10 June 2023: Database Analysis
Deciphering the Influence of Estradiol and Estrogen Receptors on Cognitive Function: A Bibliometric Analysis and Emerging Research Trends
Min Wu1BDEF, Shujie Lou1A*DOI: 10.12659/MSM.939676
Med Sci Monit 2023; 29:e939676
Abstract
BACKGROUND: The cognitive impact of estradiol (E2), a sex steroid hormone, particularly its unique characteristics mediated through different estrogen receptors (ERs), is garnering research interest to optimize estrogen replacement therapy (ERT) and mitigate adverse effects. However, a systematic bibliometric investigation elucidating the connection between E2/ERs and cognition is lacking. This study examines 3502 Web of Science Core Collection publications using CiteSpace to unveil trends in this research field.
MATERIAL AND METHODS: The primary goal was to analyze highly co-cited articles characterized by extensive citation, centrality, Sigma index, and burst strength. We identified six research themes and directions from ten distinct, highly credible clusters (Q=0.8266; S=0.978), established by frequently employed keywords. Secondly, we sought to highlight the most contributing countries, institutions, and authors in this domain.
RESULTS: The study unveiled that the ‘critical age window period’ hypothesis of ERT, hippocampus-derived E2, the mediating role of GPER, and crosstalk among ERs are the current hotspots in this field. Future research is likely to explore the links between E2/ERs and the hippocampus, various memory types, sex specificity, and receptor specificity. The United States and the University of Wisconsin have the most publications, while Scotland and Stanford University have the highest centrality. The most influential authors are Woolley CS, Frick KM, Tuscher JJ, and Espeland MA.
CONCLUSIONS: These findings inform prospective research directions and hint at potential E2 targets for cognitive enhancement.
Keywords: GPER1 Protein, Mouse, Cognition, Bibliometrics, Data Visualization, 17 beta-(arylazido-beta-alanine)estradiol-3-methyl ether, Female, Humans, Estradiol, Receptors, Estrogen, Prospective Studies
Background
Estrogens are steroid hormones mainly produced by the ovaries and adrenal glands; in addition, the brain can also produce endogenous estrogens from cholesterol [1,2]. Estrogens are known to promote female sexual characteristics and reproductive capacity, and they can cross the blood-brain barrier, affecting the brain [2–4]. Estrogens have 3 forms: estrone (E1), estradiol (E2), and estriol (E3). Because E2 is abundant and potent in the body, the term estrogens mainly refers to E2 [2,5,6]. A marked decrease in estrogens levels occurs during a 6-month period around menopause, most pronounced in E2, and during the next 3 years, the levels of E2 and E1 show an essentially parallel, moderate decline [7], but the binding affinity of E1 with intracellular estrogen receptors (ERs) is lower than that of E2 [8]. Subcutaneous injection of E2 significantly increased BrdU-labeled newborn neurons in the hippocampus dentate gyrus (DG) and activation of new neurons in response to spatial memory retrieval, but the same dose of E1 decreased neurogenesis in the DG [5]. Therefore, different estrogens may have different effects on neurogenesis and cognition in the hippocampus.
ERs are found throughout the brain and are predominantly present in the hippocampus [9], prefrontal cortex (PFC) [9], cerebellum [10], hypothalamus [11], ventral tegmental area [12], and amygdala [12], as well as in the raphe nuclei of the midbrain [12]. They exist in neurons and glial cells [13], and the hippocampus is especially rich in ERs [9,13,14]. ERs mainly include nuclear ERs (nERs) and membrane-embedded ER (mER), and nERs have 2 subtypes: ERα and ERβ [9]. In addition, orphan G protein-coupled receptor (GPCR), which binds E2 with high affinity and initiates rapid signal transduction from the plasma membrane, was first found in SKBR3 breast cancer cells [15]. This receptor was discovered and cloned in 1997 and was called GPR30, and in 2007 it was officially named G protein-coupled estrogen receptor (GPER) by the International Union of Basic and Clinical Pharmacology [16].
Articles [17,18] reported the localization of nERs on the plasma membrane by E2 treatment. The post-translational lipid modification of nERs through palmitoylation is helpful for the binding of nERs to caveolin (CAV), thereby transporting nERs to the cell membrane and activating several cell-signaling cascades [17,19–21]. nERs are also localized to mitochondria depending on the cell type [22,23]. Mitochondrial estrogen receptors (mtERs) have been found in the hippocampus, cortex, and hypothalamic of rats, which could mediate the effect of E2 on mitochondria. The density analysis of mtERs in young adult and aged rats showed that aging did not cause a decrease in the number of mtERs in different brain regions, but mtERs were differentially expressed in different brain regions, indicating that they may be involved in different functions in the brain during aging [24]. In primary rat neurons and HT22 mouse hippocampal cell line, ERβ has been shown to co-localize with mitochondria [25] and regulate transcription and protein activity in this region [26]. The mtERs can bind to the estrogen response element (ERE) located in the mitochondrial DNA, thereby increasing the expression of mitochondrial-encoded genes and the activity of electron transport chains (ETC). In addition, co-immunoprecipitation results showed that ERβ could interact with mitochondrial respiratory complex V [27,28].
Studies have shown that E2 has important effects on higher-order brain functions, including locomotor activity [12], mood [12,29], affect [30], anxiety [31,32], fear [30], and cognition [29,33–35]. Cognitive function is the ability to learn, retain, and recall information; in a broad context, cognition includes all mental abilities and processes related to knowledge, including, but not limited to, attention, memory, reasoning, comprehension, and language production [36]. The decline in E2 levels caused by perimenopause and postmenopausal can affect memory and increase the risk of Alzheimer disease (AD) [37,38]. Moreover, the prevalence of AD and related dementia is higher among women than among men worldwide, and this difference increases with age [39]. Researchers suggest that the level of E2 may be closely related to cognition [40]. This possibility provides a basis for the cognitive protection of estrogens replacement therapy (ERT), but long-term exposure to E2 is the main risk factor for breast cancer progression [41,42]. Researchers have attempted to explore the unique cognitive characteristics mediated by different ERs to improve the efficacy of ERT and reduce its adverse effects [19,43,44]. Therefore, we conducted a knowledge domain visualization (KDviz) study on E2/ERs and cognitive-related research fields for the first time to help research groups review and analyze the trends in this knowledge domain. CiteSpace is a freely available Java application for KDviz and analyzes emerging trends and changes in the scientific literature [45]. The main motivation for this work was to simplify the search for important publications in a knowledge domain’s literature so that people can search for visually salient features, such as hub nodes, in a visualized network [45].
Material and Methods
SEARCH STRATEGY:
The data for this study were obtained from articles and reviews published from 2010 to 2021 in the Web of Science Core Collection (WOSCC) database. The search keywords were established in reference to Medical Subject Heading terms from PubMed. The topic terms used were “estrogen,” “estradiol,” “estrogen receptor,” “ovariectomy,” and “postmenopausal”. This query produced 114 629 records, which comprised Set #1. Another set of topic terms consists of “cognition,” “learning and memory,” “synaptic plasticity,” “neural plasticity,” “brain health,” “hippocampal,” and “neurogenesis”. This query led to 278 454 records, which comprised Set #2. We combined Set #1 and Set #2 and obtained the final dataset, Set #3, which consists of 3502 records (Table 1). All the authors reviewed 3502 publications, including 2842 research articles (81.15%) and 660 review articles (18.85%). Of the 3502 publications, Neurosciences ranked first, with 1579 (45.09%). Endocrinology Metabolism ranked second, with 737 (21.05%). Behavioral Sciences ranked third, with 377 (10.77%). Other disciplines include Psychiatry, Biochemistry Molecular Biology, Pharmacology Pharmacy, Geriatrics Gerontology, Clinical Neurology, Obstetrics Gynecology, and Multidisciplinary Sciences. A total of 3502 references were retrieved and downloaded on September 18, 2021. All cited reference information was downloaded and saved as plain text files for data processing and analysis. Finally, the downloaded data were imported into CiteSpace for deduplication. This search strategy has some limitations. First, the retrieval strategy is limited to the WOSCC database. Secondly, the search words may not be very comprehensive, the time range of the search was limited, and non-English publications were excluded.
DATA ANALYSIS METHOD:
We used CiteSpace 5.7.R5W (http://cluster.cis.drexel.edu/~cchen/citespace/) to conduct statistical analysis of publications [46]. CiteSpace can generate a KDviz map, whose primary goal is to monitor the evolution of a knowledge domain [47]. The parameters of CiteSpace were set as follows: Method (the Log-likelihood ratio algorithm), Time slice (2010–2021), Slice length (1 year), Node type (1 selected at a time), Selection criteria (co-authors, co-cited authors, co-author’s institution, and co-author’s country are the top 20 objects in each slice length, and co-cited references and co-occurring keyword clusters set were the top 50 objects in each slice length), and Pruning (Pathfinder and Pruning the merged network) [48]. All CiteSpace analysis terms are summarized in Table 2. The most prominent feature of this software is that it can be used for collaboration network analysis of co-author, co-author’ institution, co-author’s country, co-cited analysis of the article, author, and journal, and co-occurrence analysis of term, keyword, source, and category.
We used CiteSpace to analyze co-authors, co-author’s country and institution, co-cited authors, co-cited references, and co-occurring keyword clusters. Sigma (∑) was used to detect co-authors and co-cited references. Co-authors were defined as 2 or more authors who co-created an article and were jointly responsible for the knowledge content of the article. The co-citation count is the number of times that 2 published articles are co-cited by later articles, then the 2 articles constitute the relationship of co-cited reference and the authors of the 2 articles constitute co-cited authors [49]. Co-occurring keyword clusters are a graphical representation of the number of keywords that appear [50]. When the modularity (the Q score) [51] is greater than 0.3, it is considered that the divided cluster structure is significant. The lower Q score indicates that a network cannot be simplified as a cluster with clear boundaries. When the silhouette (the S score) [45] exceeds 0.7, the cluster is considered to be highly credible. For these 2 indicators, a score close to + 1 represents the best cluster model.
The centrality of a node indicates the importance of its position in the network [45]. A common centrality index is betweenness centrality, which measures the percentage of the number of shortest paths in the network to which a given node belongs [52]. Nodes with high betweenness centrality tend to appear in the paths connecting different clusters and are considered to be key hubs [53]. Citation burst refers to the importance of nodes in time. Burst detection measures the rate of change [45], which determines whether a given frequency function has statistically significant fluctuations over a short time interval over the entire cycle [50]. Cheng et al introduced the burst detection algorithm [45]. Burst is mainly used to detect articles that attract peer scientists’ attention. Sigma combines centrality as a structural attribute with citation burst as a temporal attribute. It is calculated as (centrality + 1)burst, and the higher these values, the greater the potential influence of the authors or articles [45].
The maps consist of different nodes and links. The size of nodes is related to the number of publications and number of times co-cited. Purple outer rings represent the importance of nodes in terms of betweenness centrality (≥0.1), and the thickness of the ring is proportional to the value of the betweenness centrality. The thickness of the red outer ring of the node represents the burst strength [45]. The number and thickness of links represent the degree of cooperation between the 2 nodes and the correlation between co-cited articles. The color of the link indicates the time of the first connection [47].
Results and Discussion
ANALYSIS OF NETWORKS ACROSS COUNTRIES, INSTITUTIONS, AND AUTHORS:
We used CiteSpace 5.7.R5W software to report the countries and institutions with the most publications and the highest centrality, respectively (Figures 1A, 2A and Tables 3, 4). Specifically, the node circles of the United States, People’s Republic of China, and Canada are larger, indicating that these countries have a large number of publications (Figure 1A). However, Scotland, Switzerland, and Belgium have larger purple rings (high centrality), indicating that they have close cooperation with other countries in this field (Figure 1A). Universities are the main research institutions related to E2/ERs and cognition. The University of Wisconsin is the university with the largest number of publications (77), and the centrality in this research area was Stanford University (0.78) (Figure 2A and Tables 3, 4).
We used CiteSpace 5.7.R5W to generate the co-cited authors network and the co-authorship network (Figures 1B, 2B). Co-cited authors were ranked by number of times cited, centrality, Sigma index, and burst strength (Tables 5–8), and the authors with the most publications were listed (Table 9). Woolley CS is the author with the most citations (594), Frick KM has the highest centrality (1.27) and Sigma index (1261582180303.55), Tuscher JJ has the strongest burst strength (40.66), and Espeland MA has the most publications (38). These KDviz maps enabled us to assess the influence and centrality of some important countries, institutions, and authors.
ANALYSIS OF CO-CITED REFERENCE:
Figure 3 shows the generated map of co-cited references and Figure 4 shows the top 50 co-cited references with the strongest citation bursts sorted by burst time. The top 5 co-cited references with high times, centrality, Sigma index, and burst strength were listed (Tables 10–13). These reviews [1,36,44,54] showed that E2 could improve hippocampal formation (structural and functional plasticity), enhance hippocampus-dependent cognitive function, and provide the required cellular signaling mechanism. In addition, the type and distribution of ERs were described in detail. Luine VN et al [36] discussed the “critical age window period” hypothesis of E2 effect and new strategies for the development of ERT. Three clinical trials [55–57] showed that ERT could not prevent mild cognitive impairment (MCI) for women over 65 years old, and it may also increase the risk of dementia, which had a greater impact on the cognitive ability of women with low cognitive function. Therefore, women over 65 years old are not recommended to use ERT to prevent dementia or MCI. Subsequently, Shao HB et al [58] explored the relationship between the time of using ERT and AD, and found that ERT starting at or more than 5 years after menopause was associated with an increased risk of AD, whereas using ERT in early menopause reduced the risk of AD in women by 30%. Another cohort study [38] showed that premenopausal ovariectomy (OVX) was associated with increased risk of cognitive impairment or dementia. Henderson VW et al [59] reviewed the effects of surgical and natural menopause on cognition. Surgical menopause may be accompanied by cognitive impairment, mainly affecting verbal episodic memory. By contrast, natural menopause transition is not accompanied by substantial changes in cognitive ability. When premenopausal women undergo ERT shortly after OVX, they may gain potential short-term cognitive benefits, but elderly postmenopausal women will not. Studies [60] also found that long-term ERT immediately after OVX had a positive effect on working memory (WM) in female rats, whereas long-term hormone deprivation followed by ERT was ineffective. The above studies have shown that ERT has a neuroprotective effect on the brain of premenopausal, perimenopausal, and early postmenopausal women, suggesting that it has a critical window period to treat cognitive impairment.
Woolley CS [1] found that E2, as a neurosteroid, was produced at a high level in the brain and acutely changed neuronal physiology. Hojo et al [61] showed immunoreactions of dehydroepiandrosterone synthase (P45017a) and P450 aromatase (AROM) in the pyramidal and granular neurons of the adult rat hippocampus. P45017a and AROM transformed pregnenolone in neurons into E2, indicating that E2 can be locally synthesized in adult hippocampal neurons. Rosenfeld CS et al [62] introduced 6 AROM inhibitors – Anastrazole, 1,4,6-androstatriene-3,17-dione, Exemestane, Fadrozole, Letrozole, and Vorozole – among which, Letrozole is the most commonly used. They are mainly used for the treatment of women with breast cancer [63]. AROM inhibitors can cause low levels of brain-derived E2 synthesis and cognitive impairment, and women receiving AROM inhibitors are at higher risk of cognitive impairment [64]. Nonetheless, exercise can be implemented early to reduce this risk [65]. Scientists also reported that a primate model for Letrozole treatment reduced levels of peripheral E2 but unexpectedly increased levels of E2 in the hippocampus [66]. In OVX- and Letrozole-treated female mice, the number of dendritic spine synapses in the hippocampus decreased significantly, but not in the PFC or cerebellar cortex [67]. These results showed that the effect of Letrozole might differ by species and brain regions.
ANALYSIS OF RECENT BURST CO-CITED REFERENCES:
We list 13 recent burst co-cited references (Table 14), indicating that these articles and topics have recently received close attention. Based on these recent burst co-cited references and other current studies, researchers found that GPER upregulates ERK (extracellular signal-regulated kinase) signaling in vivo and in vitro [68–71]. However, another in vivo study [43] found no activation of ERK but did find activation of JNK (c-Jun N-terminal kinase) in the dorsal hippocampus (DH) after administration of G-1 (GPER agonists). DH infusion of G-1 can promote OR (Object Recognition) and SR (Social Recognition) but had no effect on OP (Object Placement). These experiments were completed within 40 min after G-1 infusion. Therefore, GPER can rapidly promote OR and SR in female mice [72], and OP can be mediated by nERs [73], but other studies have found that OP can be promoted by GPER activation [43].
Hadjimarkou MM et al [74] summarized agonists and antagonists for all ERs and receptor variants, and discussed crosstalk between GPER and ERα, which is conducive to future research on the mechanism by which E2/ERs improve cognition. Galea LAM et al [75] suggested that studies assessing sex differences must consider the role of other interference factors, such as reproductive and hormonal status. Luine V, Scharfman HE, and Zárate S [76–78] provided evidence for 3 findings: (1) E2 and BDNF can synergistically enhance cognitive ability; (2) E2 can promote the expression of the BDNF gene by binding to nERs and acting on the estrogen response element (ERE) of the BDNF gene; and (3) BDNF promotes the activation of intracellular signaling pathways, including ERK, phosphatidylinositol 3-kinase (PI3K), and cAMP response element-binding protein (CREB) by tropomyosin receptor kinase B (TrkB).
ANALYSIS OF CO-OCCURRING KEYWORD CLUSTERS:
Figure 5 shows 10 different clusters with high Q and S scores (Q=0.8266; S=0.978) identified in the network of co-occurring keywords indicating highly credible clusters. We divided them into 6 themes: (1) E2/ERs and hippocampus (#0 expression, #7 dendritic spine density, #8 neurogenesis and #9 hippocampal neuron); (2) The Women’s Health Initiative Memory Study (#1 women and #4 health initiative memory); (3) Memory type (#3 WM); (4) Sex specificity (#2 sex); (5) Receptor specificity (#5 receptor); (6) The animal models used in the research (#6 mice).
E2/ERS AND HIPPOCAMPUS:
E2 is involved in the influence of the CNS, including the regulation of synaptic plasticity and physiological plasticity, which is considered the key mechanism and basis for the effect of E2 on cognitive function [54,79]. Acute activation of E2 and ERβ has been shown to rapidly regulate the density of dendritic spines [80,81]. In addition, exogenous GPER expression of primary rat cortical neurons grown for 24 days in vitro has a higher density of dendritic spines than that without GPER expression [82]. In the mHippo-E14 cell line, activation of GPER also upregulates actin polymerization and synaptic proteins [83]. Hippocampal slices of nERs KO mice were bathed in 17β-estradiol-3-benthic ester (EB) and G-1, and GPER was found to regulate LTP [70].
The mTORC2 complex is a multimer kinase composed of mammalian target of rapamycin (mTOR), mlst8, msin1, and rictor [84,85]. E2-treatment induces ERK and mTOR-dependent spinogenesis in hippocampus CA1 and PFC pyramidal neurons [86], and it was recently found that aerobic exercise can also improve dendritic spine plasticity in the hippocampus and PFC of OVX mice through the BDNF/mTOR signaling pathway [87]. When rictor, the core component of mTORC2, was deleted, hippocampal actin polymerization decreased. In addition, steroid receptor coactivator-1 (SRC-1) has been shown to enhance the transcriptional activity of steroid nuclear receptors [88,89]. SRC-1 is highly expressed in the hippocampus, and it has been proved to regulate growth and aging and mediate the effect of E2 on synaptogenesis in the hippocampus [90,91]. SRC-1 is regulated upstream of mTORC2, and ERs inactivation results in a significant decrease in SRC-1 expression [91]. In OVX mice, intraperitoneally injected G-1 reversed spatial memory decline, loss of dendritic spines, and actin depolymerization, and G-15 (GPER antagonists) injected continuously for 7 days blocked the effect of G-1 [83]. Based on the above findings, we believe that ERs, mTOR, rictor, and SRC-1 may provide new targets for the prevention and treatment of E2-related cognitive impairment in elderly people by improving dendritic spine plasticity.
Hippocampus-derived E2 plays a critical role in synaptic plasticity. Lan et al [92] constructed AROM-specific RNA interference AAVs and found a critical role of hippocampus-derived E2 in regulating hippocampal structure and function. In adult female mice, intraperitoneal injection of Letrozole significantly decreased severe synapse density, spatial learning, and memory impairment in OVX mice [35,67], and Letrozole inhibits actin polymerization and synaptic plasticity in primary cultured hippocampal neurons of mice [93]. These results indicated that the content of hippocampus-derived E2 should be considered in the process of ERT, not only focusing on peripheral E2. The plasticity of hippocampal neurons includes adult hippocampus neurogenesis (the proliferation, differentiation, and migration of neural progenitor cells [NPCs] in the adult hippocampus) to replace non-functional cells, and activation of ERβ promotes neurogenesis in DG [94]. DPN (ERβ agonists) regulates proliferation, differentiation, and transcription of several key NPCs and survival regulators, including Insulin-like growth factor 2, acidic fibroblast growth factor, Midkine, Prolactin, and cyclin-dependent kinase [95–100].
THE WOMEN’S HEALTH INITIATIVE MEMORY STUDY (WHIMS):
In the last century, women’s life expectancy increased by 30 years, from 50 to 83 years old; however, the average age of spontaneous menopause remained stable, at 50–51 years [101]. As a result, women now live more years with low estrogen levels [102]. The Women’s Health Initiative (WHI) study is a large, multi-site, population-based study assessing the risks and benefits of ERT for healthy postmenopausal women [103]. WHIMS is an additional study of WHI, which aims to study the effect of postmenopausal ERT on cognition and memory in healthy women aged 65 and above at baseline. WHIMS found that older women using conjugated equine estrogen (CEE) with or without medroxyprogesterone acetate (MPA) have an increased risk of dementia and smaller hippocampal and frontal brain volumes [55,56,104]. In some WHIMS participants who participated in the auxiliary WHI cognitive aging study, compared with placebo, CEE + MPA impaired verbal memory over time [105]. The increase of these risks seems to be related to the acceleration of brain atrophy.
The Young Women’s WHIMS (WHIMSY) study investigated the cognitive function of women aged 50–55 who participated in the WHI-hormone therapy (HT) test. Unlike WHISM, the WHIMSY data showed no difference in the cognitive performance of women receiving HT compared with placebo, and no difference was found in the individual binding of CEE and CEE + MPA [106–108]. Other studies have shown that the benefits of estrogen are preserved only when ERT begins in the postmenopausal years [109–111]. These findings led to the “critical age window period” hypothesis that suggests a precise window of opportunity for beneficial ERT in postmenopausal women.
MEMORY TYPE:
In rodents, both the Morris water maze (MWM) and the radial eight-arm maze (RAM) can be used to measure hippocampal spatial memory [112,113], but the RAM can use the reference memory (RM) to reflect the long-term spatial memory and WM to evaluate short-term spatial memory [112]. Four rewarded arms and 4 no-rewarded arms are used in the RAM. RM is memory consistent across sessions (ie, location of rewarded arms), and WM is memory used within a single session (ie, which arms were already visited) [112]. In such tests, individual animals were repeatedly tested for many days, and their ability to find a platform or maze arm that remained constant was measured [114,115]. The MWM is mainly used in animals who hate the water environment and want to escape to a safe platform, whereas the motivation in the RAM maze is food. In the MWM task, there are almost an infinite number of swim path trajectories that could be used, while in the RAM task, the number of trajectories is limited; moreover, the radial arm maze has a spatial WM component not present in the MWM to reflect short-term memory [116].
WM supports many complex cognitive functions that require temporary storage and manipulation of information over short periods of time in order to make a response [117]. Some evidence shows that E2 increases WM [118–120] but does not improve RM [121,122]. In addition, E2 fluctuations at different stages of the estrous cycle, affecting the performance of tasks used to assess memory [122]. Hampson’s group found for the first time that there was a correlation between ERT and WM performance in postmenopausal women [123] and young women [124], which was later reported by other groups [125]. Recent studies [115] on the virtual MWM and the virtual RAM of young female college students showed that both high and low levels of natural-cycle E2 did not affect the WM and RM of the virtual RAM, but high levels of natural E2 promoted the spatial memory performance of the virtual MWM. This suggests that the effect of E2 on women’s spatial memory may be limited to more complex spatial memory tasks, such as the virtual MWM, which is more ambiguous because it does not have a direct path to the target location and participants can move freely in any direction.
In summary, the inconsistent results of spatial memory research may be related to species, test methods, and fluctuations and sources of E2, which need to be further explored.
SEX SPECIFICITY:
Many neurological and neurodegenerative diseases vary by sex [126]. For example, the incidence of autism spectrum disorders is much higher in boys than in girls [127], and women have a higher risk and faster progression of AD than men [128]. Many studies have shown that the brains of males and females are different at the molecular level [129].
Current studies have shown that the beneficial effects of hippocampal E2 on memory consolidation in males and females are mediated by different molecular mechanisms. In female mice, E2 upregulates the ERK/CREB signaling pathway by ERα/mGluR1 crosstalk, while E2 regulates male memory by phosphorylation of CREB independent of ERK [19,130–132]. Woolley’s group [126,133] showed that E2 rapidly suppresses inhibitory synaptic transmission through ERα/mGluR1 crosstalk and activates the phospholipase C (PLC)/IP3/Ca+ signaling pathway to mobilize endocannabinoids in the hippocampus via a sex-specific manner in females. E2 acts via ERβ to increase presynaptic glutamate release probability and through GPER to increase postsynaptic sensitivity to glutamate in females, whereas E2 plays a role through presynaptic ERα to increase glutamate release probability and postsynaptic ERβ to increase glutamate sensitivity in males [134]. Although the degree of synaptic enhancement induced by acute E2 is the same in both sexes, Ca2+/CAMKII, L-type Ca2+ channels and internally stored Ca2+ release are required for E2-induced information enhancement in males and females. However, protein kinase A (PKA) only needs to initiate enhancement in females [135].
In organotypic entorhinal-hippocampal cultures from mice, GPER mediated the increase of dendritic spine density in the stratum lacunosum-molecular layer of the hippocampus, which was sex-specific only in cultures from female mice [136]. Moreover, Letrozole treatment decreased dendritic spine density, synaptophysin, postsynaptic protein, mitochondrial volume, and LTP in female mice. Interestingly, these phenomena were sex-specific, but not found in male animals [137,138]. Studies [139] found that neurogenin 3 (Ngn3) promoted neuritogenesis in hippocampal neurons with sex differences, which was mainly related to higher expression of Ngn3 in female neurons. Therefore, female neurons have higher dendritic complexity and longer axons than male neurons.
The findings of sex differences suggest that therapeutic targets for cognitive impairment in men and women are sex-specific.
RECEPTOR SPECIFICITY:
GPER is located on the cell membrane and mediates rapid E2 signal transduction, whereas ERα and ERβ were initially proven to be transcription factors that function in a genomic way [82,140]. Unlike nERs, GPER is not a transcription factor, so it cannot directly regulate gene expression, and GPER can indirectly regulate gene expression by activating proteins that change the activity of transcription factors [141,142]. GPER widely exists in various brain regions, and it has a similar distribution pattern in the brain tissues of rats, mice, and humans [143–145]. GPER is also expressed in rats and mice hippocampal CA1–CA3 and DG areas [143,146,147]. Although GPER is localized in the plasma membrane, it can exist in the hippocampus cells’ endoplasmic reticulum or Golgi apparatus. In addition, GPER can be transferred from the plasma membrane to the perinuclear space [148]. In a virtual and biomolecular screening of about 10 000 molecules, a compound called G-1 was developed, which did not significantly bind to ERα or ERβ even at high concentrations [149]. Evidence suggests that G-1 binds explicitly to a 36-kDa splice variant of ERα (ERα36) and induces its activity to mediate non-genomic E2 signaling pathways [141]. Studies have reported that PPT (ERα agonists) also has an agonist effect on GPER at high concentrations [150], which complicates investigation of the effects of G-1 or PPT on GPER and ERα in future studies.
Different ERs may be responsible for mediating different memory tasks. ERα KO mice showed complete SR disorder, whereas ERβ KO mice showed partial SR damage [151], and selective activation of GPER can improve SR [72,152] and OR within 40 min [72], but does not affect OP [72]. Chronic (trial 4 h later) and acute (daily injection for 2 days with trial 48 h later) DPN treatment increased OR and OP performance in OVX rats, whereas acute PPT treatment did not change OR and OP performance [153]. In addition, the injection of WAY-200070 (ERβ agonists) promoted spatial learning and memory, dendritic spine density, and the level of synaptic protein in OVX rats, whereas PPT did not show a promotion effect [154].
ANIMAL MODELS USED IN THE RESEARCH:
Prange-Kiel et al [155] first proved the local synthesis of E2 in the hippocampus, and studies confirm that E2 is produced in the brain [2]. The application of Letrozole, AROM KO mouse, and various in vitro models of the hippocampus cell are valuable tools for validating the effects of neurogenic E2 on the morphology and function of hippocampal neurons.
Four transgenic mouse models that downregulate GPER have been established to assess the role of GPER in physiological function [156–159]. Studies of GPER KO mice have also identified the role of GPER in the CNS [160]. The effective interference sequence of GPER short hairpin RNA (shRNA) in cultured cortical neurons [161] and basolateral amygdala of OVX mice [162] has been reported, which can be used for subsequent studies on GPER. Genetically modified mice lacking Cyp19 gene encoding AROM have been used in many studies to detect the effects of lifelong AROM deficiency on various organs, including the brain [163–165]. A conditional KO, an adeno-associated viral vector (AAV) approach, or other targeted methods can be used to delete ERs or AROM expression in specific brain regions.
Conclusions
Through analysis, we finally draw our conclusions. First, the United States and the University of Wisconsin are the most-published countries and institutions, respectively, whereas Scotland and Stanford University have the most centrality. Woolley CS, Frick KM, Tuscher JJ, and Espeland MA are influential authors in this field. Second, the co-cited analysis shows that, in recent years, researchers focused on the “critical age window period” hypothesis of ERT, hippocampus-derived E2, the mediating role of GPER, and crosstalk between ERs. Third, we introduce through co-occurring keyword cluster analysis the 5 research directions that are most concentrated in this research field (E2 and hippocampus, WHIMS, memory type, sex specificity, receptor specificity) and animal models used in the study. These research results can help research groups determine position new directions for future research in this field and provide some potential targets for E2 to improve cognitive ability.
More and more animal studies have indicated that E2 can be used as a neuromodulator to regulate cognitive function, and the results of WHIMS show that this effect has a “critical age window period”. ERT can be implemented earlier in future research. Recent studies have revealed that the regulation of cognition by E2/ERs is closely related to the spine plasticity of neurons, involving several cell-signaling cascades and genomic effects caused by different ERs. There is increasing evidence that E2 improves cognition with sex specificity, the specificity of ERs activating intracellular signals and their effects on different memory types, ERs can crosstalk with each other, and ERs can interact with other membrane receptors. Researchers should also consider the specific effects of E2 on different cell types, the study of female animals’ cognition should exclude the effect of fluctuations in E2 levels during the estrous cycle, and sex should also be considered as a biological variable. To promote the development of this field, researchers can use more new technologies in the future, such as single-cell sequencing, omics analyses, and gene targeting.
Figures
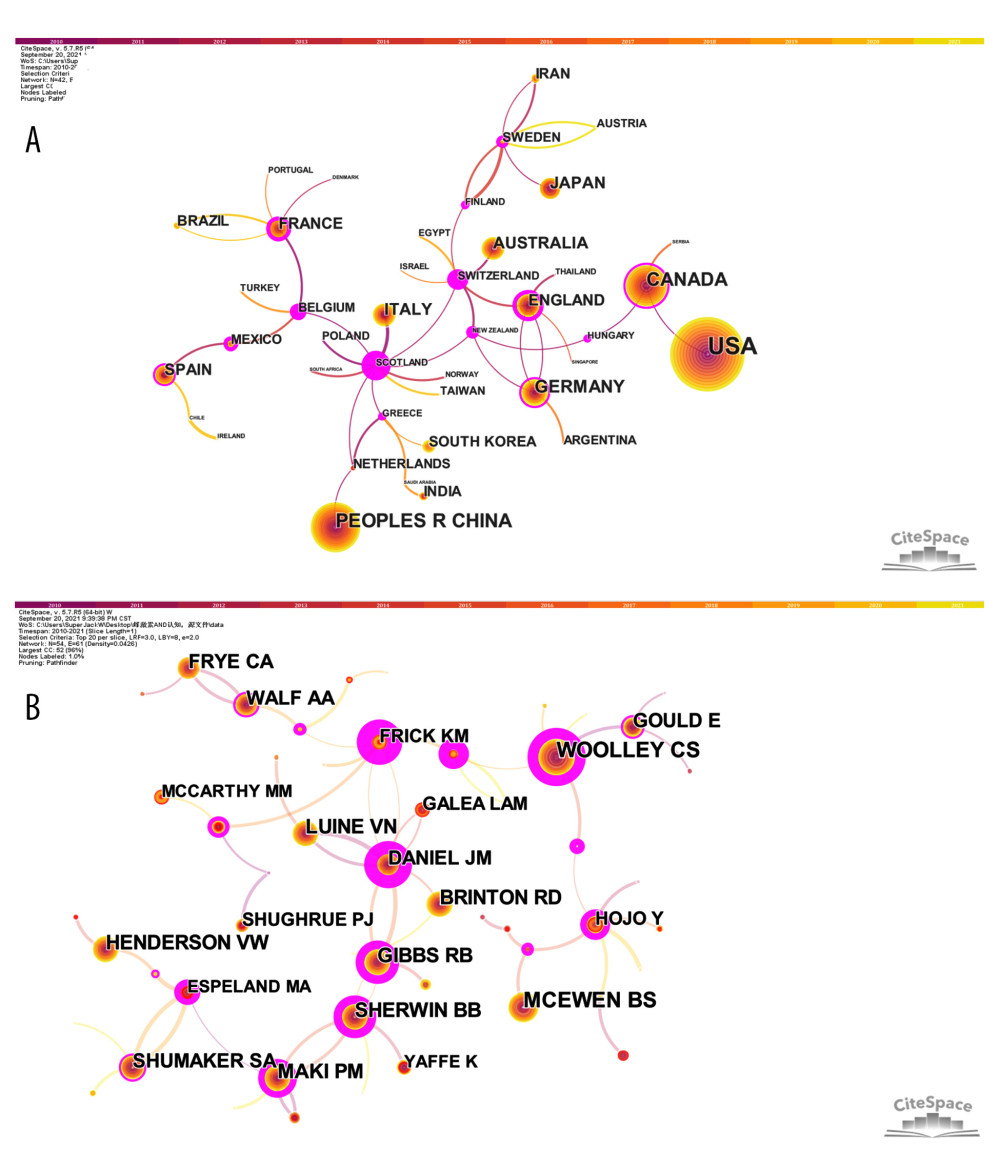 Figure 1. (A) Network map of co-authors’ countries. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Country, Top N=20, Pruning: Pathfinder and Pruning the merged network, N (nodes)=42, and E (links)=48. (B) Network map of co-cited authors. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Cited author, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=54, and E=61. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 1. (A) Network map of co-authors’ countries. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Country, Top N=20, Pruning: Pathfinder and Pruning the merged network, N (nodes)=42, and E (links)=48. (B) Network map of co-cited authors. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Cited author, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=54, and E=61. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). 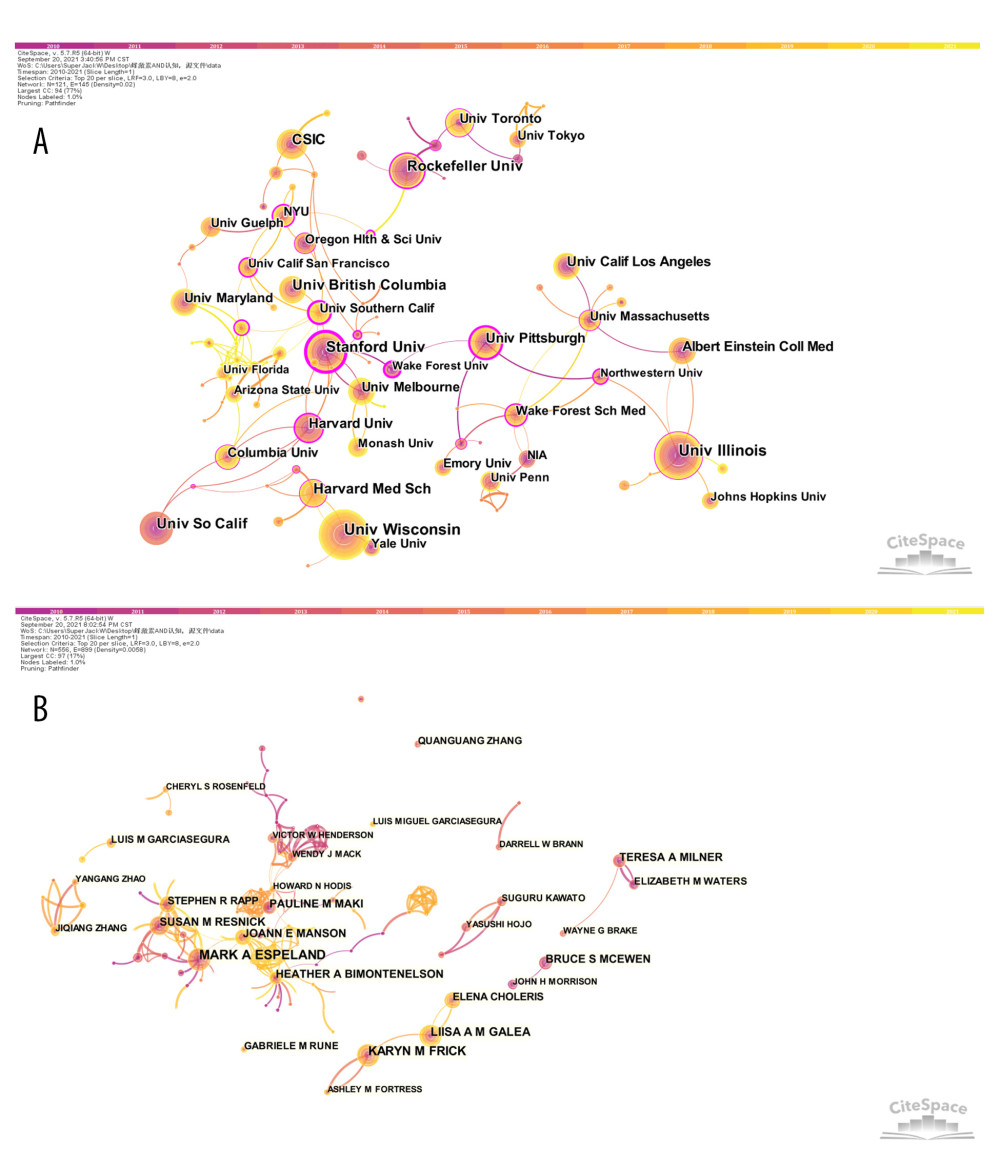 Figure 2. (A) Network map of co-authors’ institutions. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Institution, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=121, and E=145. (B) Network map of co-authors. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Author, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=556, and E=899. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 2. (A) Network map of co-authors’ institutions. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Institution, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=121, and E=145. (B) Network map of co-authors. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Author, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=556, and E=899. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). 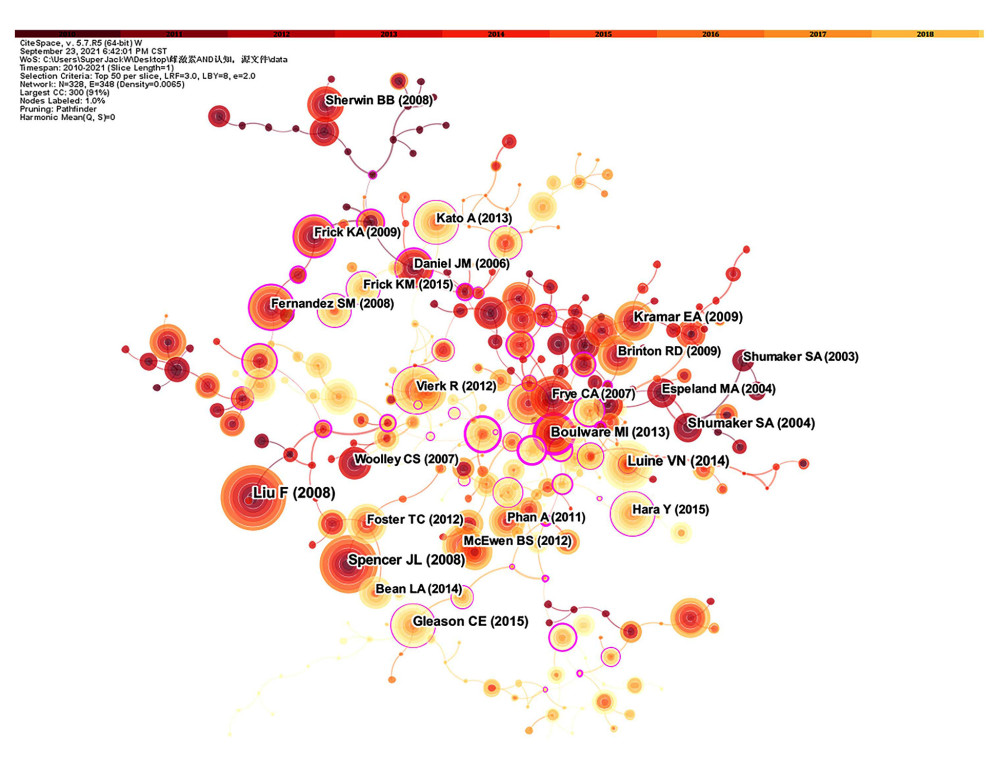 Figure 3. Network map of co-cited reference. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Reference, Top N=50, Pruning: Pathfinder and Pruning the merged network, N=328, E=348. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 3. Network map of co-cited reference. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Reference, Top N=50, Pruning: Pathfinder and Pruning the merged network, N=328, E=348. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). 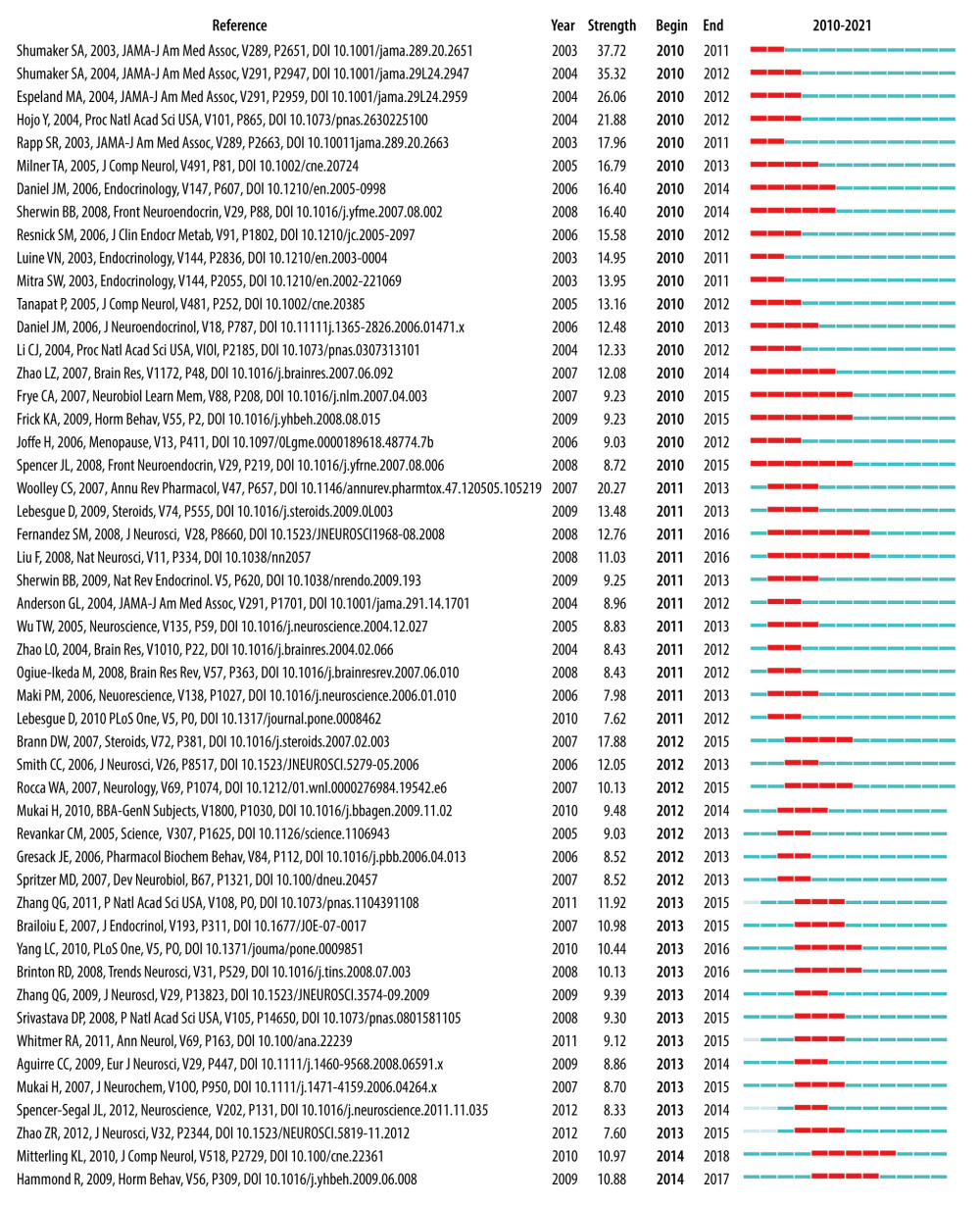 Figure 4. The top 50 co-cited references with the strongest citation bursts sorted by burst time. The burst detection was based on the citations made by the top 50 articles per year during the 12-year period, and the top 100 references with the strongest citation bursts from 2010 to 2021 were identified. Citation burst information include citation information, citation burst strength, burst start time, burst end time, and burst start-to-end time axis diagram. Red bars: Duration of burst. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 4. The top 50 co-cited references with the strongest citation bursts sorted by burst time. The burst detection was based on the citations made by the top 50 articles per year during the 12-year period, and the top 100 references with the strongest citation bursts from 2010 to 2021 were identified. Citation burst information include citation information, citation burst strength, burst start time, burst end time, and burst start-to-end time axis diagram. Red bars: Duration of burst. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). 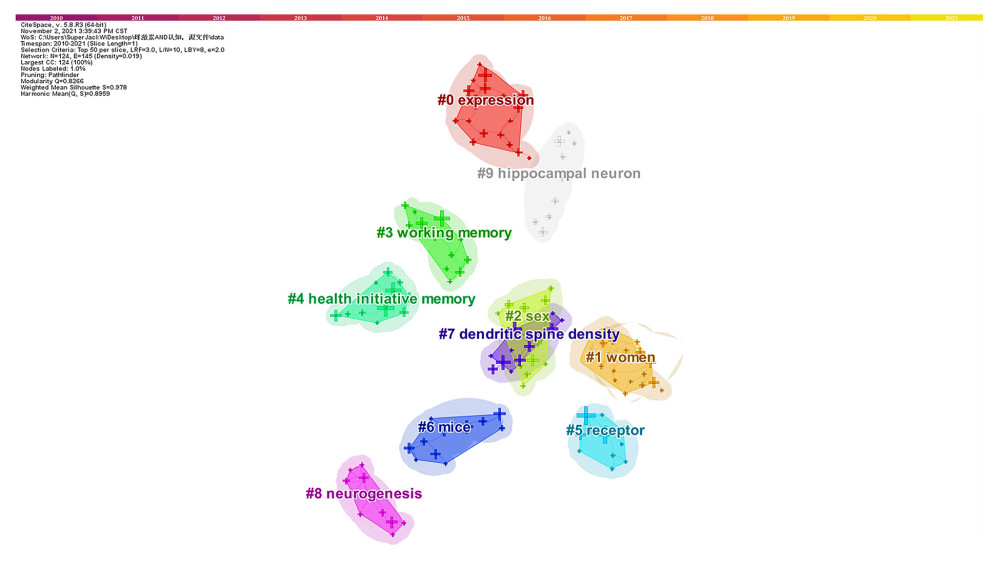 Figure 5. Network map of co-occurring keyword clusters. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: keywords, Top N=50, pathfinder and pruning the merged network, N=124, E=145, choose all in one, the keywords sources, the Log-likelihood ratio (LLR) algorithm to label clusters, the Modularity (Q=0.8266), and the Mean Silhouette (S=0.978). #0 expression (S=0.963); #1 women (S=0.956); #2 sex (S=1); #3 working memory(S=1); #4 health initiative memory (S=1); #5 receptor(S=1); #6 mice (S=0.897); #7 dendritic spine density(S=0.979); #8 neurogenesis (S=1); #9 hippocampal neuron (S=1). (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 5. Network map of co-occurring keyword clusters. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: keywords, Top N=50, pathfinder and pruning the merged network, N=124, E=145, choose all in one, the keywords sources, the Log-likelihood ratio (LLR) algorithm to label clusters, the Modularity (Q=0.8266), and the Mean Silhouette (S=0.978). #0 expression (S=0.963); #1 women (S=0.956); #2 sex (S=1); #3 working memory(S=1); #4 health initiative memory (S=1); #5 receptor(S=1); #6 mice (S=0.897); #7 dendritic spine density(S=0.979); #8 neurogenesis (S=1); #9 hippocampal neuron (S=1). (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). Tables
Table 1. Search strategy from Web of Science core collection.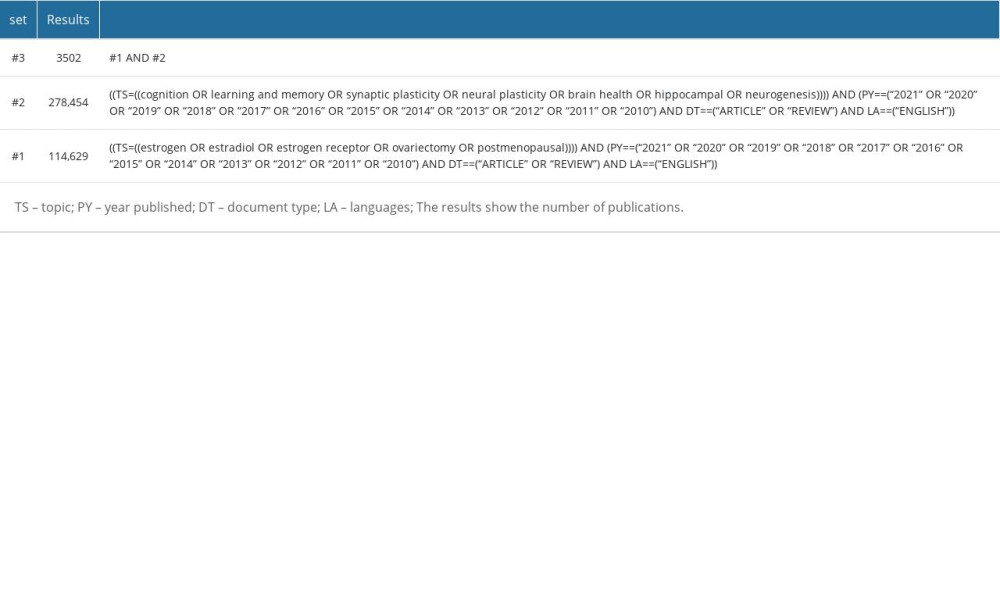 Table 2. CiteSpace analysis terms.
Table 2. CiteSpace analysis terms. Table 3. Top 5 countries and institutions ranked by number of publications.
Table 3. Top 5 countries and institutions ranked by number of publications.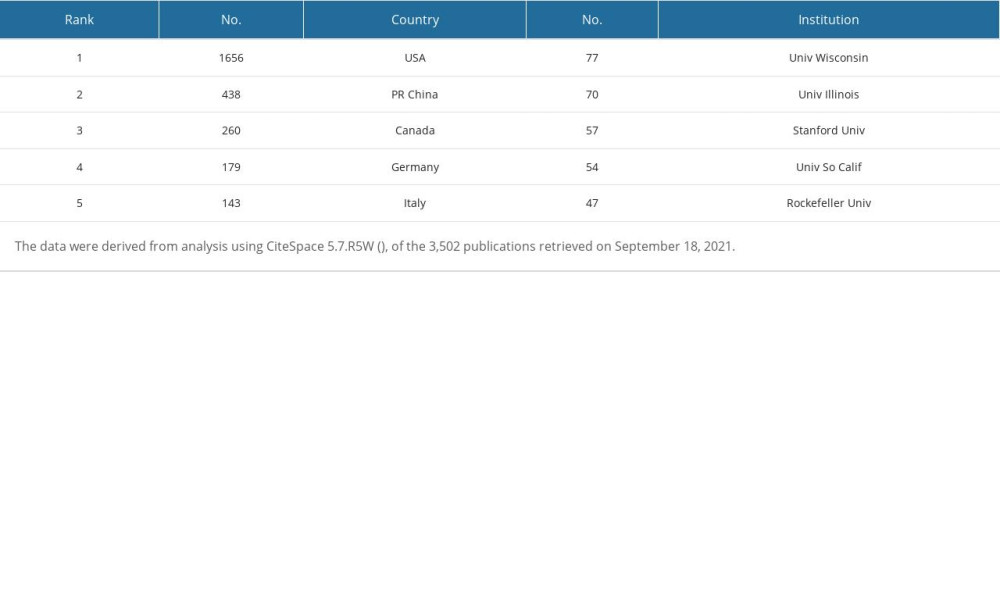 Table 4. Top 5 countries and institutions ranked by centrality.
Table 4. Top 5 countries and institutions ranked by centrality.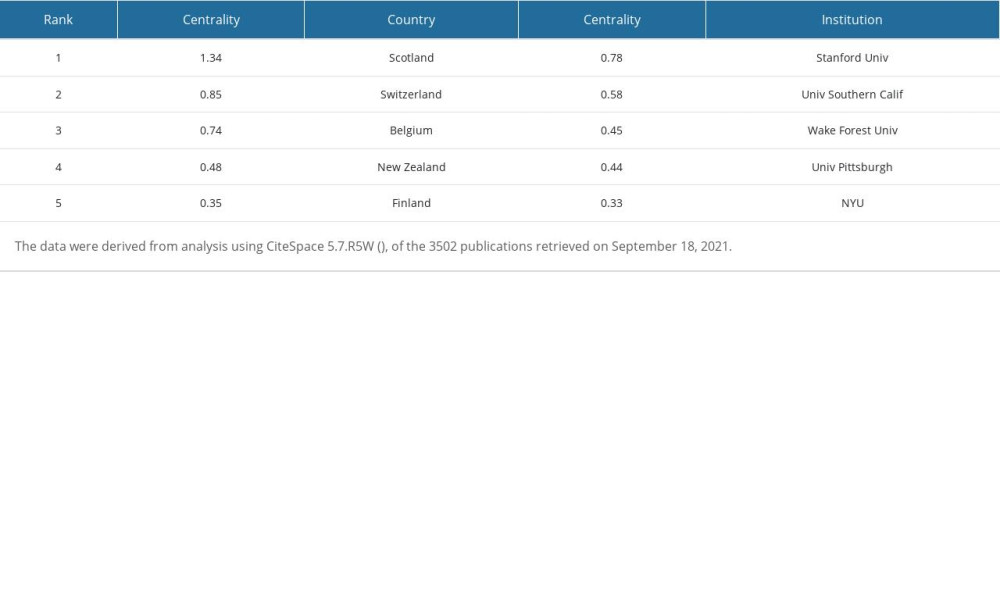 Table 5. Top 5 authors ranked by number of citations.
Table 5. Top 5 authors ranked by number of citations.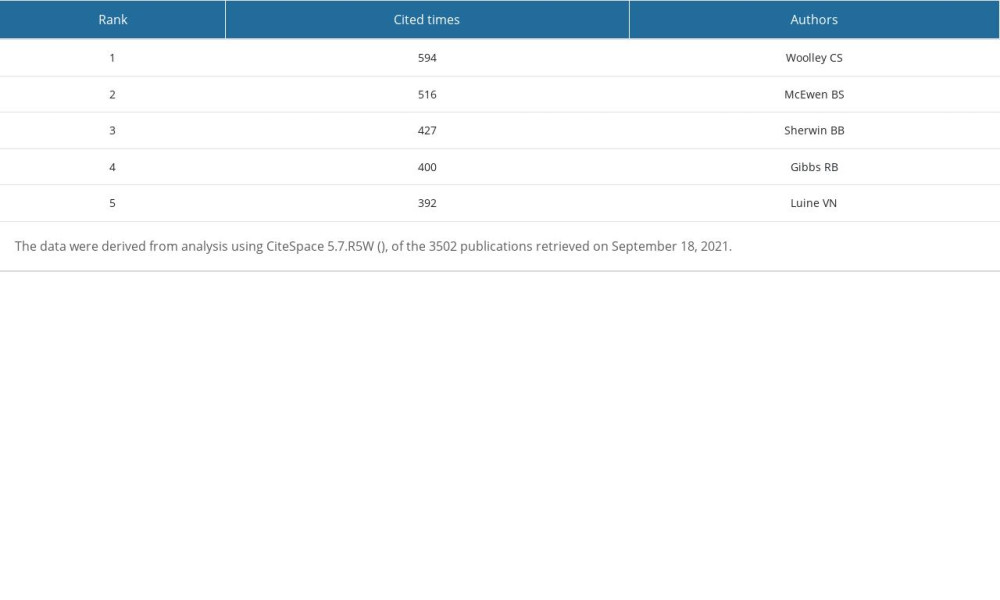 Table 6. Top 5 authors ranked by centrality.
Table 6. Top 5 authors ranked by centrality.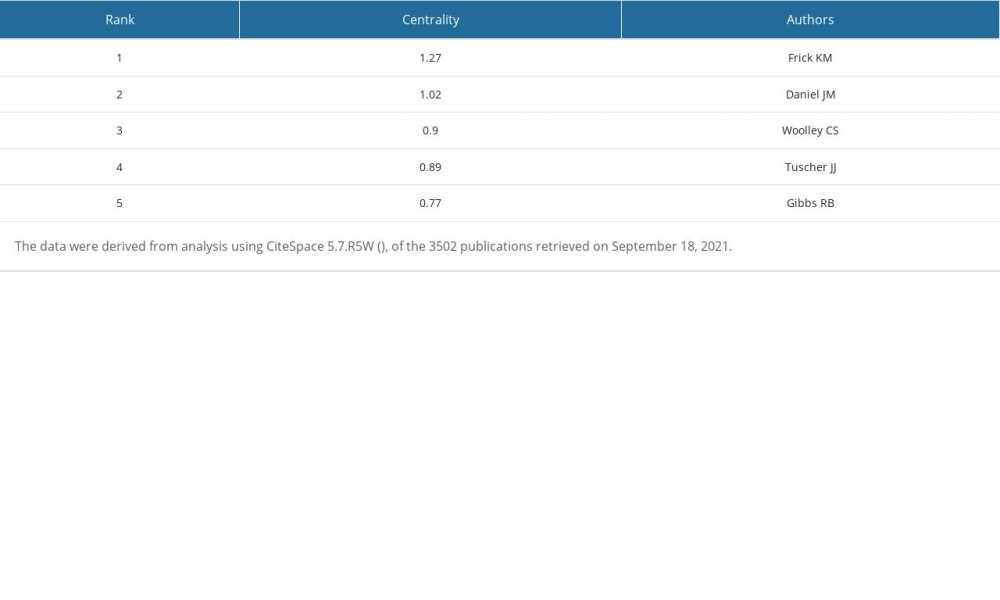 Table 7. Top 5 authors ranked by Sigma.
Table 7. Top 5 authors ranked by Sigma.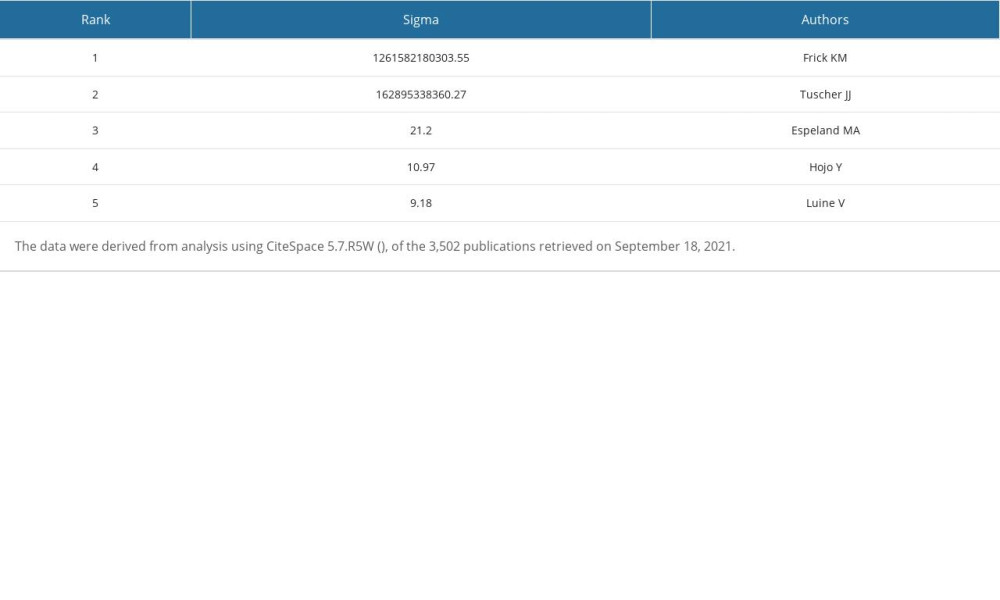 Table 8. Top 5 authors ranked by burst strength.
Table 8. Top 5 authors ranked by burst strength.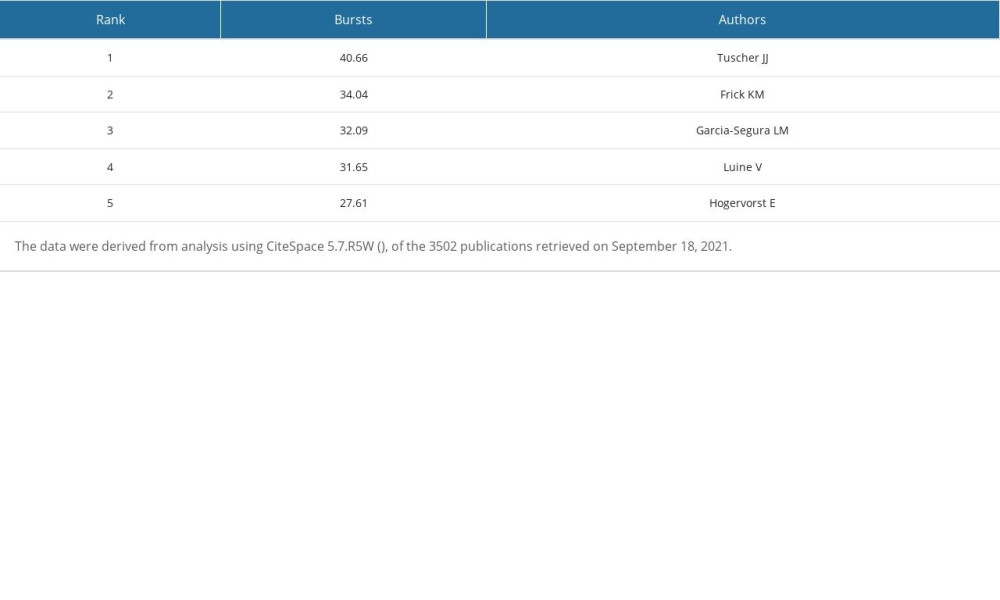 Table 9. Top 5 authors ranked by number of publications.
Table 9. Top 5 authors ranked by number of publications.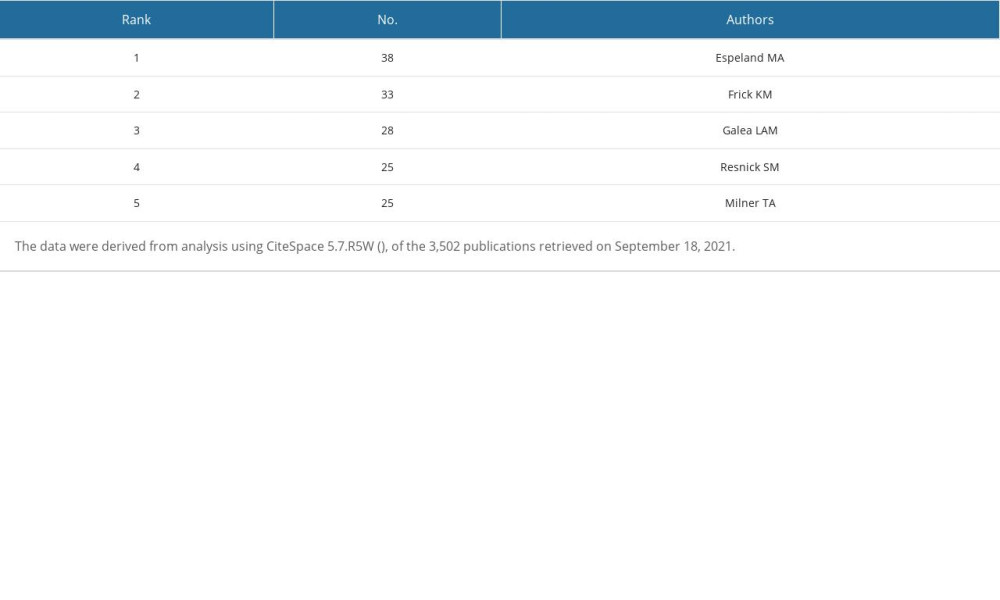 Table 10. Top 5 co-cited references ranked by number of citations.
Table 10. Top 5 co-cited references ranked by number of citations.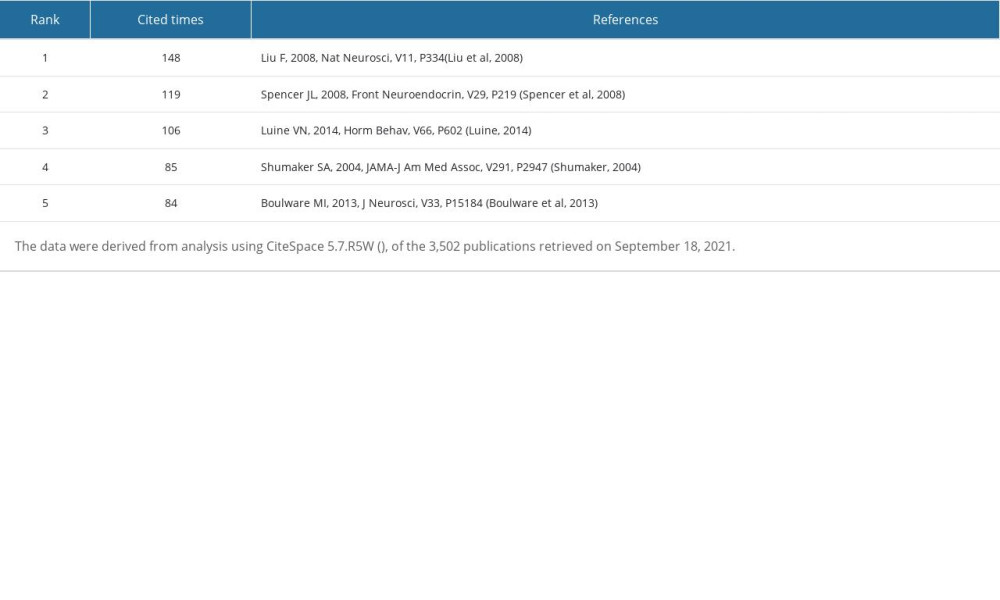 Table 11. Top 5 co-cited references ranked by centrality.
Table 11. Top 5 co-cited references ranked by centrality.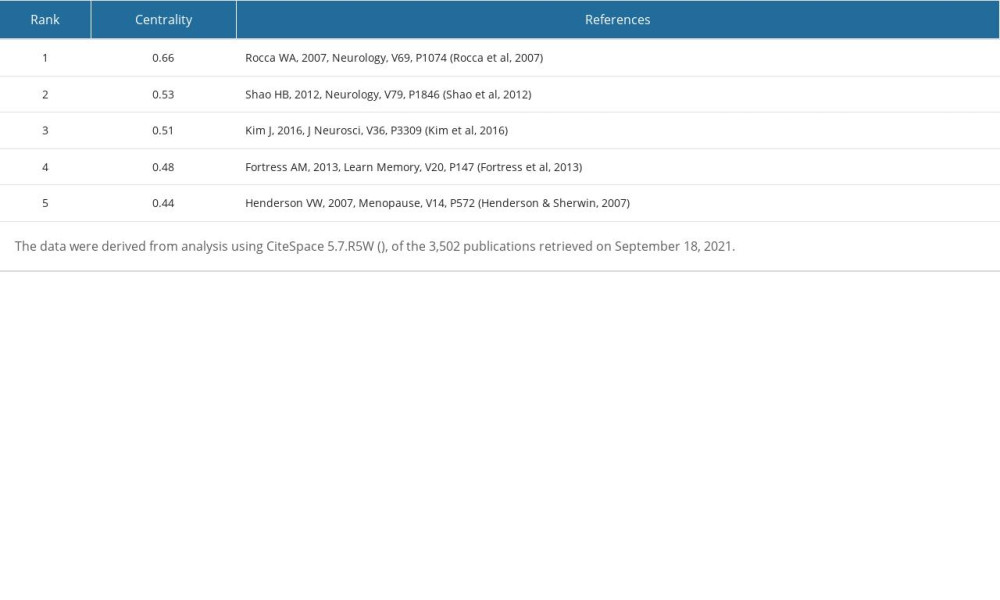 Table 12. Top 5 co-cited references ranked by Sigma.
Table 12. Top 5 co-cited references ranked by Sigma.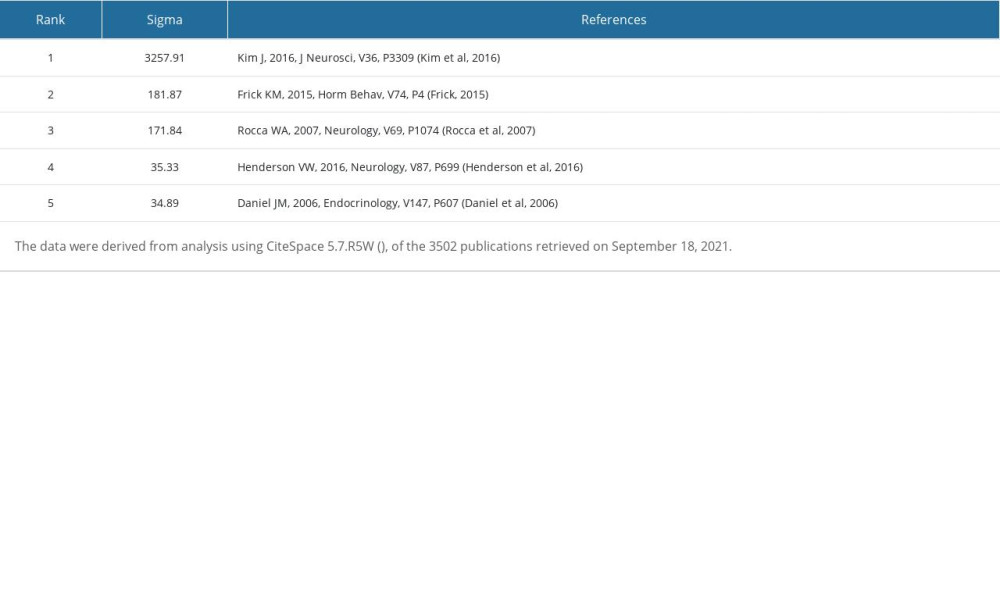 Table 13. Top 5 co-cited references with the strongest citation bursts during 2010–2021.
Table 13. Top 5 co-cited references with the strongest citation bursts during 2010–2021.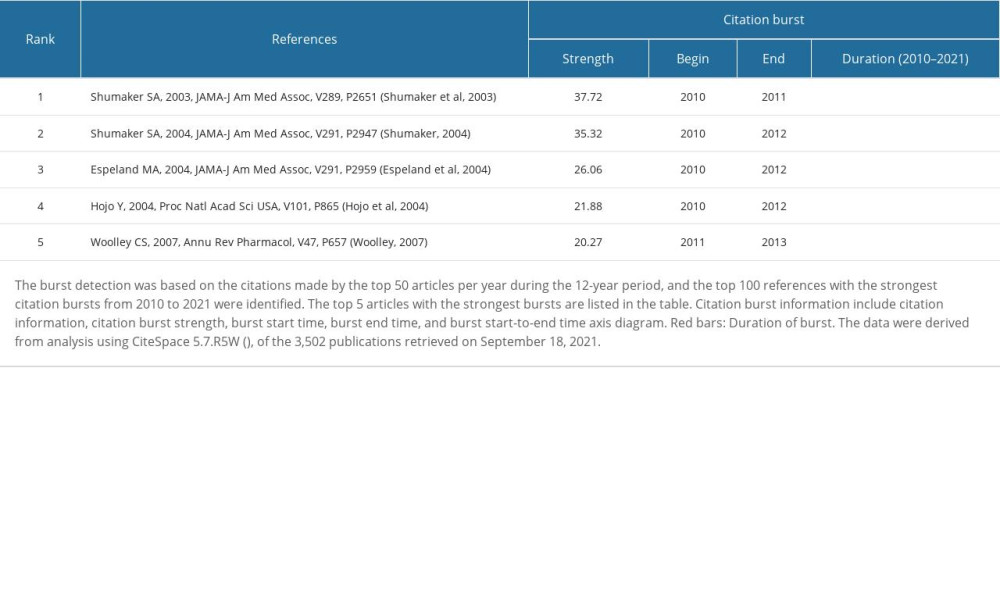 Table 14. Co-cited references with the most recent bursts from 2018.
Table 14. Co-cited references with the most recent bursts from 2018.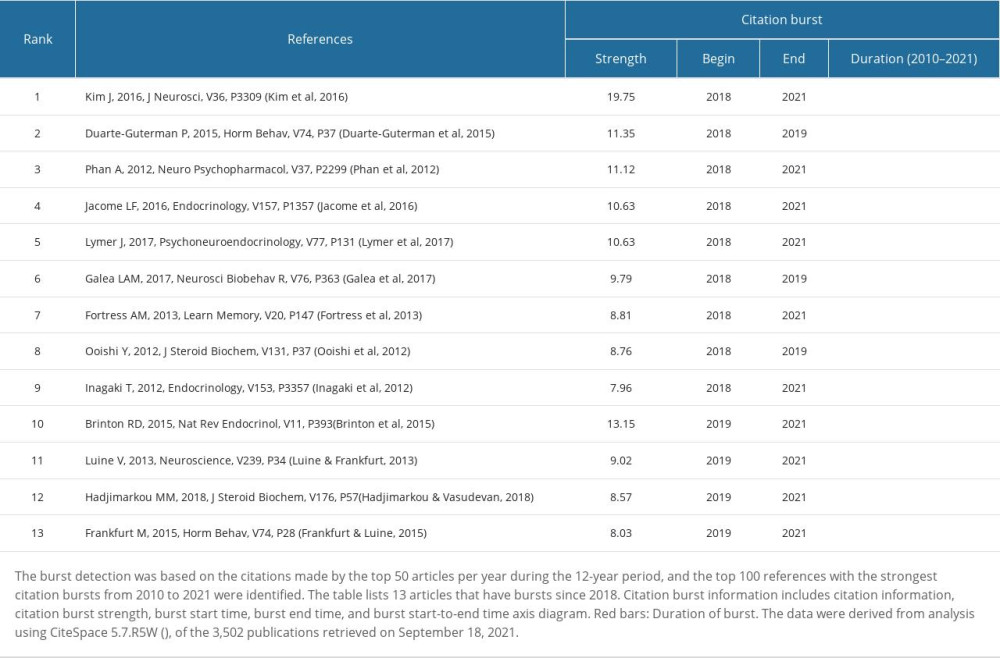
References
1. Woolley CS, Acute effects of estrogen on neuronal physiology: Annu Rev Pharmacol Toxicol, 2007; 47; 657-80
2. Brann DW, Lu Y, Wang J, Brain-derived estrogen and neural function: Neurosci Biobehav Rev, 2022; 132; 793-817
3. Beikoghli KS, Kararigas G, Oestrogenic regulation of mitochondrial dynamics: Int J Mol Sci, 2022; 23(3); 1118
4. Gegenhuber B, Tollkuhn J, Epigenetic mechanisms of brain sexual differentiation: Cold Spring Harb Perspect Biol, 2022; 14(11); a039099
5. McClure RE, Barha CK, Galea LA, 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats: Horm Behav, 2013; 63(1); 144-57
6. Rettberg JR, Yao J, Brinton RD, Estrogen: A master regulator of bioenergetic systems in the brain and body: Front Neuroendocrinol, 2014; 35(1); 8-30
7. Rannevik G, Jeppsson S, Johnell O, A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density: Maturitas, 2008; 61(1–2); 67-77
8. Loomis AK, Thomas P, Binding characteristics of estrogen receptor (ER) in Atlantic croaker (Micropogonias undulatus) testis: Different affinity for estrogens and xenobiotics from that of hepatic ER: Biol Reprod, 1999; 61(1); 51-60
9. Hara Y, Waters EM, McEwen BS, Estrogen effects on cognitive and synaptic health over the lifecourse: Physiol Rev, 2015; 95(3); 785-807
10. Belcher SM, Rapid signaling mechanisms of estrogens in the developing cerebellum: Brain Res Rev, 2008; 57(2); 481-92
11. Zigmond RE, McEwen BS, Selective retention of oestradiol by cell nuclei in specific brain regions of the ovariectomized rat: J Neurochem, 1970; 17(7); 889-99
12. McEwen BS, Alves SE, Estrogen actions in the central nervous system: Endocr Rev, 1999; 20(3); 279-307
13. Mitterling KL, Spencer JL, Dziedzic N, Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus: J Comp Neurol, 2010; 518(14); 2729-43
14. Shughrue PJ, Merchenthaler I, Estrogen is more than just a “sex hormone”: Novel sites for estrogen action in the hippocampus and cerebral cortex: Front Neuroendocrinol, 2000; 21(1); 95-101
15. Thomas P, Pang Y, Filardo EJ, Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells: Endocrinology, 2005; 146(2); 624-32
16. Alexander SP, Mathie A, Peters JA: Br J Pharmacol, 2011; 164(Suppl 1); S1-324
17. Micevych PE, Mermelstein PG, Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain: Mol Neurobiol, 2008; 38(1); 66-77
18. Sheldahl LC, Shapiro RA, Bryant DN, Estrogen induces rapid translocation of estrogen receptor beta, but not estrogen receptor alpha, to the neuronal plasma membrane: Neuroscience, 2008; 153(3); 751-61
19. Boulware MI, Heisler JD, Frick KM, The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling: J Neurosci, 2013; 33(38); 15184-94
20. Boulware MI, Kordasiewicz H, Mermelstein PG, Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons: J Neurosci, 2007; 27(37); 9941-50
21. Levin ER, Plasma membrane estrogen receptors: Trends Endocrinol Metab, 2009; 20(10); 477-82
22. Yager JD, Chen JQ, Mitochondrial estrogen receptors – new insights into specific functions: Trends Endocrinol Metab, 2007; 18(3); 89-91
23. Psarra AM, Sekeris CE, Steroid and thyroid hormone receptors in mitochondria: Iubmb Life, 2008; 60(4); 210-23
24. Alvarez-Delgado C, Mendoza-Rodríguez CA, Picazo O, Different expression of alpha and beta mitochondrial estrogen receptors in the aging rat brain: Interaction with respiratory complex V: Exp Gerontol, 2010; 45(7–8); 580-85
25. Yang SH, Liu R, Perez EJ, Mitochondrial localization of estrogen receptor beta: Proc Natl Acad Sci USA, 2004; 101(12); 4130-35
26. Arnold S, Beyer C, Neuroprotection by estrogen in the brain: The mitochondrial compartment as presumed therapeutic target: J Neurochem, 2009; 110(1); 1-11
27. Velarde MC, Mitochondrial and sex steroid hormone crosstalk during aging: Longev Healthspan, 2014; 3(1); 2
28. Lejri I, Grimm A, Eckert A, Mitochondria, estrogen and female brain aging: Front Aging Neurosci, 2018; 10; 124
29. Gillies GE, McArthur S, Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines: Pharmacol Rev, 2010; 62(2); 155-98
30. Watson CS, Alyea RA, Cunningham KA, Estrogens of multiple classes and their role in mental health disease mechanisms: Int J Womens Health, 2010; 2; 153-66
31. Mora S, Dussaubat N, Díaz-Véliz G, Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats: Psychoneuroendocrino, 1996; 21(7); 609-20
32. Walf AA, Frye CA, The use of the elevated plus maze as an assay of anxiety-related behavior in rodents: Nat Protoc, 2007; 2(2); 322-28
33. Raval AP, Borges-Garcia R, Javier MW, Periodic 17β-estradiol pretreatment protects rat brain from cerebral ischemic damage via estrogen receptor-β: PLoS One, 2013; 8(4); e60716
34. Dumitriu D, Rapp PR, McEwen BS, Estrogen and the aging brain: An elixir for the weary cortical network: Ann N Y Acad Sci, 2010; 1204; 104-12
35. Lu Y, Sareddy GR, Wang J, Neuron-derived estrogen regulates synaptic plasticity and memory: J Neurosci, 2019; 39(15); 2792-809
36. Luine VN, Estradiol and cognitive function: Past, present and future: Horm Behav, 2014; 66(4); 602-18
37. Boyle CP, Raji CA, Erickson KI, Estrogen, brain structure, and cognition in postmenopausal women: Hum Brain Mapp, 2021; 42(1); 24-35
38. Rocca WA, Bower JH, Maraganore DM, Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause: Neurology, 2007; 69(11); 1074-83
39. Prince M, Bryce R, Albanese E, The global prevalence of dementia: A systematic review and metaanalysis: Alzheimers Dement, 2013; 9(1); 63-75e2
40. Fu C, Hao W, Shrestha N, Association of reproductive factors with dementia: A systematic review and dose-response meta-analyses of observational studies: EClinicalMedicine, 2022; 43; 101236
41. Vivacqua A, Lappano R, De Marco P, G protein-coupled receptor 30 expression is up-regulated by EGF and TGF alpha in estrogen receptor alpha-positive cancer cells: Mol Endocrinol, 2009; 23(11); 1815-26
42. Ranganathan P, Nadig N, Nambiar S, Non-canonical estrogen signaling in endocrine resistance: Front Endocrinol (Lausanne), 2019; 10; 708
43. Kim J, Szinte JS, Boulware MI, 17β-Estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms: J Neurosci, 2016; 36(11); 3309-21
44. Frick KM, Molecular mechanisms underlying the memory-enhancing effects of estradiol: Horm Behav, 2015; 74; 4-18
45. Chen CM, Ibekwe-SanJuan F, Hou JH, The structure and dynamics of cocitation clusters: A multiple-perspective cocitation analysis: J Am Soc Inf Sci Technol, 2010; 61(7); 1386-409
46. Zhu Y, Chang T, Wang X, Bibliometric study of exercise and tendinopathy research from 2001 to 2020: Med Sci Monit, 2022; 28; e934016
47. Chen C, Searching for intellectual turning points: Progressive knowledge domain visualization: Proc Natl Acad Sci USA, 2004; 101; 5303-10
48. Wang H, Long T, You J, Bibliometric visualization analysis of microbiome-gut-brain axis from 2004 to 2020: Med Sci Monit, 2022; 28; e936037
49. Chen C, Dubin R, Kim MC, Emerging trends and new developments in regenerative medicine: A scientometric update (2000–2014): Expert Opin Biol Ther, 2014; 14(9); 1295-317
50. Sabe M, Pillinger T, Kaiser S, Half a century of research on antipsychotics and schizophrenia: A scientometric study of hotspots, nodes, bursts, and trends: Neurosci Biobehav Rev, 2022; 136; 104608
51. Shibata N, Kajikawa Y, Takeda Y, Detecting emerging research fronts in regenerative medicine by the citation network analysis of scientific publications: Technol Forecast Soc, 2011; 78(2); 274-82
52. Chen C, CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature: J Am Soc Inf Sci Technol, 2006; 57(3); 359-77
53. Girvan M, Newman ME, Community structure in social and biological networks: Proc Natl Acad Sci USA, 2002; 99(12); 7821-26
54. Spencer JL, Waters EM, Romeo RD, Uncovering the mechanisms of estrogen effects on hippocampal function: Front Neuroendocrinol, 2008; 29(2); 219-37
55. Shumaker SA, Legault C, Rapp SR, Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial: JAMA, 2003; 289(20); 2651-62
56. Shumaker SA, Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study: JAMA, 2004; 291(24); 2947
57. Espeland MA, Rapp SR, Shumaker SA, Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study: JAMA, 2004; 291(24); 2959-68
58. Shao H, Breitner JC, Whitmer RA, Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study: Neurology, 2012; 79(18); 1846-52
59. Henderson VW, Sherwin BB, Surgical versus natural menopause: Cognitive issues: Menopause, 2007; 14(3 Pt 2); 572-79
60. Daniel JM, Hulst JL, Berbling JL, Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation: Endocrinology, 2006; 147(1); 607-14
61. Hojo Y, Hattori TA, Enami T, Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons: Proc Natl Acad Sci USA, 2004; 101(3); 865-70
62. Rosenfeld CS, Shay DA, Vieira-Potter VJ, Cognitive effects of aromatase and possible role in memory disorders: Front Endocrinol (Lausanne), 2018; 9; 610
63. Hortobagyi GN, Stemmer SM, Burris HA, Overall survival with ribociclib plus letrozole in advanced breast cancer: N Engl J Med, 2022; 386(10); 942-50
64. Bayer J, Rune G, Schultz H, The effect of estrogen synthesis inhibition on hippocampal memory: Psychoneuroendocrino, 2015; 56; 213-25
65. Li C, Zhou C, Li R, Can exercise ameliorate aromatase inhibitor-induced cognitive decline in breast cancer patients?: Mol Neurobiol, 2016; 53(6); 4238-46
66. Gervais NJ, Remage-Healey L, Starrett JR, Adverse effects of aromatase inhibition on the brain and behavior in a nonhuman primate: J Neurosci, 2019; 39(5); 918-28
67. Zhou L, Fester L, von Blittersdorff B, Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice: Endocrinology, 2010; 151(3); 1153-60
68. Filardo EJ, Quinn JA, Bland KI, Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF: Mol Endocrinol, 2000; 14(10); 1649-60
69. Hart D, Nilges M, Pollard K, Activation of the G-protein coupled receptor 30 (GPR30) has different effects on anxiety in male and female mice: Steroids, 2014; 81; 49-56
70. Kumar A, Bean LA, Rani A, Contribution of estrogen receptor subtypes, ERα, ERβ, and GPER1 in rapid estradiol-mediated enhancement of hippocampal synaptic transmission in mice: Hippocampus, 2015; 25(12); 1556-66
71. Fortress AM, Fan L, Orr PT, Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus: Learn Mem, 2013; 20(3); 147-55
72. Lymer J, Robinson A, Winters BD, Rapid effects of dorsal hippocampal G-protein coupled estrogen receptor on learning in female mice: Psychoneuroendocrino, 2017; 77; 131-40
73. Phan A, Lancaster KE, Armstrong JN, Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice: Endocrinology, 2011; 152(4); 1492-502
74. Hadjimarkou MM, Vasudevan N, GPER1/GPR30 in the brain: Crosstalk with classical estrogen receptors and implications for behavior: J Steroid Biochem Mol Biol, 2018; 176; 57-64
75. Galea L, Frick KM, Hampson E, Why estrogens matter for behavior and brain health: Neurosci Biobehav Rev, 2017; 76(Pt B); 363-79
76. Scharfman HE, MacLusky NJ, Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS: Front Neuroendocrinol, 2006; 27(4); 415-35
77. Luine V, Frankfurt M, Interactions between estradiol, BDNF and dendritic spines in promoting memory: Neuroscience, 2013; 239; 34-45
78. Zárate S, Stevnsner T, Gredilla R, Role of estrogen and other sex hormones in brain aging. neuroprotection and DNA repair: Front Aging Neurosci, 2017; 9; 430
79. Srivastava DP, Penzes P, Rapid estradiol modulation of neuronal connectivity and its implications for disease: Front Endocrinol (Lausanne), 2011; 2; 77
80. Srivastava DP, Woolfrey KM, Jones KA, Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity: Proc Natl Acad Sci USA, 2008; 105(38); 14650-55
81. Srivastava DP, Woolfrey KM, Liu F, Estrogen receptor β activity modulates synaptic signaling and structure: J Neurosci, 2010; 30(40); 13454-60
82. Srivastava DP, Woolfrey KM, Penzes P, Insights into rapid modulation of neuroplasticity by brain estrogens: Pharmacol Rev, 2013; 65(4); 1318-50
83. Zhang YY, Liu MY, Liu Z, GPR30-mediated estrogenic regulation of actin polymerization and spatial memory involves SRC-1 and PI3K-mTORC2 in the hippocampus of female mice: Cns Neurosci Ther, 2019; 25(6); 714-733
84. Carson RP, Fu C, Winzenburger P, Deletion of Rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex: Hum Mol Genet, 2013; 22(1); 140-52
85. Masri J, Bernath A, Martin J, mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor: Cancer Res, 2007; 67(24); 11712-20
86. Tuscher JJ, Luine V, Frankfurt M, Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus: J Neurosci, 2016; 36(5); 1483-89
87. Feng Y, Tian X, Zhang M, Treadmill exercise reverses the change of dendritic morphology and activates BNDF-mTOR signaling pathway in the hippocampus and cerebral cortex of ovariectomized mice: J Mol Neurosci, 2021; 71(9); 1849-62
88. Zhao Y, Yu Y, Zhang Y, Letrozole regulates actin cytoskeleton polymerization dynamics in a SRC-1 dependent manner in the hippocampus of mice: J Steroid Biochem Mol Biol, 2017; 167; 86-97
89. Liu M, Huangfu X, Zhao Y, Steroid receptor coactivator-1 mediates letrozole induced downregulation of postsynaptic protein PSD-95 in the hippocampus of adult female rats: J Steroid Biochem Mol Biol, 2015; 154; 168-75
90. Bian C, Zhu K, Guo Q, Sex differences and synchronous development of steroid receptor coactivator-1 and synaptic proteins in the hippocampus of postnatal female and male C57BL/6 mice: Steroids, 2012; 77(1–2); 149-56
91. Xing F, Zhao Y, Zhang Y, Nuclear and membrane estrogen receptor antagonists induce similar mTORC2 activation-reversible changes in synaptic protein expression and actin polymerization in the mouse hippocampus: Cns Neurosci Ther, 2018; 24(6); 495-507
92. Lan Z, Meng Z, Lian B, Hippocampal aromatase knockdown aggravates ovariectomy-induced spatial memory impairment, Aβ accumulation and neural plasticity deficiency in adult female mice: Neurochem Res, 2021; 46(5); 1188-202
93. Feng Y, Shi R, Hu J, Effects of neural-derived estradiol on actin polymerization and synaptic plasticity-related proteins in prefrontal and hippocampal cells of mice: Steroids, 2022; 177; 108935
94. Clark JA, Alves S, Gundlah C, Selective estrogen receptor-beta (SERM-beta) compounds modulate raphe nuclei tryptophan hydroxylase-1 (TPH-1) mRNA expression and cause antidepressant-like effects in the forced swim test: Neuropharmacology, 2012; 63(6); 1051-63
95. Ouchi Y, Banno Y, Shimizu Y, Reduced adult hippocampal neurogenesis and working memory deficits in the Dgcr8-deficient mouse model of 22q11.2 deletion-associated schizophrenia can be rescued by IGF2: J Neurosci, 2013; 33(22); 9408-19
96. Ferrón SR, Radford EJ, Domingo-Muelas A, Differential genomic imprinting regulates paracrine and autocrine roles of IGF2 in mouse adult neurogenesis: Nat Commun, 2015; 6; 8265
97. Furutachi S, Matsumoto A, Nakayama KI, p57 controls adult neural stem cell quiescence and modulates the pace of lifelong neurogenesis: Embo J, 2013; 32(7); 970-81
98. Larsen CM, Grattan DR, Prolactin, neurogenesis, and maternal behaviors: Brain Behav Immun, 2012; 26(2); 201-9
99. Ma DK, Jang MH, Guo JU, Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis: Science, 2009; 323(5917); 1074-77
100. Zou P, Muramatsu H, Miyata T, Midkine, a heparin-binding growth factor, is expressed in neural precursor cells and promotes their growth: J Neurochem, 2006; 99(6); 1470-79
101. Pompili A, Arnone B, Gasbarri A, Estrogens and memory in physiological and neuropathological conditions: Psychoneuroendocrino, 2012; 37(9); 1379-96
102. Goveas JS, Espeland MA, Woods NF, Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: The Women’s Health Initiative Memory Study: J Am Geriatr Soc, 2011; 59(1); 57-66
103. Rossouw JE, Anderson GL, Prentice RL, Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial: JAMA, 2002; 288(3); 321-33
104. Coker LH, Espeland MA, Rapp SR, Postmenopausal hormone therapy and cognitive outcomes: The Women’s Health Initiative Memory Study (WHIMS): J Steroid Biochem Mol Biol, 2010; 118(4–5); 304-10
105. Zhang T, Casanova R, Resnick SM, Effects of hormone therapy on brain volumes changes of postmenopausal women revealed by optimally-discriminative voxel-based morphometry: PLoS One, 2016; 11(3); e0150834
106. Espeland MA, Shumaker SA, Leng I, Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years: JAMA Intern Med, 2013; 173(15); 1429-36
107. Mehta JM, Chester RC, Kling JM, The timing hypothesis: hormone therapy for treating symptomatic women during menopause and its relationship to cardiovascular disease: J Womens Health (Larchmt), 2019; 28(5); 705-11
108. Maki PM, Hormone therapy and cognitive function: Is there a critical period for benefit?: Neuroscience, 2006; 138(3); 1027-30
109. Sherwin BB, Estrogen and cognitive aging in women: Neuroscience, 2006; 138(3); 1021-26
110. Ma Y, Liu M, Yang L, Loss of estrogen efficacy against hippocampus damage in long-term OVX mice is related to the reduction of hippocampus local estrogen production and estrogen receptor degradation: Mol Neurobiol, 2020; 57(8); 3540-51
111. Jew K, Herr D, Wong C, Selective memory and behavioral alterations after ambient ultrafine particulate matter exposure in aged 3xTgAD Alzheimer’s disease mice: Part Fibre Toxicol, 2019; 16(1); 45
112. Xu H, Baracskay P, O’Neill J, Assembly responses of hippocampal CA1 place cells predict learned behavior in goal-directed spatial tasks on the radial eight-arm maze: Neuron, 2019; 101(1); 119-132e4
113. Gerlai R, Behavioral tests of hippocampal function: simple paradigms complex problems: Behav Brain Res, 2001; 125(1–2); 269-77
114. Morgan D: Water maze tasks in mice: Special reference to Alzheimer’s transgenic mice, 2009, Boca Raton (FL), CRC Press/Taylor & Francis
115. Patel SA, Frick KM, Newhouse PA, Estradiol effects on spatial memory in women: Behav Brain Res, 2022; 417; 113592
116. Hampson E, Estrogens, aging, and working memory: Curr Psychiatry Rep, 2018; 20(12); 109
117. Daniel JM, Fader AJ, Spencer AL, Estrogen enhances performance of female rats during acquisition of a radial arm maze: Horm Behav, 1997; 32(3); 217-25
118. Bimonte HA, Denenberg VH, Estradiol facilitates performance as working memory load increases: Psychoneuroendocrino, 1999; 24(2); 161-73
119. Nelson BS, Springer RC, Daniel JM, Antagonism of brain insulin-like growth factor-1 receptors blocks estradiol effects on memory and levels of hippocampal synaptic proteins in ovariectomized rats: Psychopharmacology (Berl), 2014; 231(5); 899-907
120. Fader AJ, Johnson PE, Dohanich GP, Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze: Pharmacol Biochem Behav, 1999; 62(4); 711-17
121. Gibbs RB, Johnson DA, Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats: Endocrinology, 2008; 149(6); 3176-83
122. Pompili A, Tomaz C, Arnone B, Working and reference memory across the estrous cycle of rat: A long-term study in gonadally intact females: Behav Brain Res, 2010; 213(1); 10-8
123. Duff SJ, Hampson E, A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy: Horm Behav, 2000; 38(4); 262-76
124. Duff SJ, Hampson E, A sex difference on a novel spatial working memory task in humans: Brain Cogn, 2001; 47(3); 470-93
125. Keenan PA, Ezzat WH, Ginsburg K, Prefrontal cortex as the site of estrogen’s effect on cognition: Psychoneuroendocrino, 2001; 26(6); 577-90
126. Tabatadze N, Huang G, May RM, Sex differences in molecular signaling at inhibitory synapses in the hippocampus: J Neurosci, 2015; 35(32); 11252-65
127. Christensen DL, Baio J, Van Naarden BK, Prevalence and characteristics of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2012: MMWR Surveill Summ, 2016; 65(3); 1-23
128. Tschanz JT, Corcoran CD, Schwartz S, Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: The Cache County Dementia Progression study: Am J Geriatr Psychiatry, 2011; 19(6); 532-42
129. Woolley CS, His and Hers: Sex differences in the brain: Cerebrum, 2021; 2021; cer-02-21
130. Fan L, Zhao Z, Orr PT, Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation: J Neurosci, 2010; 30(12); 4390-400
131. Frick KM, Kim J, Mechanisms underlying the rapid effects of estradiol and progesterone on hippocampal memory consolidation in female rodents: Horm Behav, 2018; 104; 100-10
132. Koss WA, Haertel JM, Philippi SM, Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17β-Estradiol: eNeuro, 2018; 5(5); 0267-18
133. Huang GZ, Woolley CS, Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism: Neuron, 2012; 74(5); 801-8
134. Oberlander JG, Woolley CS, 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females: J Neurosci, 2016; 36(9); 2677-90
135. Jain A, Huang GZ, Woolley CS, Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus: J Neurosci, 2019; 39(9); 1552-65
136. Li X, Johann S, Rune GM, Sex-specific regulation of spine density and synaptic proteins by G-protein-coupled estrogen receptor (GPER)1 in developing hippocampus: Neuroscience, 2021; 472; 35-50
137. Chang PK, Boridy S, McKinney RA, Letrozole potentiates mitochondrial and dendritic spine impairments induced by β amyloid: J Aging Res, 2013; 2013; 538979
138. Vierk R, Glassmeier G, Zhou L, Aromatase inhibition abolishes LTP generation in female but not in male mice: J Neurosci, 2012; 32(24); 8116-26
139. Ruiz-Palmero I, Ortiz-Rodriguez A, Melcangi RC, Oestradiol synthesized by female neurons generates sex differences in neuritogenesis: Sci Rep, 2016; 6; 31891
140. Fester L, Prange-Kiel J, Zhou L, Estrogen-regulated synaptogenesis in the hippocampus: Sexual dimorphism in vivo but not in vitro: J Steroid Biochem Mol Biol, 2012; 131(1–2); 24-29
141. Kang L, Zhang X, Xie Y, Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling: Mol Endocrinol, 2010; 24(4); 709-21
142. Pupo M, Pisano A, Lappano R, Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts: Environ Health Perspect, 2012; 120(8); 1177-82
143. Brailoiu E, Dun SL, Brailoiu GC, Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system: J Endocrinol, 2007; 193(2); 311-21
144. Owman C, Nilsson C, Lolait SJ, Cloning of cDNA encoding a putative chemoattractant receptor: Genomics, 1996; 37(2); 187-94
145. Hazell GG, Yao ST, Roper JA, Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues: J Endocrinol, 2009; 202(2); 223-36
146. Waters EM, Thompson LI, Patel P, G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus: J Neurosci, 2015; 35(6); 2384-97
147. Matsuda K, Sakamoto H, Mori H, Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation: Neurosci Lett, 2008; 441(1); 94-99
148. Vajaria R, Vasudevan N, Is the membrane estrogen receptor, GPER1, a promiscuous receptor that modulates nuclear estrogen receptor-mediated functions in the brain?: Horm Behav, 2018; 104; 165-72
149. Bologa CG, Revankar CM, Young SM, Virtual and biomolecular screening converge on a selective agonist for GPR30: Nat Chem Biol, 2006; 2(4); 207-12
150. Petrie WK, Dennis MK, Hu C, G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth: Obstet Gynecol Int, 2013; 2013; 472720
151. Choleris E, Ogawa S, Kavaliers M, Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice: Genes Brain Behav, 2006; 5(7); 528-39
152. Gabor C, Lymer J, Phan A, Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice: Physiol Behav, 2015; 149; 53-60
153. Jacome LF, Gautreaux C, Inagaki T, Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines: Neurobiol Learn Mem, 2010; 94(4); 488-98
154. Liu F, Day M, Muñiz LC, Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory: Nat Neurosci, 2008; 11(3); 334-43
155. Prange-Kiel J, Wehrenberg U, Jarry H, Para/autocrine regulation of estrogen receptors in hippocampal neurons: Hippocampus, 2003; 13(2); 226-34
156. Wang C, Dehghani B, Magrisso IJ, GPR30 contributes to estrogen-induced thymic atrophy: Mol Endocrinol, 2008; 22(3); 636-48
157. Mårtensson UE, Salehi SA, Windahl S, Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice: Endocrinology, 2009; 150(2); 687-98
158. Otto C, Fuchs I, Kauselmann G, GPR30 does not mediate estrogenic responses in reproductive organs in mice: Biol Reprod, 2009; 80(1); 34-41
159. Isensee J, Meoli L, Zazzu V, Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice: Endocrinology, 2009; 150(4); 1722-30
160. Prossnitz ER, Hathaway HJ, What have we learned about GPER function in physiology and disease from knockout mice?: J Steroid Biochem Mol Biol, 2015; 153; 114-26
161. Liu SB, Zhang N, Guo YY, G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors: J Neurosci, 2012; 32(14); 4887-900
162. Liu SB, Tian Z, Guo YY, Activation of GPR30 attenuates chronic pain-related anxiety in ovariectomized mice: Psychoneuroendocrino, 2015; 53; 94-107
163. Fisher CR, Graves KH, Parlow AF, Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene: Proc Natl Acad Sci USA, 1998; 95(12); 6965-70
164. Nemoto Y, Toda K, Ono M, Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice: J Clin Invest, 2000; 105(12); 1819-25
165. Hill RA, Boon WC, Estrogens, brain, and behavior: Lessons from knockout mouse models: Semin Reprod Med, 2009; 27(3); 218-28
Figures
 Figure 1. (A) Network map of co-authors’ countries. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Country, Top N=20, Pruning: Pathfinder and Pruning the merged network, N (nodes)=42, and E (links)=48. (B) Network map of co-cited authors. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Cited author, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=54, and E=61. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 1. (A) Network map of co-authors’ countries. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Country, Top N=20, Pruning: Pathfinder and Pruning the merged network, N (nodes)=42, and E (links)=48. (B) Network map of co-cited authors. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Cited author, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=54, and E=61. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). Figure 2. (A) Network map of co-authors’ institutions. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Institution, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=121, and E=145. (B) Network map of co-authors. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Author, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=556, and E=899. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 2. (A) Network map of co-authors’ institutions. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Institution, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=121, and E=145. (B) Network map of co-authors. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Author, Top N=20, Pruning: Pathfinder and Pruning the merged network, N=556, and E=899. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). Figure 3. Network map of co-cited reference. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Reference, Top N=50, Pruning: Pathfinder and Pruning the merged network, N=328, E=348. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 3. Network map of co-cited reference. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: Reference, Top N=50, Pruning: Pathfinder and Pruning the merged network, N=328, E=348. Above the picture is the timeline with color (2010–2021). The color of a link represents the earliest time slice in which the connection was first made. A node of high betweenness centrality is usually one that connects 2 or more large groups of nodes. A node with a strong betweenness centrality score has a great influence on a network. High betweenness centrality is represented by the thickness of a purple ring. Citation burst is revealed by the presence of red tree rings. The thicker the red tree rings, the more burst for the corresponding node. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). Figure 4. The top 50 co-cited references with the strongest citation bursts sorted by burst time. The burst detection was based on the citations made by the top 50 articles per year during the 12-year period, and the top 100 references with the strongest citation bursts from 2010 to 2021 were identified. Citation burst information include citation information, citation burst strength, burst start time, burst end time, and burst start-to-end time axis diagram. Red bars: Duration of burst. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 4. The top 50 co-cited references with the strongest citation bursts sorted by burst time. The burst detection was based on the citations made by the top 50 articles per year during the 12-year period, and the top 100 references with the strongest citation bursts from 2010 to 2021 were identified. Citation burst information include citation information, citation burst strength, burst start time, burst end time, and burst start-to-end time axis diagram. Red bars: Duration of burst. (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). Figure 5. Network map of co-occurring keyword clusters. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: keywords, Top N=50, pathfinder and pruning the merged network, N=124, E=145, choose all in one, the keywords sources, the Log-likelihood ratio (LLR) algorithm to label clusters, the Modularity (Q=0.8266), and the Mean Silhouette (S=0.978). #0 expression (S=0.963); #1 women (S=0.956); #2 sex (S=1); #3 working memory(S=1); #4 health initiative memory (S=1); #5 receptor(S=1); #6 mice (S=0.897); #7 dendritic spine density(S=0.979); #8 neurogenesis (S=1); #9 hippocampal neuron (S=1). (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA).
Figure 5. Network map of co-occurring keyword clusters. Time slicing: January 2010 to September 2021, Slice length: 1 year, Node types: keywords, Top N=50, pathfinder and pruning the merged network, N=124, E=145, choose all in one, the keywords sources, the Log-likelihood ratio (LLR) algorithm to label clusters, the Modularity (Q=0.8266), and the Mean Silhouette (S=0.978). #0 expression (S=0.963); #1 women (S=0.956); #2 sex (S=1); #3 working memory(S=1); #4 health initiative memory (S=1); #5 receptor(S=1); #6 mice (S=0.897); #7 dendritic spine density(S=0.979); #8 neurogenesis (S=1); #9 hippocampal neuron (S=1). (Software: CiteSpace 5.7.R5W, Drexel University, Philadelphia, USA). Tables
 Table 1. Search strategy from Web of Science core collection.
Table 1. Search strategy from Web of Science core collection. Table 2. CiteSpace analysis terms.
Table 2. CiteSpace analysis terms. Table 3. Top 5 countries and institutions ranked by number of publications.
Table 3. Top 5 countries and institutions ranked by number of publications. Table 4. Top 5 countries and institutions ranked by centrality.
Table 4. Top 5 countries and institutions ranked by centrality. Table 5. Top 5 authors ranked by number of citations.
Table 5. Top 5 authors ranked by number of citations. Table 6. Top 5 authors ranked by centrality.
Table 6. Top 5 authors ranked by centrality. Table 7. Top 5 authors ranked by Sigma.
Table 7. Top 5 authors ranked by Sigma. Table 8. Top 5 authors ranked by burst strength.
Table 8. Top 5 authors ranked by burst strength. Table 9. Top 5 authors ranked by number of publications.
Table 9. Top 5 authors ranked by number of publications. Table 10. Top 5 co-cited references ranked by number of citations.
Table 10. Top 5 co-cited references ranked by number of citations. Table 11. Top 5 co-cited references ranked by centrality.
Table 11. Top 5 co-cited references ranked by centrality. Table 12. Top 5 co-cited references ranked by Sigma.
Table 12. Top 5 co-cited references ranked by Sigma. Table 13. Top 5 co-cited references with the strongest citation bursts during 2010–2021.
Table 13. Top 5 co-cited references with the strongest citation bursts during 2010–2021. Table 14. Co-cited references with the most recent bursts from 2018.
Table 14. Co-cited references with the most recent bursts from 2018. Table 1. Search strategy from Web of Science core collection.
Table 1. Search strategy from Web of Science core collection. Table 2. CiteSpace analysis terms.
Table 2. CiteSpace analysis terms. Table 3. Top 5 countries and institutions ranked by number of publications.
Table 3. Top 5 countries and institutions ranked by number of publications. Table 4. Top 5 countries and institutions ranked by centrality.
Table 4. Top 5 countries and institutions ranked by centrality. Table 5. Top 5 authors ranked by number of citations.
Table 5. Top 5 authors ranked by number of citations. Table 6. Top 5 authors ranked by centrality.
Table 6. Top 5 authors ranked by centrality. Table 7. Top 5 authors ranked by Sigma.
Table 7. Top 5 authors ranked by Sigma. Table 8. Top 5 authors ranked by burst strength.
Table 8. Top 5 authors ranked by burst strength. Table 9. Top 5 authors ranked by number of publications.
Table 9. Top 5 authors ranked by number of publications. Table 10. Top 5 co-cited references ranked by number of citations.
Table 10. Top 5 co-cited references ranked by number of citations. Table 11. Top 5 co-cited references ranked by centrality.
Table 11. Top 5 co-cited references ranked by centrality. Table 12. Top 5 co-cited references ranked by Sigma.
Table 12. Top 5 co-cited references ranked by Sigma. Table 13. Top 5 co-cited references with the strongest citation bursts during 2010–2021.
Table 13. Top 5 co-cited references with the strongest citation bursts during 2010–2021. Table 14. Co-cited references with the most recent bursts from 2018.
Table 14. Co-cited references with the most recent bursts from 2018. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








