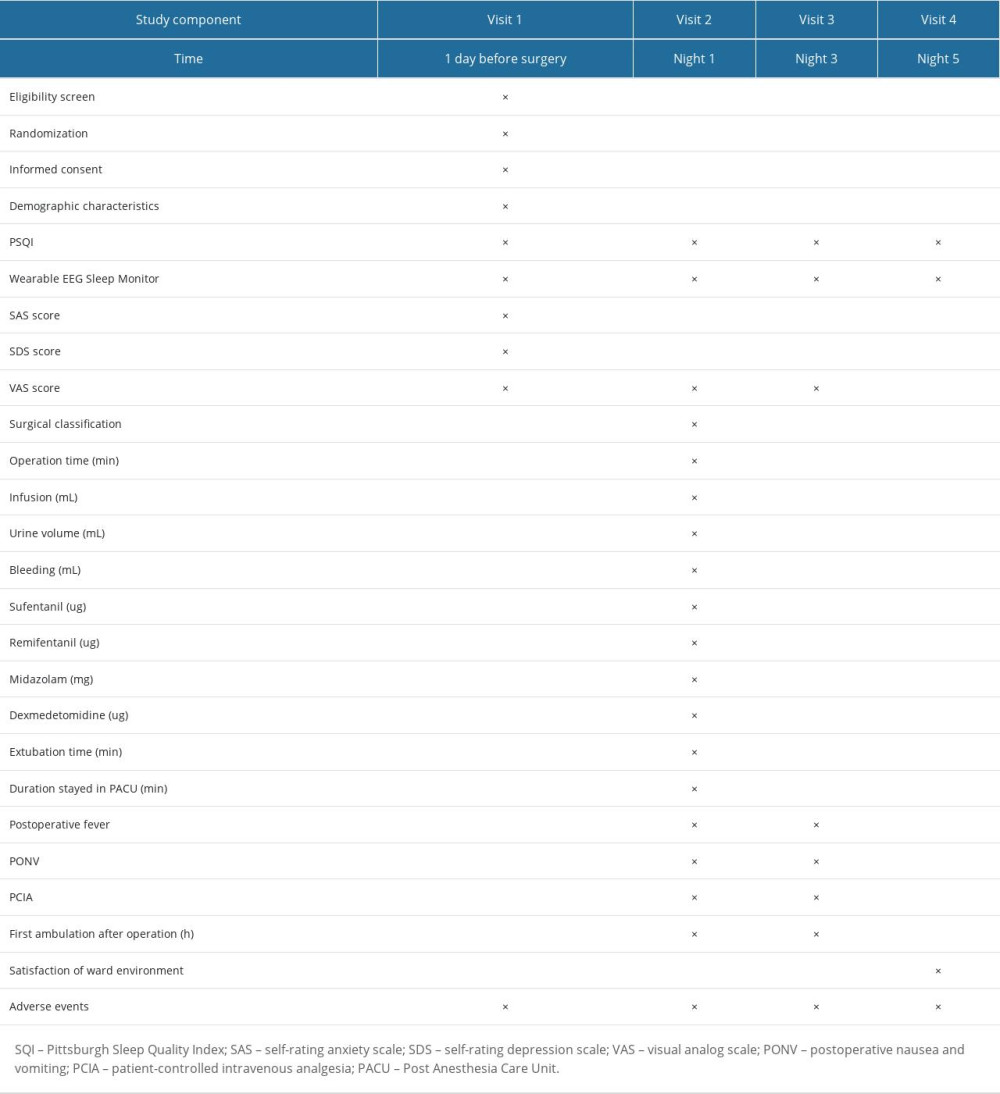
One night before surgery, the researchers will use the subjective evaluation scale, the PSQI, to evaluate the sleep quality of all participants in the past 1 month (if the score exceeds 5 points or the patient has been diagnosed previously with a sleep disorder, we will exclude the patient). Then, the wearable EEG sleep monitor will be used for bedside sleep detection throughout the night. Operation of the device is as follows: first scrub the forehead and bilateral mastoid skin with water or alcohol when patients want to sleep at night, then the EEG electrodes are placed on the forehead (FPZ) 1 to 2 cm above the bilateral eyebrows (left FP1, right FP2) and on the bilateral mastoid (left C1, right C2); finally, the switch button on the device is turned on (Figure 1A, 1B). At this point, the EEG monitoring starts and is recorded as the start of sleep. When patients wake up in the morning, they will remove the electrodes and turn off the switch, which is the end of sleep time. The patients will repeat the same method on the first, third, and fifth days after surgery. In addition, the patient will have another PSQI assessment on the fifth day after surgery.
DATA SOURCES AND COLLECTION:
After obtaining informed consent from the participants, we will obtain data through the hospital electronic medical record and follow-ups. The collected data will be recorded on the case report form by certificated clinical research coordinators. The potential risk factors for sleep disturbances are based on physiological and pathological characteristics of humans, perioperative interventions, and related literature, which include the following: (1) Preoperative variables: sex, age, body mass index, American Society of Anesthesiologists physical status class, smoking and drinking history, education level, economic status, obstructive sleep apnea syndrome, hypertension, diabetes mellitus, coronary heart disease, cerebral embolism, preoperative numerical rating scale score, preoperative self-rating anxiety scale score, preoperative self-rating depression scale score, restless leg syndrome, or periodic limb movement; (2) Intraoperative variables: surgery classification, operation time, type of anesthesia (general anesthesia, intraspinal anesthesia), amount of fluid infusion, blood loss, urine volume, dosage of midazolam, dexmedetomidine and opioids, time from the end of the surgery to the removal of the tracheal intubation, duration of stay in Postanesthesia Care Unit; and (3) Postoperative variables: postoperative fever, postoperative nausea and vomiting, the use of patient-controlled intravenous analgesia, postoperative numerical rating scale score, time of first ambulation after surgery, satisfaction of ward environment (1, very dissatisfied; 2, not satisfied; 3, average; 4, satisfied; 5, very satisfied) (Table 1).
PERIOPERATIVE SLEEP QUALITY EVALUATION:
The sleep data obtained through the wearable EEG sleep monitor will be calculated by a cloud server that will automatically generate a sleep report. The sleep report will be analyzed and reported by the same expert in our research team. Sleep efficiency detected by the wearable sleep EEG monitor of less than 80% will be diagnosed as sleep disturbance (sleep efficiency refers to sleep time as a percentage of total sleep time). Also, we will collect the PSQI questionnaire, and scores greater than 5 will be diagnosed as sleep disturbance.
PRIMARY OUTCOMES:
The primary outcomes are the incidence of sleep disorder measured using wearable the EEG sleep monitor at the first, third, and fifth postoperative nights. Patients will be following the hospital policy of lights-on (6: 00 a.m.) and lights-off (8: 00 p.m.) times. Each patient will be placed in a standard room (eg, light, temperature) that is free from noise, television, mobile phone, and computer.
SECONDARY OUTCOMES:
The secondary outcomes are factors related to sleep disturbance and changes of sleep structure on the first, third, and fifth nights after surgery. Sleep disturbance includes all preoperative, intraoperative, and postoperative variables. Changes of sleep structure include changes in preoperative and postoperative stage 2 sleep, slow wave sleep, and REM percentages.
SAMPLE SIZE CALCULATION:
This study will use the events per variable method for sample size calculation. The sample size is calculated from the primary outcome. It is hypothesized that there are 10 variables related to postoperative sleep disturbance, and the required positive sample size is 100. Previous studies reported that the incidence of postoperative sleep disorders is 49.7%, and is even up to 95% in cardiac surgery [30,31]. We assume that if the incidence of sleep disorders is 60%, then 167 patients are required. Taking into account a 20% dropout rate, the final total sample size of this study is 208 patients.
SAFETY AND ADVERSE EVENT REPORTING:
All adverse events (described as unfavorable or unintended signs, symptoms, or diseases occurring during the trial) related to the intervention will be monitored by team members during the entire study period. Since the wearable EEG Sleep Monitor is achieved by placing electrodes on the patient’s forehead and bilateral mastoid behind the ears, some patients can experience a slight discomfort, such as skin redness, allergy, or even breakage. Furthermore, no blood, urine, or other specimen collection or invasive procedures are involved in this study; therefore, the occurrence of serious adverse events will be nearly impossible in this trial.
STATISTICAL ANALYSIS:
Statistical analysis will be done using SPSS 20.0 software (IBM Corp, Armonk, NY, USA). All continuous variables will be expressed as mean±standard deviation for a normal distribution or median (interquartile range) for a skewed distribution. For categorical variables, the data will be presented as numbers and percentages. Multivariate logistic regression will be used to analyze the influencing factors of perioperative sleep disturbance. The statistical significance will be set at
The primary outcome is the incidence of sleep disorders on the first, third, and fifth nights after surgery. We will analyze the influencing factors related to postoperative sleep disturbance as one of the secondary outcomes. First, univariate analysis will be applied to select non-zero characteristic variables, followed by multivariate logistic regression analysis to obtain independent risk factors for postoperative sleep disturbance. The other secondary outcome is the change of sleep structure on the first, third, and fifth postoperative nights. Stage 2 sleep, slow wave sleep, and REM percentages will be analyzed with the independent-sample
Discussion
In recent years, with the proposal of enhanced recovery after surgery, postoperative sleep disturbance has received more attention [32]. However, the mechanism of postoperative sleep is complex, and most current studies are limited to the impact of a single factor on perioperative sleep, such as the relationship between sleep disturbance and pain, mental disorders, and analgesics. In addition, these studies use mostly subjective evaluation scales to analyze sleep quality. The assessment methods are highly subjective and easily affected by outside influences. The purpose of this study, therefore, is to exploit an objective assessment method, the wearable EEG sleep monitor, to assess the incidence of postoperative sleep disturbance and related influencing factors in patients undergoing non-cardiac surgery; the wearable EEG sleep monitor has the advantages of being simple, convenient, inexpensive, and reliable, in comparison to the polysomnography [33]. Compared with subjective evaluation scales, the wearable monitor can analyze sleep parameters of total sleep time, rapid eye movement, slow wave sleep, non-rapid eye movement, and sleep efficiency. The principle of the device is to calculate and analyze EEG signals through a cloud server to obtain relevant sleep data. EEG signals, which directly represent brain activity, are reliable to analyze sleep [34]. Berthomier et al found that the single-channel sleep EEG analysis provides reliable sleep scoring, which is 82.9% consistent with expert manual scoring of polysomnograms [35]. There is also a correlation between sleep stages and EEG power in different frequency bands [36].
There are some limitations in this study. First, the study will be conducted in only 1 medical institution, and it would be better to conduct the study in multiple medical institutions. Second, some factors may not be considered in this study since the risk factors for postoperative sleep disturbance are complex and some risk factors may be removed owing to a relatively small sample size. Third, in our hospital, due to restriction of the hospitalization system, the patients are not allowed to be admitted many days prior to surgery; thus, we will take the sleep quality on the night before surgery as the baseline sleep quality, which may affect the validity of outcomes. However, once the patients enter the study, we will also use the PSQI to evaluate the sleep quality in the last 1 month. If the score exceeds 5 points or the patient has been previously diagnosed with sleep disorders, we will exclude the patient from the study. This measure helps to overcome the limitation of measuring baseline sleep on only 1 night before surgery. Last, previous studies have shown that postoperative sleep disturbances take 7 days or even longer to recover [37]. However, due to the limitation of equipment and hospitalization time of patients in this study, the postoperative observation time is only until the fifth postoperative day; it is necessary to conduct a longer follow-up in the future.
Conclusions
Postoperative sleep disorders are often neglected, but they are closely related to postoperative cognitive dysfunction, postoperative pain sensitization, postoperative paroxysmal hypoxemia, and cardiovascular adverse events caused by sympathetic nervous system excitement. It is an important issue for medical staff to assess the sleep quality of perioperative patients, evaluate the multi-dimensional influencing factors of sleep disorders, and formulate individualized sleep management measures to upgrade patients’ sleep.
References
1. Li Y, Zhao L, Yang C, Development and validation of a clinical prediction model for sleep disorders in the ICU: A retrospective cohort study: Front Neurosci, 2021; 15; 644845
2. Tsukinaga A, Mihara T, Takeshima T, Effect of melatonin and melatonin agonists on postoperative sleep quality in adult patients: A protocol for systematic review and meta-analysis with trial sequential analysis: BMJ Open, 2021; 11(9); e047858
3. Chouchou F, Khoury S, Chauny JM, Postoperative sleep disruptions: A potential catalyst of acute pain?: Sleep Med Rev, 2014; 18(3); 273-82
4. Rosenberg-Adamsen S, Kehlet H, Dodds C, Rosenberg J, Postoperative sleep disturbances: Mechanisms and clinical implications: Br J Anaesth, 1996; 76(4); 552-59
5. Altman MT, Knauert MP, Pisani MA, Sleep disturbance after hospitalization and critical illness: A systematic review: Annals ATS, 2017; 14(9); 1457-68
6. Sipilä RM, Kalso EA, Sleep well and recover faster with less pain – a narrative review on sleep in the perioperative period: J Clin Med, 2021; 10(9); 2000
7. Wang X, Hua D, Tang X, The role of perioperative sleep disturbance in postoperative neurocognitive disorders: Nat Sci Sleep, 2021; 13; 1395-410
8. Su X, Meng ZT, Wu XH, Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: A randomised, double-blind, placebo-controlled trial: Lancet, 2016; 388(10054); 1893-902
9. Jaussent I, Dauvilliers Y, Ancelin ML, Insomnia symptoms in older adults: Associated factors and gender differences: Am J Geriatr Psychiatry, 2011; 19(1); 88-97
10. Ellis BW, Dudley HA, Some aspects of sleep research in surgical stress: J Psychosom Res, 1976; 20(4); 303-8
11. Luo M, Song B, Zhu J, Sleep disturbances after general anesthesia: Current perspectives: Front Neurol, 2020; 11; 629
12. Su X, Wang DX, Improve postoperative sleep: What can we do?: Curr Opin Anaesthesiol, 2018; 31(1); 83-88
13. Nelson LE, Franks NP, Maze M, Rested and refreshed after anesthesia? Overlapping neurobiologic mechanisms of sleep and anesthesia: Anesthesiology, 2004; 100(6); 1341-42
14. Cronin AJ, Keifer JC, Davies MF, Postoperative sleep disturbance: Influences of opioids and pain in humans: Sleep, 2001; 24(1); 39-44
15. Dimsdale JE, Norman D, DeJardin D, Wallace MS, The effect of opioids on sleep architecture: J Clin Sleep Med, 2007; 3(1); 33-36
16. Shin S, Kim SH, Jeon B, Objective assessment of sleep patterns among night-shift workers: A scoping review: Int J Environ Res Public Health, 2021; 18(24); 13236
17. Fabbri M, Beracci A, Martoni M, Measuring subjective sleep quality: A review: Int J Environ Res Public Health, 2021; 18(3); 1082
18. Buysse DJ, Reynolds CF, Monk TH, The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research: Psychiatry Res, 1989; 28(2); 193-213
19. Spira AP, Beaudreau SA, Stone KLOsteoporotic Fractures in Men Study, Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men: J Gerontol A Biol Sci Med Sci, 2012; 67(4); 433-39
20. Heinze N, Hussain SF, Castle CL, The impact of COVID-19 on sleep quality in people living with disabilities: Front Psychol, 2021; 12; 786904
21. Slyepchenko A, Allega OR, Leng X, Association of functioning and quality of life with objective and subjective measures of sleep and biological rhythms in major depressive and bipolar disorder: Aust N Z J Psychiatry, 2019; 53(7); 683-96
22. Sadeghniiat-Haghighi K, Yazdi Z, Firoozeh M, Comparison of two assessment tools that measure insomnia: The insomnia severity index and polysomnography: Indian J Psychol Med, 2014; 36(1); 54-57
23. Rundo JV, Downey R, Polysomnography: Handbook of clinical neurology [Internet], 2019; 381-92, Elsevier [cited 2022 Dec 24] Available from: https://linkinghubelsevier.com/retrieve/pii/B9780444640321000254
24. Chinoy ED, Cuellar JA, Huwa KE, Performance of seven consumer sleep-tracking devices compared with polysomnography: Sleep, 2021; 44(5); zsaa291
25. Imtiaz SA, A systematic review of sensing technologies for wearable sleep staging: Sensors, 2021; 21(5); 1562
26. Wu L, Wang S, Wang Y, Prediction of hemodynamic reactivity by electroencephalographically derived pain threshold index in children undergoing general anesthesia: A prospective observational study: J Pain Res, 2019; 12; 3245-55
27. Wang R, Deng Y, Zhou S, Zhang J, EEG-derived pain threshold index for prediction of postoperative pain in patients undergoing laparoscopic urological surgery: A comparison with surgical pleth index: J Clin Monit Comput, 2021; 35(6); 1395-402
28. Song B, Li Y, Teng X, Comparison of morning and evening operation under general anesthesia on intraoperative anesthetic requirement, postoperative sleep quality, and pain: A randomized controlled trial: Nat Sci Sleep, 2020; 12; 467-75
29. Song B, Li Y, Teng X, The effect of intraoperative use of dexmedetomidine during the daytime operation vs the nighttime operation on postoperative sleep quality and pain under general anesthesia: Nat Sci Sleep, 2019; 11; 207-15
30. Lin D, Huang X, Sun Y, Perioperative sleep disorder: A review: Front Med, 2021; 8; 640416
31. Ghorbani A, Hajizadeh F, Sheykhi MR, Mohammadpoor asl A, The effects of deep-breathing exercises on postoperative sleep duration and quality in patients undergoing coronary artery bypass graft (CABG): A randomized clinical trial: J Caring Sci, 2019; 8(4); 219-24
32. Rampes S, Ma K, Divecha YA, Postoperative sleep disorders and their potential impacts on surgical outcomes: J Biomed Res, 2019; 34(4); 271-80
33. Wright KP, Bogan RK, Wyatt JK, Shift work and the assessment and management of shift work disorder (SWD): Sleep Med Rev, 2013; 17(1); 41-54
34. Ling H, Luyuan Y, Xinxin L, Bingliang D, Staging study of single-channel sleep EEG signals based on data augmentation: Front Public Health, 2022; 10; 1038742
35. Berthomier C, Drouot X, Herman-Stoïca M, Automatic analysis of single-channel sleep EEG: Validation in healthy individuals: Sleep, 2007; 30(11); 1587-95
36. Mann K, Röschke J, Different phase relationships between EEG frequency bands during NREM and REM sleep: Sleep, 1997; 20(9); 753-56
37. Chung F, Liao P, Yegneswaran B, Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea: Anesthesiology, 2014; 120(2); 287-98
In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952