03 April 2023: Clinical Research
Evaluation of a Previously Developed Predictive Model for Infective Endocarditis in 320 Patients Presenting with Fever at 4 Centers in Japan Between January 2018 and December 2020
Shun YamashitaDOI: 10.12659/MSM.939640
Med Sci Monit 2023; 29:e939640
Abstract
BACKGROUND: In our previous single-center study, we developed an infective endocarditis (IE) prediction model among patients with undiagnosed fever (UF) based on 5 factors that can be obtained on admission: ambulance transfer, presence of cardiac murmur or pleural effusion, blood neutrophil percentage, and platelet count. The present study aimed to retrospectively evaluate the prediction model for IE in 320 patients presenting with fever at 4 university hospitals in Japan from January 2018 to December 2020.
MATERIAL AND METHODS: Patients aged ≥20 years admitted to 4 hospitals with I-330 (IE) or R-50-9 (UF) according to the International Statistical Classification of Diseases and Related Health Problems-10 were enrolled. More than 2 physicians at each hospital reviewed the patient diagnoses using the modified Duke criteria, allocating “definite IE” to IE group (n=119) and “non-definite IE” to UF group (n=201). Five factors on admission were analyzed by multivariate logistic regression. The discriminative ability and calibration of the model were evaluated using the area under the curve (AUC) and the shrinkage coefficient, respectively.
RESULTS: A total of 320 patients were enrolled. The odds ratios (95% confidence intervals) were as follows: ambulance transfer 1.81 (0.91-3.55); cardiac murmur 13.13 (6.69-27.36); pleural effusion 2.34 (0.62-2.42); blood neutrophil percentage 1.09 (1.06-1.14); and platelet count 0.96 (0.93-0.99). The AUC was 0.783 (0.732-0.834) with a shrinkage coefficient of 0.961.
CONCLUSIONS: The IE prediction model is useful to estimate the probability of IE immediately after admission for fever in patients aged ≥20 years.
Keywords: Clinical Decision Rules, Endocarditis, Fever, multicenter study, Validation study, Humans, Japan, Endocarditis, Bacterial, Hospitals, University
Background
Diagnosing infective endocarditis (IE) is difficult due to its non-specific manifestations and physical and laboratory findings [1]. When using the modified Duke criteria (m-DC), various advanced medical modalities, including multiple blood cultures, transthoracic or transesophageal echocardiography, computed tomography (CT), and magnetic reasoning image (MRI), are required [2]. Thus, reaching a definitive IE diagnosis is almost impossible at medical facilities that do not have access to these modalities. It is also impractical to perform these examinations on all patients with fever of unknown cause, even in hospitals that provide high-level medical services, because of the burden on patients and medical staff, as well as the high medical cost. In such situations, antibiotics may be administered before obtaining blood culture samples [3,4], possibly making diagnosis more difficult and with a poorer prognosis [4,5].
Therefore, developing prediction models that are easier to use than the m-DC and that are usable before performing examinations with advanced medical modalities is desirable. Previous prediction models can only be used after detection of specific bacteria, such as
Material and Methods
ETHICAL CONSIDERATIONS:
The Ethics Committee of Saga University Hospital approved the study (file number: 2020-11-R-10), which was conducted in accordance with Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan and the Declaration of Helsinki. The requirement for written informed consent was waived by the Ethics Committee of Saga University Hospital due to the retrospective study design. All patients were informed about the purpose of the study via the website of the clinical research center of each university hospital and provided consent as per the comprehensive agreement method. Patient anonymity was protected. This study is registered in the University hospital Medical Information Network (UMIN) at
STUDY DESIGN AND PARTICIPANTS:
This study was a multi-center retrospective study for external validation study of our previous study published in 2021 [11]. The participants in the previous study were not included in the present study. Among patients aged ≥20 years admitted to 4 university hospitals in Japan owing to fever from January 2018 to December 2020, patients categorized as I-330 (IE) and R-50-9 (UF) according to the International Statistical Classification of Diseases and Related Health Problems-10 were enrolled. All patients without a body temperature of >37°C before admission were excluded. Among patients coded as I-330, those with a nosocomial onset or referred for valve surgery were excluded. Additionally, among patients coded as R-50-9, those with a diagnosis before admission or who had not been examined by chest X-ray, blood tests, or urinalysis on admission were excluded. More than 2 physicians at each hospital reviewed the diagnoses using the m-DC, dividing patients into the IE group and the group with fever caused by other diseases (UF group).
Among patients coded as I-330, those diagnosed with “definite IE” were allocated to the IE group. Patients showing accumulation to the prosthetic valve on 18F-fluorodeoxyglucose positron emission tomography CT (18F-FDG PET-CT), which was judged by the radiologist, were allocated to the IE group, even when the result of the m-DC was not definite, because 18F-FDG PET-CT is diagnostically more useful in such patients [13–15]. Patients coded as I-330 without a definite diagnosis of IE were excluded from the analysis because they could have been given the code due to lack of the required examinations with the m-DC, such as multiple blood cultures, rheumatoid factor, urinalysis, echocardiography, CT, MRI, and fundoscopy, which meant that the reviewed diagnosis was “not definite.”
Among patients coded as R-50-9, those not diagnosed with definite IE were allocated to the UF group. Patients diagnosed with definite IE were allocated to the IE group, except those with a definite diagnosis of non-infectious disease, who were excluded. The final diagnoses of patients in the UF group were determined by reviewing all medical records, consequently identifying the causes of hospitalization from their codes. If the causative disease was unknown, “fever of unknown origin” was the final diagnosis.
SETTING:
This study was performed at 4 university hospitals in Japan: Saga University Hospital, Dokkyo University Hospital, Juntendo University Hospital, and Toho University Hospital. Saga University Hospital is the only university hospital in Saga Prefecture, a rural city with 811 000 population in Japan, which has 602 inpatient beds. Dokkyo University Hospital is one of 3 university hospitals in Tochigi Prefecture, a suburban city with 1 933 000 population in Japan, which has 1195 inpatient beds. Juntendo University Hospital and Toho University Hospital are 2 of 15 university hospitals in Tokyo prefecture, urban area with 14 048 000 population in Japan, which have 1036 and 916 inpatient beds, respectively. These 4 university hospitals have 29, 31, 31, and 34 departments, respectively, including the departments of general medicine, cardiology, or cardiovascular surgery, and provide high-level medical services, mainly for patients in acute disease phases.
DATA SOURCES:
Two or 3 physicians from the department of general medicine at each hospital reviewed the medical records and referral letters from previous doctors to collect the data of potentially eligible patients. The survey items are shown in Supplementary Table 1. Prolonged antimicrobial use for chronic diseases was excluded from the definition of antibiotic administration before obtaining blood cultures. The presence of pleural effusion or pulmonary edema on chest X-ray or chest CT on admission was determined by a radiology specialist. If interpretation by a radiologist was not possible, it was determined by 2 general physicians (GPs). When 2 GPs agreed on the presence of pleural effusion or pulmonary edema, it was determined as “present.” When 2 GPs disagreed, a decision was reached by consensus.
STATISTICAL ANALYSIS:
Every examination required for the m-DC evaluation was not necessarily performed on all patients; rather, each examination was performed according to the clinical decision. In cases where some of the examinations were not performed, the results of these examinations required by the major criteria or minor criteria were judged as “no abnormalities.” Values for other missed survey items were excluded from the univariate and multivariate analyses. Categorical variables are expressed as percentages and were compared using the χ2 test. Continuous variables are expressed as median and interquartile range (IQR) and were compared using the Mann-Whitney U test. Statistical significance was set at p<0.05. The multivariate logistic regression analysis with the forced-entry method was performed on 5 predictive factors of the model developed in our previous study: ambulance transfer, presence of cardiac murmur, presence of pleural effusion, blood neutrophil percentage, and platelet count [11]. Subsequently, the odds ratio (OR) and 95% confidence interval (CI) were calculated for each factor. Additionally, the predictive probability of definite IE determined by the m-DC was calculated. The model formula developed in our previous study was used without modification in the present study. The predictive performance and calibration of the model were evaluated using the area under the curve (AUC) and shrinkage coefficient, respectively. The stratum-specific likelihood ratio was also calculated. Finally, we calculated the probability of definite IE, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each cut-off point corresponding to a sensitivity of 90%, Youden’s index, or a specificity of 90% of the prediction model in the present study and in our previous study [11]. A subgroup analysis was performed by comparing the AUC values of the prediction model between the IE group and the UF group divided by the presence of the survey items that were not used in the prediction model. SPSS (version 25; IBM Corp., Armonk, NY, USA) was used for statistical analysis.
We calculated a sample size of 20 patients based on an effect size of 0.30 (predicted AUC: 0.8; null hypothesis AUC: 0.50), an alpha error of 0.05, and a beta error of 0.20, which were estimated by the AUC of 0.893 reported previously [11]. However, we adopted 100 for each group commonly required in external validation [16].
Results
PATIENT ENROLLMENT AND ALLOCATION:
Patient enrollment and allocation to either the IE or UF group is shown in Figure 1. Ninety-seven of 264 patients coded as I-330, who were without a fever of ≥37°C (n=55), with a nosocomial onset (n=23), and with a referral for valve surgery (n=19) were excluded. Of the remaining 167 patients, 112 were diagnosed with definite IE and were allocated to the IE group. Of the 112 patients, 1 was diagnosed with definite IE because 18F-FDG PET-CT showed accumulation to the prosthetic valve, despite the result of “non-definite” according to the m-DC. Fifty-five patients coded as I-330 without a “definite” diagnosis were excluded. Similarly, 140 of 348 patients coded as R-50-9, who were without a fever of ≥37°C (n=40), in whom the cause of fever had been identified before admission (n=82), and without blood tests, urinalysis, or chest X-ray before admission (n=18), were excluded. Of the remaining 208 patients, 7 were diagnosed with definite IE and were allocated to the IE group. Thus, 201 patients were allocated to the UF group. A total of 320 patients (119 patients in the IE group and 201 patients in the UF group) were included. The number of patients included from each hospital is shown in Supplementary Table 2.
PATIENTS’ CHARACTERISTICS:
Patients’ characteristics are shown in Table 1. The ratio of males (67% vs 48%, p=0.001) and the rates of 30-day mortality (11% vs 3%, p=0.002), in-hospital mortality (18% vs 3%, p<0.001), IE history (9% vs 1%, p<0.001), prosthetic valve replacement (30% vs 7%, p<0.001), chronic dermatological disorder (13% vs 5%, p=0.007), dental problems within 6 months (50% vs 33%, p=0.021), cardiac murmur on admission (64% vs 12%, p<0.001), pulmonary edema on admission (13% vs 4%, p=0.001), and pleural effusion on admission (39% vs 22%, p=0.001) were significantly higher in the IE group than in UF group. Additionally, the duration of hospital stay was longer in the IE group (46 [IQR: 25-60] vs 15 [IQR: 9–25] days, p<0.001). Conversely, the percentage of patients administered antibiotics before obtaining blood culture samples (19% vs 32%, p=0.018) and the percentage of patients that used steroids before admission (3% vs 10%, p=0.031) were significantly higher in the UF group. Vital signs and laboratory findings on admission in both groups are shown in Table 2. The IE group had a significantly higher respiratory rate (20 [IQR: 16–24] vs 19 [16–21] beats/min, p=0.036), white blood cell (WBC) count (9.9×103 [IQR: 7.7–14.2] vs 8.4×103 [5.1–12.5]/μL, p=0.001), blood neutrophil percentage (86.8 [IQR: 81.7–91.7] vs 79.2 [IQR: 69.8–86.1]%, p<0.001), serum total bilirubin concentration (0.7 [IQR: 0.5–1.1] vs 0.7 [0.4–0.9] mg/dL, p=0.013), serum lactate dehydrogenase concentration (275.0 [IQR: 223.0–367.0] vs 238.5 [IQR: 180.0–299.5] IU/L, p<0.001), blood urea nitrogen concentration (21.0 [IQR: 13.0–35.1] vs 15.0 [IQR: 11.0–23.2] mg/dL, p<0.001), and serum creatinine concentration (0.9 [IQR: 0.7–1.5] vs 0.8 [IQR: 0.6–1.1], p<0.002). Systolic blood pressure (116 [IQR: 105–127] vs 121 [108–137] mmHg, p=0.016), platelet count (15.1×104 [IQR: 11.2–21.7] vs 20.5×104 [14.7–31.5]/μL, p<0.001), and serum albumin concentration (2.9 [IQR: 2.5–3.4] vs 3.1 [2.6–3.6] g/dL, p=0.021) were significantly lower in the IE group. The differences between the 2 groups in the results of the m-DC, causative organisms, and the execution rate of each examination required by the m-DC in each group are shown in Supplementary Tables 3–5. A breakdown of the final diagnoses in the UF group is shown in Supplementary Table 6.
PERFORMANCE OF THE PREDICTIVE MODEL FOR IE:
The results of the multivariate logistic regression analysis of predictive factors are shown in Table 3. The presence of cardiac murmur (OR 13.13, 95% CI 6.69–27.36, p<0.001), blood neutrophil percentage (OR 1.09, 95% CI 1.06–1.14, p<0.001), and platelet count (OR 0.96, 95% CI 0.93–0.99, p=0.003) were significant factors. No significant differences were observed in ambulance transfer (OR 1.81, 95% CI 0.91–3.55, p=0.089) or presence of pleural effusion (OR 2.34, 95% CI 0.62–2.42, p=0.565). This study demonstrated the excellent discriminative ability of the model with an AUC of 0.783 (95% CI 0.732–0.834; Figure 2). The model was well calibrated, with a shrinkage coefficient of 0.963 (Figure 3). The stratum-specific likelihood ratio was 0.1–3.4, which increased as the score increased (Table 4). The cut-off values with a sensitivity of 90%, Youden’s index, and a specificity of 90% were −1.75, −0.72, and 3.15, respectively. The PPV and NPV derived from each cut-off value were 52% and 90%, 59% and 88%, and 66% and 69%, respectively (Table 5). The PPV and NPV calculated from the cut-off values of −1.68, −1.1, and −0.38 derived in our previous study were 53% and 90%, 55% and 88%, and 60% and 87%, respectively (Table 5).
SUBGROUP ANALYSIS:
To identify the factors that could improve the performance of the IE prediction model, subgroup analyses were performed on the survey items with more than 10 cases, including the types of cardiac valve (native or prosthetic), use of steroids or immunosuppressant agents, and presence of glomerulonephritis as a complication, in both the IE group and the UF group. The AUC of the prediction model was >0.8 in patients with native cardiac valves (AUC: 0.820, 95%CI 0.768–0.872) and without glomerulonephritis (AUC: 0.822, 95%CI 0.760–0.884) (Table 6).
Discussion
In hospitals without advanced medical modalities, antibiotics may be administered to patients with fever without typical symptoms or findings of IE before obtaining blood culture samples, which makes it difficult to make a precise diagnosis [4,5]. Our previous study in Japan from 2007 to 2017 revealed that antibiotics were administered to 43.2% of patients before obtaining blood culture samples [17]. In addition, a previous study reported that early diagnosis and provision of treatment opportunities is essential for patients with IE in the current era [18]. Therefore, development of the IE prediction model, which is easier to use than the m-DC and can be used at an early stage prior to examinations with advanced medical modalities, is required. Existing models can be used only after a specific bacterial strain is detected on blood culture [6–10], which led us to develop the prediction model for definite IE diagnosed with the m-DC among patients with UF [11]. The present study was performed to validate the applicability of our model in the multi-center setting, revealing a high AUC and good calibration. The results suggest that our model has broad utility in many hospitals. Additionally, the presence of cardiac murmur, blood neutrophil percentage, and platelet count were particularly important, because they were significant factors for the IE prediction in the present study as well as in our previous study [11]. Therefore, the use of our model in patients with UF could make it possible to predict IE immediately after admission, which would prevent antimicrobial administration before obtaining blood cultures and help physicians to select patients to undergo investigation with advanced medical modalities.
Our prediction model demonstrated a high AUC, although it was slightly lower than in our previous study [11]. Ambulance transfer was not an independent factor in the present study because the percentage of patients transferred by ambulance in the IE group was lower (39% vs 51%) and that in the UF group was higher (26% vs 1%) than in the previous study [11]. In addition to realizing the seriousness of their symptoms, various socioemotional factors, including strong anxiety, difficulty in accessing hospitals solely by the will of the patients themselves without an ambulance, or their desire to avoid causing inconvenience to family members or caregivers, could also be reasons for patients choosing to call for an ambulance [12]. Hospital accessibility could also depend on the area of residence (eg, urban vs rural) [12]. This study was a multi-center retrospective study involving hospitals at various cities in Japan: a large metropolitan city, Tokyo; a medium-scale city, Tochigi; and a typical rural city, Saga. However, our previous study was conducted only in a rural city [11]. Therefore, hospital accessibility may have differed between the present study and our previous study. Additionally, despite an equivalent in-hospital mortality rate between the IE group in the present study and our previous study (18% vs 17%), pulmonary edema on admission (13% vs 29%), pleural effusion on admission (39% vs 64%), pulse rate (90 bpm vs 102 bpm), respiratory rate (20 breaths/min vs 23 breaths/min), serum WBC count (9.9×103/μL vs 13.5×103/μL), blood neutrophil percentage (86.8% vs 89.6%), and serum C-reactive protein (CRP) concentration (8.3 mg/dL vs 10.4 mg/mL) in the IE group were lower than in the previous study [11]. Accordingly, patients in the IE group in the present study might have had a less severe condition on admission, possibly resulting in fewer ambulance transfers. However, the serum WBC count (8.4×103/μL vs 10.5×103/μL) and CRP concentration on admission (8.6 mg/dL vs 11.7 mg/mL) in the UF group in the present study were lower than in our previous study [11]. Additionally, pulmonary edema on admission (4% vs 1%), pleural effusion (22% vs 29%), pulse rate (91 bpm vs 90 bpm), respiratory rate (19 breaths/min vs 18 breaths/min), and in-hospital mortality (3% vs 6%) were the same in the UF group in both studies. Accordingly, the severity in the UF group in the present study might not have been higher; rather, it may have been equal or lower. Nevertheless, the percentage of ambulance transfers in the UF group was higher than in our previous study, which might have been influenced by various socioemotional factors other than disease severity.
The fact that pleural effusion was not an independent factor in the present study. unlike in our previous study. could be another reason for the lower AUC in this study. Pleural effusion increases with the elevation in hydrostatic pressure due to heart failure, liver cirrhosis, or renal failure; the decrease in oncotic pressure due to hypoalbuminemia; and increased vascular permeability [19,20]. The percentage of patients with pleural effusion in the present study was lower in the IE group (39% vs 64%) and the same in the UF group (22% vs 29%) compared with our previous study [11]. Fewer patients might have been affected by increased vascular permeability or decreased oncotic pressure in the IE group, resulting in a lower percentage of patients with pleural effusion, which was suggested by the fact that the IE group had a higher serum albumin concentration (2.9 vs 2.5 g/dL) and a lower serum WBC count and CRP concentration than the IE group in the previous study [11]. Consequently, pleural effusion was not an independent factor for our prediction model in the present study.
The difference in the severity of the condition on admission between the present study and our previous study could have caused the difference in the AUC. Our prediction model used data obtained on admission. However, the condition of some patients with few symptoms on admission can worsen with time [21]. A lower percentage of patients transferred by ambulance and the presence of pleural effusion might indicate a less severe condition in patients in the IE group in the present study, which could have influenced the performance of our model.
Finally, the subgroup analysis showed a higher AUC with the IE prediction model in patients with native valve than in the overall patients in this study. The IE prediction model, which was developed in our previous study and externally validated in this study, can estimate the possibility of definite IE according to the m-DC. However, the sensitivity and NPV of the m-DC for diagnosis patients with IE with prosthetic valves were low at 50–60% [14]. This may be the cause of the low AUC of the IE prediction model when used in patients with prosthetic valves. Therefore, selectively using this prediction model in patients with native cardiac valves would help to improve the discriminatory ability of the model.
This study has some limitations that should be noted. Although this was a multi-center retrospective study conducted at 4 university hospitals in Japan, the relatively small number of participating hospitals may not be representative of hospitals all over Japan, especially in terms of the severity of the condition on admission and the percentage of patients transferred by ambulance. Furthermore, Japan is the most aged society in the world. Accordingly, the age distribution, types of causative disease, disease severity, percentage of patients transferred by ambulance, and healthcare insurance system in Japan differ from other countries [22,23], which could have introduced selection bias. Moreover, a previous study reported that there are geographic differences in the characteristics of patients with IE between countries [18]. Therefore, multi-center studies involving many different types of hospitals worldwide are ideal for further model generalization.
Conclusions
In this multi-center, retrospective, external validation study performed at university hospitals in Japan, our IE prediction model among patients aged ≥20 years with UF was well-validated. The model makes it possible to predict IE among patients with UF at an earlier stage after admission, preventing antibiotic administration before obtaining blood culture samples or helping physicians to determine the indications for advanced medical modalities.
Figures
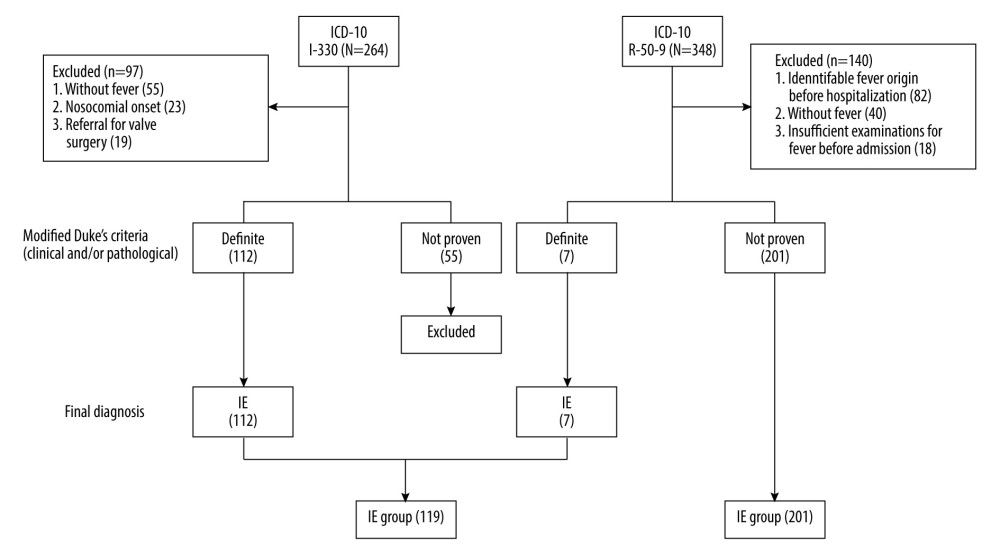 Figure 1. Enrollment and allocation flow diagramNinety-seven of 264 patients were categorized as I-330 corresponding to infective endocarditis (IE) according to the International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10), who were without a fever of ≥37°C (n=55), with a nosocomial onset (n=23) or being referred for valve surgery (n=19), were excluded. Of the remaining 167 patients, 112 were diagnosed with definite IE according to the modified Duke criteria (m-DC) and were allocated to the IE group. One of those 112 patients was diagnosed with definite IE despite the result of “non-definite” according to the m-DC, because 18F-fluorodeoxyglucose positron emission tomography with computed tomography showed accumulation to the prosthetic valve. Similarly, 140 of 348 patients categorized as R-50–9 corresponding to undiagnosed fever (UF) with ICD-10, who were without a fever of ≥37°C (n=40), in whom the cause of fever was identified before admission (n=82), or who did not undergo blood tests, urinalysis, or chest X-ray before admission (n=18), were excluded. Of the remaining 208 patients, 7 were allocated to the IE group with definite IE according to the m-DC, resulting in 201 patients allocated to the UF group. Finally, a total of 320 patients were included (119 patients in the IE group and 201 patients in the UF group). This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002).
Figure 1. Enrollment and allocation flow diagramNinety-seven of 264 patients were categorized as I-330 corresponding to infective endocarditis (IE) according to the International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10), who were without a fever of ≥37°C (n=55), with a nosocomial onset (n=23) or being referred for valve surgery (n=19), were excluded. Of the remaining 167 patients, 112 were diagnosed with definite IE according to the modified Duke criteria (m-DC) and were allocated to the IE group. One of those 112 patients was diagnosed with definite IE despite the result of “non-definite” according to the m-DC, because 18F-fluorodeoxyglucose positron emission tomography with computed tomography showed accumulation to the prosthetic valve. Similarly, 140 of 348 patients categorized as R-50–9 corresponding to undiagnosed fever (UF) with ICD-10, who were without a fever of ≥37°C (n=40), in whom the cause of fever was identified before admission (n=82), or who did not undergo blood tests, urinalysis, or chest X-ray before admission (n=18), were excluded. Of the remaining 208 patients, 7 were allocated to the IE group with definite IE according to the m-DC, resulting in 201 patients allocated to the UF group. Finally, a total of 320 patients were included (119 patients in the IE group and 201 patients in the UF group). This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002). ![Receiver operating characteristic curve and the area under the curveThe model performance was evaluated with the area under the curve (AUC), which was 0.783 (95% confidence interval [CI] 0.732–0.834), showing good discriminative ability. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002) and SPSS (version 25; IBM Corp., Armonk, NY, USA).](https://jours.isi-science.com/imageXml.php?i=medscimonit-29-e939640-g002.jpg&idArt=939640&w=1000) Figure 2. Receiver operating characteristic curve and the area under the curveThe model performance was evaluated with the area under the curve (AUC), which was 0.783 (95% confidence interval [CI] 0.732–0.834), showing good discriminative ability. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002) and SPSS (version 25; IBM Corp., Armonk, NY, USA).
Figure 2. Receiver operating characteristic curve and the area under the curveThe model performance was evaluated with the area under the curve (AUC), which was 0.783 (95% confidence interval [CI] 0.732–0.834), showing good discriminative ability. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002) and SPSS (version 25; IBM Corp., Armonk, NY, USA). 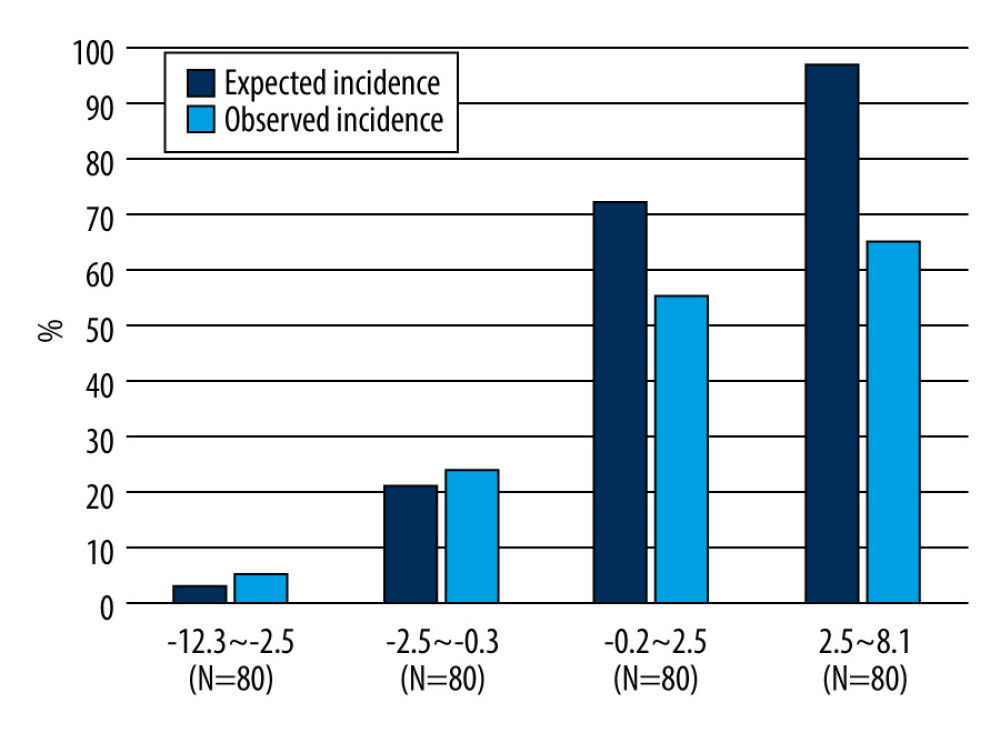 Figure 3. Predicted and observed rates of infective endocarditis (IE) in quadrisect groups according to the scores of the external validation cohort in the present studyBoth the predicted probability of IE calculated with our prediction model and the actual observed ratio of IE increased as the score of our model increased. The higher the score, the higher the predicted probability became compared with the actual observed rate. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002).
Figure 3. Predicted and observed rates of infective endocarditis (IE) in quadrisect groups according to the scores of the external validation cohort in the present studyBoth the predicted probability of IE calculated with our prediction model and the actual observed ratio of IE increased as the score of our model increased. The higher the score, the higher the predicted probability became compared with the actual observed rate. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002). Tables
Table 1. Univariate analysis of patient characteristics.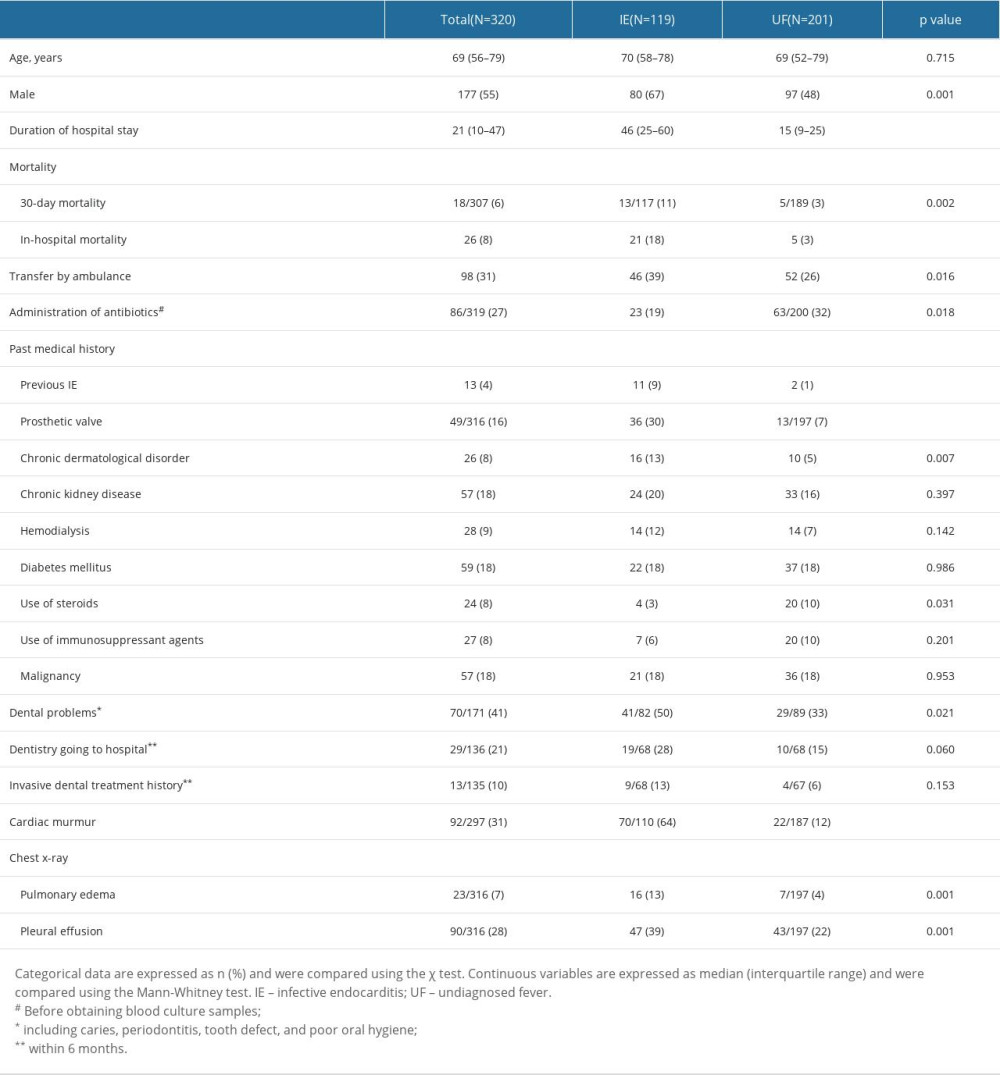 Table 2. Univariate analysis of vital signs and laboratory findings on admission.
Table 2. Univariate analysis of vital signs and laboratory findings on admission.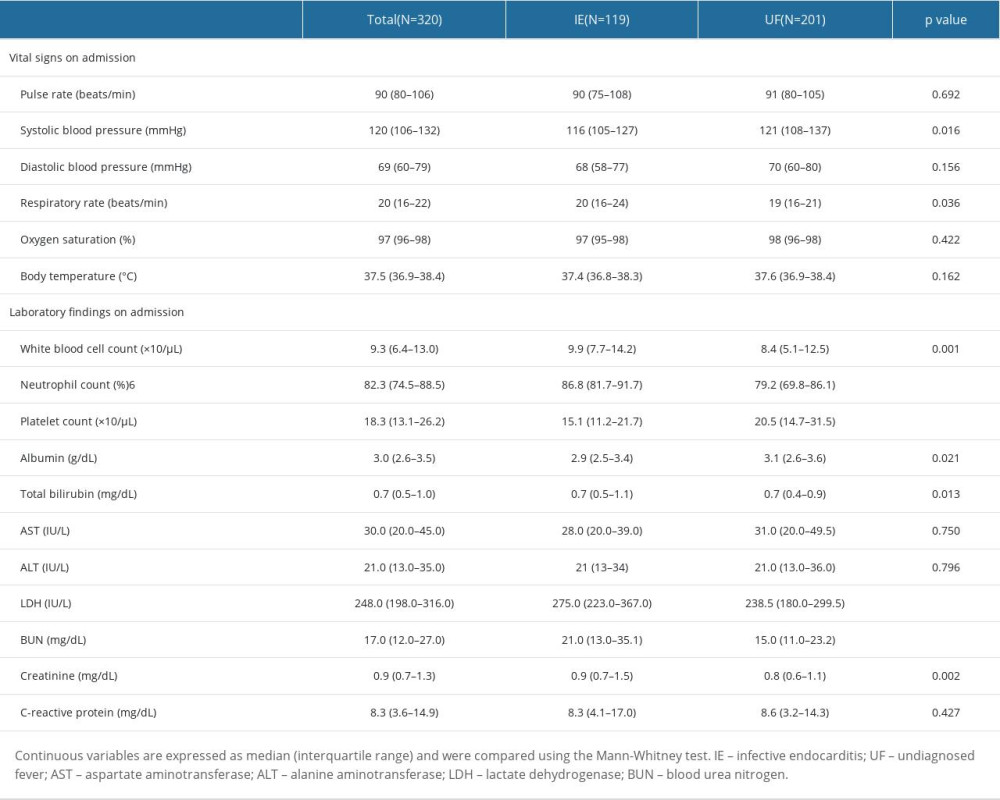 Table 3. External validation of the prediction model by multivariate logistic regression.
Table 3. External validation of the prediction model by multivariate logistic regression.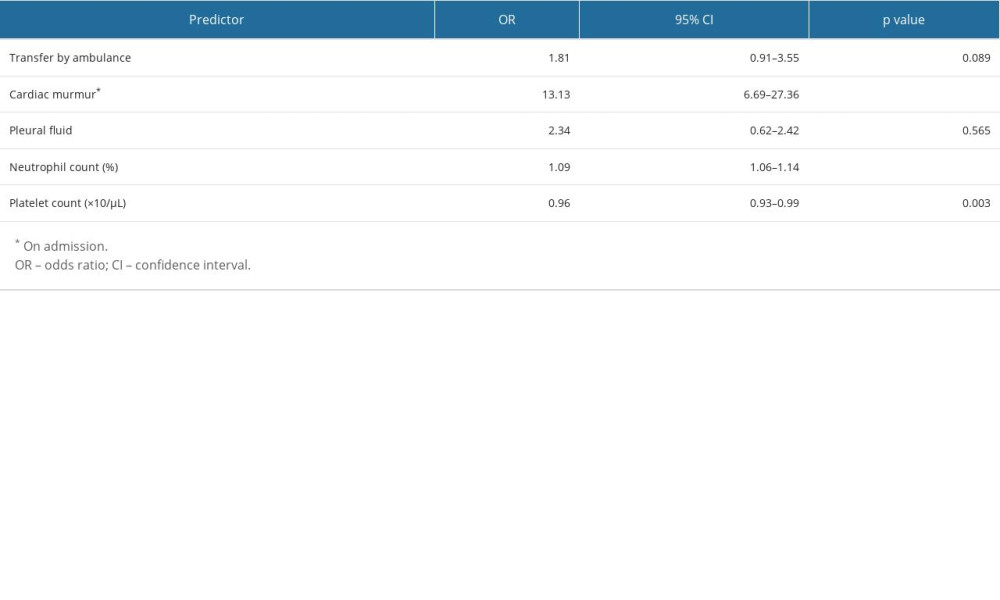 Table 4. Stratified likelihood ratio of the prediction model in the present cohort.
Table 4. Stratified likelihood ratio of the prediction model in the present cohort.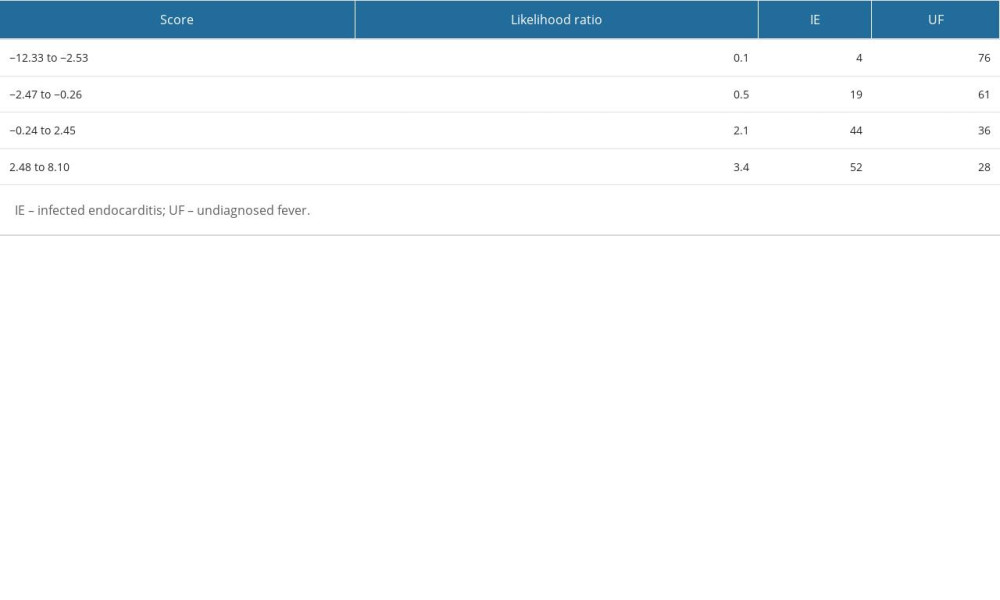 Table 5. Validation of the prediction model with the cut-off points determined in the present study and the original study.
Table 5. Validation of the prediction model with the cut-off points determined in the present study and the original study.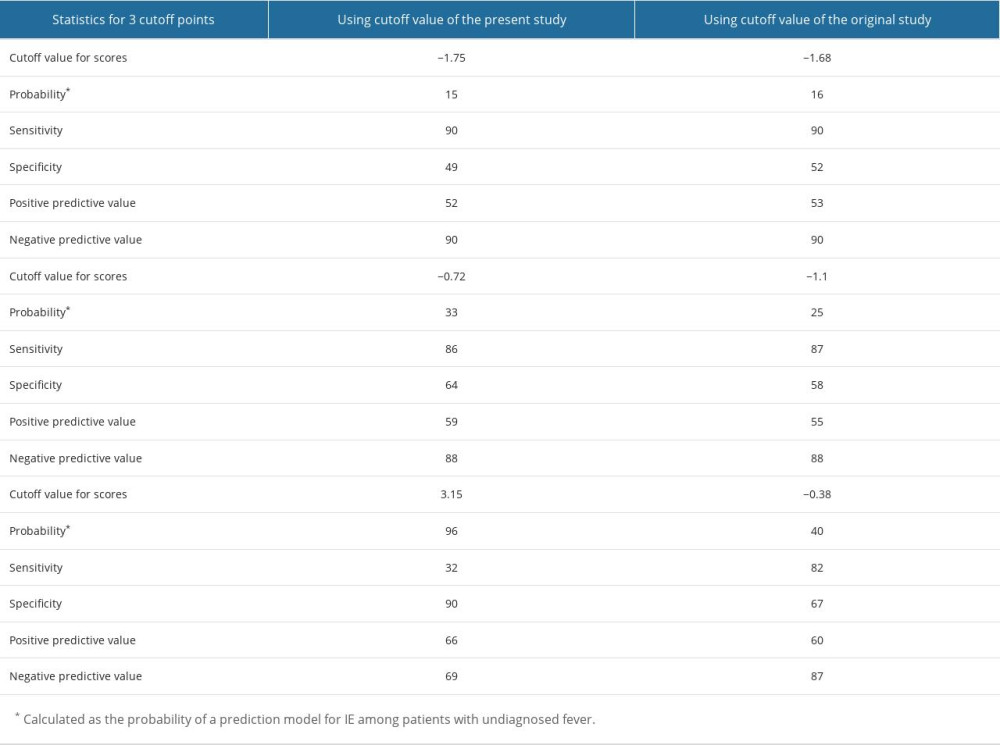 Table 6. Subgroup analysis to improve the performance of the prediction model.
Table 6. Subgroup analysis to improve the performance of the prediction model.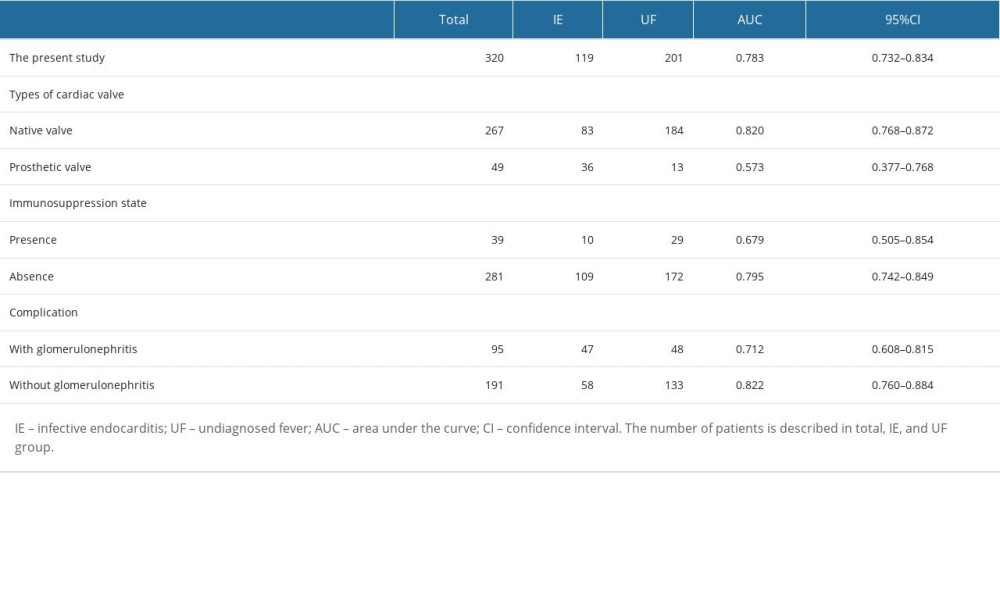 Supplementary Table 1. Survey items.
Supplementary Table 1. Survey items. Supplementary Table 2. The number of patients in each university hospital.
Supplementary Table 2. The number of patients in each university hospital. Supplementary Table 3. Modified Duke criteria in the infective endocarditis group and the undiagnosed fever group.
Supplementary Table 3. Modified Duke criteria in the infective endocarditis group and the undiagnosed fever group.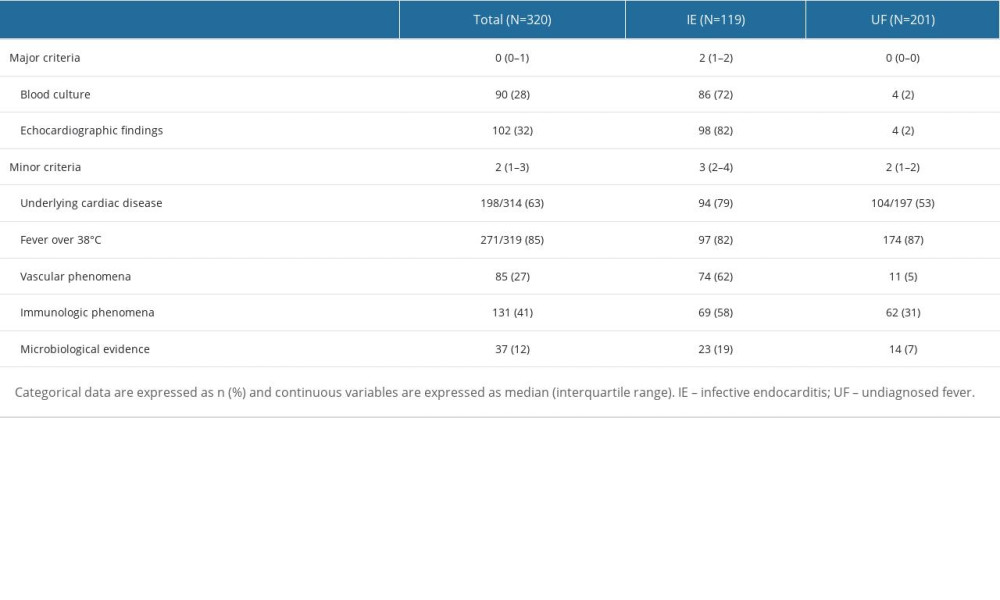 Supplementary Table 4. Causative bacteria in the infective endocarditis group and the undiagnosed fever group.
Supplementary Table 4. Causative bacteria in the infective endocarditis group and the undiagnosed fever group.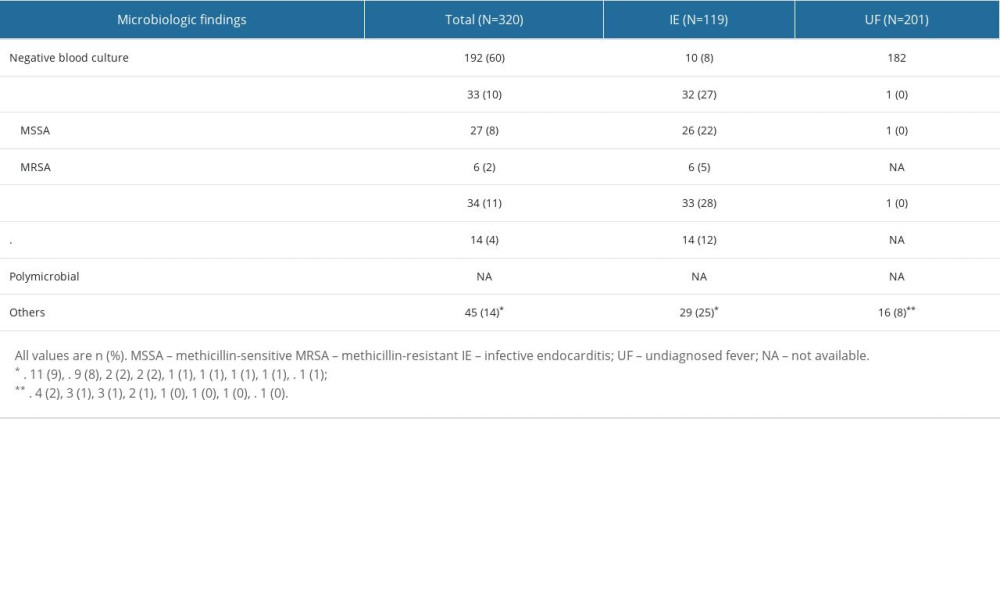 Supplementary Table 5. Execution rate of each examination in the infective endocarditis group and the undiagnosed fever group.
Supplementary Table 5. Execution rate of each examination in the infective endocarditis group and the undiagnosed fever group. Supplementary Table 6. Breakdown of final diagnoses in undiagnosed fever group.
Supplementary Table 6. Breakdown of final diagnoses in undiagnosed fever group.
References
1. Cahill TJ, Prendergast BD, Infective endocarditis: Lancet, 2016; 387(10021); 882-93
2. Li JS, Sexton DJ, Nettles R, Proposed modifications to the duke criteria for the diagnosis of infective endocarditis: Clin infect Dis, 2000; 30(4); 633-38
3. Jingushi N, Iwata M, Terasawa T, Clinical features of patients with infective endocarditis presenting to the emergency department: A retrospective case series: Nagoya J Med Sci, 2017; 79(4); 467-76
4. Fukuchi T, Iwata K, Ohji G, Failure of early diagnosis of infective endocarditis in Japan – a retrospective descriptive analysis: Medicine (Baltimore), 2014; 93(27); e237
5. Nishiguchi S, Nishino K, Kitagawa I, Tokuda Y, Factors associated with delayed diagnosis of infective endocarditis. A retrospective cohort study in a teaching hospital in Japan: Medicine (Baltimore), 2020; 99(30); e21418
6. Bai AD, Agarwal A, Steinberg M: Clin Microbiol Infect, 2017; 23(12); 900-6
7. Palraj BR, Baddour LM, Hess EP: Clin Infect Dis, 2015; 61(1); 18-28
8. Gow N, Lowe BS, Freeman J, Roberts S: N Z Med J, 2015; 128(1416); 28-35
9. Sunnerhagen T, Törnell A, Vikbrant M, HANDOC: A handy score to determine the need for echocardiography in non-β-hemolytic streptococcal bacteremia: Clin Infect Dis, 2018; 66(5); 693-98
10. Bouza E, Kestler M, Beca T, The NOVA score: A proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia [published erratum appears in Clin Infect Dis. 2015;61(2):297]: Clin Infect Dis, 2015; 60(4); 528-35
11. Yamashita S, Tago M, Motomura S, Development of a clinical prediction model for infective endocarditis among patients with undiagnosed fever: A pilot case-control study: Int J Gen Med, 2021; 14; 4443-51
12. Booker MJ, Purdy S, Shaw ARG, Seeking ambulance treatment for ‘primary care’ problems: A qualitative systematic review of patient, carer and professional perspectives: BMJ Open, 2017; 7(8); e016832
13. Vincent LL, Otto CM, Infective endocarditis: Update on epidemiology, outcomes, and management: Curr Cardiol Rep, 2018; 20(10); 86
14. Pizzi MN, Roque A, Fernández-Hidalgo N: Circulation, 2015; 132(12); 1113-26
15. Habib G, Lancellotti P, Antunes MJ, 2015 ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM): Eur Heart J, 2015; 36(44); 3075-128
16. Vergouwe Y, Steyerberg EW, Eijkemans MJC, Habbema JDF, Substantial effective sample sizes were required for external validation studies of predictive logistic regression models: J Clin Epidemiol, 2005; 58(5); 475-83
17. Yamashita S, Tokushima M, Nakashima T, Clinical status quo of infective endocarditis in a university hospital in Japan: A single-hospital-based retrospective cohort study: Intern Med, 2020; 59(12); 1497-507
18. Murdoch DR, Corey GR, Hoen B: Arch Intern Med, 2009; 169(5); 463-73
19. Jany B, Welte T, Pleural effusion in adults – etiology, diagnosis, and treatment: Dtsch Arztebl Int, 2019; 116(21); 377-86
20. Beaudoin S, Gonzalez AV, Evaluation of the patient with pleural effusion: CMAJ, 2018; 190(10); E291-95
21. Wu HP, Yang WC, Wu KH, Diagnosing appendicitis at different time points in children with right lower quadrant pain: Comparison between Pediatric Appendicitis Score and the Alvarado score: World J Surg, 2012; 36(1); 216-21
22. Arai H, Ouchi Y, Toba K, Japan as the front-runner of super-aged societies: Perspectives from medicine and medical care in Japan: Geriatr Gerontol Int, 2015; 15(6); 673-87
23. Shibuya K, Hashimoto H, Ikegami N, Future of Japan’s system of good health at low cost with equity: Beyond universal coverage: Lancet, 2011; 378(9798); 1265-73
Figures
 Figure 1. Enrollment and allocation flow diagramNinety-seven of 264 patients were categorized as I-330 corresponding to infective endocarditis (IE) according to the International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10), who were without a fever of ≥37°C (n=55), with a nosocomial onset (n=23) or being referred for valve surgery (n=19), were excluded. Of the remaining 167 patients, 112 were diagnosed with definite IE according to the modified Duke criteria (m-DC) and were allocated to the IE group. One of those 112 patients was diagnosed with definite IE despite the result of “non-definite” according to the m-DC, because 18F-fluorodeoxyglucose positron emission tomography with computed tomography showed accumulation to the prosthetic valve. Similarly, 140 of 348 patients categorized as R-50–9 corresponding to undiagnosed fever (UF) with ICD-10, who were without a fever of ≥37°C (n=40), in whom the cause of fever was identified before admission (n=82), or who did not undergo blood tests, urinalysis, or chest X-ray before admission (n=18), were excluded. Of the remaining 208 patients, 7 were allocated to the IE group with definite IE according to the m-DC, resulting in 201 patients allocated to the UF group. Finally, a total of 320 patients were included (119 patients in the IE group and 201 patients in the UF group). This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002).
Figure 1. Enrollment and allocation flow diagramNinety-seven of 264 patients were categorized as I-330 corresponding to infective endocarditis (IE) according to the International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10), who were without a fever of ≥37°C (n=55), with a nosocomial onset (n=23) or being referred for valve surgery (n=19), were excluded. Of the remaining 167 patients, 112 were diagnosed with definite IE according to the modified Duke criteria (m-DC) and were allocated to the IE group. One of those 112 patients was diagnosed with definite IE despite the result of “non-definite” according to the m-DC, because 18F-fluorodeoxyglucose positron emission tomography with computed tomography showed accumulation to the prosthetic valve. Similarly, 140 of 348 patients categorized as R-50–9 corresponding to undiagnosed fever (UF) with ICD-10, who were without a fever of ≥37°C (n=40), in whom the cause of fever was identified before admission (n=82), or who did not undergo blood tests, urinalysis, or chest X-ray before admission (n=18), were excluded. Of the remaining 208 patients, 7 were allocated to the IE group with definite IE according to the m-DC, resulting in 201 patients allocated to the UF group. Finally, a total of 320 patients were included (119 patients in the IE group and 201 patients in the UF group). This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002).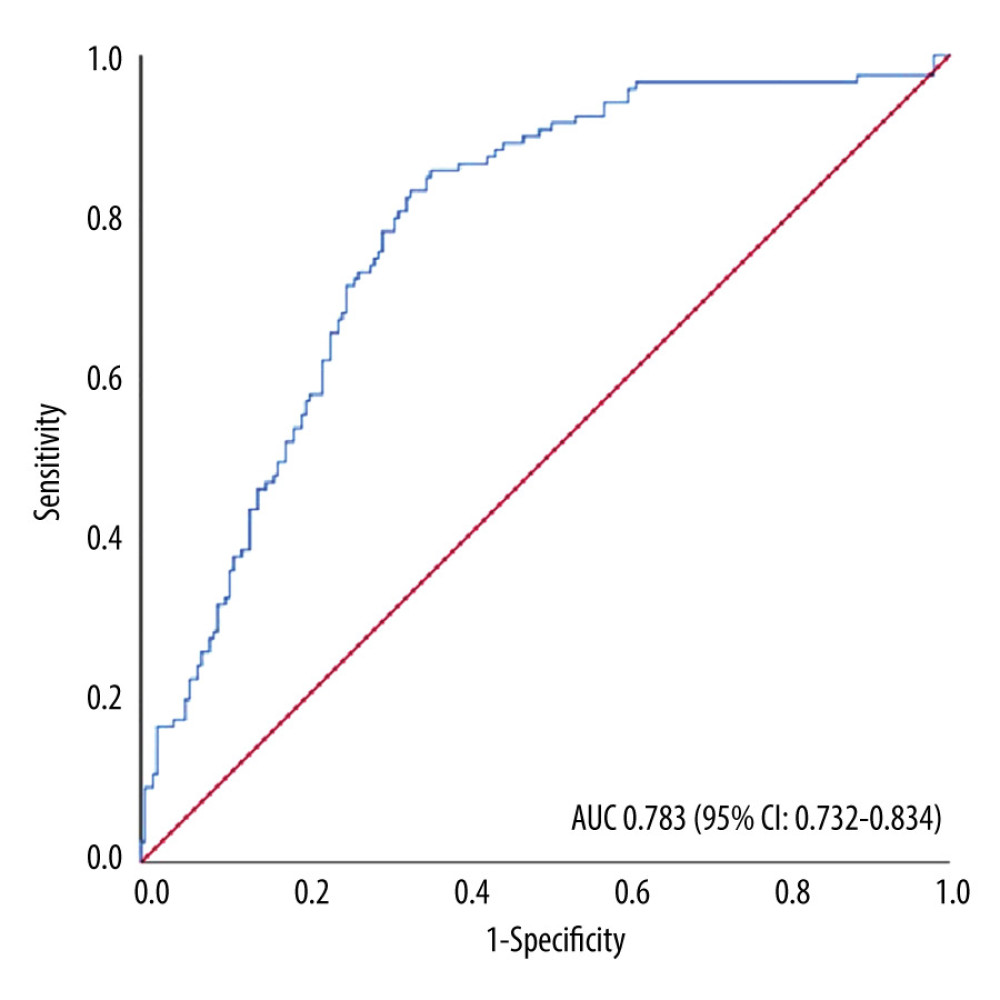 Figure 2. Receiver operating characteristic curve and the area under the curveThe model performance was evaluated with the area under the curve (AUC), which was 0.783 (95% confidence interval [CI] 0.732–0.834), showing good discriminative ability. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002) and SPSS (version 25; IBM Corp., Armonk, NY, USA).
Figure 2. Receiver operating characteristic curve and the area under the curveThe model performance was evaluated with the area under the curve (AUC), which was 0.783 (95% confidence interval [CI] 0.732–0.834), showing good discriminative ability. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002) and SPSS (version 25; IBM Corp., Armonk, NY, USA). Figure 3. Predicted and observed rates of infective endocarditis (IE) in quadrisect groups according to the scores of the external validation cohort in the present studyBoth the predicted probability of IE calculated with our prediction model and the actual observed ratio of IE increased as the score of our model increased. The higher the score, the higher the predicted probability became compared with the actual observed rate. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002).
Figure 3. Predicted and observed rates of infective endocarditis (IE) in quadrisect groups according to the scores of the external validation cohort in the present studyBoth the predicted probability of IE calculated with our prediction model and the actual observed ratio of IE increased as the score of our model increased. The higher the score, the higher the predicted probability became compared with the actual observed rate. This figure was created using Microsoft® PowerPoint® for Microsoft 365 MSO (version 2301 build 16.0.16026.20002). Tables
 Table 1. Univariate analysis of patient characteristics.
Table 1. Univariate analysis of patient characteristics. Table 2. Univariate analysis of vital signs and laboratory findings on admission.
Table 2. Univariate analysis of vital signs and laboratory findings on admission. Table 3. External validation of the prediction model by multivariate logistic regression.
Table 3. External validation of the prediction model by multivariate logistic regression. Table 4. Stratified likelihood ratio of the prediction model in the present cohort.
Table 4. Stratified likelihood ratio of the prediction model in the present cohort. Table 5. Validation of the prediction model with the cut-off points determined in the present study and the original study.
Table 5. Validation of the prediction model with the cut-off points determined in the present study and the original study. Table 6. Subgroup analysis to improve the performance of the prediction model.
Table 6. Subgroup analysis to improve the performance of the prediction model. Table 1. Univariate analysis of patient characteristics.
Table 1. Univariate analysis of patient characteristics. Table 2. Univariate analysis of vital signs and laboratory findings on admission.
Table 2. Univariate analysis of vital signs and laboratory findings on admission. Table 3. External validation of the prediction model by multivariate logistic regression.
Table 3. External validation of the prediction model by multivariate logistic regression. Table 4. Stratified likelihood ratio of the prediction model in the present cohort.
Table 4. Stratified likelihood ratio of the prediction model in the present cohort. Table 5. Validation of the prediction model with the cut-off points determined in the present study and the original study.
Table 5. Validation of the prediction model with the cut-off points determined in the present study and the original study. Table 6. Subgroup analysis to improve the performance of the prediction model.
Table 6. Subgroup analysis to improve the performance of the prediction model. Supplementary Table 1. Survey items.
Supplementary Table 1. Survey items. Supplementary Table 2. The number of patients in each university hospital.
Supplementary Table 2. The number of patients in each university hospital. Supplementary Table 3. Modified Duke criteria in the infective endocarditis group and the undiagnosed fever group.
Supplementary Table 3. Modified Duke criteria in the infective endocarditis group and the undiagnosed fever group. Supplementary Table 4. Causative bacteria in the infective endocarditis group and the undiagnosed fever group.
Supplementary Table 4. Causative bacteria in the infective endocarditis group and the undiagnosed fever group. Supplementary Table 5. Execution rate of each examination in the infective endocarditis group and the undiagnosed fever group.
Supplementary Table 5. Execution rate of each examination in the infective endocarditis group and the undiagnosed fever group. Supplementary Table 6. Breakdown of final diagnoses in undiagnosed fever group.
Supplementary Table 6. Breakdown of final diagnoses in undiagnosed fever group. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








