08 June 2023: Clinical Research
Analysis of Factors Related to Type 2 Diabetic Peripheral Neuropathy Based on Flash Glucose Monitoring System
Qian Ma1ABCDEF*DOI: 10.12659/MSM.939157
Med Sci Monit 2023; 29:e939157
Abstract
BACKGROUND: Diabetic peripheral neuropathy is a common, serious microvascular complication of type-2 diabetes, greatly impacting patients' quality of life. Due to the lack of effective clinical treatments to delay or reverse the progression of diabetic peripheral neuropathy, early and comprehensive management of risk factors becomes crucial in preventing this complication and improving clinical prognosis. This study aimed to identify the risk factors associated with peripheral neuropathy in patients with type-2 diabetes mellitus, using flash glucose monitoring data gathered in a retrospective clinical study.
MATERIAL AND METHODS: The study encompassed 325 patients with type-2 diabetes mellitus who were diagnosed and treated at Chu Hsien-I Memorial Hospital of Tianjin Medical University between February 2019 and May 2021. All patients underwent continuous flash glucose monitoring for 14 days. Based on the occurrence of diabetic peripheral neuropathy, patients were categorized into two groups: the diabetic peripheral neuropathy group (150 patients) and the non-diabetic peripheral neuropathy group (175 patients). Risk factors for diabetic peripheral neuropathy were analyzed by comparing the clinical data, biochemical indicators, and blood glucose fluctuation data between the two groups.
RESULTS: Spearman correlation analysis demonstrated that factors such as smoking, duration of diabetes, fasting blood glucose (FBG), 2-hour postprandial glucose (2hPG), glycated hemoglobin (HbA1c), insulin resistance index, mean blood glucose (MBG), coefficient of variation, standard deviation, mean amplitude of glycemic excursion, mean of daily differences, and time above range were positively correlated with diabetic peripheral neuropathy. Conversely, time in range was negatively associated with diabetic peripheral neuropathy. Multivariate logistic regression analysis identified smoking, duration of diabetes, insulin resistance index, and time in range as related factors of diabetic peripheral neuropathy.
CONCLUSIONS: Smoking, duration of diabetes, insulin resistance index, and time in range were identified as significant risk factors for type-2 diabetic peripheral neuropathy.
Keywords: Diabetes Complications, Humans, Diabetes Mellitus, Type 2, Blood Glucose, Diabetic Neuropathies, Glucose, Blood Glucose Self-Monitoring, Retrospective Studies, Quality of Life
Background
Type 2 diabetes mellitus (T2DM) is a complex, multisystem, chronic, metabolic disease. Long-term exposure to hyperglycemia and poor blood glucose management are the main causes of chronic complications [1]. Diabetic peripheral neuropathy (DPN) is one of the most common and serious microvascular complications of type 2 diabetes. The disease has hidden onset, complex etiology, and high disability and death rates, which seriously affects quality of life [2,3]. Previous studies have found that older age, long course of diabetes, hyperglycemia, hypertension, dyslipidemia, urine microalbumin/urine creatinine ratio (UACR), insulin resistance index (HOMA-IR), C-P level, and mean amplitude of glycemic excursion (MAGE) are associated with the occurrence of DPN [4–8]. According to literature, appropriate interventions for high-risk DPN patients can reduce the incidence of ulcers by 60% and the risk of amputation by 85% [9]. Therefore, early effective control of risk factors for DPN and individualized diabetes management plans are important for preventing DPN occurrence and improving clinical prognosis.
The importance of blood glucose fluctuations in diabetes management cannot be ignored. Compared with persistent hyperglycemia, blood glucose fluctuations have a greater impact on oxidative stress and vascular endothelial cells, and play a more important role in promoting the occurrence and development of chronic complications of diabetes [10,11]. This study used the recently developed flash glucose monitoring (FGM) system to observe the blood glucose fluctuation of DPN to explore the blood glucose fluctuation indicators and clinical factors related to DPN.
Material and Methods
CLINICAL DATA:
This study enrolled 325 patients with T2DM (diagnosed according to standards established by the WHO in 1999) treated at Chu Hsien-I Memorial Hospital of Tianjin Medical University from February 2020 to May 2021. The patients were aged 18~75 years old, 170 were males and 155 were females. All patients wore a FGM glucose level detector for 14 days.
DIAGNOSTIC CRITERIA FOR DPN:
The diagnostic criteria of DPN refer to the standards established by the American Diabetes Association [12] as follow: (1) Diabetes history was clear; (2) Diabetic neuropathy occurred at or after diagnosis; (3) Clinical symptoms and signs were consistent with DPN; (4) Pain, numbness, paresthesia, other clinical symptoms were present, and at least 1 of the 5 examinations (ankle reflex, needle tingling, vibration sensation, temperature sensation, and pressure sensation) was abnormal, or no there were no clinical symptoms but results of at least 2 of the 5 examinations were abnormal.
EXCLUSION CRITERIA FOR DPN:
Exclusion criteria were: (1) Pregnant and lactating women; (2) Neuropathy caused by non-diabetic causes such as cervical and lumbar diseases, cerebrovascular diseases and Guillain-Barre syndrome (GBS); (3) Chemotherapeutic drugs or other drugs that affect the nervous system; (4) Arteriovenous vascular diseases (eg, arteriosclerosis obliterans of lower limbs and Takayasu arteritis); (5) Endocrine diseases (eg, thyroid diseases, adrenocortical hyperthyroidism).
DATA COLLECTION:
Age, smoking status, diabetes course, body mass index (BMI), HbA1c, systolic blood pressure (SBP), diastolic blood pressure (DBP), FBG, 2hPG, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-L), low-density lipoprotein cholesterol (LDL-L), HOMA-IR, diabetes medication, and other basic information were recorded. The dynamic blood glucose data were collected by having patients continuously wear a 14-day FGM blood glucose monitoring device produced by Abbott, UK.
INSTRUMENT AND CALCULATION FORMULA:
The FGM system in this study consisted of 3 parts: scanning detector, sensor, and FreeStyle Libre software. A scanning detector is used to activate sensors on patients, detect glucose, and to obtain sensor data and system information. The sensor package includes a sensor assembly package and sensor applicator. After wearing the sensor, the glucose readings were stored every 15 min and 96 blood glucose values were recorded every 24 h for 14 days. FreeStyle Libre software was installed on a computer, upload the data to the computer through the scanning detector, and ambulatory glucose profile (AGP) report. The blood glucose monitoring indicators and calculation formula provided by the AGP report are as follows:
Note:
λ
STATISTICAL ANALYSIS:
SPSS 23.0 statistical software was used for data analysis, The measurement data are expressed as mean±standard deviation (χ̄±s). The 2 samples were compared by independent
Results
COMPARISON OF CLINICAL BASELINE BHARACTERISTICS:
Comparison of 2 groups, the duration of diabetes, smoking, FBG, 2hPG, HbA1c, and HOMA-IR in group DPN were higher than those in non-DPN group, the difference was statistically significant (P<0.05). The number of cases, gender, age, hypertension, BMI, SBP, DBP, TC, TG, HDL-C, and LDL-C in the 2 groups were not significantly different (P>0.05). In terms of medication, there was no significant difference in the proportion of insulin (except GLP1 agonist), hypoglycemic drugs (Include SGLT2 inhibitor), and statins between the 2 groups (P>0.05) (Table 1).
COMPARISON OF FGM BLOOD GLUCOSE PARAMETERS BETWEEN THE 2 GROUPS:
The MBG, SDBG, CV, MAGE, MODD, and TAR of the DPN group were higher than those of the non-DPN group, and TIR was lower than that of the non-DPN group. There were statistically significant differences in the above (P<0.05) (Table 2).
SPEARMAN CORRELATION ANALYSIS AND UNIVARIATE LOGISTIC REGRESSION ANALYSIS BETWEEN CLINICAL VARIABLES AND DPN:
Spearman correlation analysis showed that gender, age, BMI, SBP, DBP, TC, TG, HDL-C, and LDL-C were negative for clinical indicators. Smoking, diabetes duration, FBG, 2hPG, HbA1c, and HOMA-IR were positively correlated with DPN. For FGM parameters, MBG, CV, SDBG, MAGE, MODD, and tar were positively correlated with DPN, and TIR was negatively correlated with DPN (Table 3). Univariate logistic regression analysis showed that diabetes course, smoking, FBG, 2hPG, HbA1c, HDL-C, LDL-C, HOMA-IR, MBG, CV, SDBG, MAGE, MODD, TAR, and TIR were related factors of DPN (Table 4).
MULTIPLE LOGISTIC REGRESSION ANALYSIS OF RELATED FACTORS AFFECTING DPN:
The univariate variables related to DPN, diabetes course, smoking, FBG, 2hPG, HbA1c, HDL-C, LDL-C, HOMA-IR, MBG, SDBG, CV, MAGE, MODD, TAR, and TIR as independent variables. Taking whether T2DM is concurrent with DPN as the dependent variable, multivariate logistic regression analysis showed that smoking (OR=4.235, 95% CI: 2.151–8.339, P=0.000), diabetes course (OR=1.103, 95% CI: 1.028–1.185, P=0.007), HOMA-IR (OR=1.366, 95% CI: 1.093–1.707, P=0.006), and TIR (OR=0.915, 95% CI: 0.853–0.982, P=0.014) were related factors of TIR (Table 5).
Discussion
Diabetic peripheral neuropathy (DPN) is a heterogeneous group of diseases caused by nerve dysfunction in diabetic patients. Its pathogenesis is complex, which is mostly considered to be related to polyol pathway activation, oxidative stress, long-term low-grade inflammation, immune system damage, excessive glycosylation of nerve tissue, and lack of neurotrophic factors. The study found with the extension of diabetes, about 50% of diabetic patients will develop peripheral neuropathy. Intensive glycemic control is the key therapeutic measure to reduce the risk of DPN [13]. In this study, the incidence of DPN was 46.2%, and the course of diabetes was a related factor of DPN. Comparing the 2 groups of patients, it was found that the course of diabetes in the DPN group was longer than that in the non-DPN group, which was similar to the above report. HbA1c is the criterion standard for evaluating long-term blood glucose control. The study reported that poor blood glucose control (HbA1c >6.5%) increased the risk of DPN by more than 5 times; when HbA1c <7%, the incidence of DPN decreased by 60%. At the same time, it was found that the increase of HbA1c variability was also closely related to DPN [14–16]. This study showed that FBG, 2hPG, and HbA1c were positively correlated with DPN. Therefore, clinical diabetes management should strengthen the monitoring frequency of blood glucose and strictly control the level of blood glucose. Lowering blood glucose and reaching the normal level is an important measure to prevent the occurrence of DPN.
Smoking is one of the risk factors that can be changed, affecting many chronic diseases such as diabetes. Studies have confirmed that the adverse effects of smoking on diabetes are associated not only with macrovascular complications, but also with microvascular complications. Smoking is a risk factor for diabetic neuropathy and is positively correlated with the incidence of diabetic neuropathy [17]. Another study found that long-term smoking significantly increased insulin resistance in patients with type 2 diabetes [18]. Insulin resistance (IR) is one of the significant characteristics of T2DM. Its cause is the insufficient insulin metabolic effects in target tissues, including glucose utilization in skeletal muscle, inhibition of glucose production in the liver, and inhibition of lipolysis in adipose tissue, resulting in the compensatory increase of insulin [19]. A 6-year follow-up study found that DPN in patients with type 2 diabetes was independently related to IR and not related to blood glucose control levels [20]. Metabolic syndrome is a high-risk factor that increases the risk of T2DM. Studies have found that in metabolic syndrome, neurons also produce insulin resistance, resulting in neuronal damage and the development of DPN [21]. In this study, smoking and HOMA-IR are factors related to DPN, suggesting that in diabetes management, we should strengthen the measures of quitting smoking for diabetic patients and use hypoglycemic drugs with increased insulin sensitivity and lower insulin resistance to improve insulin resistance, thereby reducing the risk of DPN.
Blood glucose fluctuation is an important and independent risk factor for diabetes and its complications. In 2020, the ADA guide used TIR in CGM core indicators as a new glycemic control standard to help diabetic patients rebuild their blood glucose homeostasis individually [22]. TIR is defined as the amount of time when the glucose level is within the target range (usually between 3.9 mmol/l and 10.0 mmol/l), which can directly reflect the 24h blood glucose fluctuation of patients, and better reflect the occurrence of hypoglycemia and the degree of blood glucose variation at the same HbA1c [23]. “Time in range” includes TIR, TAR, and TBR. The combination of the 3 can more comprehensively reflect the overall blood glucose situation. With the application of CGM technology, the limitations of HbA1c become more and more obvious. Since HbA1c mainly reflects hyperglycemia and cannot provide information about hypoglycemia and blood glucose variability, the emergence of TIR is a good supplement to HbA1c. Previous studies have found that TIR is linearly correlated with HbA1c, and for every 10% increase in TIR, HbA1c decreases by about 0.5~0.8% [24]. However, another study reported that there was no significant correlation between TIR and HbA1c, indicating that the 2 reflected a different emphasis on blood glucose information [25]. TIR is one of the key indicators for evaluating intra-day blood glucose fluctuation and daytime blood glucose fluctuation, and is closely related to diabetic macrovascular complications and microvascular complications. In an observational study of 105 patients with T2DM, Mayeda [3] reported that in T2DM and T2DM chronic kidney disease (CKD), the risk of DPN increased with the decrease of TIR, and the prevalence of DPN was negatively and significantly correlated with TIR. In this study, TIR was negatively correlated with DPN, and TIR was the related factor of DPN. It indicates that in the treatment of diabetes, blood glucose control should be strengthened, blood glucose attainment time should be increased, the occurrence time of hyperglycemia and hypoglycemia will be reduced, and the fluctuation of blood glucose will be an important therapeutic measure to prevent the occurrence of DPN.
Previous studies [4–6] have shown that hyperglycemia, hypertension, dyslipidemia, increased HbA1c, and MAGE are important factors affecting DPN in patients with type 2 diabetes. The results of this study are indeed different, perhaps because DPN is heterogeneous and related to long-term blood glucose control. A single short-term study can not fully and accurately reflect a variety of clinical factors related to DPN. In addition, the blood glucose fluctuation index monitored by FGM in this study is inconsistent with previous studies, which may have contributed to the difference in results.
Conclusions
In conclusion, our study shows that smoking, diabetes course, HOMA-IR, and TIR are risk factors for type 2 diabetic peripheral neuropathy. FGM is the latest glycemic monitoring device in clinical practice. It can carry out continuous 14-day blood glucose monitoring without blood calibration, has high accuracy, and provides more comprehensive blood glucose information and short-term daily blood glucose fluctuation data. It has become an important auxiliary means for clinical diabetes management [26]. This study provides valuable information for clinicians to assess the risk of diabetic complications through use of the FGM glycemic index. However, since this was a single-center, retrospective study with a small sample size, no subjects with GLP1 agonists were selected, and the research results have certain limitations. Large-scale, multi-center, prospective studies are needed to provide new ideas for clinical prevention and treatment of diabetes and its complications.
Tables
Table 1. Comparison of clinical data and biochemical indicators χ̄±s, n/n, M (QL, QU).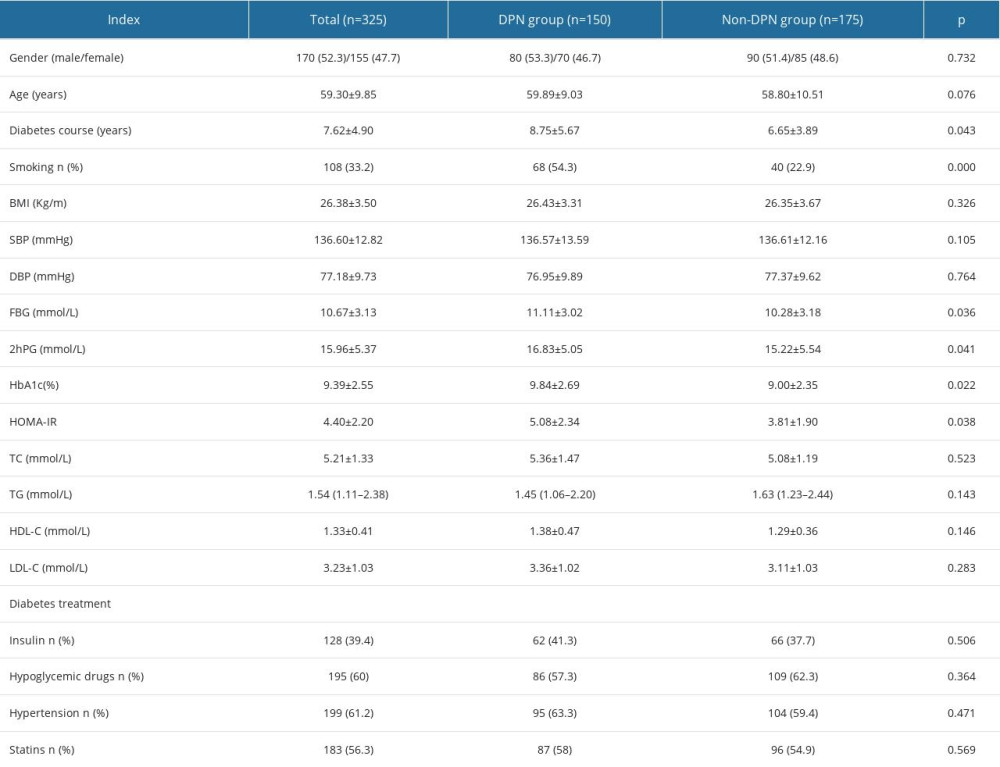 Table 2. Comparison of FGM blood glucose parameters between 2 groups [χ̄±s, n/n, M(QL, QU)].
Table 2. Comparison of FGM blood glucose parameters between 2 groups [χ̄±s, n/n, M(QL, QU)].![Comparison of FGM blood glucose parameters between 2 groups [χ̄±s, n/n, M(QL, QU)].](https://jours.isi-science.com/imageXml.php?i=t2-medscimonit-29-e939157.jpg&idArt=939157&w=1000) Table 3. Spearman correlation analysis between clinical variables and DPN (n=150).
Table 3. Spearman correlation analysis between clinical variables and DPN (n=150).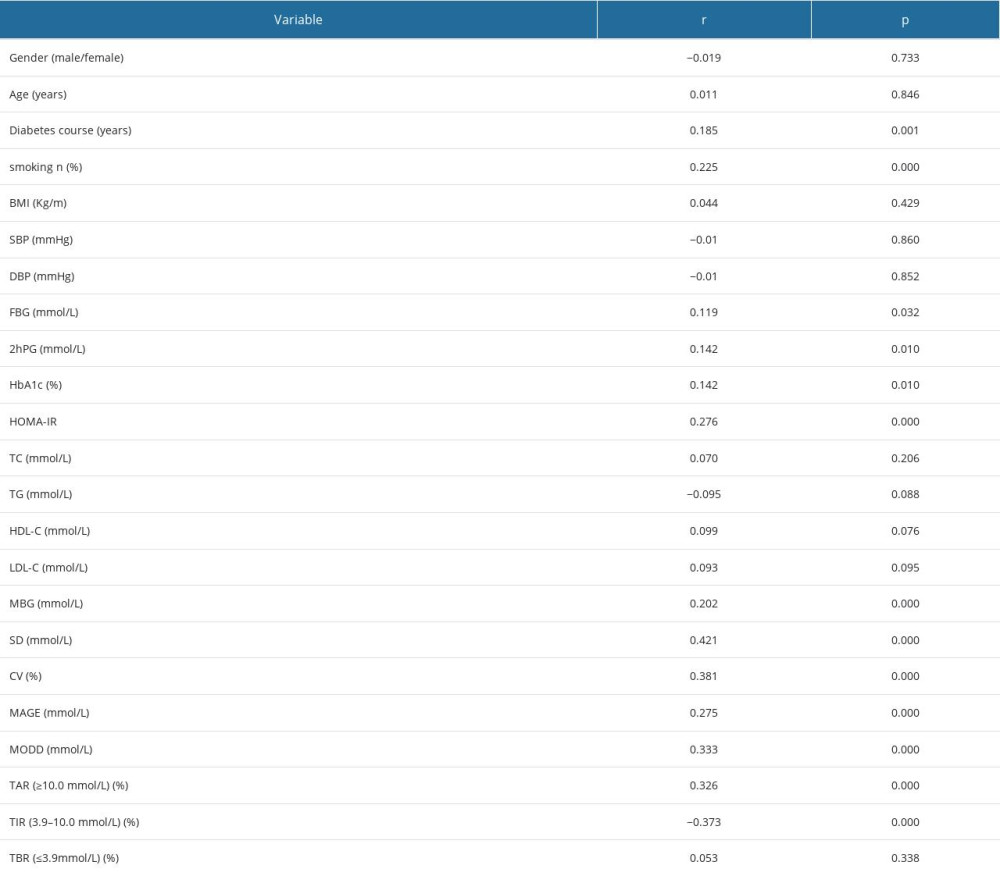 Table 4. Univariate logistic regression between clinical variables and DPN (n=150).
Table 4. Univariate logistic regression between clinical variables and DPN (n=150).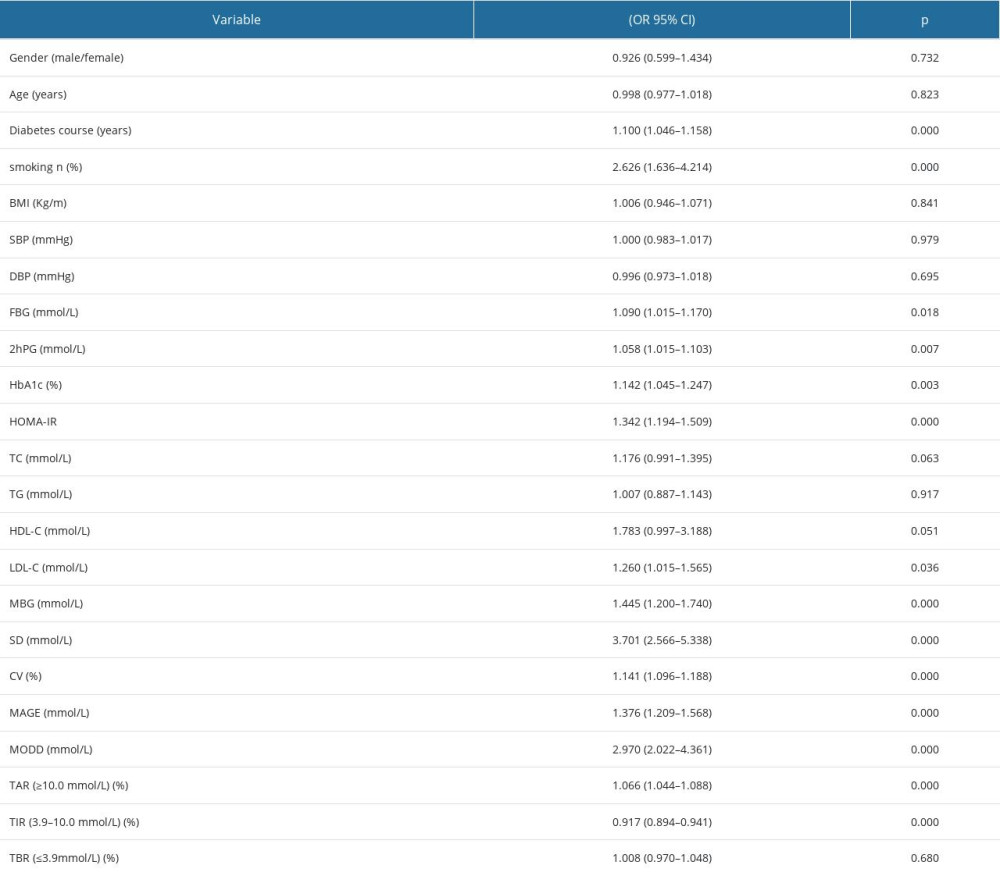 Table 5. Multivariate logistic regression analysis of related factors of DPN.
Table 5. Multivariate logistic regression analysis of related factors of DPN.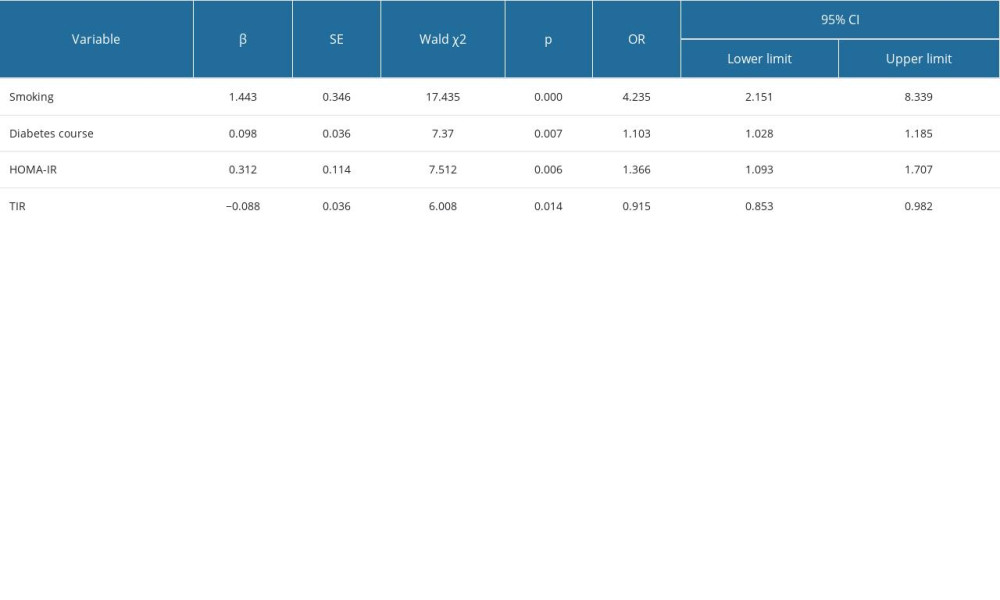
References
1. Faselis C, Katsimardou A, Imprialos K, Microvascular complications of type 2 diabetes mellitus: Curr Vasc Pharmacol, 2020; 18(2); 117-24
2. Amara F, Hafez S, Orabi A, Review of diabetic polyneuropathy: Pathogenesis, diagnosis and management according to the consensus of Egyptian experts: Curr Diabetes Rev, 2019; 15(4); 340-45
3. Mayeda L, Katz R, Ahmad I, Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease: BMJ Open Diabetes Res Care, 2020; 8(1); e000991
4. Gebabo TF, Zewdie TH, Shagaro SS, Determinants of peripheral neuropathy among diabetic patients under follow-up in chronic care clinics of public hospitals at Gamo and Gofa zones, southern Ethiopia: PLoS One, 2021; 16(2); e0246722
5. Pai YW, Lin CH, Lin SY, Reconfirmation of newly discovered risk factors of diabetic peripheral neuropathy in patients with type 2 diabetes: A case-control study: PLoS One, 2019; 14(7); e0220175
6. Hu YM, Zhao LH, Zhang XL, Association of glycaemic variability evaluated by continuous glucose monitoring with diabetic peripheral neuropathy in type 2 diabetic patients: Endocrine, 2018; 60(2); 292-300
7. Wan H, Jiang X, Wang L, Correlation analysis of newly diagnosed type 2 diabetes C-peptide level and type 2 diabetes peripheral neuropathy: Chinese Journal of Diabetes, 2017; 25(06); 514-17
8. Liu S, Zheng H, Zhu X, Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients: Diabetes Res Clin Pract, 2017; 130; 90-97
9. Liu X, Xu Y, An M, The risk factors for diabetic peripheral neuropathy: A meta-analysis: PLoS One, 2019; 14(2); e0212574
10. Costantino S, Paneni F, Battista R, Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA(1c) Levels: Diabetes, 2017; 66(9); 2472-82
11. Prázný M, Škrha J, Šoupal JShort-term and long-term glycemic variability and its relationship to microvascular complications of diabetes: Vnitr Lek, 2016; 62(11 Suppl 4); S85-93 [in Czech]
12. Pop-Busui R, Boulton AJ, Feldman EL, Diabetic neuropathy: A position statement by the American Diabetes Association: Diabetes Care, 2017; 40(1); 136-54
13. Tang HY, Jiang AJ, Ma JL, Understanding the signaling pathways related to the mechanism and treatment of diabetic peripheral neuropathy: Endocrinology, 2019; 160(9); 2119-27
14. Su JB, Zhao LH, Zhang XL, HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients: Cardiovasc Diabetol, 2018; 17(1); 47
15. Jun JE, Lee SE, Lee YB, Glycated albumin and its variability as an indicator of cardiovascular autonomic neuropathy development in type 2 diabetic patients: Cardiovasc Diabetol, 2017; 16(1); 127
16. Lee WJ, Jang S, Lee SH, Correlation between the severity of diabetic peripheral polyneuropathy and glycosylated hemoglobin levels: A quantitative study: Ann Rehabil Med, 2016; 40(2); 263-70
17. Al-Mahroos F, Al-Roomi K, Diabetic neuropathy, foot ulceration, peripheral vascular disease and potential risk factors among patients with diabetes in Bahrain: A nationwide primary care diabetes clinic-based study: Ann Saudi Med, 2007; 27(1); 25-31
18. Śliwińska-Mossoń M, Milnerowicz H, The impact of smoking on the development of diabetes and its complications: Diab Vasc Dis Res, 2017; 14(4); 265-76
19. Mokáň M, Galajda P, Primary and secondary insulin resistance: Vnitr Lek, 2019; 65(4); 264-72
20. Cho YN, Lee KO, Jeong J, The role of insulin resistance in diabetic neuropathy in Koreans with type 2 diabetes mellitus: A 6-year follow-up study: Yonsei Med J, 2014; 55(3); 700-8
21. Han L, Ji L, Chang J, Peripheral neuropathy is associated with insulin resistance independent of metabolic syndrome: Diabetol Metab Syndr, 2015; 7; 14
22. American Diabetes Association, 2. Classification and diagnosis of diabetes: Standards of medical care indDiabetes – 2020: Diabetes Care, 2020; 43(Suppl 1); S14-31
23. Vigersky RA, Mcmahon C, The relationship of hemoglobin A1C to time-in-range in patients with diabetes: Diabetes Technol Ther, 2019; 21(2); 81-85
24. Battelino T, Danne T, Bergenstal RM, Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the International Consensus on Time in Range: Diabetes Care, 2019; 42(8); 1593-603
25. Beck RW, Bergenstal RM, Cheng P, The relationships between time in range, hyperglycemia metrics, and HbA1c: J Diabetes Sci Technol, 2019; 13(4); 614-26
26. Ji L, Guo X, Guo L, A multicenter evaluation of the performance and usability of a novel glucose monitoring system in Chinese adults with diabetes: J Diabetes Sci Technol, 2017; 11(2); 290-95
Tables
 Table 1. Comparison of clinical data and biochemical indicators χ̄±s, n/n, M (QL, QU).
Table 1. Comparison of clinical data and biochemical indicators χ̄±s, n/n, M (QL, QU).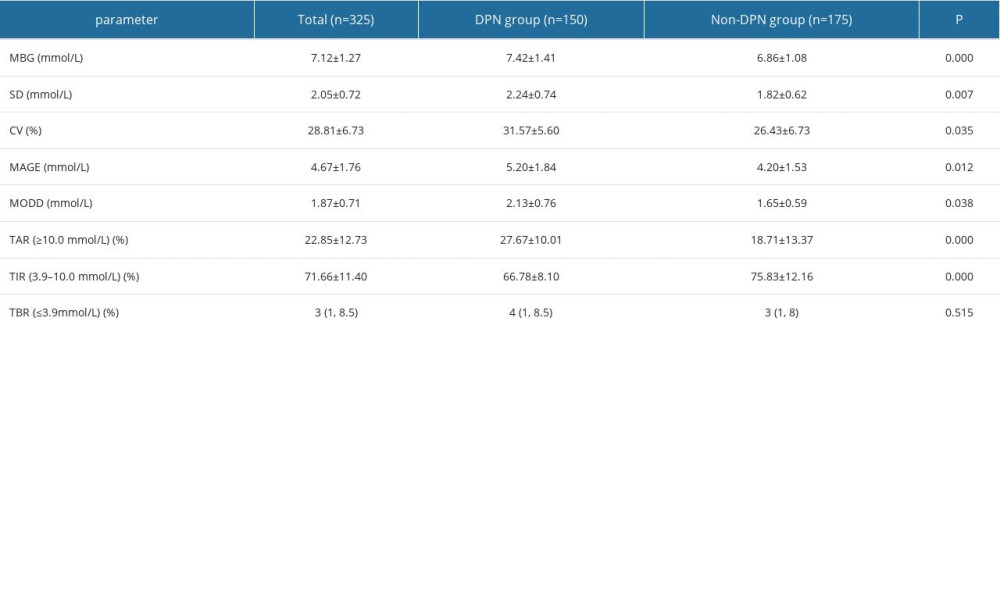 Table 2. Comparison of FGM blood glucose parameters between 2 groups [χ̄±s, n/n, M(QL, QU)].
Table 2. Comparison of FGM blood glucose parameters between 2 groups [χ̄±s, n/n, M(QL, QU)]. Table 3. Spearman correlation analysis between clinical variables and DPN (n=150).
Table 3. Spearman correlation analysis between clinical variables and DPN (n=150). Table 4. Univariate logistic regression between clinical variables and DPN (n=150).
Table 4. Univariate logistic regression between clinical variables and DPN (n=150). Table 5. Multivariate logistic regression analysis of related factors of DPN.
Table 5. Multivariate logistic regression analysis of related factors of DPN. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








