07 March 2023: Clinical Research
Splenic Artery Steal Syndrome in Patients with Liver Cirrhosis: A Retrospective Clinical Study
Wei Mao1ABEF, Xinhua Jiang1BCF, Sixuan Guo2BCDF, Xuguang Hu3AEF*, Yehong Yan4AFDOI: 10.12659/MSM.938998
Med Sci Monit 2023; 29:e938998
Abstract
BACKGROUND: Splenic artery steal syndrome (SASS) can aggravate liver damage in patients with cirrhosis. This study explored whether SASS could be an effective therapeutic target for improving hepatic artery perfusion and liver function in patients with decompensated cirrhosis.
MATERIAL AND METHODS: Based on inclusion and exclusion criteria, 87 patients with hepatitis B cirrhosis and portal hypertension hypersplenism admitted to our General Surgery Department for splenectomy and pericardial devascularization surgery were selected. A total of 35 cases met the diagnostic criteria of SASS and were assigned to the SASS group; the remaining 52 cases were assigned to the control group. The indicators before, during, and after surgery were compared between the 2 groups.
RESULTS: There were no significant differences in preoperative and intraoperative indicators between SASS group and control group (P>0.05). The MELD score 7 days after surgery and the hepatic artery diameter and hepatic artery velocity 14 days after surgery in both groups were significantly better than before surgery. The MELD score 7 days after surgery in the SASS group was significantly better than that in the control group, and the hepatic artery diameter and hepatic artery velocity 14 days after surgery in the SASS group were significantly better than those in the control group (P<0.05).
CONCLUSIONS: Splenectomy and pericardial devascularization surgery was an effective treatment to redirect blood flow to the hepatic artery for cirrhotic patients diagnosed with SASS. The introduction of cirrhotic SASS into clinical practice may benefit more patients with cirrhotic portal hypertension and hypersplenism.
Keywords: Hepatic Artery, Liver Cirrhosis, Splenectomy, Splenic Artery, Humans, hypersplenism, Hypertension, Portal
Background
In clinical practice, some patients with chronic viral hepatitis and cirrhosis progress slowly despite the effective application of antiviral therapy. Some patients gradually develop splenomegaly, hypersplenism, gastroesophageal varices, and even rupture and bleeding, and some even gradually develop chronic liver failure. Obviously, these are not caused by the hepatitis virus. Portal hypertension in cirrhosis can lead to a series of hemodynamic abnormalities such as splenic congestion and swelling, hypersplenism, portal vein thickening, esophageal and gastric varices, and increased blood flow in splenic vessels. After some patients underwent splenectomy because of severe hypersplenism, all indicators of liver function improve to varying degrees. In response to this phenomenon, some scholars have conducted in-depth studies and proposed the concept of splenic artery steal syndrome (SASS). However, most of the clinical diagnosis and treatment of SASS are for patients after liver transplantation. Meanwhile, the concept of SASS in patients with cirrhosis has been controversial, and relevant studies are rare. Since 2019, we have introduced the concept of SASS into clinical practice. The patients with portal hypertension and hypersplenism in cirrhosis who were about to undergo surgery were re-evaluated and screened according to the diagnostic criteria of SASS, confirming that SASS can be an effective therapeutic target for improving liver function in patients with decompensated cirrhosis.
Material and Methods
GENERAL DATA:
We enrolled 87 patients with hepatitis B cirrhosis and portal hypertension hypersplenism admitted to our General Surgery Department for splenectomy and pericardial devascularization surgery from January 2020 to December 2021, including 54 males and 33 females, aged 32–69 years, weighing 52–86 kg. A total of 35 cases (24 males and 11 females) met the diagnostic criteria and were assigned to the SASS group; the remaining 52 cases (30 males and 22 females) were assigned to the control group. All cases had preoperative Child-Turcotte-Pugh (CTP) classification A or B class, American Society of Anesthesiologists (ASA) classification I or II class, and all underwent open splenectomy and pericardial devascularization surgery. All patients were assessed using an automatic blood and biochemical analysis instrument (Mindray, China) to detect liver function indicators before and after surgery. The diameter and flow velocity of hepatic artery were measured by color Doppler ultrasound (Mindray, China) before and after surgery. All patients had signed a preoperative informed consent for surgery and a scientific pan-informed consent form. This study was approved by the Medical Ethics Committee of Nanchang University Infectious Diseases Hospital (No. 2022-12).
The diagnostic criteria for SASS were: 1) The presence of significant splenomegaly; 2) Significant thickening of the splenic artery (greater than 4 mm or greater than 1.5 times the diameter of the hepatic artery); 3) Patency of the hepatic artery without obstruction, but slender caliber, slow blood flow, and delayed filling of the hepatic parenchyma in the arterial phase; 4) Thickening of the splenic artery with rapid blood flow and early filling of the splenic parenchyma with contrast medium; 5) The splenic vein and portal vein were visualized early after imaging, even in the arterial phase, due to the high velocity of blood flow in the splenic artery.
INCLUSION CRITERIA:
1) Preoperative diagnosis was clear, and open splenectomy and pericardial devascularization surgery was required; 2) Preoperative assessment of heart, lung, brain, liver and kidney function was normal, no malignant tumor, and systemic condition could tolerate surgery; 3) Imaging data was perfect, and could be screened according to SASS diagnostic criteria; 4) No combined tuberculosis, acquired immunodeficiency syndrome (AIDS), or other systemic diseases; 5) Clear consciousness without cognitive impairment, and able to cooperate with the investigation and evaluation.
EXCLUSION CRITERIA:
1) Preoperative liver function CTP classification C class, or assessed to be unsuitable for surgery or unable to tolerate surgery; 2) Consciousness impairment or cognitive dysfunction, unable to cooperate with examination and treatment; 3) Presence of a malignant tumor or severe malnutrition; 4) Postoperative liver failure or required intensive care treatment, or unplanned secondary surgery after the operation; 5) Incomplete clinical data.
SURGICAL METHOD:
All patients were operated on under general anaesthesia with tracheal intubation, and the same anaesthetic induction drug and anaesthetic maintenance drug were used during surgery. After successful anaesthesia, the left upper abdomen was opened with a subcostal incision, and the splenic artery was found at the superior margin of the pancreas and ligated with 3-0 Prolene sutures. The spleen was resected according to the conventional method, and the pericardial devascularization surgery was performed. The patient was awake and returned to the ward. Postoperative symptomatic treatment with routine liver protection was performed.
OBSERVATION INDICATORS AND STATISTICAL METHODS:
The preoperative indicators of patients in both groups were counted, including sex, age, height, weight, CTP classification, and ASA classification, and the preoperative liver function indicators were recorded to calculate the preoperative Model for End-stage Liver disease (MELD) score.
The intraoperative and postoperative indicators of patients in both groups were counted, including surgery duration, intraoperative blood loss, intraoperative blood transfusion, and postoperative liver function at 3 days and 7 days, and the MELD score was calculated separately.
Preoperative ultrasound was used to assess the proper hepatic artery internal diameter and its flow velocity.
All data were statistically processed using SPSS 19.0 software. The
Results
There were no significant differences in preoperative and intraoperative indicators, including sex, age, height, weight, CTP classification, ASA classification, surgery duration, intraoperative blood loss and intraoperative blood transfusion, between the 2 groups (
There were no significant differences in preoperative hepatic artery diameter, preoperative hepatic artery velocity, preoperative MELD score, or MELD score 3 days after surgery between the 2 groups (
Discussion
Cirrhosis is the pathological process of various chronic liver diseases progressing to the middle and late stages, characterized by diffuse fibrosis of the liver, pseudolobule of liver formation, and proliferation of blood vessels inside and outside the liver. The compensated stage may have no obvious clinical symptoms, while the decompensated stage is characterized by portal hypertension and severe impairment of liver function [1]. China has more than 400 million patients with various types of liver disease, including about 7 million cases of liver cirrhosis [2], mainly due to cirrhosis caused by viral hepatitis. Clinically, patients often start to consult the doctor only after they have already developed complications related to cirrhosis, and by then they are often in the decompensated stage of cirrhosis, and the median survival period of the decompensated stage of cirrhosis is only 1.8 years, which is much lower than the 12 years for patients in the compensated stage [3]. Therefore, early and aggressive intervention in such patients will help improve their clinical prognosis.
SASS was first described by Manner et al [4] in 1991, and was named by Langer et al [5] in 1992. The post-orthotopic liver transplant patients included in the study had rapidly increasing transaminase levels, and arteriography demonstrated significantly decreased perfusion to the graft despite a normal appearance of the hepatic artery. Delayed filling of contrast in the intrahepatic arterial branches was also seen compared to more prompt filling of the prominent splenic artery and intrasplenic branches. The authors surmised that blood flow was “stolen” from the hepatic artery by the splenic artery. This phenomenon was named SASS and resulted in decreased perfusion of the hepatic artery, ischemia and hypoxia of the transplanted liver, damage to the bile duct epithelium and hepatocytes, and ultimately loss of function of the transplanted liver. SASS has been a controversial diagnosis [6] and has been described as “non-occlusive hepatic artery hypoperfusion syndrome” [7]. No consensus about SASS has been reached at present. Therefore, it is necessary for scholars to study it. We hope that with more and more relevant studies, the diagnosis and treatment of SASS will reach a consensus. In China, it was first reported by Liu et al in 2003 [8,9]. In 2011, Liu et al [10] were the first to propose the concept of “cirrhotic SASS”, which was the first time the concept of SASS was used outside of liver transplantation internationally, pointing out that it was the result of chronic liver damage and could aggravate the pathological process of liver damage. We also confirmed the prevalence of SASS in patients with decompensated cirrhosis through 3D CT angiography and angiography, and the liver function indicators and CTP classification of cirrhotic patients improved significantly after the correction of SASS. It was proposed that SASS was an effective therapeutic target for improving liver function in patients with decompensated cirrhosis [11]. Although SASS was not a completely new concept for clinicians, few relevant studies have been reported in recent years, indicating that this new target has not attracted sufficient attention from clinicians.
SASS is prevalent in patients with cirrhotic portal hypertension and has the same hemodynamic characteristics as SASS after liver transplantation. In patients with SASS, the splenic artery is thicker in diameter and has a relatively lower vascular resistance, thus shunting more blood from the abdominal trunk, resulting in reduced blood flow to the hepatic artery and a long-term ischemic and hypoxic state in the liver. Eventually, the liver damage slowly progresses in some patients despite effective antiviral therapy. The consequences of SASS are positively correlated with the hepatic artery ischemia time. Therefore, SASS should be avoided and diagnosed and treated as early as possible. Grieser et al noted that CT-determined splenic volumes greater than 829 ml had a 75% accuracy for the prediction of SASS [12]. Other studies found that CT-measured splenic artery diameters of over 4 mm and differences of 6 mm between splenic and hepatic artery diameters were preoperative predictors of SASS [13–15]. In 2011, Liu et al summarized the diagnostic criteria for SASS as follows: 1) The presence of significant splenomegaly; 2) Significant thickening of the splenic artery (greater than 4 mm or greater than 1.5 times the diameter of the hepatic artery); 3) Patency of the hepatic artery without obstruction, but small caliber, slow blood flow, and delayed filling of the hepatic parenchyma in the arterial phase; 4) Thickening of the splenic artery with rapid blood flow and early filling of the splenic parenchyma with contrast medium; 5) The splenic vein and portal vein were visualized early after imaging, even in the arterial phase, due to the high velocity of blood flow in the splenic artery (Figures 1, 2). Cirrhotic SASS required early treatment, and the commonly used treatments included splenectomy, splenic artery ligation and interventional embolization [16–20]. Many institutions reported that SASS should be treated by coil embolization, but patients with SASS usually have a thick splenic artery, the diameter of which is more than 5 mm. The fast splenic arterial flow often pushes the coil into the branch of the splenic artery, which can induce local ischemic necrosis of the spleen, infection, and septicemia [16–18]. The incidence of infection after embolization was reported as 50% [17]. Splenectomy combined with pericardial devascularization is the most common and effective method in clinical practice, which not only reduces portal vein pressure but also reduces the risk of variceal rupture and bleeding [21].
Before splenectomy combined with pericardial devascularization surgery, the clinician must make a thorough evaluation of the patient. Identification of the extent of splenomegaly and hypersplenism is an important part of the evaluation. There is currently no consensus on splenomegaly classification methods. Chinese surgeons commonly use splenomegaly classification of 3 grades: for splenomegaly grade I, the patient lies down and deeply inhales, and the spleen edge is within 2 cm under the left costal margin; for splenomegaly grade II, the spleen edge is less than 2 cm above the left costal margin and does not reach the umbilical horizontal line; and for splenomegaly grade III, the spleen edge is over the umbilical horizontal line or exceeds the ventral midline [22]. On this basis, Jiang Hongchi et al [23] further proposed splenomegaly grade IV, in which the splenic margin exceeds the level of the anterior superior iliac spine to reach the pelvic cavity, also known as super-giant splenomegaly, and on this basis determined the surgical indications of splenomegaly at all levels. The European Association for Endoscopic Surgery (EAES) defined splenomegaly as splenomegaly with a maximum diameter of >15 cm, macrosplenia with a maximum diameter of >20 cm, or weight of >1000 g, and super macrosplenia with a maximum diameter of >22 cm or weight of >1600 g [24]. Linguraru et al [25] proposed that the average volume of splenomegaly was 1004.75±644.27 ml, and suggested a volume threshold of 314.47 ml/430.84 ml to define mild/severe splenomegaly, respectively. Indiran et al [26] proposed the Splenic Index, which refers to the length×width×thickness of the spleen measured on CT, and splenomegaly was defined as splenomegaly with Splenic Index >480 cm3. In addition, there are many grading methods, each with its own advantages and disadvantages, and these various systems are difficult to unify. However, there are individual differences in the same spleen weight or volume among people of different heights and weights, so the evaluation of spleen size based on spleen weight or volume may lack accuracy and clinical significance.
The classification of hypersplenism has received little worldwide attention. According to the white blood cell count (WBC) and platelet count (PLT), Chinese scholars [27] classified hypersplenism into 3 grades: mild, moderate, and severe [28], with mild (WBC: 3.0~4.0×109/L, PLT: 70~100×109/L), moderate (WBC: 2.0~3.0×109/L, PLT: 30~70×109/L), and severe (WBC: <2.0×109/L, PLT <30×109/L). Hou et al [29] proposed 6 indexes, including white blood cells, red blood cells, platelets, splenic vein diameter, splenic length, and splenic thickness, as the basis for grading. Mild hypersplenism was defined when white blood cells were 3.0~4.0×109, red blood cells were 2.5~3.5×1012, platelets were 7.0~10.0×109, splenic veins were less than 1.0 cm, splenic length was less than 12 cm, and thickness was less than 6 cm. Moderate hypersplenism was defined as white blood cells 2.0~3.0×109, red blood cells 1.5~2.5×1012, platelets 5.0~7.0×109, splenic vein 1.0~1.5 cm, splenic length 12~16 cm, and splenic thickness 6~8 cm. Severe hypersplenism was defined when the white blood cells were less than 2.0×109, red blood cells were less than 1.5×1012, platelets were less than 5.0×109, splenic veins were more than 1.5 cm, splenic length was more than 16 cm, and splenic thickness was more than 8 cm. We used this grading method and only considered surgery for patients with moderate or higher hypersplenism. After splenectomy, the distal ligated splenic artery no longer competed to shunt the hepatic artery blood flow, which significantly increased the hepatic arterial blood flow, improved the blood and oxygen supply of hepatocytes, improved liver function, improved liver regeneration ability, and effectively controlled symptoms of hypersplenism [30,31]. However, pericardial devascularization can block the main portal collateral veins, ensure more portal blood flow into the liver, and greatly improve the blood supply of the liver.
In view of the shortcomings of Child-Turcotte-Pugh (CTP) scoring, the Mayo Clinic research team proposed the Model for End-stage Liver Disease (MELD) scoring system from 2000 to 2001, which was initially used to predict the prognosis of patients with portal hypertension undergoing transjugular intrahepatic portacaval shunt (TIPS). In 2002, the United States officially began using the MELD scoring system as the standard for adult liver transplantation. Since then, scholars around the world have begun to verify its value in judging the liver reserve function of patients with end-stage liver disease. The MELD score is significantly better than the CTP score in predicting the short-term survival rate of patients with cirrhosis who are undergoing TIPS, indicating that the MELD score can better predict the short-term survival rate of patients with chronic severe liver disease [32,33]. In our study, we used the MELD scoring system to assess the liver function after surgery.
As can be seen from our results, during the period after splenectomy and pericardial devascularization surgery, the hepatic blood perfusion of SASS patients significantly increased, MELD score decreased, and liver function improved. Better perfusion of the liver after surgery was mainly due to 2 main effects. Firstly, it resulted in the redirection of blood flow from the splenic flow territory to the hepatic artery territory increasing blood pressure in the common hepatic artery. Secondly, another mechanism has been discussed in the literature, called hepatic artery buffer response (HABR) [34,35]. The unique dual blood supply of the liver is due to the fact that there are 2 major vessels in the liver – the portal vein and the common hepatic artery – providing a balanced supply of blood. This double blood supply ensures adequate blood supply, about 1500 ml per minute, equivalent to a quarter of the heart’s output. Portal hyperperfusion also leads to a decelerated arterial inflow with an increase in the hepatic resistive index (RI) in Doppler ultrasound [36]. A potential physiological explanation might be the adenosine washout into portal blood leading to decreased adenosine concentrations around hepatic arterial resistance vessels, with consecutive arteriolar vasoconstriction and reduced arterial blood flow [37,38]. This condition can be reversed by reduction of portal hyperperfusion via splenectomy [39]. A decrease in flow to the spleen automatically results in a lower blood volume in the portal vein. Through a negative feedback mechanism, this finally leads to vasodilatation of hepatic arteries and improved arterial blood flow to the liver. Therefore, splenectomy and pericardial devascularization surgery was an effective treatment to redirect blood flow to the hepatic artery (Figure 3). However, the number of samples included in the research was limited, and more research is needed to verify the results of this paper.
Conclusions
We propose that SASS may be an effective therapeutic target for decompensated cirrhosis patients. We found that the blood supply to the hepatic artery was increased and the liver function was improved for the cirrhotic patients diagnosed with SASS after splenectomy and pericardial devascularization surgery. Moreover, we hope to introduce the concept of “cirrhotic SASS” into clinical practice and to promote more clinician attention and research. With further research and more cases, the clinical application of SASS could benefit more patients with cirrhosis.
Figures
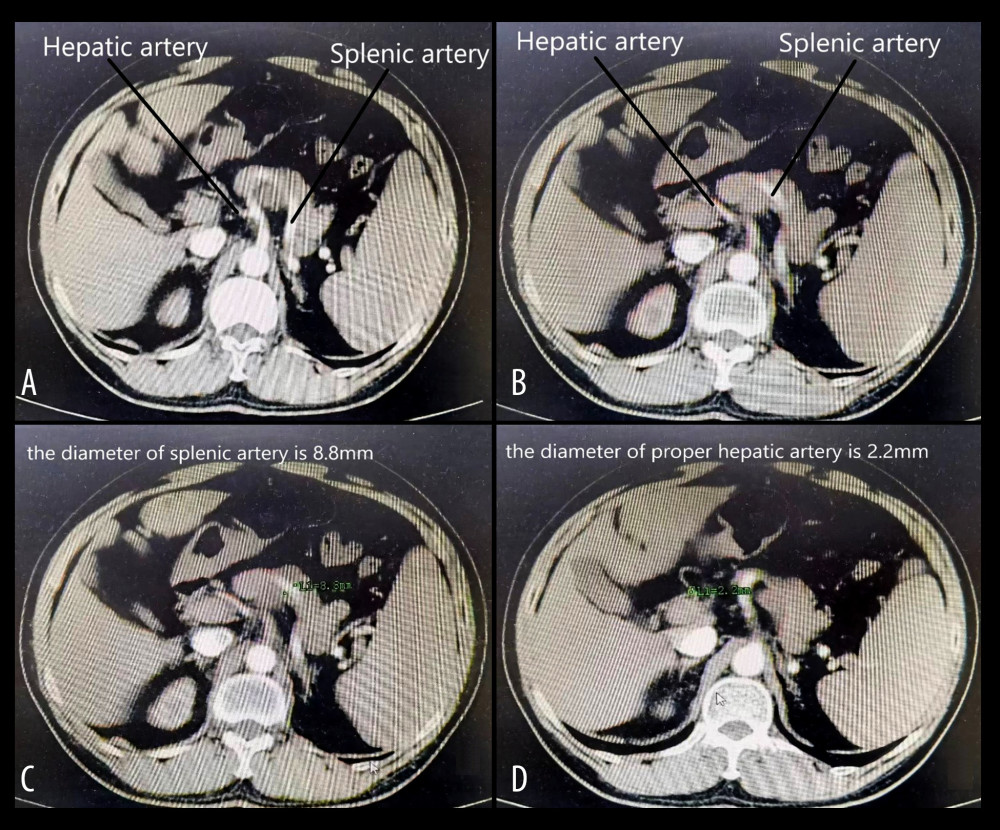 Figure 1. (A, B) The splenic artery is much thicker than the hepatic artery, which conforms to the diagnostic criteria of SASS. (C, D) The diameter of the splenic artery is 8.8 mm, and the diameter of the hepatic artery is 2.2 mm, corresponding to the diagnostic criteria of SASS. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC).
Figure 1. (A, B) The splenic artery is much thicker than the hepatic artery, which conforms to the diagnostic criteria of SASS. (C, D) The diameter of the splenic artery is 8.8 mm, and the diameter of the hepatic artery is 2.2 mm, corresponding to the diagnostic criteria of SASS. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC). 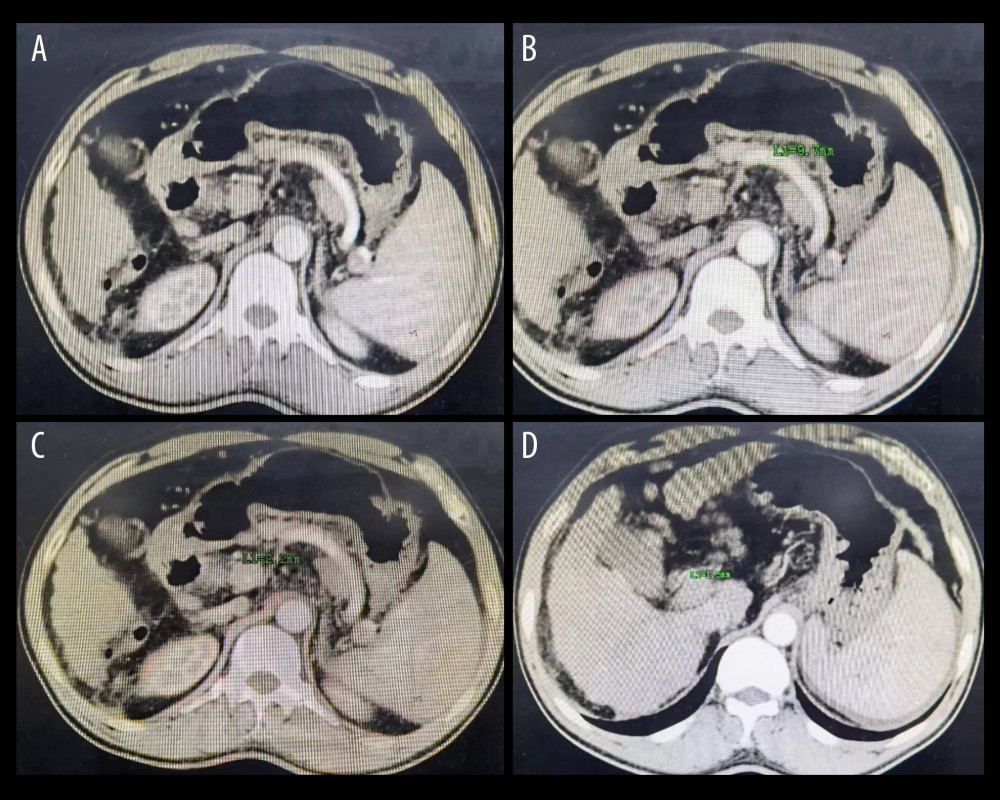 Figure 2. (A) The splenic artery is much thicker than the hepatic artery. (B) The diameter of the splenic artery is 9.7 mm. (C) The diameter of the common hepatic artery is 2.2 mm. (D) The diameter of the proper hepatic artery is 1.5 mm. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC).
Figure 2. (A) The splenic artery is much thicker than the hepatic artery. (B) The diameter of the splenic artery is 9.7 mm. (C) The diameter of the common hepatic artery is 2.2 mm. (D) The diameter of the proper hepatic artery is 1.5 mm. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC). 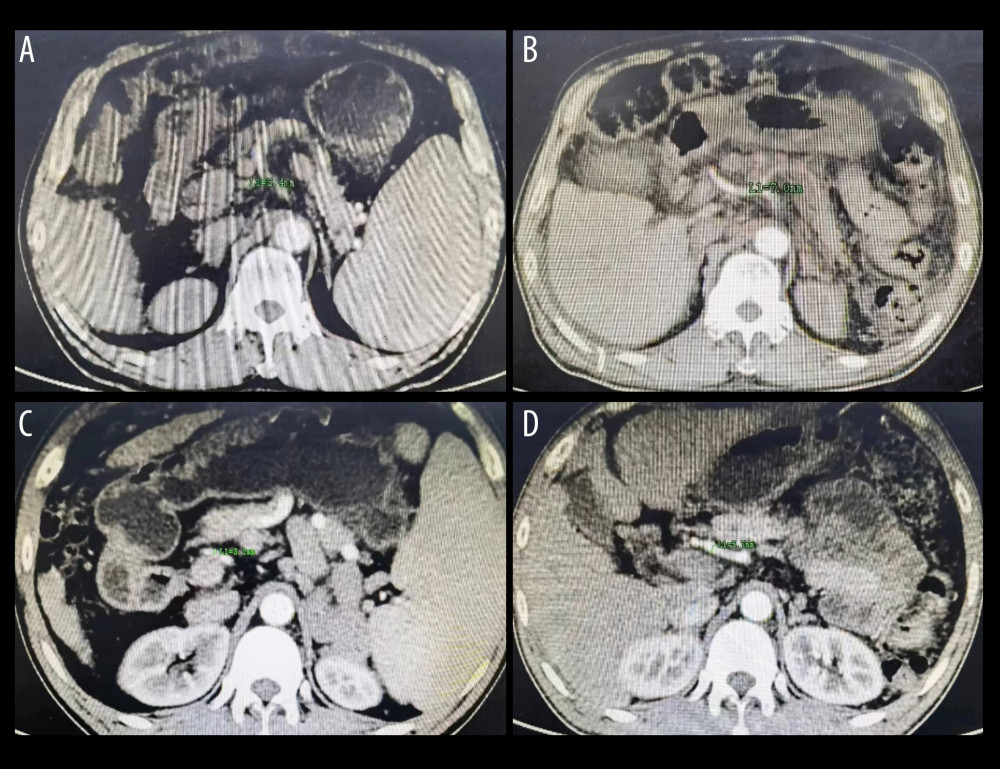 Figure 3. (A) The diameter of hepatic artery is 3.4 mm before splenectomy. (B) The same patient as in Figure A, the diameter of hepatic artery is 7.0 mm 5 months postoperatively. (C) The diameter of hepatic artery is 3.1 mm before splenectomy. (D) The same patient as in Figure C, the diameter of hepatic artery is 5.7 mm 6 months postoperatively. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC).
Figure 3. (A) The diameter of hepatic artery is 3.4 mm before splenectomy. (B) The same patient as in Figure A, the diameter of hepatic artery is 7.0 mm 5 months postoperatively. (C) The diameter of hepatic artery is 3.1 mm before splenectomy. (D) The same patient as in Figure C, the diameter of hepatic artery is 5.7 mm 6 months postoperatively. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC). References
1. Chinese guidelines on the management of liver cirrhosis: Zhonghua Gan Zang Bing Za Zhi, 2019; 27(11); 846-65
2. Xiao J, Wang F, Wong NK, Global liver disease burdens and research trends: Analysis from a Chinese perspective: J Hepatol, 2019; 71(1); 212-21
3. D’Amico G, Pasta L, Morabito A, Competing risks and prognostic stages of cirrhosis: A 25-year inception cohort study of 494 patients: Aliment Pharmacol Ther, 2014; 39(10); 1180-93
4. Manner M, Otto G, Senninger N, Arterial steal: An unusual cause for hepatic hypoperfusion after liver transplantation: Transpl Int, 1991; 4(2); 122-24
5. Langer R, Langer M, Scholz A, The splenic steal syndrome and the gastroduodenal steal syndrome in patients before and after liver transplantation: Aktuelle Radiol, 1992; 2(2); 55-58
6. Quintini C, Hirose K, Hashimoto K, “Splenic artery steal syndrome” is a misnomer: The cause is portal hyperperfusion, not arterial siphon: Liver Transpl”, 2008; 14(3); 374-79
7. Saad WE, Nonocclusive hepatic artery hypoperfusion syndrome (splenic steal syndrome) in liver transplant recipients: Semin Intervent Radiol, 2012; 29(2); 140-46
8. Liu QD, Zhou NX, Wang MQ, Diagnosis and treatment of splenic artery steal syndrome after liver transplantation: Journal of Digestive Surgery, 2004; 3(4); 232-36
9. Liu QD, Zhou NX, Wang MQ, Splenic artery steal syndrome after liver transplantation: Chinese Journal of Surgery, 2005; 43(15); 989-90
10. Liu QD, Song Y, Qiao HQ, Splenic arterial steal syndrome-an ignored concept: Journal of Regional Anatomy and Operative Surgery, 2011; 20(1); 1-2
11. Liu QD, Song Y, Zhou NX, Splenic arterial steal syndrome: A neglected therapeutic target for liver diseases: Journal of Clinical Hepatol, 2011; 27(3); 241-44
12. Grieser C, Denecke T, Steffen IG, Multidetector computed tomography for preoperative assessment of hepatic vasculature and prediction of splenic artery steal syndrome in patients with liver cirrhosis before transplantation: Eur Radiol, 2010; 20(1); 108-17
13. Mogl MT, Nüssler NC, Presser SJ, Evolving experience with prevention and treatment of splenic artery syndrome after orthotopic liver transplantation: Transpl Int, 2010; 23(8); 831-41
14. Dokmak S, Aussilhou B, Belghiti J, Liver transplantation and splenic artery steal syndrome: the diagnosis should be established preoperatively: Liver Transpl, 2013; 19(6); 667-68
15. Kirbas I, Ulu EM, Ozturk A, Multidetector computed tomographic angiography findings of splenic artery steal syndrome in liver transplantation: Transplantat Proc, 2007; 39(4); 1178-80
16. Uflacker R, Selby JB, Chavin K, Transcatheter splenic artery occlusion for treatment of splenic artery steal syndrome after orthotopic liver transplantation: Cardiovasc Intervent Radiol, 2002; 25(4); 300-6
17. Nüssler NC, Settmacher U, Haase R, Diagnosis and treatment of arterial steal syndromes in liver transplant recipients: Liver Transplant, 2003; 9(6); 596-602
18. Sevmis S, Boyvat F, Aytekin C, Arterial steal syndrome after orthotopic liver transplantation: Transplant Proc, 2006; 38(10); 3651-55
19. Madoff DC, Denys A, Wallace MJ, Splenic arterial interventions: Anatomy, indications, technical considerations, and potential complications: Radiographics, 2005; 25(Suppl 1); S191-211
20. Alvarez D, Gerona S, Waisburg Z, Splanchnic hyperemia after liver transplantation in patients with end-stage liver disease: Liver Transpl Surg, 1998; 4(4); 300-3
21. Kawanaka H, Akahoshi T, Kinjo N, Effect of laparoscopic splenectomy on portal haemodynamics in patients with liver cirrhosis and portal hypertension: Br J Surg, 2014; 101(12); 1585-93
22. Liu KP, Ma Y, Jiang HC, Suggestion of grade judgment and selection of surgical methods for splenomegaly: Chinese Journal of Practical Surgery, 2019; 39(3); 200-2
23. Jiang HC, Zhou MH, Progress and prospect of clinical research in spleen surgery: Chinese Journal of Practical Surgery, 2020; 40(1); 53-57
24. Habermalz B, Sauerland S, Decker G, Laparoscopic splenectomy: The clinical practice guidelines of the European Association for Endoscopic Surgery (EAES): Surg Endosc, 2008; 22(4); 821-48
25. Linguraru MG, Sandberg JK, Jones EC, Assessing splenomegaly: Automated volumetric analysis of the spleen: Acad Radiol, 2013; 20(6); 675-84
26. Indiran V, Singh NV, Prasad TR, Does coronal oblique length of spleen on CT reflect splenic index?: Abdom Radiol (NY), 2017; 42(5); 1444-48
27. Yang Y, Liu MQ, Tian MG, The expression of P-STAT3, VEGF and BFGF in the spleen of patients with portal hypertension: Ningxia Medical Journal, 2018; 40(4); 292-95
28. Gao YJ, Gao ZL, Sun WJQuantitative assessment of hepatic and splenic blood flow status in patients with hypersplenism of different degrees based on multi-slice spiral CT whole-liver perfusion imaging: Zhonghua Gan Zang Bing Za Zhi, 2020; 28(4); 326-31
29. Hou Y, Yang JS, Sun GX, Clinical study on classification diagnosis and treatment of hypersplenism in portal hypertension: Chinese Journal of Laboratory Diagnosis, 2009; 13(10); 1446-47
30. Liu Q, Current research situation of splenic artery steal syndrome: Medical Recapitulate, 2011; 17(24); 3768-70
31. Liu L, Yang L, Chen HY, Changes of hemodynamics after splenectomy combined with pericardial devascularization in patients with cirrhotic portal hypertention complicated by splenic artery steal syndrome: Journal of Practical Hepatology, 2019; 22(6); 884-87
32. Ruf A, Dirchwolf M, Freeman RB, From Child-Pugh to MELD score and beyond: Taking a walk down memory lane: Ann Hepatol, 2022; 27(1); 100535
33. Álvarez-López P, Campos-Varela I, Quiroga S, Spontaneous portosystemic shunt embolization in liver transplant recipients with recurrent hepatic encephalopathy: Ann Hepatol, 2022; 27(3); 100687
34. Lautt WW, Relationship between hepatic blood flow and overall metabolism: the hepatic arterial buffer response: Fed Proc, 1983; 42(6); 1662-66
35. Richter S, Vollmar B, Mücke I, Hepatic arteriolo-portal venular shunting guarantees maintenance of nutritional microvascular supply in hepatic arterial buffer response of rat livers: J Physiol, 2001; 531(Pt 1); 193-201
36. Jakab F, Ráth Z, Schmal F, The interaction between hepatic arterial and portal venous blood flows; simultaneous measurement by transit time ultrasonic volume flowmetry: Hepatogastroenterology, 1995; 42(1); 18-21
37. Ezzat WR, Lautt WW, Hepatic arterial pressure-flow autoregulation is adenosine mediated: Am J Physiol, 1987; 252(4 Pt 2); H836-45
38. Jakab F, Sugár I, Ráth Z, The relationship between portal venous and hepatic arterial blood flow. I. Experimental liver transplantation: HPB Surg, 1996; 10(1); 21-26
39. Lo CM, Liu CL, Fan ST, Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation: Liver Transpl, 2003; 9(6); 626-28
Figures
 Figure 1. (A, B) The splenic artery is much thicker than the hepatic artery, which conforms to the diagnostic criteria of SASS. (C, D) The diameter of the splenic artery is 8.8 mm, and the diameter of the hepatic artery is 2.2 mm, corresponding to the diagnostic criteria of SASS. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC).
Figure 1. (A, B) The splenic artery is much thicker than the hepatic artery, which conforms to the diagnostic criteria of SASS. (C, D) The diameter of the splenic artery is 8.8 mm, and the diameter of the hepatic artery is 2.2 mm, corresponding to the diagnostic criteria of SASS. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC). Figure 2. (A) The splenic artery is much thicker than the hepatic artery. (B) The diameter of the splenic artery is 9.7 mm. (C) The diameter of the common hepatic artery is 2.2 mm. (D) The diameter of the proper hepatic artery is 1.5 mm. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC).
Figure 2. (A) The splenic artery is much thicker than the hepatic artery. (B) The diameter of the splenic artery is 9.7 mm. (C) The diameter of the common hepatic artery is 2.2 mm. (D) The diameter of the proper hepatic artery is 1.5 mm. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC). Figure 3. (A) The diameter of hepatic artery is 3.4 mm before splenectomy. (B) The same patient as in Figure A, the diameter of hepatic artery is 7.0 mm 5 months postoperatively. (C) The diameter of hepatic artery is 3.1 mm before splenectomy. (D) The same patient as in Figure C, the diameter of hepatic artery is 5.7 mm 6 months postoperatively. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC).
Figure 3. (A) The diameter of hepatic artery is 3.4 mm before splenectomy. (B) The same patient as in Figure A, the diameter of hepatic artery is 7.0 mm 5 months postoperatively. (C) The diameter of hepatic artery is 3.1 mm before splenectomy. (D) The same patient as in Figure C, the diameter of hepatic artery is 5.7 mm 6 months postoperatively. Figure generated by HUAWEI version P40pro. (HUAWEI Corp, Shenzhen, PRC). Tables
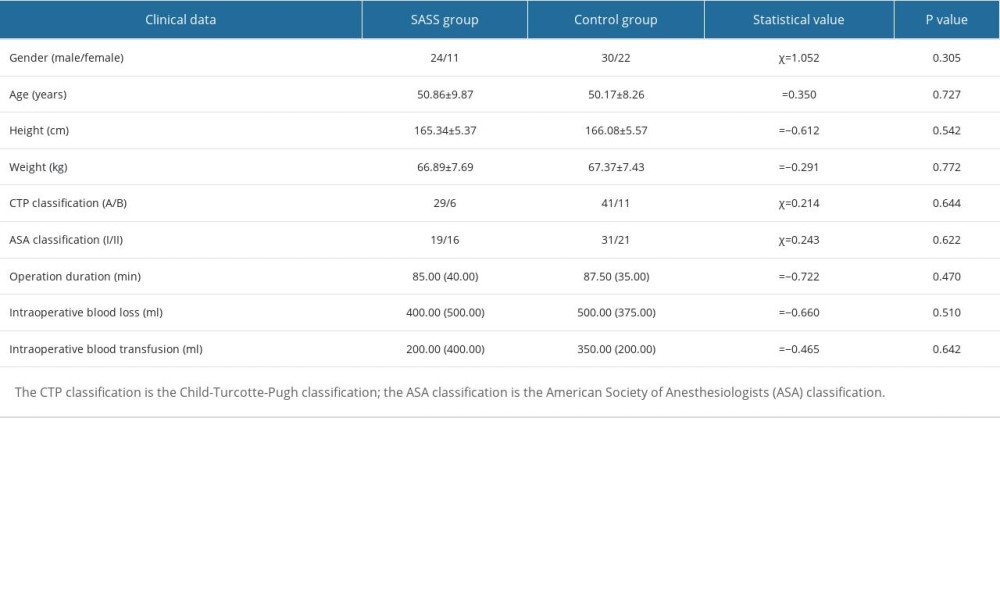 Table 1. Comparison of preoperative and postoperative clinical data between the 2 groups.
Table 1. Comparison of preoperative and postoperative clinical data between the 2 groups.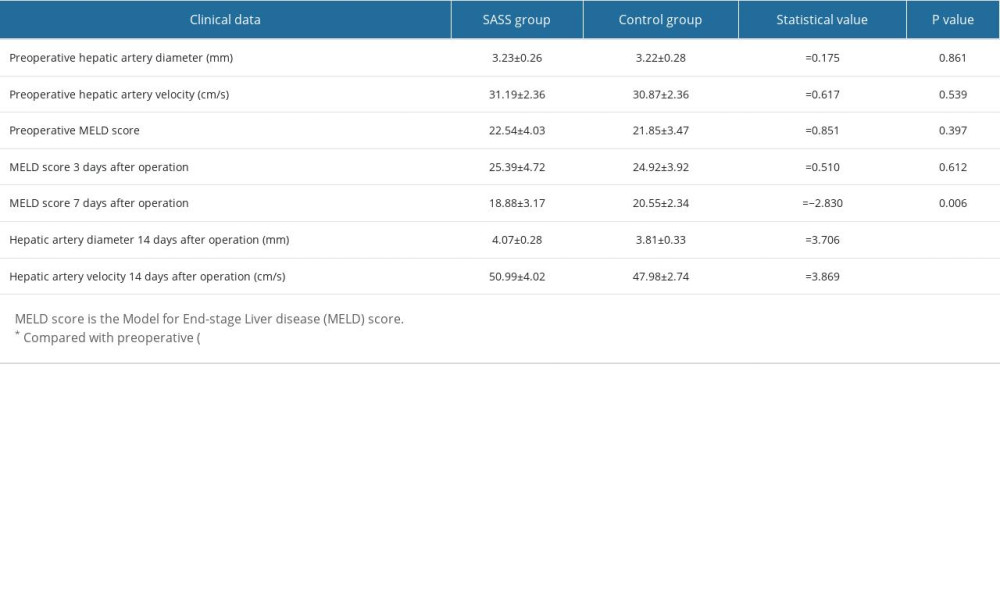 Table 2. Comparison of liver-related data between the 2 groups.
Table 2. Comparison of liver-related data between the 2 groups. Table 1. Comparison of preoperative and postoperative clinical data between the 2 groups.
Table 1. Comparison of preoperative and postoperative clinical data between the 2 groups. Table 2. Comparison of liver-related data between the 2 groups.
Table 2. Comparison of liver-related data between the 2 groups. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








