19 June 2023: Clinical Research
B-Type Natriuretic Peptide Can Distinguish Ischemic Stroke Subtypes Better than Other Cardiac Biomarkers
Ping Liang12ABCEF, Shuang Han12ADG, Yucen Jiang12B, Huikai Miao3BD*, Xiaowei Qi12ADGDOI: 10.12659/MSM.939829
Med Sci Monit 2023; 29:e939829
Abstract
BACKGROUND: The primary benefit to patients of being able to distinguish among subtypes of ischemic stroke (IS) is creation of a better treatment decision-making process. Current classification methods are complex and time-consuming, requiring hours to days. Blood-based cardiac biomarker measurements have the potential to improve mechanism classification of ischemic stroke.
MATERIAL AND METHODS: In this study, 223 patients with IS were selected as the case group and 75 healthy people who underwent physical examination at the same time were selected as the control group. The chemiluminescent immunoassay (CLIA) method established in this study was used to quantitatively detect plasma B-type natriuretic peptide (BNP) levels in subjects. All subjects were assessed for serum creatine kinase isoenzyme-MB (CK-MB), cardiac troponin I (cTnI), and myoglobin (MYO) after admission. We investigated the effectiveness of BNP and other cardiac biomarkers in the diagnosis of different subtypes of IS.
RESULTS: The levels of the 4 cardiac biomarkers were increased in IS patients. BNP could better diagnose different types of IS compared to other cardiac biomarkers, and BNP combined with other cardiac biomarkers was better than a single indicator in diagnosing IS.
CONCLUSIONS: Compared with other cardiac biomarkers, BNP is a better marker for the diagnosis of different subtypes of ischemic stroke. Routine screening for BNP in IS patients is recommended to improve the treatment decision-making process and minimize the time to thrombosis, thereby providing a more precise treatment for patients with different subtypes of stroke.
Keywords: Atrial Natriuretic Factor Receptor B, Clinical Laboratory Techniques, ischemic stroke, Luminescence, Humans, Natriuretic Peptide, Brain, biomarkers, Stroke, Creatine Kinase, MB Form
Background
Stroke is the second leading cause of disability and death in the world [1]. Ischemic stroke (IS) accounts for 87% of all strokes and is further subtyped into cardioembolic, atherosclerosis, lacunar, other causes, and cryptogenic strokes [2]. The primary value of the classification of patients with ischemic stroke lies in a better treatment decision-making process. Different subtypes have different treatments. Anticoagulant therapy is the best treatment for cardiogenic embolic stroke, and antiplatelet therapy is recommended for atherosclerotic and other non-embolic stroke [3]. “Time is brain” because delays in diagnostic time are associated with worsening patient outcomes [4]. Currently, the neuroimaging of acute stroke is still dominated by computed tomography (CT) [5]. Non-enhanced CT scans of the head are rapid, sensitive, and cost-effective in excluding intracranial hemorrhage, which is usually sufficient to make a thrombolytic decision. However, the sensitivity and specificity of CT scans for acute ischemia are relatively poor [6], and even after comprehensive examination, as many as one-third of patients still have unknown causes [7]. Magnetic resolution imaging (MRI) technology has higher sensitivity compared with CT. However, its main disadvantages include high cost, unavailability in the acute phase, and MRI detection is not available for patients with metal implants (pacemakers, implantable defibrillators) or claustrophobia [8]. In addition, the pathophysiological process of stroke involving brain injury and repair needs further exploration.
Biomarkers are objective indicators used to evaluate therapeutic responses and predict outcomes. Blood-based biomarker detection has the advantages of convenience, early use, and non-invasiveness. Blood biomarker measurement is expected to improve the classification mechanism of ischemic stroke [9]. The number of candidate blood markers for stroke is increasing. Different stroke subtypes have different pathologic bases, such as atherosclerosis and cardiac embolism. While there are currently no biomarkers that provide precise information on the cause of stroke, several biomarkers linked to heart disease have recently been discovered. Peddada et al [10] reported a higher risk of in-hospital death in IS patients with abnormal cardiac troponin (cTn) levels. However, the prognostic value of elevated cTn levels in stroke patients remains controversial. It has been found that creatine kinase-MB (CK-MB) is elevated in some stroke patients, but there is no clear evidence that the elevation is caused by myocardial injury [11]. Myoglobin (MYO) is also a marker of myocardial injury, but there have been few studies in IS patients [12]. As a cardiac hormone, B-type natriuretic peptide (BNP) has shown good value in the diagnosis of cardiogenic stroke [13,14], but its utility in the diagnosis of other stroke subtypes remains unclear.
The present study focused on BNP and established a chemiluminescent immunoassay (CLIA) method to quantitatively detect plasma BNP levels in subjects and explore its relationship with different IS subtypes in combination with other cardiac biomarkers to find the best biomarker that can distinguish among IS subtypes.
Material and Methods
MAIN REAGENTS AND MATERIALS:
BNP antigen was purchased from Lanyu Biotechnology (Nanjing, China). BNP antibody was purchased from Tansheng Technology (Chongqing, China). Enterprise reference products, substrates, biotinylated diluents, and enzyme conjugate diluents were provided by Baiming Biotechnology (Wuxi, China). Chemiluminescence immunoluminescence instrument was purchased from Antu Experimental Instrument (Zhengzhou, China). The electronic balance was purchased from Sartorius (Germany). We purchased pH meters from Mettler Toledo (Switzerland).
PREPARATION OF BIOTINYLATED ANTIBODY:
BNP antibody and Sulfo-NHS-LC-Biotin solids were dissolved in PBS buffer and the concentration was adjusted to 1 mg/mL. The dissolved antibody solution and biotin solution were sent into a centrifuge tube for vortex shaking, sealed with a sealing film, and placed in a homogenizer at 37°C in a constant-temperature incubator for 1 hour. After incubation was complete, dialysis was performed in a 14 000 Da dialysis bag by PBS buffer for 4 hours, then the PBS buffer was replaced and dialysis was continued overnight (16–18 hours).
PREPARATION OF HORSERADISH PEROXIDASE (HRP) BINDING ANTIBODY:
Various amounts of BNP antibody and EDC were completely dissolved in separated 0.05 M sodium bicarbonate solutions, then the solutions were mixed after the solute dissolved completely. HRP was dissolved in 0.1 M phosphate buffer (pH 7.2). The resulting mixed solution was added to the HRP solution and the pH was lowered to 5.8 with diluted hydrochloric acid. Then, the mixture was incubated for 5 hours at room temperature. Finally, the impurities were removed with a desalination column [15].
REACTION PROCESS:
We added 50 μL sample and 60 μL HRP-labeled antibody to the microplates pre-coated with captured antibody. The microplates were incubated for 15 minutes at 37°C, and then washed 3 times using washing buffer. Finally, the substrate was added and the relative light units (RLU) was measured.
STUDY POPULATION:
Using the American Heart Association/American Stroke Association (AHA/ASA) 2018 Guidelines [16], 223 patients with IS were selected as the case group and 75 healthy people who underwent physical examination at the same time were selected as the control group. Age, sex distribution, and body mass index were comparable between the 2 groups. The CLIA method developed for this study was used to quantitatively detect plasma BNP levels in subjects. A complete stroke etiology evaluation was completed to determine and classify ischemic stroke etiology by the Trial of Org 10172 in Acute Stroke Treatment (TOAST) [2] criteria for each enrolled patient. The clinical evaluation included neuroimaging (MRI or CT brain), cerebrovascular imaging (MRI angiogram or CT angiogram of the head and neck), cardiac assessment (echocardiography, electrocardiogram), and vascular risk factor screening (chronic history and laboratory tests). The severity of neurological impairment was assessed using the National Institutes of Health Stroke Scale (NIHSS).
As defined by the TOAST classification, the subtypes of large-artery atherosclerosis (LAA) and cardioembolism (CE) include clinical findings (cortical or brainstem injury or cerebellar dysfunction) and brain imaging findings (infarction >1.5 cm). In “large-artery atherosclerosis”, intracranial or extracranial artery stenosis greater than 50% should be demonstrated. In “cardiogenic embolism”, it is necessary to have high- or moderate-risk cardiogenic embolism. Diagnosis of “small-vessel occlusion” (SVO) requires traditional clinical lacunar syndrome with no evidence of cerebral cortical dysfunction, normal CT/MRI findings, or associated brain stem or subcortical hemisphere lesions less than 1.5 cm in diameter [17]. We excluded patients whose stroke was due to another determined cause per the TOAST criteria (dissection, vasculitis, hypercoagulable state) or was cryptogenic, as well as patients with cerebral hemorrhage, hemorrhagic infarction, mental illness, or impaired consciousness [18]. The study population was not affected by malignancies, systemic infections or autoimmune diseases. This study was approved by the institutional ethics committee.
Venous blood was collected from all subjects at admission, and some of the blood samples were immediately sent to the laboratory department of the hospital for detection of cTnI (Access hsTnI kit, Catalog Number: 23379), CK-MB (Access CK-MB kit, Catalog Number: 386371) and MYO (Access Myoglobin kit, Catalog Number: 973243) content using a Beckman automatic chemiluminescence immunoanalyzer (DXI 800). The remaining blood samples were used for the BNP detection test. The CLIA method developed for this study was used to quantitatively detect plasma BNP levels in subjects.
DATA ANALYSIS:
SPSS 25.0 was used to analyze the data. GraphPad Prism 9.0 (Figures 1, 2 and Supplementary Figures 1–6) and PowerPoint (Supplementary Figure 7) were used for graph drawing. Receiver operating characteristic (ROC) curves were used to analyze the effect of BNP and other cardiac biomarkers in predicting different types of IS. Univariable and multivariable logistic regression were used to analyze the independent influencing factors of IS patients. P<0.05 indicated that the difference was statistically significant.
Results
OPTIMIZATION OF WORKING CONCENTRATION AND TIME OF ANTIBODY:
A square array experiment was conducted with biotin-labeled antibody and HRP-labeled antibody, and a series of calibrators were measured, as shown in Supplementary Figure 1. When BNP-biotin was 5 ug/mL and BNP-HRP was 1 ug/mL (Condition 9), the RLU was separated by an obvious gradient. Therefore, this combination was selected as the working concentration of antibody in this study. We measured the variations of RLU with reaction times for different concentrations of calibrators. The results showed that the 2 concentrations of calibrators showed the same trend, and the RLU reached stability after the reaction time reached 15 minutes. As shown in Supplementary Figure 2, RLU was relatively stable within 0 to 3 minutes after substrate addition, and began to decline when the action times exceeded 3 minutes, so it was appropriate to detect RLU within 3 minutes after substrate addition.
EVALUATION OF METHOD PERFORMANCE:
The method performance was evaluated according to the EP guidelines of the Clinical and Laboratory Standards Institute (CLSI). The limits of detection (LOD) in 3 experiments were 1.21 pg/mL, 1.12 pg/mL, and 1.44 pg/mL, so the LOD in this study was set to 1.5 pg/mL, as shown in Supplementary Table 1. The bias of the quality control products measured in this study was between −2.28~1.40%, as shown in Supplementary Table 2. The intra-assay and inter-assay coefficients of variation (CV) measured in this study were lower than 10% (Supplementary Table 3). A high-concentration sample was gradient diluted with a low-concentration sample for the linearity test. The linear correlation coefficient of BNP reagent measured in this study was 0.9958 (Supplementary Figure 4). The reagent had good thermal stability, with signal retention ≥70% after 7 days at 37°C (Supplementary Table 4).
CLINICAL EVALUATION:
A schematic diagram of the CLIA method established in this experiment is shown in Supplementary Figure 7. The test result showed good correlation between the developed method and Beckman-Coulter (sample range: 5~4456 pg/mL). The correlation coefficient was 0.9884 and the slope was 0.9750 (Supplementary Figure 5).
BASELINE CHARACTERISTICS OF THE STUDY POPULATION:
A total of 293 patients with suspected stroke were evaluated during the study period. Of these, 52 had intracerebral hemorrhage, 11 had other stroke-like pathologic processes, 5 ischemic stroke patients met the exclusion criteria, and in 2 we were unable to obtain plasma samples. Therefore, the final number of patients included was 223. The enrolled IS patients included 67 SVO patients, 104 LAA patients, and 52 CE patients. Age, sex, and BMI were comparable between groups (P>0.05). After statistical analysis of the results, it was found that the proportion of hypertension and diabetes history in IS patients was significantly higher than that in healthy subjects. According to the NIHSS score, among the IS patients, the CE group was the most serious (P<0.001). See Table 1.
CARDIAC BIOMARKERS ASSOCIATED WITH VASCULAR RISK FACTORS AND STROKE SEVERITY:
We analyzed the effects of vascular risk factors on cardiac biomarker levels, and the results showed that hypertension increased the levels of BNP, cTnI, and MYO (all P<0.05); coronary heart disease and previous stroke increased BNP, CK-MB, cTnI and MYO levels (all P<0.05); and diabetes mellitus increased cTnI levels (P=0.006). In addition, we found a slight association between the severity of neurological deficits at admission (NIHSS scores) and cardiac biomarkers: BNP (Supplementary Figure 5A, r=0.345, P<0.001); CK-MB (Supplementary Figure 5B, r=0.317, P<0.001); cTnI (Supplementary Figure 5C, r=0.337, P<0.001), and MYO (Supplementary Figure 5D, r=0.399, P<0.001).
COMPARISON OF BNP AND OTHER CARDIAC MARKERS BETWEEN THE EXPERIMENTAL GROUP AND THE CONTROL GROUP:
The levels of the 4 cardiac biomarkers were higher in IS patients than in healthy people (all P<0.001), as shown in Table 2. The levels of BNP, CK-MB, cTnI, and MYO were lowest in the SVO group and highest in the CE group, and the differences were statistically significant. There was no significant difference in CK-MB and MYO levels between the control group and the non-CE group (Table 2, Figure 1).
ESTABLISHING THE JOINT DIAGNOSIS MODEL OF BNP, CK-MB, CTNI, AND MYO:
We performed diagnostic tests on the 4 cardiac biomarkers according to clinical classification, and the results showed that BNP had the highest AUCs in diagnosing patients in the SVO, LAA, and CE groups, which were SVO (AUC=0.725,95% CI: 0.64–0.81); LAA (AUC=0.808, 95% CI: 0.75–0.87); and CE (AUC=0.924, 95% CI: 0.87–0.98). This showed that BNP had good utility in the diagnosis of 3 clinical subtypes of IS. The regression models constructed in combination with other cardiac biomarkers were as follows:
In 3 clinical subtypes, AUCs were increased after BNP combined with other cardiac biomarkers, which indicated that combined diagnosis using these 4 cardiac biomarkers is better than using a single indicator (Table 3, Figures 2, 3).
INFLUENTIAL FACTORS OF ISCHEMIC STROKE:
Univariable and multivariable logistic regression analyses were performed on the influencing factors of patients with different subtypes of ischemic stroke. The results showed that after multivariate adjustment, hypertension history, plasma BNP, cTnI, uric acid (UA), and total cholesterol (TC) levels were independent risk factors for SVO. History of hypertension, plasma BNP, cTnI, UA, and apolipoprotein A1 (apo-A1) levels were independent risk factors for LAA. Plasma BNP, cTnI, UA, and apo-A1 levels were independent risk factors for CE (all P<0.05). See Tables 4–6.
Discussion
It is clear that early classification of IS patients can help to better treat patients and improve their prognosis. However, current methods for classifying mechanisms of ischemic stroke are complex, time-consuming (taking hours to days), and require specialized personnel. The currently preferred method for diagnosis of suspected stroke patients is CT. However, it is less sensitive to new or smaller ischemic lesions. MRI is more sensitive than CT, but it has many limitations, including high cost, limited availability, and longer examination time [19]. Blood-based cardiac biomarker detection has the advantages of convenience, early performance, and non-invasiveness, which is expected to improve the classification mechanism of ischemic stroke, and can be considered for early detection of high-risk patients and rapid development of treatment strategies [20,21].
BNP is secreted by ventricles after myocardial cells overstretch. It is mainly used as a cardiovascular biomarker for diagnosis and monitoring of heart failure [22,23]. In the present study, BNP was found to be a better predictor of different subtypes of IS than were other cardiac biomarkers. It has promising potential to be a good marker for patients with different subtypes of IS. The BNP assay kit developed in this study has the advantages of fast detection, high accuracy, simple operation, and low cost. It is particularly suitable for point-of-care testing (POCT) and small clinical laboratory applications.
In addition, we also conducted a combined diagnosis, and the results showed that BNP combined with other cardiac biomarkers was superior to a single indicator in the diagnosis of IS and its subtypes. We also noted that cTnI and MYO had good diagnostic effects for IS patients in the CE group except for BNP. When cTnI levels exceeded 0.005 ng/mL, CE could be predicted (sensitivity 0.885, specificity 0.907). Some studies have reported that cTnI is more common in CE because they have evidence of conditions such as atrial fibrillation and ischemic heart disease [24]. When MYO levels exceeded 36.5 ng/mL, CE can be predicted (sensitivity 0.846, specificity 0.96). There are few studies on MYO in IS subtypes, and an increased sample size may be considered to further explore the value of MYO in the diagnosis of different subtypes of IS. Although CK-MB levels were elevated in patients with IS, its diagnostic value in IS was not found in this study, which may be related to the study population.
The influencing factors of ischemic stroke patients with different subtypes were slightly different. cTnI levels and UA levels, in addition to BNP, were also common factors in the 3 subtypes of IS patients. Cardiac troponin is an important biomarker of acute myocardial infarction (AMI) and has been routinely studied in the prognosis of IS patients [25]. However, the prognostic value of elevated cTnI levels in stroke patients remains controversial. UA is a catabolism product of purine nucleotides, which are major components of DNA, RNA, and cellular energy stores. UA levels decline rapidly after acute stroke, which is associated with poor stroke outcomes [26]. History of hypertension was an independent factor in the non-CE groups (SVO and LAA), while apo-A1 levels were an independent factor in the LAA and CE groups. Hypertension is the most important risk factor for all types of stroke: IS, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH) [27]. Chronic hypertension can cause IS through a variety of mechanisms. First of all, high blood pressure causes great pressure on the endothelium, which is an important factor in the formation of atherosclerosis. In addition, endothelial injury and altered hemocyte-endothelial interactions can lead to local thrombosis and ischemic lesions [28]. Apolipoprotein A is related to IS caused by atherosclerosis. It has been reported that apolipoprotein A can distinguish atherothrombotic stroke from lacunar infarction, and is related to the severity of atherosclerotic stroke [29]. In addition, the SVO group was also affected by total cholesterol levels, possibly related to sample collection.
In this study, we established a CLIA method to quantitatively detect plasma BNP levels in subjects, and discussed the diagnostic ability of BNP in different subtypes of IS patients compared with other cardiac biomarkers. The results showed that the levels of 4 biomarkers were elevated in IS patients, with the highest biomarker levels in the CE group. In addition, BNP was a better predictor of different subtypes of IS than were other cardiac biomarkers. BNP is a better marker for different subtypes of IS patients in comparison with the others mentioned above. Routine screening for BNP in IS patients is recommended to improve the treatment decision-making process and minimize the time to thrombosis, thereby providing a more precise treatment for patients with various subtypes of stroke.
This study has some shortcomings. First, the sample size was 223 cases, which is small. In the future, larger-sample, multicenter, and multistage studies will be used to explore the diagnostic value of BNP, so as to further optimize the diagnostic efficacy. Secondly, the diagnostic ability of BNP levels in other subtypes of stroke needs to be studied to benefit a broader range of patients.
Conclusions
In this study, we established a CLIA method to quantitatively detect plasma BNP levels in subjects, and discussed the diagnostic ability of BNP in different subtypes of IS patients compared with other cardiac biomarkers. The results showed that the levels of the 4 biomarkers were elevated in IS patients, with the highest biomarker levels in the CE group. In addition, BNP was a better predictor of different subtypes of IS than were other cardiac biomarkers. BNP is a better marker for different subtypes of IS patients in comparison with the other markers mentioned above. Routine screening for BNP in IS patients is recommended to improve the treatment decision-making process and minimize the time to thrombosis, thereby providing more precise treatment for patients with various subtypes of stroke.
Figures
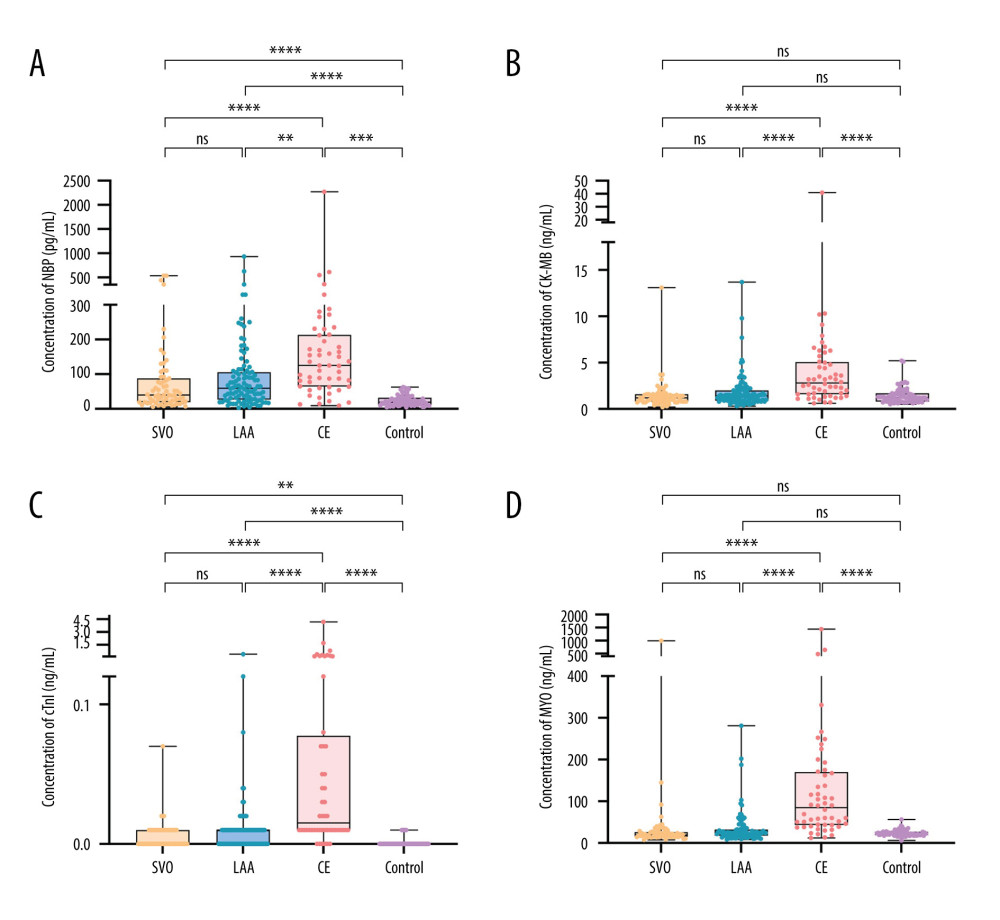 Figure 1. Levels distribution of BNP, CK-MB, cTnI, and MYO in IS group and control group. (A) BNP levels in IS group and control group. (B) CK-MB levels in IS group and control group. (C) cTnI levels in IS group and control group. (D) MYO levels in IS group and control group. * P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001; ns, nonsignificant.
Figure 1. Levels distribution of BNP, CK-MB, cTnI, and MYO in IS group and control group. (A) BNP levels in IS group and control group. (B) CK-MB levels in IS group and control group. (C) cTnI levels in IS group and control group. (D) MYO levels in IS group and control group. * P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001; ns, nonsignificant. 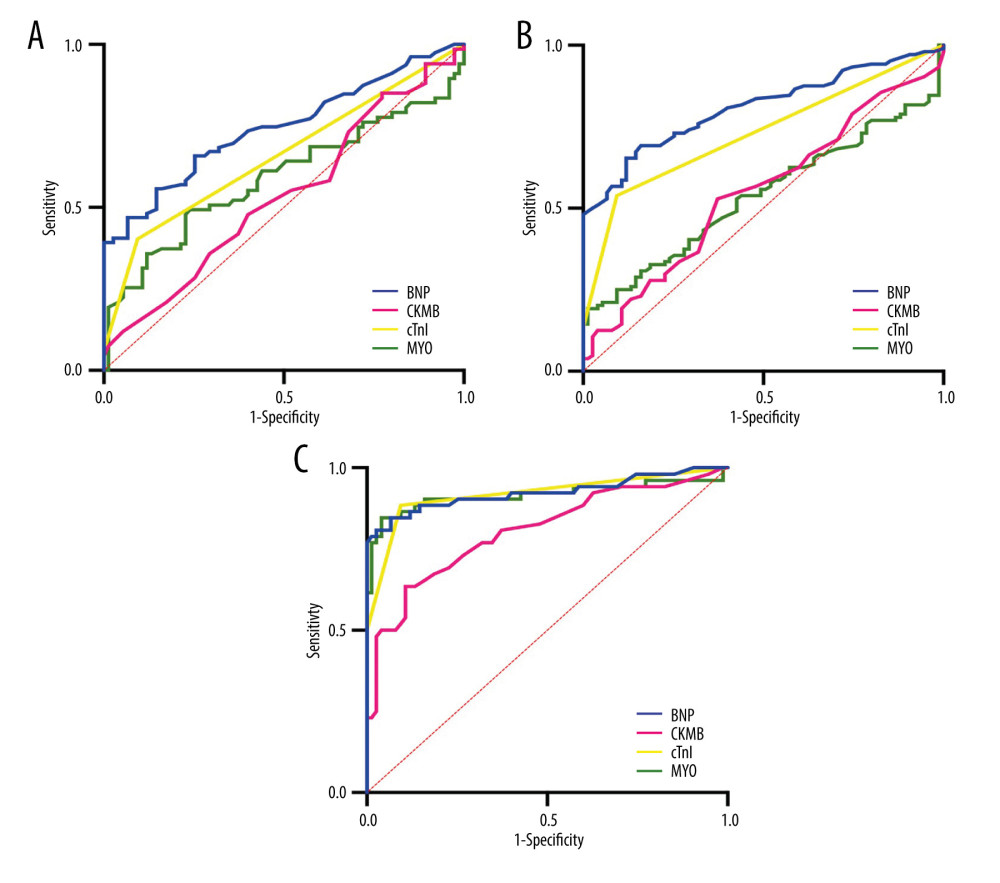 Figure 2. Diagnostic value of 4 cardiac biomarkers for different subtypes of IS. (A) ROC curves of 4 cardiac biomarkers in the SVO group; (B) ROC curves of 4 cardiac biomarkers in the LAA group; (C) ROC curves of 4 cardiac biomarkers in CE group.
Figure 2. Diagnostic value of 4 cardiac biomarkers for different subtypes of IS. (A) ROC curves of 4 cardiac biomarkers in the SVO group; (B) ROC curves of 4 cardiac biomarkers in the LAA group; (C) ROC curves of 4 cardiac biomarkers in CE group. 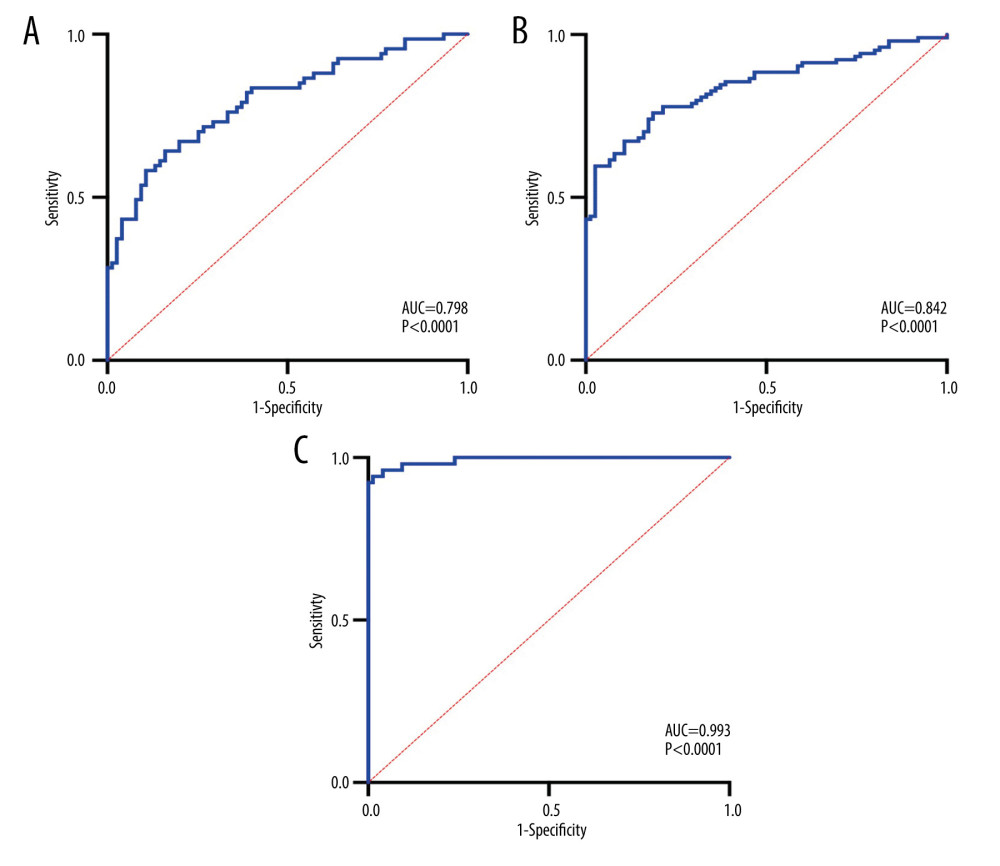 Figure 3. Combined diagnostic value of 4 cardiac biomarkers for different subtypes of IS. (A) ROC curve for the combined diagnosis of SVO with 4 cardiac biomarkers; (B) ROC curve for the combined diagnosis of LAA with 4 cardiac biomarkers; (C) ROC curve for the combined diagnosis of CE with 4 cardiac biomarkers.
Figure 3. Combined diagnostic value of 4 cardiac biomarkers for different subtypes of IS. (A) ROC curve for the combined diagnosis of SVO with 4 cardiac biomarkers; (B) ROC curve for the combined diagnosis of LAA with 4 cardiac biomarkers; (C) ROC curve for the combined diagnosis of CE with 4 cardiac biomarkers. Tables
Table 1. Baseline characteristics of the study population.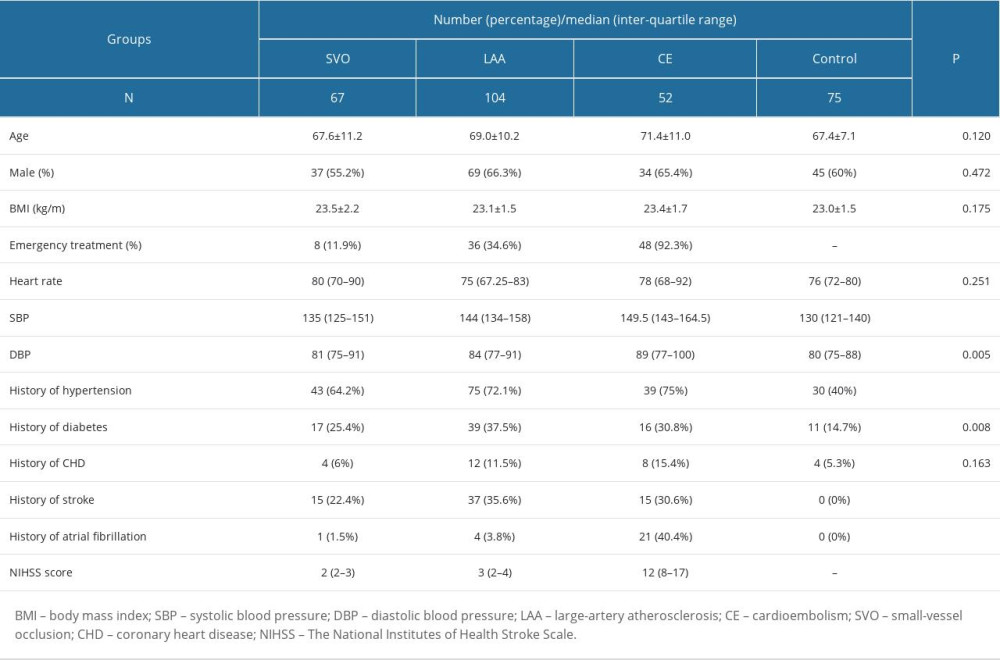 Table 2. Levels of BNP and other cardiac biomarkers in IS patients and healthy people.
Table 2. Levels of BNP and other cardiac biomarkers in IS patients and healthy people.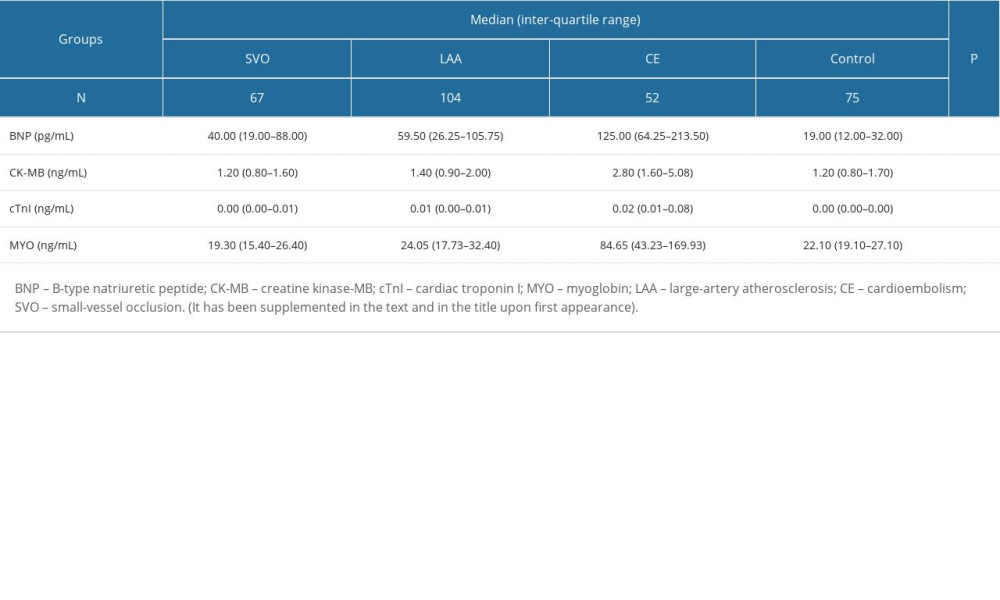 Table 3. Diagnostic test results of IS patients.
Table 3. Diagnostic test results of IS patients.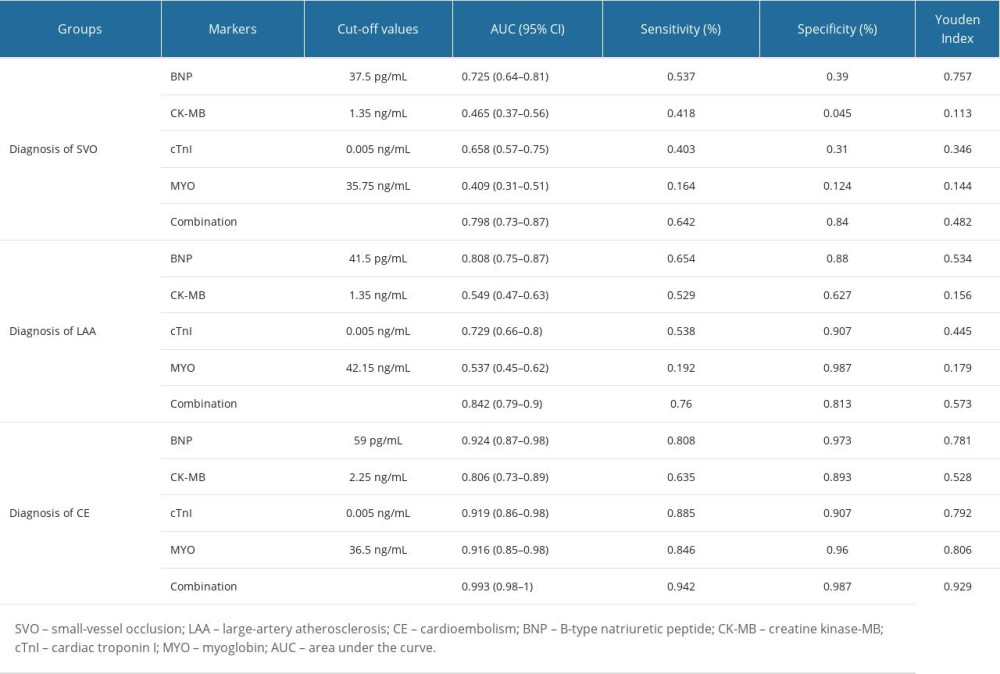 Table 4. Influential factors of small-vessel occlusion ischemic stroke.
Table 4. Influential factors of small-vessel occlusion ischemic stroke.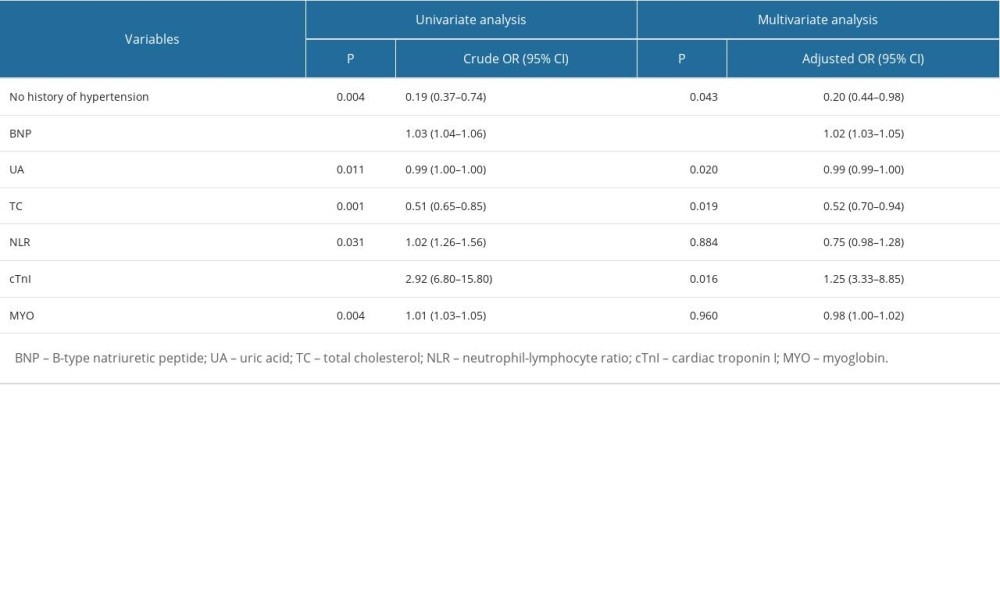 Table 5. Influential factors of large-artery atherosclerosis ischemic stroke.
Table 5. Influential factors of large-artery atherosclerosis ischemic stroke.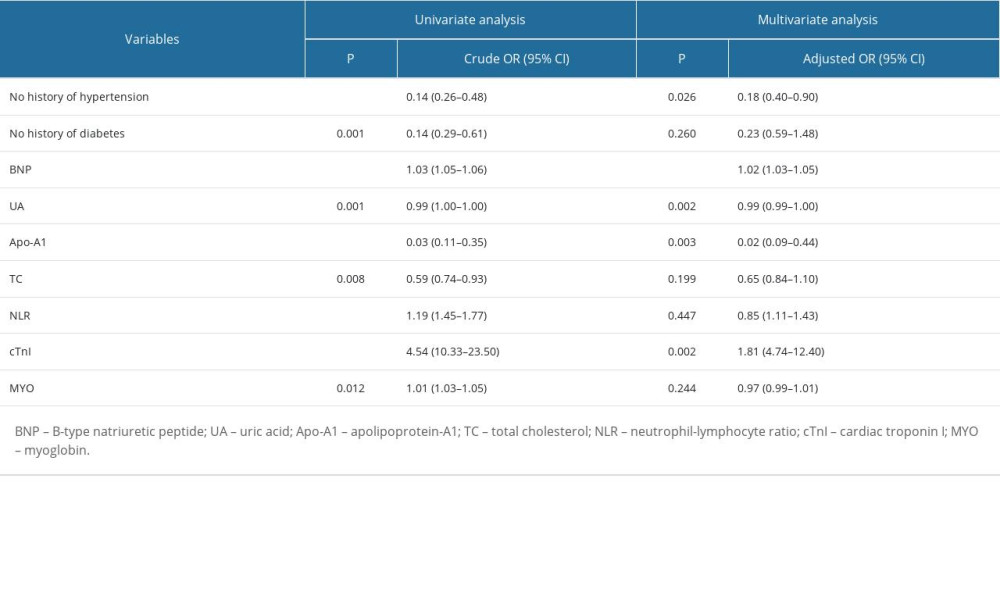 Table 6. Influential factors of cardioembolism ischemic stroke.
Table 6. Influential factors of cardioembolism ischemic stroke.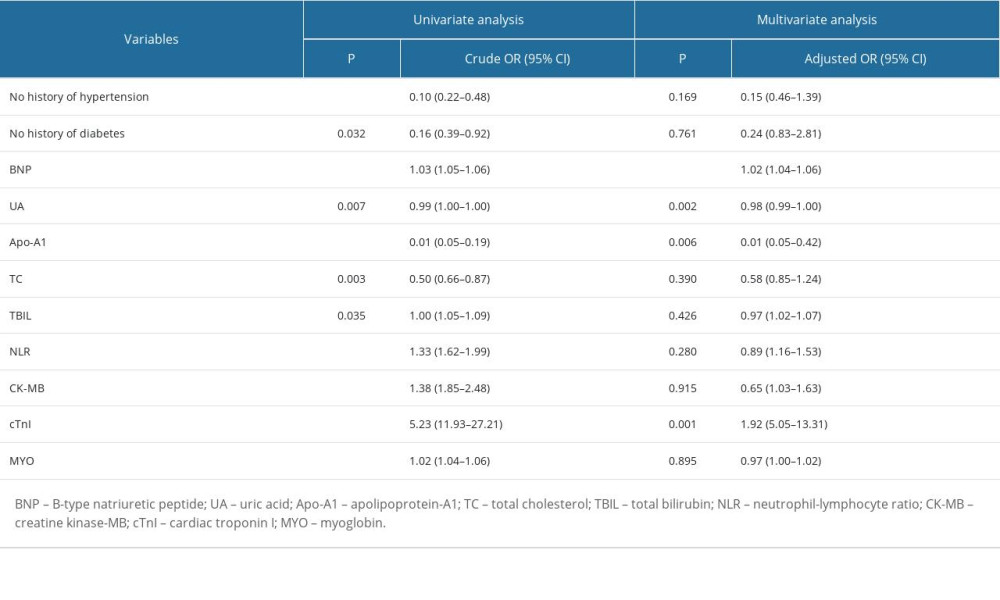 Supplementary Table 1. Verification results of the limit of detection.
Supplementary Table 1. Verification results of the limit of detection.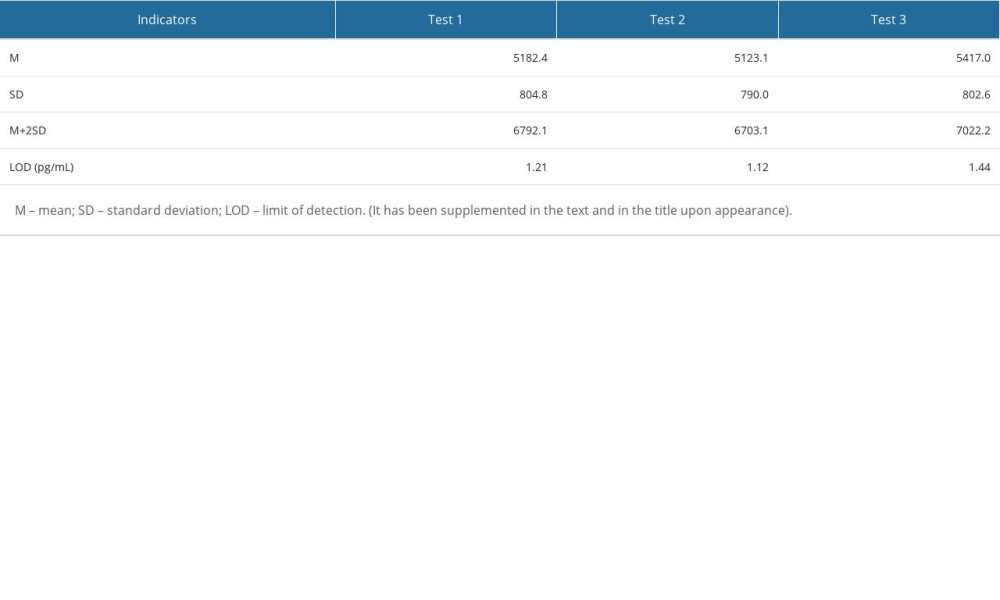 Supplementary Table 2. Verification results of accuracy.
Supplementary Table 2. Verification results of accuracy.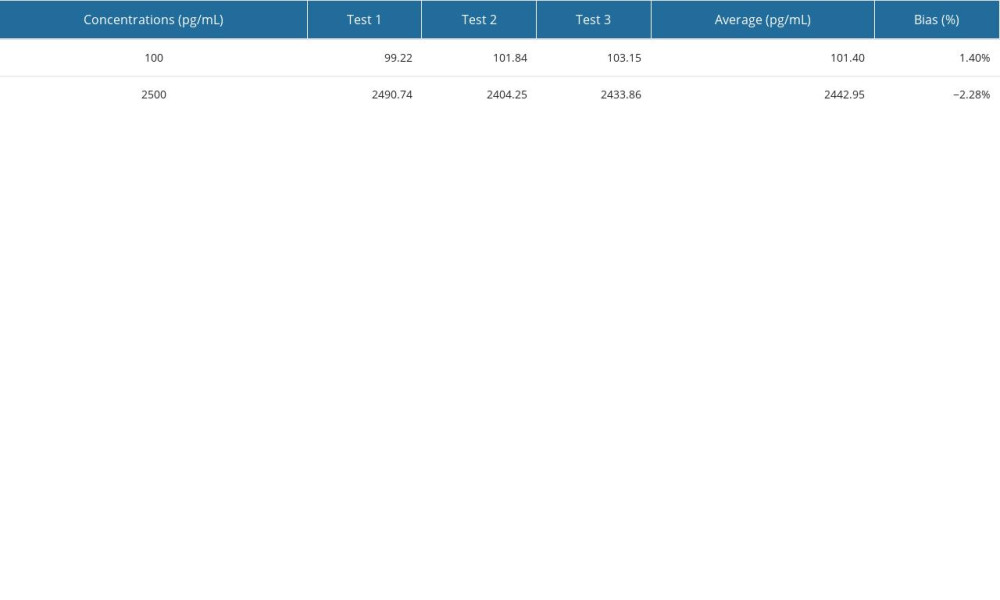 Supplementary Table 3. Verification results of precision.
Supplementary Table 3. Verification results of precision.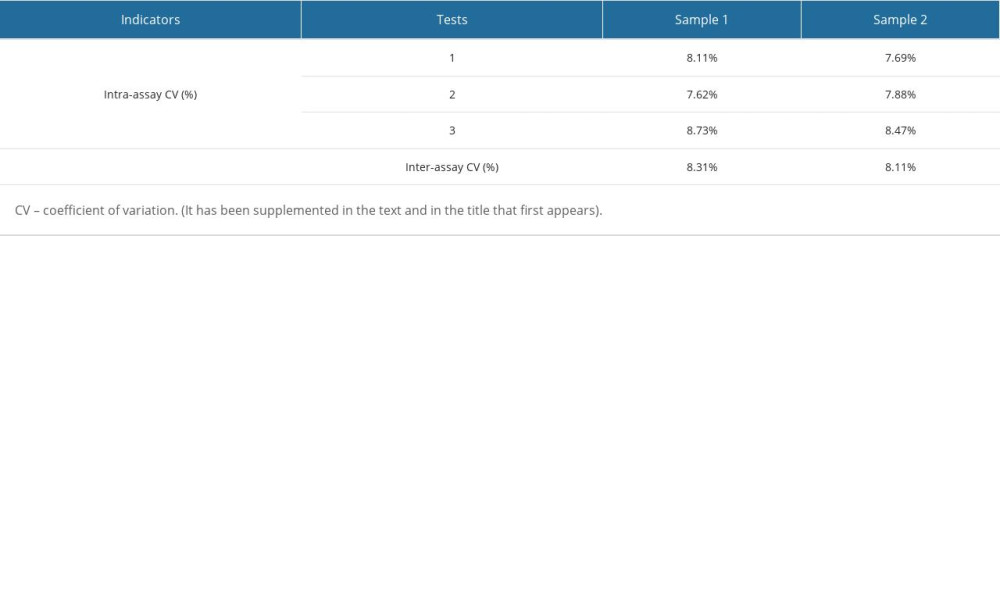 Supplementary Table 4. Verification results of thermal stability.
Supplementary Table 4. Verification results of thermal stability.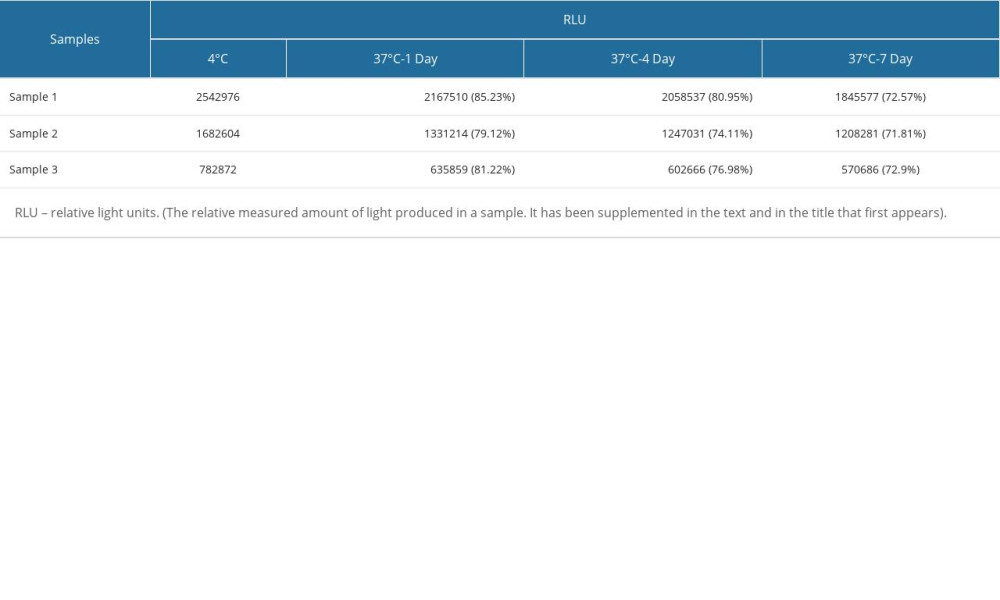
References
1. GBD 2016 Stroke Collaborators, Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016: Lancet Neurol, 2019; 18(5); 439-58
2. Adams HP, Bendixen BH, Kappelle LJ, Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment: Stroke, 1993; 24(1); 35-41
3. Kernan WN, Ovbiagele B, Black HR, Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2014; 45(7); 2160-236
4. Campbell BCV, De Silva DA, Macleod MR, Ischaemic stroke: Nat Rev Dis Primers, 2019; 5(1); 70
5. Brazzelli M, Shuler K, Quayyum Z, Clinical and imaging services for TIA and minor stroke: Results of two surveys of practice across the UK: BMJ Open, 2013; 3(8); e003359
6. Hurford R, Sekhar A, Hughes TAT, Muir KW, Diagnosis and management of acute ischaemic stroke: Pract Neurol, 2020; 20(4); 304-16
7. Yaghi S, Bernstein RA, Passman R, Cryptogenic stroke: Research and practice: Circ Res, 2017; 120(3); 527-40
8. Wijerathne H, Witek MA, Baird AE, Soper SA, Liquid biopsy markers for stroke diagnosis: Expert Rev Mol Diagn, 2020; 20(8); 771-88
9. Simpkins AN, Janowski M, Oz HS, Biomarker application for precision medicine in stroke: Transl Stroke Res, 2020; 11(4); 615-27
10. Peddada K, Cruz-Flores S, Goldstein LB, Ischemic stroke with troponin elevation: Patient characteristics, resource utilization, and in-hospital outcomes: Cerebrovasc Dis, 2016; 42(3–4); 213-23
11. Suleiman HM, Aliyu IS, Abubakar SA, Cardiac Troponin T and creatine kinase MB fraction levels among patients with acute ischemic stroke in Nigeria: Niger J Clin Pract, 2017; 20(12); 1618-21
12. Santamarina E, Penalba A, García-Berrocoso T, Biomarker level improves the diagnosis of embolic source in ischemic stroke of unknown origin: J Neurol, 2012; 259(12); 2538-45
13. Llombart V, Antolin-Fontes A, Bustamante A, B-type natriuretic peptides help in cardioembolic stroke diagnosis: Pooled data meta-analysis: Stroke, 2015; 46(5); 1187-95
14. Chaudhuri JR, Sharma VK, Mridula KR, Association of plasma brain natriuretic peptide levels in acute ischemic stroke subtypes and outcome: J Stroke Cerebrovasc Dis, 2015; 24(2); 485-91
15. Han S, Ying Z, Ren H, The development of novel N-terminal pro-peptide of type I chemiluminescence assay: Clin Lab, 2021; 67(9) 2021.210210
16. Powers WJ, Rabinstein AA, Ackerson T, 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2018; 49(3); e46-e110
17. Montaner J, Perea-Gainza M, Delgado P, Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers: Stroke, 2008; 39(8); 2280-87
18. Kalani R, Krishnamoorthy S, Deepa D, Apolipoproteins B and A1 in ischemic stroke subtypes: J Stroke Cerebrovasc Dis, 2020; 29(4); 104670
19. Powers WJ, Rabinstein AA, Ackerson T, Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2019; 50(12); e344-e418
20. Fonseca AC, Coelho P, Update on biomarkers associated to cardioembolic stroke: A narrative review: Life (Basel), 2021; 11(5); 448
21. Harpaz D, Seet RCS, Marks RS, Tok AIY, Blood-based biomarkers are associated with different ischemic stroke mechanisms and enable rapid classification between cardioembolic and atherosclerosis etiologies: Diagnostics (Basel), 2020; 10(10); 804
22. Balion C, McKelvie R, Don-Wauchope AC, B-type natriuretic peptide-guided therapy: A systematic review: Heart Fail Rev, 2014; 19(4); 553-64
23. Pathak A, Pandey SP, Madhukar P, Blood biomarkers for the differentiation of cardiac ischemic stroke subtypes: A systematic review: Cardiovasc Hematol Disord Drug Targets, 2019; 19(3); 215-27
24. Radhakrishnan S, Moorthy S, Gadde S, Madhavan K, Role of cardiac biomarkers in the assessment of acute cerebrovascular accident: J Neurosci Rural Pract, 2021; 12(1); 106-11
25. Batal O, Jentzer J, Balaney B, The prognostic significance of troponin I elevation in acute ischemic stroke: J Crit Care, 2016; 31(1); 41-47
26. Amaro S, Jiménez-Altayó F, Chamorro Á, Uric acid therapy for vasculoprotection in acute ischemic stroke: Brain Circ, 2019; 5(2); 55-61
27. Dubow J, Fink ME, Impact of hypertension on stroke: Curr Atheroscler Rep, 2011; 13(4); 298-305
28. Johansson BB, Hypertension mechanisms causing stroke: Clin Exp Pharmacol Physiol, 1999; 26(7); 563-65
29. Zambrelli E, Emanuele E, Marcheselli S, Apo(a) size in ischemic stroke: Relation with subtype and severity on hospital admission: Neurology, 2005; 64(8); 1366-70
Figures
 Figure 1. Levels distribution of BNP, CK-MB, cTnI, and MYO in IS group and control group. (A) BNP levels in IS group and control group. (B) CK-MB levels in IS group and control group. (C) cTnI levels in IS group and control group. (D) MYO levels in IS group and control group. * P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001; ns, nonsignificant.
Figure 1. Levels distribution of BNP, CK-MB, cTnI, and MYO in IS group and control group. (A) BNP levels in IS group and control group. (B) CK-MB levels in IS group and control group. (C) cTnI levels in IS group and control group. (D) MYO levels in IS group and control group. * P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001; ns, nonsignificant. Figure 2. Diagnostic value of 4 cardiac biomarkers for different subtypes of IS. (A) ROC curves of 4 cardiac biomarkers in the SVO group; (B) ROC curves of 4 cardiac biomarkers in the LAA group; (C) ROC curves of 4 cardiac biomarkers in CE group.
Figure 2. Diagnostic value of 4 cardiac biomarkers for different subtypes of IS. (A) ROC curves of 4 cardiac biomarkers in the SVO group; (B) ROC curves of 4 cardiac biomarkers in the LAA group; (C) ROC curves of 4 cardiac biomarkers in CE group. Figure 3. Combined diagnostic value of 4 cardiac biomarkers for different subtypes of IS. (A) ROC curve for the combined diagnosis of SVO with 4 cardiac biomarkers; (B) ROC curve for the combined diagnosis of LAA with 4 cardiac biomarkers; (C) ROC curve for the combined diagnosis of CE with 4 cardiac biomarkers.
Figure 3. Combined diagnostic value of 4 cardiac biomarkers for different subtypes of IS. (A) ROC curve for the combined diagnosis of SVO with 4 cardiac biomarkers; (B) ROC curve for the combined diagnosis of LAA with 4 cardiac biomarkers; (C) ROC curve for the combined diagnosis of CE with 4 cardiac biomarkers. Tables
 Table 1. Baseline characteristics of the study population.
Table 1. Baseline characteristics of the study population. Table 2. Levels of BNP and other cardiac biomarkers in IS patients and healthy people.
Table 2. Levels of BNP and other cardiac biomarkers in IS patients and healthy people. Table 3. Diagnostic test results of IS patients.
Table 3. Diagnostic test results of IS patients. Table 4. Influential factors of small-vessel occlusion ischemic stroke.
Table 4. Influential factors of small-vessel occlusion ischemic stroke. Table 5. Influential factors of large-artery atherosclerosis ischemic stroke.
Table 5. Influential factors of large-artery atherosclerosis ischemic stroke. Table 6. Influential factors of cardioembolism ischemic stroke.
Table 6. Influential factors of cardioembolism ischemic stroke. Table 1. Baseline characteristics of the study population.
Table 1. Baseline characteristics of the study population. Table 2. Levels of BNP and other cardiac biomarkers in IS patients and healthy people.
Table 2. Levels of BNP and other cardiac biomarkers in IS patients and healthy people. Table 3. Diagnostic test results of IS patients.
Table 3. Diagnostic test results of IS patients. Table 4. Influential factors of small-vessel occlusion ischemic stroke.
Table 4. Influential factors of small-vessel occlusion ischemic stroke. Table 5. Influential factors of large-artery atherosclerosis ischemic stroke.
Table 5. Influential factors of large-artery atherosclerosis ischemic stroke. Table 6. Influential factors of cardioembolism ischemic stroke.
Table 6. Influential factors of cardioembolism ischemic stroke. Supplementary Table 1. Verification results of the limit of detection.
Supplementary Table 1. Verification results of the limit of detection. Supplementary Table 2. Verification results of accuracy.
Supplementary Table 2. Verification results of accuracy. Supplementary Table 3. Verification results of precision.
Supplementary Table 3. Verification results of precision. Supplementary Table 4. Verification results of thermal stability.
Supplementary Table 4. Verification results of thermal stability. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








