15 April 2023: Clinical Research
Comparison of Extravillous Intermediate Trophoblast Invasion Depth and Distribution Pattern Between Placenta Accreta and Non-Accreta
Dodi Suardi1ABDE, Huda ToriqDOI: 10.12659/MSM.939125
Med Sci Monit 2023; 29:e939125
Abstract
BACKGROUND: Placenta accreta spectrum (PAS) is a complex obstetric complication that poses a major risk for life-threatening hemorrhage. The pathogenesis of PAS is known to be related to placentogenesis, trophoblastic cells invasion, and previous obstetrical procedures that cause uterine wall defects. However, the precise mechanism of this disease has not been fully explained. This study aimed to evaluate the differences in maximum depth of invasion and distribution pattern of implantation site intermediate trophoblasts between PAS and non-accreta cases.
MATERIAL AND METHODS: This was an observational, analytic, cross-sectional study that utilized paraffin block specimen of peripartum hysterectomy performed in Hasan Sadikin General Hospital Bandung from 2018 to 2020. Sixty-four samples were obtained, then classified as PAS and non-accreta (normal placenta). Implantation site-intermediate trophoblasts were identified using CD-146 staining. Maximum invasion depth of intermediate trophoblasts was measured in micrometers, while the distribution pattern was assessed and classified into 2 groups: confluent and scattered.
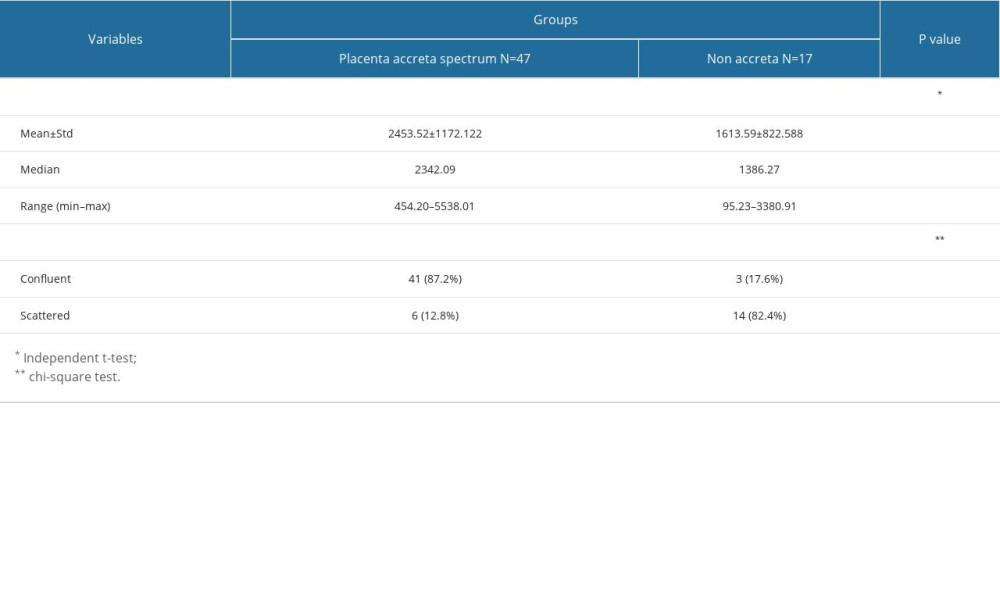
RESULTS: We found that the maximum invasion depth of the intermediate trophoblasts was significantly higher in the PAS group compared to that of the non-accreta group (2453.52±1172.122 µm vs 1613.59±822.588 µm, P=0.009). The confluent distribution pattern was significantly more common in the PAS group compared to that of the non-accreta group (87.2% vs 17.6%, P=0.0001).
CONCLUSIONS: The findings of our study suggested that implantation site intermediate trophoblasts play a role in the pathophysiology of placenta accreta. Further studies are needed to determine factors that affect trophoblast invasion leading to placenta accreta spectrum.
Keywords: Hysterectomy, Placenta Accreta, Trophoblasts, CD146 Antigen, Pregnancy, Female, Humans, Myometrium, Cross-Sectional Studies, Uterus, Placenta
Background
According to FIGO consensus guidelines, placenta accreta spectrum (PAS) is defined as a spectrum of abnormal placentation disorders that range from (1) placenta adherent or vera, in which villi attach to myometrial surface without invading it; (2) placenta increta, where villi penetrate into the myometrium until the uterine serosa; and (3) placenta percreta, in which villous tissue penetrates through the uterine serosa and may even reach surrounding pelvic tissues, vessels, and organs [1–3].
Placenta accreta spectrum is a high-risk condition associated with various maternal morbidity and mortality, in particular life-threatening hemorrhage, which often necessitates hysterectomy. Management of PAS requires multidiscipline collaboration and is recommended to be performed in a tertiary hospital where sub-specialists are available. For such cases, hysterectomy is often required to save the patient [1,4,5].
There are several theories regarding the etiology of placenta accreta spectrum, but the most favored one is a defect in the decidua layer associated with previous uterine surgeries. However, this theory fails to explain the rare occurrence of placenta accreta spectrum in women without prior uterine surgery or curettage [5]. Placenta accreta spectrum development is a multifactorial process. Some of the underlying mechanisms responsible for placenta accreta spectrum include angiogenesis, proliferation, and trophoblast invasion [6].
Trophoblast cells consist of 3 main subpopulations: cytotrophoblasts, syncytiotrophoblasts, and intermediate trophoblasts. Compared to other types, intermediate trophoblasts are considered highly invasive, and they gain their invasive property by expressing cell adhesion molecule and producing matrix-degrading proteases. Intermediate trophoblast cells invade both decidua and myometrium, and thus can be found in the superficial one-third of myometrium, even in normal pregnancy. Melanoma cell adhesion molecule (Mel-CAM) or CD146 is a cell adhesion molecule belonging to the immunoglobulin gene group that is expressed by intermediate trophoblasts and therefore is a useful marker for identifying implantation site intermediate trophoblasts [7,8]. In this study, we examined the implantation site intermediate trophoblasts of PAS and non-accreta (normal placenta) cases. We compared the maximum depth and distribution pattern of intermediate trophoblasts in the myometrial implantation site between PAS and non-accreta cases.
Material and Methods
CASE SELECTION:
This was an observational, analytical, cross-sectional study that analyzed archival formalin-fixed paraffin-embedded (FFPE) tissue blocks of patients who underwent peripartum hysterectomy between 2018 to 2020 at our center. All patients consented to the use of their anonymized medical data for research purposes. The cases were categorized into 2 groups: PAS and non-accreta groups based on previous clinical and pathological findings (Figures 1, 2). Placenta accreta spectrum was defined based on FIGO 2018 consensus guidelines, which stated PAS as a spectrum of abnormal placentation disorders, that ranges from placenta adherent or vera, placenta increta, and placenta percreta [1–3]. On the other hand, the placenta non-accreta group consisted of normal placenta specimens.
We excluded cases complicated by preeclampsia because previous studies have reported failure of intermediate trophoblast Mel-Cam (CD146) expression and invasion on preeclampsia cases [9]. The corresponding paraffin blocks were then collected. Sixty-four qualified cases were collected for this study, consisting of 47 PAS cases and 17 non-accreta cases.
HEMATOXYLIN AND EOSIN STAINING:
Formalin-fixed paraffin-embedded blocks were first cut into 4–5-μm ribbons, placed on a slide, cleaned with xylene, and then rehydrated with ethanol and 70% alcohol solutions. After that, the slides were rinsed under running tap water at room temperature and then stained with hematoxylin solution for 3 minutes. We then rinsed the slides again under running tap water at room temperature and stained them with working eosin solution Y for 2 minutes. Afterward, the slides were dehydrated using ethanol accordingly and cleared 3 times with xylene for 2 minutes per change. Then, we placed a drop of mounting medium over the tissue and added a cover slip over each slide.
IMMUNOHISTOCHEMISTRY:
For immunohistochemical staining, we used labeled streptavidin-biotin immunoperoxidase complex method with One-step Neopoly Detection Kits (Biogear Scientific, BioVentures, Inc., Iowa, USA). Paraffin blocks were cut into 4-μm slices, cleaned using xylol, and rehydrated using an alcohol solution. The antigens were obtained using a decloaking device for 45–60 minutes at 98°C. The primary antibody used was CD146 antibody (rabbit polyclonal antihuman CD146, Cat. No. GTX108777, GeneTex, Shenzhen, China) with 1: 3000 dilution, overnight at 4. We used negative control and internal positive control from the same sample slide from skin tissue (Figure 3). Secondary antibody and counterstaining were conducted in accordance with the streptavidin-biotin immunoperoxidase complex label method using One-step Neopoly Detection Kits (Biogear Scientific, BioVentures, Inc., Iowa, USA). The positive control used was rat liver.
MAXIMUM INVASION DEPTH OF INTERMEDIATE TROPHOBLASTS:
The implantation site was then examined under a microscope by experienced pathologists. CD146 expression on intermediate trophoblast cytoplasmic membranes was evaluated digitally using an Olympus BX51 optical microscope equipped with an Olympus XC10 digital camera (Figure 4). The program used was dotSlide version 2.0. Maximum invasion depth of intermediate trophoblasts was defined as the distance between CD146-positive intermediate trophoblasts in the deepest myometrial area and the myometrial border in the corresponding area (in micrometer).
DISTRIBUTION PATTERN OF INTERMEDIATE TROPHOBLAST:
Distribution pattern of intermediate trophoblasts was classified as ‘confluent’ when the intermediate trophoblast formed a diffuse sheet-like arrangements, continuous cell cords, or large cluster occupying more than two-thirds of the captured fields, or as ‘scattered’ when the intermediate trophoblasts were individually distributed or formed small groups of 5 or fewer cells.
STATISTICAL ANALYSIS:
Data collected were analyzed using SPSS for Windows, version 25. Normality testing was carried out using the Shapiro-Wilk test. The unpaired
Results
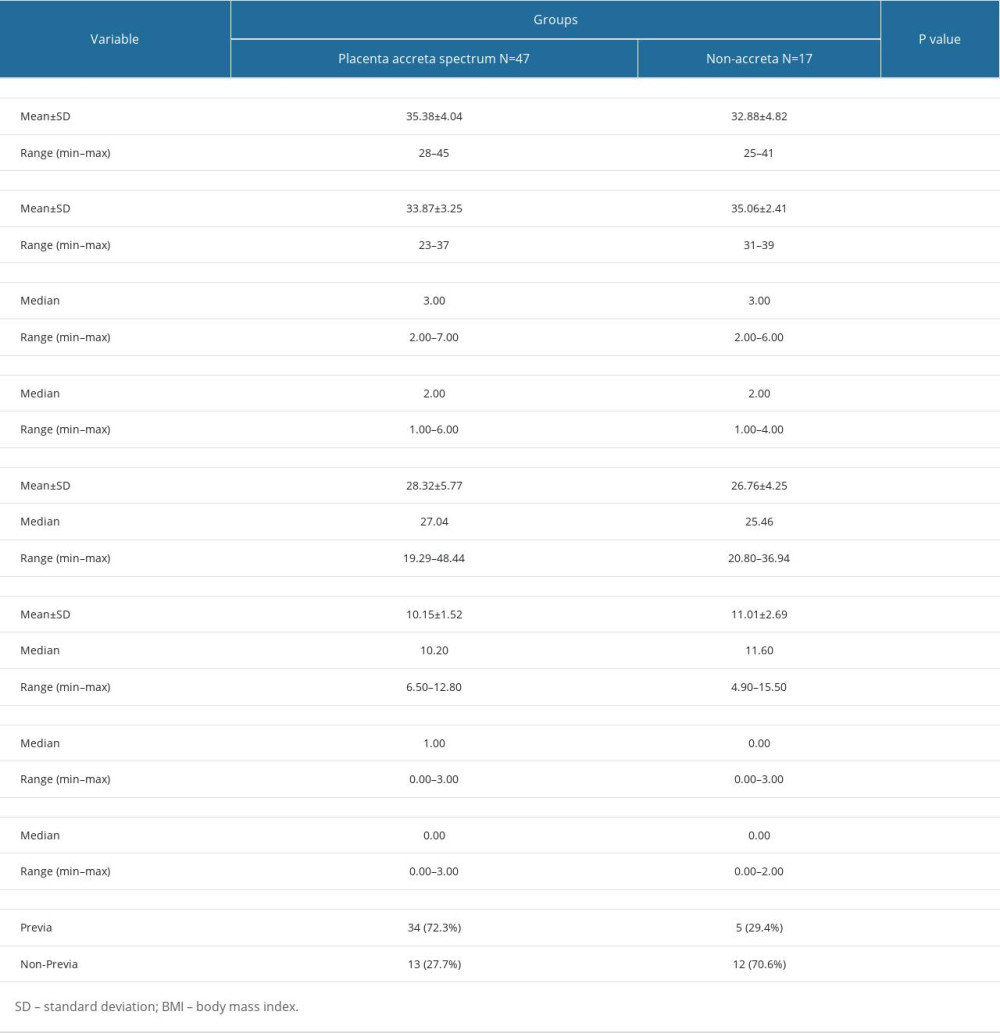
A total of 64 cases were included in this study, consisting of 47 placenta accreta spectrum cases and 17 non-accreta cases. Demographic characteristics of the patients are listed in Table 1. There were no significant differences in age, gestational age, gravidity, parity, BMI, hemoglobin level, cesarean section history, and curettage history among those with PAS and those without PAS. Placenta previa was significantly more common in the placenta accreta group compared to the non-accreta group (P=0.005).
Table 2 demonstrates the maximum invasion depth and distribution pattern of intermediate trophoblasts from PAS and non-accreta group. Maximum invasion depth of the intermediate trophoblasts was significantly higher in the PAS group (range=454.20 to 5538.01 μm, mean=2453.52±1172.122 μm) compared to that of the non-accreta group (range= 95.23–3380.91 μm, mean=1613.59±822.588) (
Discussion
Intermediate trophoblasts invade the superficial myometrium in both normal and abnormal pregnancies. Density and depth of invasion of the intermediate (extravillous) trophoblast cells are greatest in the central placental bed, and both decrease as they approach the placental margin. Regulation of intrauterine migration allows trophoblastic invasion to peak around the 12th week of gestation, followed by a slow decrease. This spatial control function of the invasion process limits the depth of invasion of intermediate trophoblast cells to the proximal one-third of the myometrium. Deregulation of this process causes various types of pregnancy complications such as intrauterine growth restriction, preeclampsia, placenta accrete spectrum, gestational trophoblastic disease, and miscarriage [8]. Several previous studies have found that excessive trophoblast invasion contributed to PAS development [8,10,11].
In accordance with previous studies, we found that the invasion depth of intermediate trophoblasts was significantly higher in the PAS group compared to the non-accreta group. It is not fully known what caused deeper intermediate trophoblast invasion in the PAS group. One study suggested that increased trophoblast invasion might be a compensatory mechanism to insufficient placental circulation [12]. Other studies believed that defective interaction between maternal tissue and migratory trophoblast along with abnormal uteroplacental circulation are responsible for the deeper trophoblast invasion into the uterus [13,14].
In normal pregnancy, intermediate trophoblasts are usually found scattered in the superficial layer of the myometrium, while in placenta accreta they are more frequently found in higher numbers [10]. Consistent with those findings, we observed a significantly higher proportion of confluent intermediate trophoblasts in the PAS group. The increased extravillous intermediate trophoblast invasion within the myometrium in placenta accreta suggests a pathophysiologic role in development of the disease.
Risk factors for developing placenta accreta spectrum include history of previous cesarean section, advanced maternal age, multiparity, and placenta previa [5,15]. In our study, there were no significant differences in age, gravidity, parity, BMI, hemoglobin level, cesarean section history, and curettage history between those with PAS and those without PAS. Placenta previa was the only variable found to be significantly more common in the PAS group compared to the non-accreta group (
Conclusions
In conclusion, we observed significantly different depths of intermediate trophoblasts and their distribution pattern between PAS and non-accreta, suggesting the role of intermediate trophoblasts in the pathophysiology of accreta. Further studies should be done to determine factors affecting immediated trophoblast invasion, which can lead to placenta accreta spectrum.
Figures
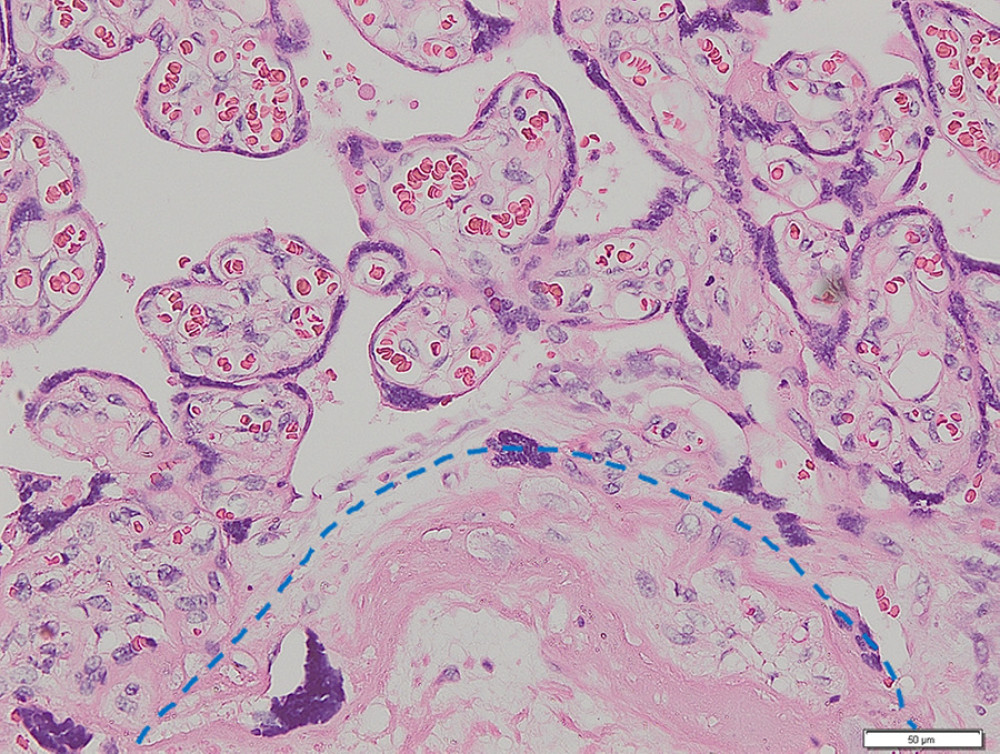 Figure 1. Placenta non-accreta. Area of chorionic villi covered by syncytiotrophoblast and cytotrophoblast is shown above the dashed line, while the myometrial area is shown below the dashed line (hematoxylin and eosin staining, magnification ×200).
Figure 1. Placenta non-accreta. Area of chorionic villi covered by syncytiotrophoblast and cytotrophoblast is shown above the dashed line, while the myometrial area is shown below the dashed line (hematoxylin and eosin staining, magnification ×200). 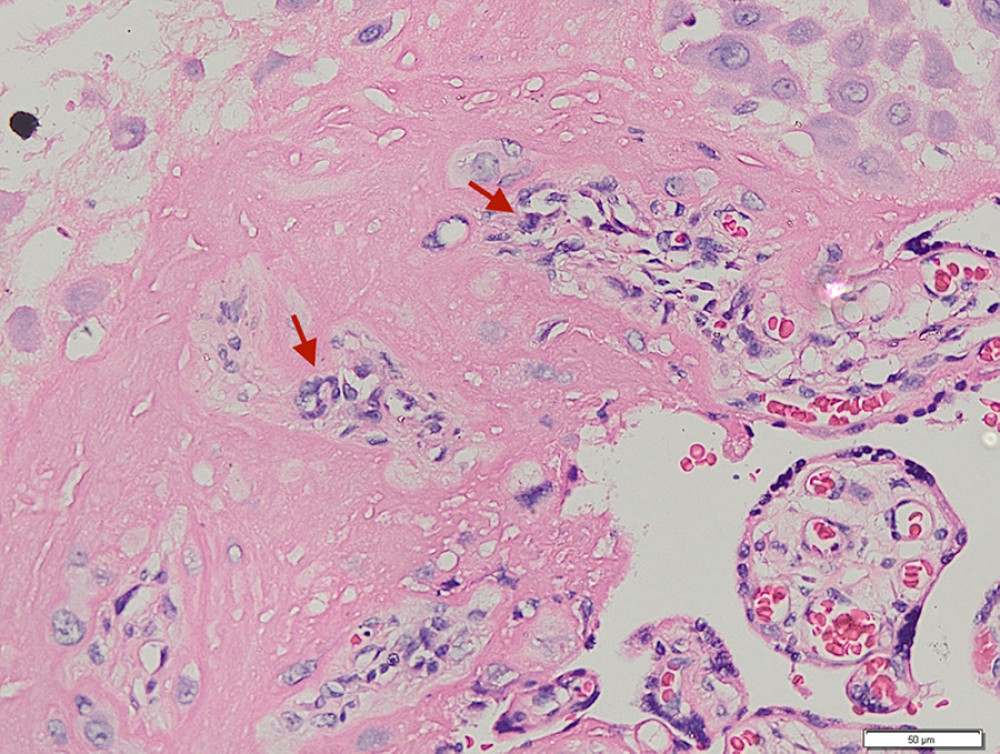 Figure 2. Placenta accreta. Groups of intermediate trophoblastic cells invaded the myometrium (arrows), and the villi are located outside and inside the myometrium (hematoxylin and eosin staining, magnification ×200).
Figure 2. Placenta accreta. Groups of intermediate trophoblastic cells invaded the myometrium (arrows), and the villi are located outside and inside the myometrium (hematoxylin and eosin staining, magnification ×200). 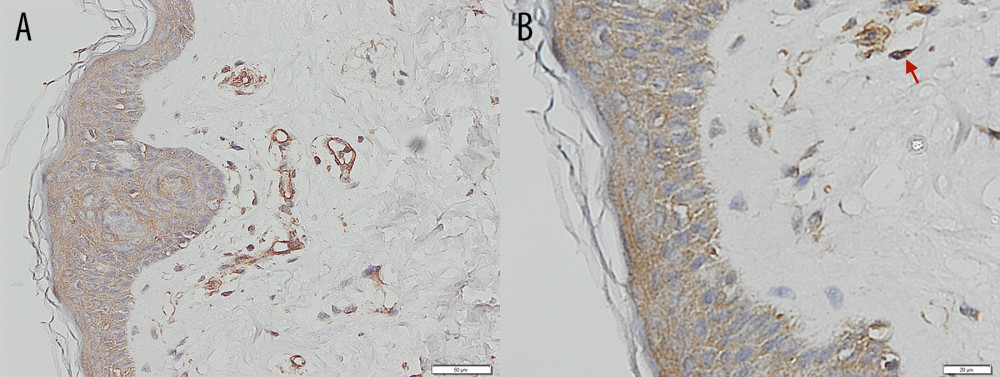 Figure 3. Immunohistochemical staining – negative control of CD146 from skin. (A) Magnification ×200. (B) Magnification ×400, with the positive internal control revealed from the vessels’ endothelial cells (arrows).
Figure 3. Immunohistochemical staining – negative control of CD146 from skin. (A) Magnification ×200. (B) Magnification ×400, with the positive internal control revealed from the vessels’ endothelial cells (arrows). 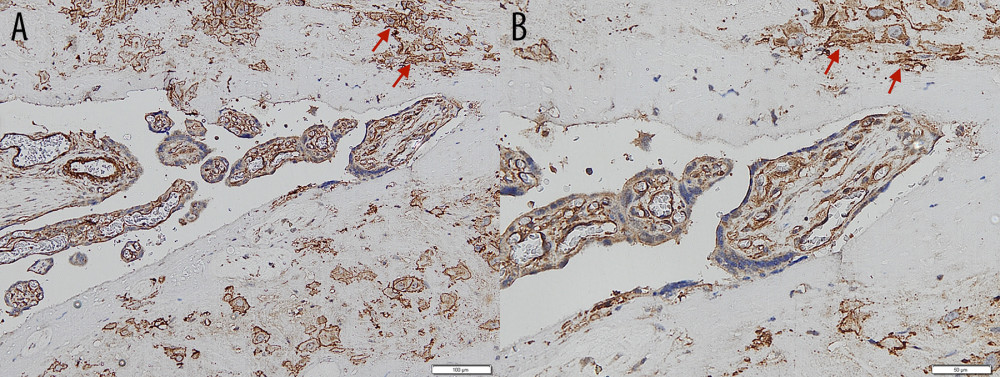 Figure 4. Immunohistochemical staining – intermediate trophoblastic cells showing reactivity to CD146 staining between the myometrium (arrows). (A) Magnification ×100. (B) Magnification ×200.
Figure 4. Immunohistochemical staining – intermediate trophoblastic cells showing reactivity to CD146 staining between the myometrium (arrows). (A) Magnification ×100. (B) Magnification ×200. 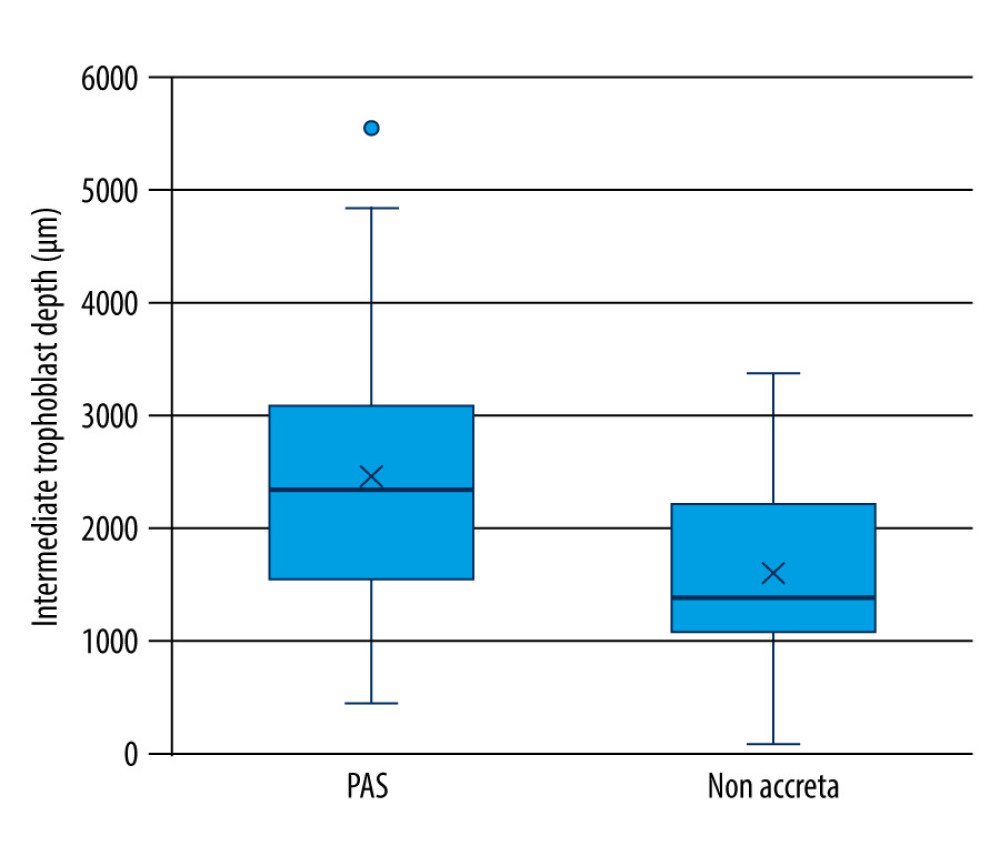 Figure 5. Maximum invasion depth of intermediate trophoblasts in placenta accreta spectrum and non-accreta cases, showing a significant difference.
Figure 5. Maximum invasion depth of intermediate trophoblasts in placenta accreta spectrum and non-accreta cases, showing a significant difference. 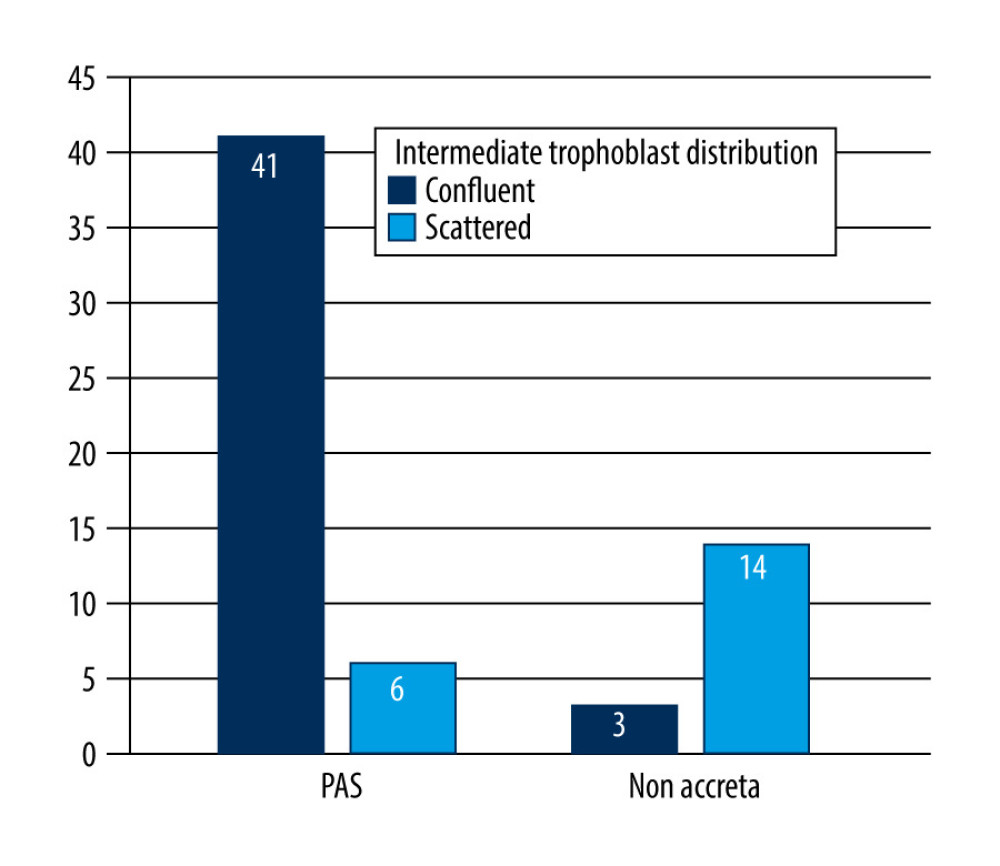 Figure 6. Comparison of intermediate trophoblast cell distribution pattern between the placenta accreta spectrum and non-accreta groups.
Figure 6. Comparison of intermediate trophoblast cell distribution pattern between the placenta accreta spectrum and non-accreta groups. References
1. Jauniaux E, Collins S, Burton GJ, Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging: Am J Obstet Gynecol, 2018; 218(1); 75-87
2. Jauniaux E, Chantraine F, Silver RM, Langhoff-Roos J, FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology: Int J Gynecol Obstet, 2018; 140(3); 265-73
3. Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders: Int J Gynecol Obstet, 2019; 146(1); 20-24
4. El Gelany S, Mosbeh MH, Ibrahim EM, Placenta Accreta Spectrum (PAS) disorders: Incidence, risk factors and outcomes of different management strategies in a tertiary referral hospital in Minia, Egypt: A prospective study: BMC Pregnancy Childbirth, 2019; 19(1); 313
5. Cahill AG, Beigi R, Heine RP, Placenta accreta spectrum: Am J Obstet Gynecol, 2018; 219(6); B2-B16
6. Bartels HC, Postle JD, Downey P, Brennan DJ, Placenta accreta spectrum: A review of pathology, molecular biology, and biomarkers: Dis Markers, 2018; 2018; 1507674
7. Cierna Z, Varga I, Danihel L, Intermediate trophoblast – a distinctive, unique and often unrecognized population of trophoblastic cells: Ann Anat, 2016; 204; 45-50
8. Silva JF, Serakides R, Intrauterine trophoblast migration: A comparative view of humans and rodents: Cell Adh Migr, 2016; 10(1–2); 88-110
9. Liu Q, Yan X, Li Y, Pre-eclampsia is associated with the failure of melanoma cell adhesion molecule (MCAM/CD146) expression by intermediate trophoblast: Lab Investig, 2004; 84; 221-28
10. Kim K-R, Jun S-Y, Kim J-Y, Ro JY, Implantation site intermediate trophoblasts in placenta cretas: Mod Pathol, 2004; 17; 1483-90
11. Stanek J, Drummond Z, Occult placenta asccreta: The missing link in the diagnosis of abnormal placentation: Pediatr Dev Pathol, 2007; 10(4); 266-73
12. Tantbirojn P, Crum CP, Parast MM, Pathophysiology of placenta creta: The role of decidua and extravillous trophoblast: Placenta, 2008; 29(7); 639-45
13. Benirschke K, Burton GJ, Baergen RN, Pathology of the human placenta, sixth edition: Pathol Hum Placenta, 2012; 1-941
14. Khong TY, Robertson WB, Placenta creta and placenta praevia creta: Placenta, 1987; 8(4); 399-409
15. Thurn L, Lindqvist PG, Jakobsson M, Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: Results from a large population-based pregnancy cohort study in the Nordic countries: BJOG, 2016; 123(8); 1348-55
16. Silver RM, Landon MB, Rouse DJ, Maternal morbidity associated with multiple repeat cesarean deliveries: Obstet Gynecol, 2006; 107(6); 1226-32
17. Biswas R, Sawhney H, Dass R, Histopathological study of placental bed biopsy in placenta previa: Acta Obstet Gynecol Scand, 1999; 78(3); 173-79
Figures
 Figure 1. Placenta non-accreta. Area of chorionic villi covered by syncytiotrophoblast and cytotrophoblast is shown above the dashed line, while the myometrial area is shown below the dashed line (hematoxylin and eosin staining, magnification ×200).
Figure 1. Placenta non-accreta. Area of chorionic villi covered by syncytiotrophoblast and cytotrophoblast is shown above the dashed line, while the myometrial area is shown below the dashed line (hematoxylin and eosin staining, magnification ×200). Figure 2. Placenta accreta. Groups of intermediate trophoblastic cells invaded the myometrium (arrows), and the villi are located outside and inside the myometrium (hematoxylin and eosin staining, magnification ×200).
Figure 2. Placenta accreta. Groups of intermediate trophoblastic cells invaded the myometrium (arrows), and the villi are located outside and inside the myometrium (hematoxylin and eosin staining, magnification ×200). Figure 3. Immunohistochemical staining – negative control of CD146 from skin. (A) Magnification ×200. (B) Magnification ×400, with the positive internal control revealed from the vessels’ endothelial cells (arrows).
Figure 3. Immunohistochemical staining – negative control of CD146 from skin. (A) Magnification ×200. (B) Magnification ×400, with the positive internal control revealed from the vessels’ endothelial cells (arrows). Figure 4. Immunohistochemical staining – intermediate trophoblastic cells showing reactivity to CD146 staining between the myometrium (arrows). (A) Magnification ×100. (B) Magnification ×200.
Figure 4. Immunohistochemical staining – intermediate trophoblastic cells showing reactivity to CD146 staining between the myometrium (arrows). (A) Magnification ×100. (B) Magnification ×200. Figure 5. Maximum invasion depth of intermediate trophoblasts in placenta accreta spectrum and non-accreta cases, showing a significant difference.
Figure 5. Maximum invasion depth of intermediate trophoblasts in placenta accreta spectrum and non-accreta cases, showing a significant difference. Figure 6. Comparison of intermediate trophoblast cell distribution pattern between the placenta accreta spectrum and non-accreta groups.
Figure 6. Comparison of intermediate trophoblast cell distribution pattern between the placenta accreta spectrum and non-accreta groups. In Press
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952