07 June 2023: Database Analysis
Database Analysis
Bin Zhu1AE*, MingNan Cao1BF, Qiao Wu2BC, Kejia Liu3BC, Wei Guo4AD, Zhigang Zhao1ADDOI: 10.12659/MSM.939546
Med Sci Monit 2023; 29:e939546
Abstract
BACKGROUND: Long-term aspirin treatment was recommended for secondary prevention of cardiovascular and cerebrovascular disease. However, some studies reveal low-dose aspirin (LDA) can raise serum uric acid (SUA) levels. Thus, the purpose of this study was to analyze whether LDA intake is associated with hyperuricemia.
MATERIAL AND METHODS: Data was collected from the National Health and Nutrition Examination Survey (NHANES) between 2011 and 2018. All participants over 40 years old and who selected “preventive aspirin use” were included in the study. Logistic regression analyses were used to evaluate the relationship between LDA intake and hyperuricemia. The stratified analysis was based on race and estimated glomerular filtration rate (eGFR).
RESULTS: A total of 3540 participants were included in the study. Of them, 805 (22.7%) took LDA, and 190 (31.6%) had hyperuricemia. There was no significant association between hyperuricemia and LDA intake (OR=1.22, 95% CI: 0.97-1.54) after adjusting for confounding factors. However, further subgroup analysis by age showed a significant association between LDA intake and hyperuricemia (OR=3.44, 95% CI: 1.88-6.27) among those 40 to 50 years of age. After adjusting for confounding factors, the relationship was still significant (OR=2.28, 95% CI: 1.10-4.73); we also found that race (Hispanic American, OR=1.84, 95% CI: 1.11-3.06) and eGFR under 60 mL/min/1.73 m² (OR=1.94, 95% CI: 1.04-3.62) may play important roles in the development of hyperuricemia.
CONCLUSIONS: LDA does not increase the hyperuricemia risk in people over 40 years. However, those aged between 40 and 50 years, Hispanic American, and with impaired renal function should have careful evaluation during LDA treatment.
Keywords: Aspirin, ethnicity, Glomerular Filtration Rate, hyperuricemia, Humans, Adult, Middle Aged, Child, Preschool, Nutrition Surveys, Cross-Sectional Studies, Uric Acid, Risk Factors
Background
Aspirin is a nonsteroidal anti-inflammatory drug that irreversibly inhibits cyclooxygenase 1 (COX-1) and plays a role in thrombus formation in the arteries [1]. It is now widely used as secondary prevention among patients with established cerebrovascular, coronary, or peripheral artery disease to lower the risk of adverse health outcomes [2,3]. As a cornerstone of cardiovascular disease prevention, low-dose aspirin (LDA) from 75 to 325 mg per day is commonly used by elderly people for the prevention of thrombosis [4]. It is estimated that over 30% of adults take aspirin for cardiovascular and cerebrovascular disease prevention in the United States [5,6].
However, despite its popularity and benefits in preventing recurrent cardiovascular events, some studies have revealed that aspirin at low or high doses can have a different effect on serum uric acid (SUA) levels. It is reported that a low dose of aspirin can promote renal uric acid reabsorption and a high dose inhibits reabsorption [7,8]. Due to this biphasic effect on SUA, there can be a certain risk of hyperuricemia for people who take aspirin for thrombosis prophylaxis.
It is worth noting that hyperuricemia is a critical issue affecting individuals worldwide [10]. Diseases such as cardiovascular disease, chronic kidney disease, and diabetes are reported to be associated with hyperuricemia [11,12]. Considering the large number of people taking LDA as primary and secondary prevention and the severity of hyperuricemia, further research to study the relationship between taking aspirin and hyperuricemia is warranted. Thus, the purpose of the present study was to assess the interaction between hyperuricemia and LDA intake for primary and secondary prevention of cardiovascular and cerebrovascular diseases in US adults. To accomplish this, we conducted cross-sectional analyses of data from the 2011–2018 National Health and Nutrition Examination Survey (NHANES).
Material and Methods
STUDY POPULATION:
Data for this study were collected from the NHANES, which is freely available from the website (
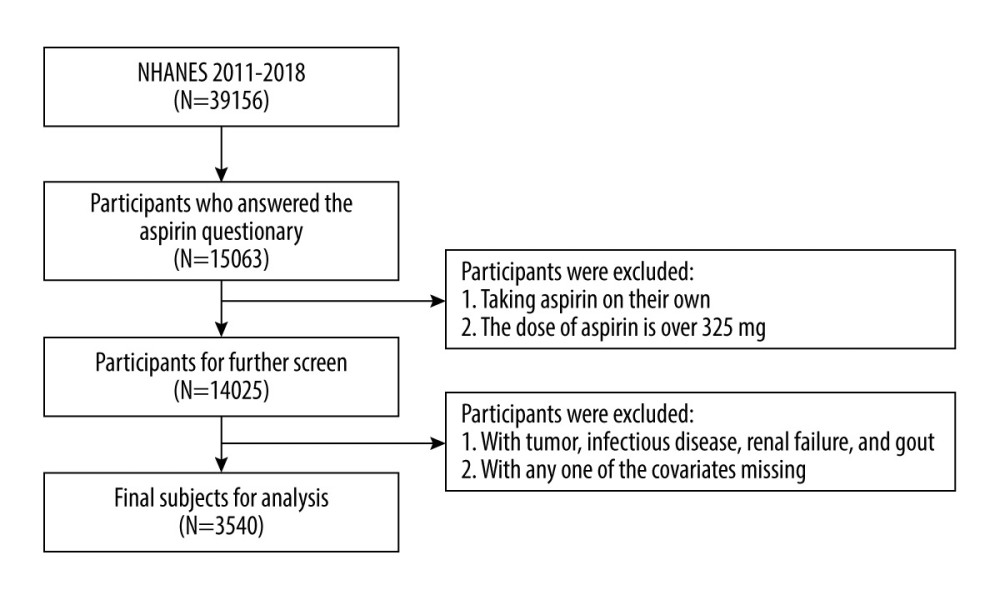
We included NHANES data between 2011 and 2018, covering 4 periods (2011–2012, 2013–2014, 2015–2016, and 2017–2018), to obtain a large sample size for analysis. All patients over 40 years of age who answered “preventive aspirin use” on the questionnaire were included in the analysis. The exclusion criteria were as follows: (1) aspirin dose over 325 mg per day; (2) taking aspirin every other day, another schedule, or don’t know; (3) patients with malignant tumors, infectious diseases, renal failure, or history of gout were excluded from this study; and (4) patients with any one of the missing covariates that were used in Tables 1 and 2 were deleted from the analysis.
Sociodemographic information including age, sex, race, and laboratory parameters was collected from the database. The body mass index (BMI) was calculated by dividing a person’s weight (in kilograms) by height (in meters squared).
DEFINITION AND ASSESSMENT OF VARIABLES:
Participants with hyperuricemia were defined as SUA ≥6.0 mg/dL in women and ≥7.0 mg/dL in men from the examination data [13]. Hypertension was defined as a mean blood pressure value of 3 measurements ≥130 mmHg for systolic blood pressure or ≥80 mmHg for diastolic blood pressure [14]. Additionally, people who had been prescribed any antihypertensive medicines or self-reported having hypertension were classified as hypertension. Diabetes was determined based on the participants who were previously diagnosed with type 2 diabetes by doctors, had fasting blood glucose levels greater than or equal to 7.0 mmol/L (126 mg/dL), or used insulin or any other anti-hypoglycemic drugs. Participants’ estimated glomerular filtration rate (eGFR) level was calculated with the Chronic Kidney Disease-Epidemiology (CKD-EPI) creatinine equation (2021) [15] and defined as a normal renal function (eGFR ≥90 mL/min/1.73 m2), mild impaired renal function (60 mL/min/1.73 m2≤ eGFR <90 mL/min/1.73 m2), and impaired renal function (eGFR <60 mL/min/1.73 m2). The determination of stroke and coronary heart disease was based on relative self-reported questions.
Smoking status, drinking status, and physical activity status information were obtained from the corresponding questionnaire data and defined following the relative criteria. Drinking status was classified as excessive alcohol consumption or not. Participants who ever had 4/5 or more alcoholic beverages every day during the past 12 months were considered to have excessive alcohol consumption. While smoking status was classified as “never smoked” (smoked <100 cigarettes in the lifetime), “former smoker” (ever smoked more than 100 cigarettes but quit smoking), or “current smoker” (smoked more than 100 cigarettes and still smoking now) based on their self-report of the number of cigarettes at the time of this survey. Physical activity status was defined as “yes” or “no” according to the questions about whether they engaged in regular, moderate, or vigorous recreational activities.
STATISTICAL ANALYSIS:
All statistical analyses were conducted using R software (version 4.2.0). Continuous variables were presented as mean±standard deviation, while categorical variables were described as cases (n) and percentages (%). Baseline characteristics were compared using Pearson chi-squared tests or 2-tailed
Results
BASELINE CHARACTERISTIC:
A total of 15 063 participants over 40 years of age answered “preventive aspirin use” on the questionnaire between 2011 and 2018. After screening with the inclusion and exclusion criteria, there were 3540 participants remaining for further analysis. The participant selection process is illustrated in Figure 1. Of the included participants, 601 (17%) were diagnosed with hyperuricemia according to their SUA levels and 2939 (83%) had normal SUA levels. Of the participants with hyperuricemia, 297 (49.4%) were men and 304 (50.6%) were women, and in the no hyperuricemia population, there were 1342 men (45.7%) and 1597 women (54.3%) enrolled. Among all the participants, 805 (22.7%) were taking LDA and 2735 (77.3%) were not taking LDA. Of all participants taking LDA, 190 (31.6%) had hyperuricemia and 615 (20.9%) did not have hyperuricemia. Of participants who did not take LDA, 411 (68.4%) had hyperuricemia and 2324 (79.1%) did not have hyperuricemia. The basic demographic characteristics are shown in Tables 1 and 2.
LDA INTAKE AND HYPERURICEMIA BASED ON AGE AND RACE:
We used logistic regression analysis to detect the relationship between LDA intake and hyperuricemia. No significant association was observed among the total cohort either in the crude model (OR=1.75, 95% CI: 1.44–2.12) or in the model adjusted for the confounding factors (OR=1.22, 95% CI: 0.97–1.54). However, when the cohort was stratified by age, we found a significant association between LDA intake and hyperuricemia in the crude model (OR=3.44, 95% CI: 1.88–6.27) among participants aged 40 to 50 years. After adjusting for confounding factors, the relationship was still significant (OR=2.28, 95% CI: 1.10–4.73). Nevertheless, no significant associations were observed either in the crude model or the adjusted model in the participants older than 50 years (Table 3).
A stratified analysis based on race was also conducted, and the results revealed a significant association between hyperuricemia and LDA intake in Hispanic Americans in the crude model (OR=2.14, 95% CI: 1.40–3.26). Once adjusted for confounding factors, there was still a significant relationship between LDA intake and hyperuricemia (OR=1.84, 95% CI: 1.11–3.06). Nevertheless, no significant association was observed in non-Hispanic Americans (OR=1.05, 95% CI: 0.70–1.57), Black (OR=1.14, 95% CI: 0.74–1.77), or other races (OR=1.42, 95% CI: 0.70–2.90) (Table 4).
RENAL FUNCTION AND HYPERURICEMIA IN PARTICIPANTS TAKING LDA:
Because uric acid is excreted mainly through the kidney, we conducted logistic regression based on renal function, which was evaluated by eGFR. There was a significant correlation between LDA intake and hyperuricemia in those with impaired renal function (eGFR <60 mL/min/1.73 m2; OR=1.95, 95% CI: 1.15–3.32), and the association still existed after adjusting for the confounding factors (OR=1.94, 95% CI: 1.04–3.62). However, this interaction was not observed in those with normal (eGFR ≥90 mL/min/1.73 m2; OR=1.40, 95% CI: 0.94–2.09) and mild impairment of renal function (60 mL/min/1.73 m2≤ eGFR <90 mL/min/1.73 m2; OR=1.00, 95% CI: 0.71–1.41) (Table 4).
Discussion
Aspirin is the cornerstone of antithrombotic management in patients with cardiovascular and cerebrovascular diseases [16]. Although major guidelines provide conflicting recommendations for its use in primary prevention, aspirin is still the most widely used preventative drug worldwide [17]. Our studyrevealed that LDA intake did not increase the hyperuricemia risk in people over 40 years of age. However, people between 40 and 50 years of age should be aware of the hyperuricemia risk of LDA. In addition, race (Hispanic American) and renal function (eGFR <60 mL/min/1.73 m2) should be evaluated when starting aspirin as primary or secondary prevention. We believe these results are worthy of special attention.
LDA has been extensively studied for its efficacy, with fewer studies focused on its relationship with hyperuricemia, which is associated with increased cardiovascular risk and aspirin resistance [18]. Although LDA had been reported to be associated with an increase in SUA levels [19], it is crucial to find out whether taking LDA can cause hyperuricemia. Short-term LDA treatment (100 mg/day for 2 weeks) can affect renal function in elderly patients, which can affect the excretion of uric acid [20]. However, Li et al reported that taking LDA did not influence SUA and urinary uric acid excretion in elderly patients with atherosclerotic cardiovascular disease in an Asian population [21], while Zhang et al also reported a negative association between LDA intake and hyperuricemia in people over 60 years of age [22]. Because the participants in the study by Zhang et al were over 60 years of age, some of their results are consistent with those of the present study.
In addition, our study revealed for the first time that people aged between 40 and 50 years should pay attention to the SUA increasing effect of LDA. Dietary habits and living habits may contribute to this, as it has been reported that obesity, excessive intake of red meat, alcoholic beverages, and eating outside the home are recognized as causal factors for hyperuricemia [23]. Older adults tend to consume less energy-dense sweets and fast foods and eat more healthily, which may help in lowing the morbidity of hyperuricemia [24].
Ethnicity is also an important risk factor that people need to pay attention to, especially for the Hispanic White population. There is not a clear mechanistic explanation for this association in only the Hispanic White population; however, studies had found that SUA concentrations exhibit a strong genetic predisposition, with a heritability estimate ranging from 40% to 70% [25]. Many genes, such as urate transporter 1 (
Renal function is another key risk factor people should give careful consideration. Uric acid is filtered by the glomeruli and reabsorbed by the proximal tubule. Nearly 70% of the uric acid is excreted, and short-term LDA intake had been reported to affect the renal tubular by decreasing uric acid excretion [29,30]. Thus, hyperuricemia is common among patients with chronic kidney disease [31]. In our study, we also found that people with impaired renal function (eGFR <60 mL/min/1.73 m2) had an increased risk of hyperuricemia.
Although findings from the present study identified the potential risk factors of hyperuricemia for those taking LDA as primary and secondary prevention, the study did have limitations. First, as this was a cross-sectional study, we cannot exclude the influence of confounding factors such as combined medicine or genotypes on the results. In addition, diet exposure and dietary supplementation, which are reported to be associated with hyperuricemia, were not included in our analysis [32]. Second, since this was a retrospective cohort study, participants with missing values that were incorporated in the analysis were deleted from the study, and this led to a greatly decreased number of enrolled participants, which may have caused bias. Third, the sample size of this study was too small to draw a definite conclusion. In addition, other studies have reported the opposite conclusion, that gout was more prevalent in adults self-reporting Black race [33]. However, the discovery of the risk factors in our study is worth further examination in clinical practice.
Conclusions
Our study revealed that LDA intake does not increase the hyperuricemia risk in people over 40 years of age. However, those aged between 40 and 50 years, Hispanic American, and with impaired renal function should be given careful evaluation during aspirin treatment. Our findings may provide a straightforward illustration to be used as a reference for the public to choose antiplatelet medicines. Nevertheless, these results should be validated in a large cohort study in the future.
Tables
Table 1. Demographic characteristics of participants with hyperuricemia and without hyperuricemia in the study.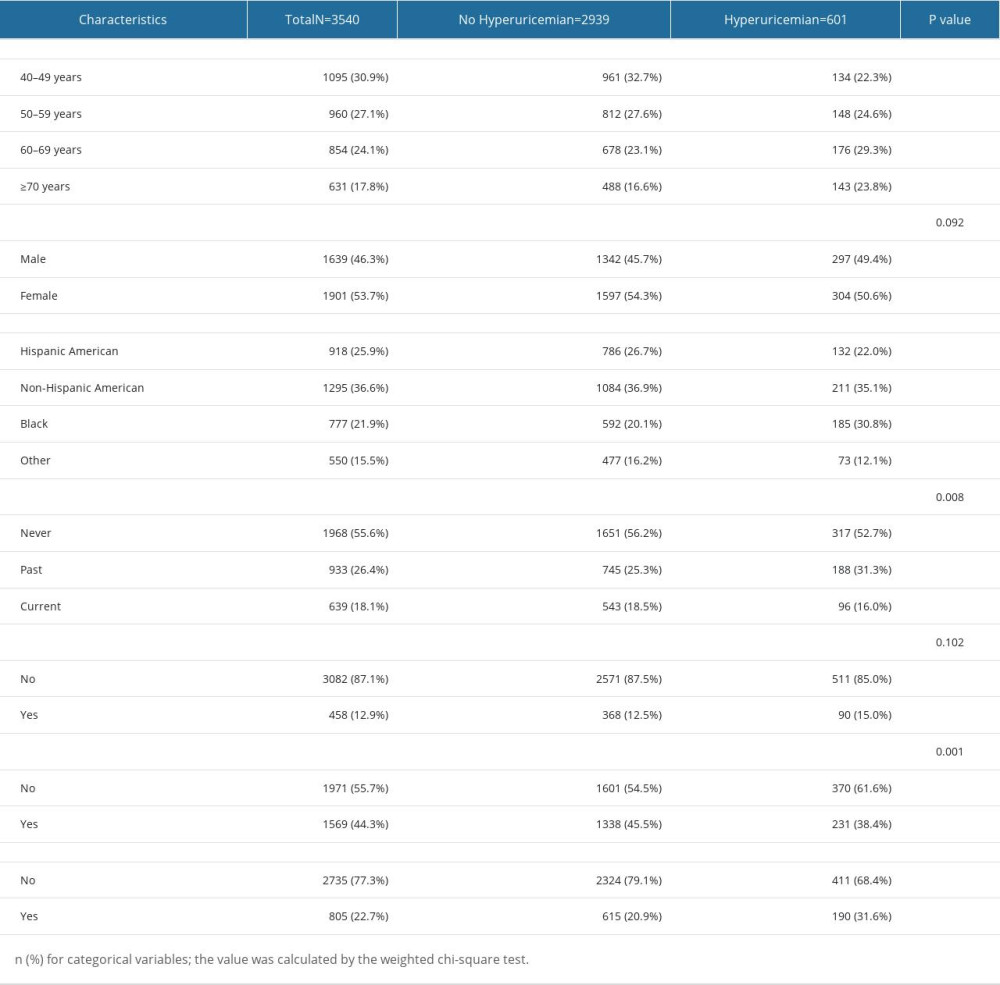 Table 2. Laboratory blood test parameters and past medical history of participants in the 2 groups.
Table 2. Laboratory blood test parameters and past medical history of participants in the 2 groups.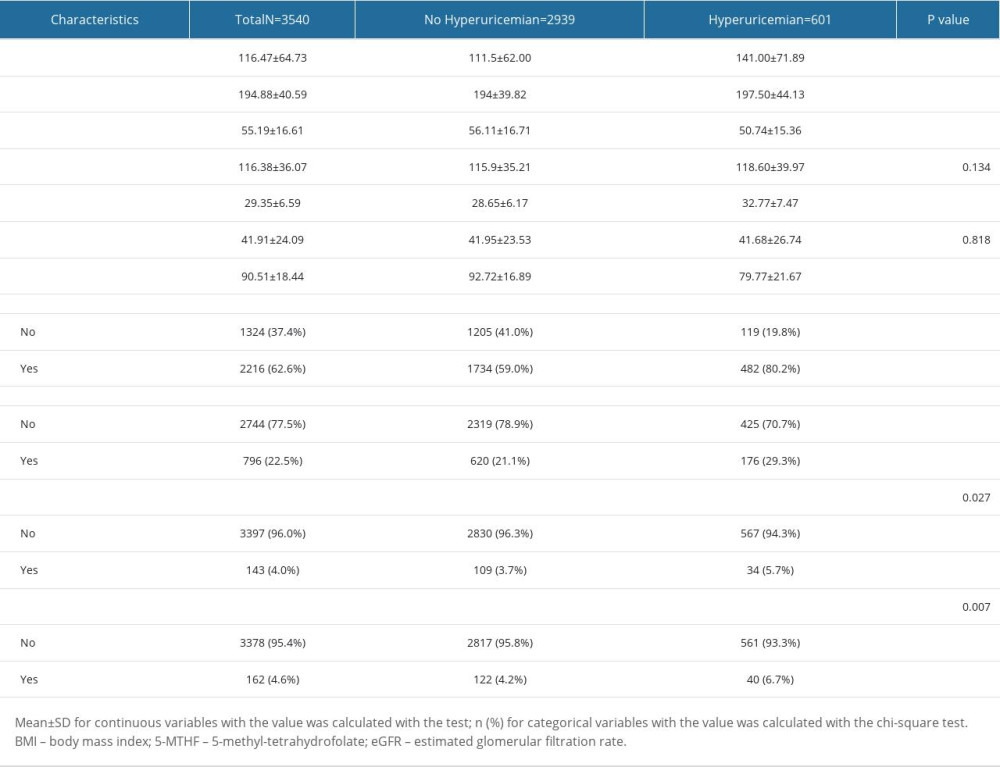 Table 3. Association between low-dose aspirin intake and hyperuricemia based on age. The confounding factors were adjusted by logistic regression analysis in different models.
Table 3. Association between low-dose aspirin intake and hyperuricemia based on age. The confounding factors were adjusted by logistic regression analysis in different models.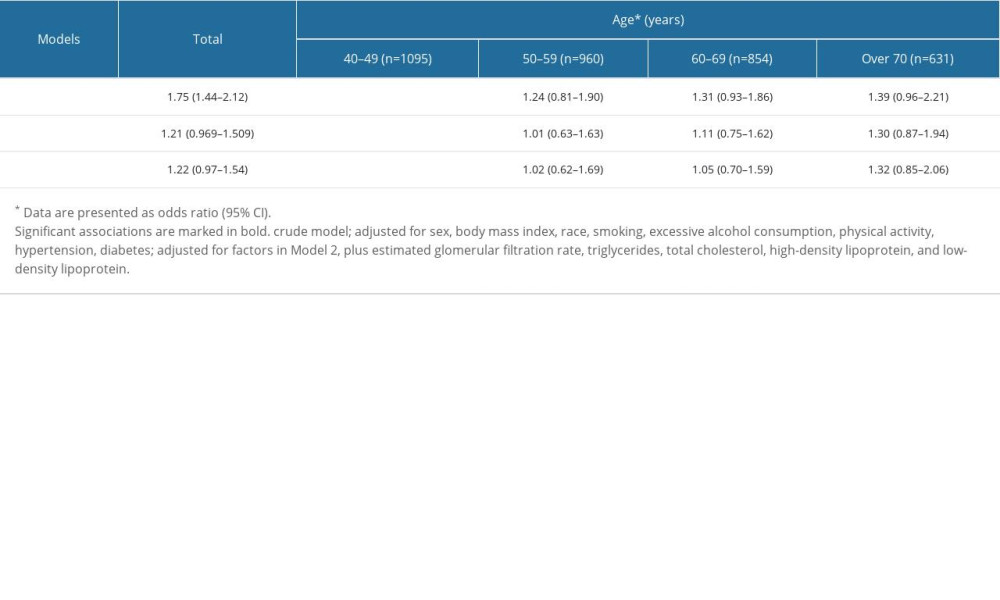 Table 4. Association between low-dose aspirin intake and hyperuricemia based on race and eGFR, respectively. Confounding factors were adjusted by logistic regression analysis in different models.
Table 4. Association between low-dose aspirin intake and hyperuricemia based on race and eGFR, respectively. Confounding factors were adjusted by logistic regression analysis in different models.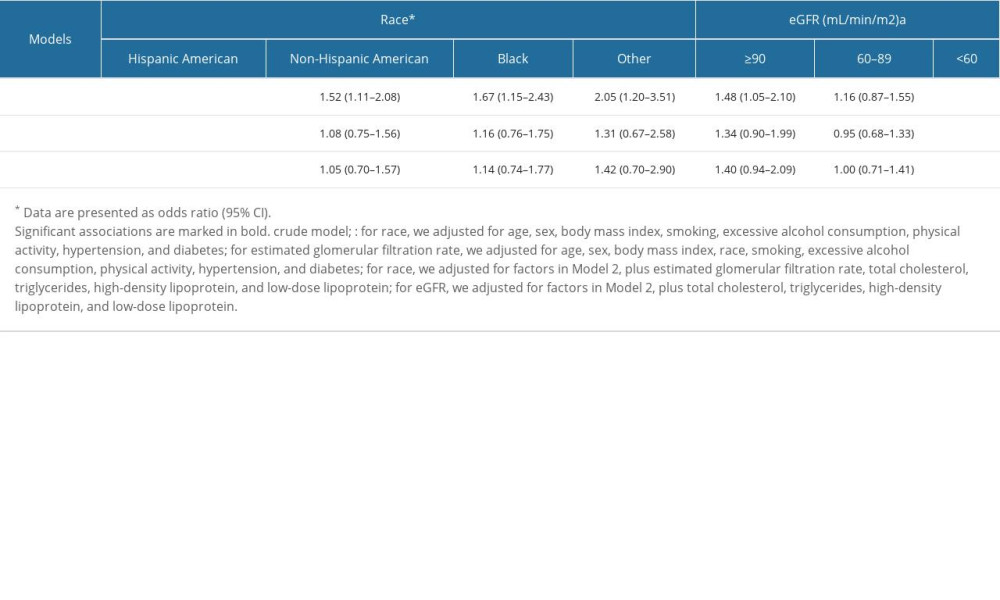
References
1. Capodanno D, Angiolillo DJ, Aspirin for primary cardiovascular risk prevention and beyond in diabetes mellitus: Circulation, 2016; 134(20); 1579-94
2. Davidson KW, Barry MJ, Mangione CM, Aspirin use to prevent cardiovascular disease: US preventive services task force recommendation statement: JAMA, 2022; 327(16); 1577-84
3. Xian Y, Xu H, Matsouaka R, Analysis of prescriptions for dual antiplatelet therapy after acute ischemic stroke: JAMA Netw Open, 2022; 5(7); e2224157
4. Fisher M, Knappertz V, The dose of aspirin for the prevention of cardiovascular and cerebrovascular events: Curr Med Res Opin, 2006; 22(7); 1239-48
5. Luepker RV, Eder M, Finnegan JR, Association of a community population and clinic education intervention program with guideline-based aspirin use for primary prevention of cardiovascular disease: A nonrandomized controlled trial: JAMA Netw Open, 2022; 5(5); e2211107
6. Stuntz M, Bernstein B, Recent trends in the prevalence of low-dose aspirin use for primary and secondary prevention of cardiovascular disease in the united states, 2012–2015: Prev Med Rep, 2017; 5; 183-86
7. Ben SC, Slim R, Fathallah N, Hmouda H, Drug-induced hyperuricaemia and gout: Rheumatology (Oxford), 2017; 56(5); 679-88
8. Leung N, Yip K, Pillinger MH, Toprover M, Lowering and raising serum urate levels: Off-label effects of commonly used medications: Mayo Clin Proc, 2022; 97(7); 1345-62
9. Bardin T, Richette P, Impact of comorbidities on gout and hyperuricaemia: An update on prevalence and treatment options: BMC Med, 2017; 15(1); 123
10. Si K, Wei C, Xu L, Hyperuricemia and the risk of heart failure: Pathophysiology and therapeutic implications: Front Endocrinol (Lausanne), 2021; 12; 770815
11. Fang J, Alderman MH, Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National health and nutrition examination survey: JAMA, 2000; 283(18); 2404-10
12. Yu J, Zheng H, Zhang P, Associations between dietary iron intake from different sources and the risk of hyperuricemia among us adults: A cross-sectional study: Food Nutr Res, 2020; 64; 1-10
13. Whelton PK, Carey RM, Aronow WS, 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College Of Cardiology/American Heart Association task force on clinical practice guidelines: Circulation, 2018; 138(17); e426-e83
14. Inker LA, Eneanya ND, Coresh J, New creatinine- and cystatin C-based equations to estimate GFR without race: N Engl J Med, 2021; 385(19); 1737-49
15. Veronese N, Demurtas J, Thompson T, Effect of low-dose aspirin on health outcomes: An umbrella review of systematic reviews and meta-analyses: Br J Clin Pharmacol, 2020; 86(8); 1465-75
16. Marquis-Gravel G, Roe MT, Harrington RA, Revisiting the role of aspirin for the primary prevention of cardiovascular disease: Circulation, 2019; 140(13); 1115-24
17. Bjornstad P, Laffel L, Lynch J, Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the treatment options for type 2 diabetes in adolescents and youth (today) study: Diabetes Care, 2019; 42(6); 1120-28
18. Liu J, Chen L, Yuan H, Survey on uric acid in chinese subjects with essential hypertension (success): A nationwide cross-sectional study: Ann Transl Med, 2021; 9(1); 27
19. Segal R, Lubart E, Leibovitz A, Early and late effects of low-dose aspirin on renal function in elderly patients: Am J Med, 2003; 115(6); 462-66
20. Li JR, Fan Y, Liu ML, Association between low-dose aspirin and uric acid in the elderly: An observational retrospective cross-sectional study: Int J Gen Med, 2021; 14; 3635-43
21. Zhang P, Wang H, Chen XH, Effect of low-dose aspirin on serum uric acid levels in chinese individuals over 60: Subanalysis of a multicentre randomized clinical trial: Eur Rev Med Pharmacol Sci, 2020; 24(5); 2719-24
22. Cui N, Dong X, Liao W, Association of eating out frequency and other factors with serum uric acid levels and hyperuricemia in Chinese population: Eur J Nutr, 2022; 61(1); 243-54
23. Drewnowski A, Shultz JM, Impact of aging on eating behaviors, food choices, nutrition, and health status: J Nutr Health Aging, 2001; 5(2); 75-79
24. Dong Z, Zhou J, Xu X, Genetic variants in two pathways influence serum urate levels and gout risk: A systematic pathway analysis: Sci Rep, 2018; 8(1); 3848
25. Ichida K, Matsuo H, Takada T, Decreased extra-renal urate excretion is a common cause of hyperuricemia: Nat Commun, 2012; 3; 764
26. Park JH, Jo YI, Lee JH, Renal effects of uric acid: Hyperuricemia and hypouricemia: Korean J Intern Med, 2020; 35(6); 1291-304
27. Krishnan E, Gout in african americans: Am J Med, 2014; 127(9); 858-64
28. Marangella M, Uric acid elimination in the urine. Pathophysiological implications: Contrib Nephrol, 2005; 147; 132-48
29. Yanai H, Adachi H, Hakoshima M, Katsuyama H, Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease: Int J Mol Sci, 2021; 22(17); 9221
30. Sato Y, Feig DI, Stack AG, The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD: Nat Rev Nephrol, 2019; 15(12); 767-75
31. Zheng Z, Harman JL, Coresh J, The dietary fructose: Vitamin C intake ratio is associated with hyperuricemia in African-American adults: J Nutr, 2018; 148(3); 419-26
32. Mccormick N, Lu N, Yokose C, Racial and sex disparities in gout prevalence among US adults: JAMA Netw Open, 2022; 5(8); e2226804
Tables
 Table 1. Demographic characteristics of participants with hyperuricemia and without hyperuricemia in the study.
Table 1. Demographic characteristics of participants with hyperuricemia and without hyperuricemia in the study. Table 2. Laboratory blood test parameters and past medical history of participants in the 2 groups.
Table 2. Laboratory blood test parameters and past medical history of participants in the 2 groups. Table 3. Association between low-dose aspirin intake and hyperuricemia based on age. The confounding factors were adjusted by logistic regression analysis in different models.
Table 3. Association between low-dose aspirin intake and hyperuricemia based on age. The confounding factors were adjusted by logistic regression analysis in different models. Table 4. Association between low-dose aspirin intake and hyperuricemia based on race and eGFR, respectively. Confounding factors were adjusted by logistic regression analysis in different models.
Table 4. Association between low-dose aspirin intake and hyperuricemia based on race and eGFR, respectively. Confounding factors were adjusted by logistic regression analysis in different models. Table 1. Demographic characteristics of participants with hyperuricemia and without hyperuricemia in the study.
Table 1. Demographic characteristics of participants with hyperuricemia and without hyperuricemia in the study. Table 2. Laboratory blood test parameters and past medical history of participants in the 2 groups.
Table 2. Laboratory blood test parameters and past medical history of participants in the 2 groups. Table 3. Association between low-dose aspirin intake and hyperuricemia based on age. The confounding factors were adjusted by logistic regression analysis in different models.
Table 3. Association between low-dose aspirin intake and hyperuricemia based on age. The confounding factors were adjusted by logistic regression analysis in different models. Table 4. Association between low-dose aspirin intake and hyperuricemia based on race and eGFR, respectively. Confounding factors were adjusted by logistic regression analysis in different models.
Table 4. Association between low-dose aspirin intake and hyperuricemia based on race and eGFR, respectively. Confounding factors were adjusted by logistic regression analysis in different models. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952