16 July 2023: Clinical Research
A Nomogram for Identifying HR+/Her2− Breast Cancer Patients with Positive Sentinel Lymph Nodes and Omitted Axillary Lymph Node Dissection Who Need Abemaciclib Therapy
Hanzhao Yang1CDE, Yadong Sun1BCD, Peili Wang1BD, Jianghua Qiao1BC, Lianfang Li1B, Zhenduo Lu1B, Xianfu Sun1B, Chongjian Zhang1B, Xiuchun Chen1B, Min Yan1B, Shude Cui1B, Chengzheng Wang1ADF, Zhenzhen Liu1AEDOI: 10.12659/MSM.940124
Med Sci Monit 2023; 29:e940124
Abstract
BACKGROUND: The efficacy of abemaciclib in high-risk patients with early-stage HR+/Her2- breast cancer has been verified by MonarchE. However, accurately determining the number of axillary lymph node (ALN) metastases remains challenging. The Z0011 trial changed the axillary management strategy, eliminating the need for axillary lymph node dissection (ALND) in patients with 1-2 sentinel lymph node (SLN) metastases. Therefore, further exploration is needed to identify patients who could benefit from abemaciclib therapy.
MATERIAL AND METHODS: This retrospective study included cT1-2N0M0 HR+/Her2- patients with 1-2 positive SLNs who underwent ALND. Clinicopathological data were collected, and logistic regression analyses identified independent predictors for ≥4 positive ALNs. A predictive nomogram was developed, and discrimination and calibration were evaluated using the C-index and calibration curve. Clinical efficacy was assessed using decision curve analysis (DCA).
RESULTS: We enrolled 444 patients, with 77 (17.3%) having ≥4 positive ALNs. Independent predictors for ≥4 positive ALNs included abnormal ALN on ultrasound, mammographic calcifications, T stage, and the number of positive SLNs. The nomogram demonstrated an AUC of 0.777 (95% CI: 0.735-0.815, P<0.001), and internal validation showed good calibration and discrimination (C-index, 0.802; 95% CI: 0.779-0.824). DCA revealed a positive net benefit for risk levels ranging from 5% to 54%.
CONCLUSIONS: This nomogram is a convenient and reliable tool to predict the risk of ≥4 positive ALNs in HR+/Her2- patients. It aids in protocol selection by identifying SLN-positive patients who may benefit from abemaciclib therapy without ALND.
Keywords: Abemaciclib, Breast Neoplasms, nomograms, Humans, Female, sentinel lymph node, Sentinel Lymph Node Biopsy, Retrospective Studies, Lymph Node Excision, Lymph Nodes, Lymphatic Metastasis, Axilla
Background
Breast cancer is the most frequent malignancy in women, and axillary lymph node dissection (ALND) was the standard treatment for positive ALNs in patients. The ACOSOG Z0011 [1] and AMAROS [2] studies proved that ALND did not improve the overall survival rates in cT1-2 patients with clinically negative axilla and 1–2 SLN metastases. As regards locoregional lymph node recurrence, the Z0011 trial showed that the sentinel lymph node biopsy (SLNB) group had a cumulative incidence of axillary recurrence of 1.5% compared to 0.5% in the ALND group. Similarly, patients undergoing ALND had a 5-year axillary recurrence rate of 0.43% compared to 1.19% in patients undergoing radiotherapy in the axilla. The above data demonstrated that ALND presented no advantage in extending overall survival and controlling locoregional recurrence. Hence, most early-stage breast-conserving patients with negative lymph nodes avoid taking ALND. HR+/Her2− breast cancer is the most common subtype among early-stage patients [3], accounting for about 60% of all breast cancers. Despite the more favorable prognosis than Her2 and triple-negative breast cancer, patients still have a 40% risk of developing long-term recurrence, and the risk rises along with increased anatomical risk [4]. Patients with high recurrence risk should be treated as early as possible to prevent recurrence and metastasis. MonarchE [5] is a global, randomized, III-stage trial that proved that standard endocrine treatment + abemaciclib (an inhibitor of cyclin-dependent kinases 4 and 6) could improve the therapeutic outcomes of early-stage patients with high-risk HR+/Her2− breast cancer. The 4-year absolute invasive disease-free survival (iDFS) benefit was 6.4% (Abemaciclib group (85.8% [95% CI 84.2–87.3] vs 79.4% in the standard endocrine treatment group [95% CI 77.5–81.1]). The risk of an IDFS event was reduced by 33.6%. Patients included in MonarchE had positive ALNs ≥4 or had 1–3 positive ALNs and at least 1 high-risk clinical feature: tumor ≥5 cm or histological grade 3 or Ki-67 ≥20%. Since the Z0011 trial, omitting ALND has been extensively applied in breast-conserving patients with 1–2 positive SLNs. This leads to a new problem of how to decide if a patient needs abemaciclib therapy when they have 1–2 positive SLNs without high-risk clinical features? Is ALND essential to confirm axillary nodal metastatic burden? It was shown that 13.0–18.4% of patients who fulfilled the Z0011 criteria had more than 4 positive ALNs [1,2,6]. Therefore, we need to identify the risk of ALN metastases ≥4 in HR+/Her2− breast cancer patients with 1–2 positive SLNs and omitted ALND without high-risk tumor characteristics. These patients can probably benefit from abemaciclib therapy to escalate systemic therapy and de-escalate local surgical treatment. The present study aimed to analyze single-center retrospective data via univariate and multivariate regression analysis and construct a nomogram to select patients suitable for abemaciclib therapy.
Material and Methods
STATISTICAL ANALYSIS:
Patient clinical data were acquired, including age, BMI, menopausal status, unifocal/multifocal tumors, ALN ultrasound (US-ALN), mammographic calcifications, surgical method, pathological type, histological grade, tumor size, lymphovascular invasion (LVI), positive and negative SLN number, N classification, progesterone receptor, and Her2 expression levels. Clinicopathological factors were analyzed as categorical variables to explore their correlation with positive ALNs ≥4. Categorical variables were analyzed using univariate logistic regression analysis, from which the obtained significant variables were analyzed in multivariate logistic regression analysis to confirm independent predictors for positive ALNs ≥4. A nomogram was constructed using the “rms” package of R software. Discrimination and calibration were employed to evaluate the performance of the nomogram. Internal validation was performed using a 1000 bootstrap resampling method, with the C-index calculated and the calibration curve plotted. The discrimination ability was evaluated by the area under the ROC curve and C-index. Decision curve analysis (DCA) was used to evaluate the clinical efficacy and net benefit of the nomogram. All statistical analyses were conducted using SPSS 25.0 (IBM Corporation, Armonk, NY, USA) and R 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna).
Results
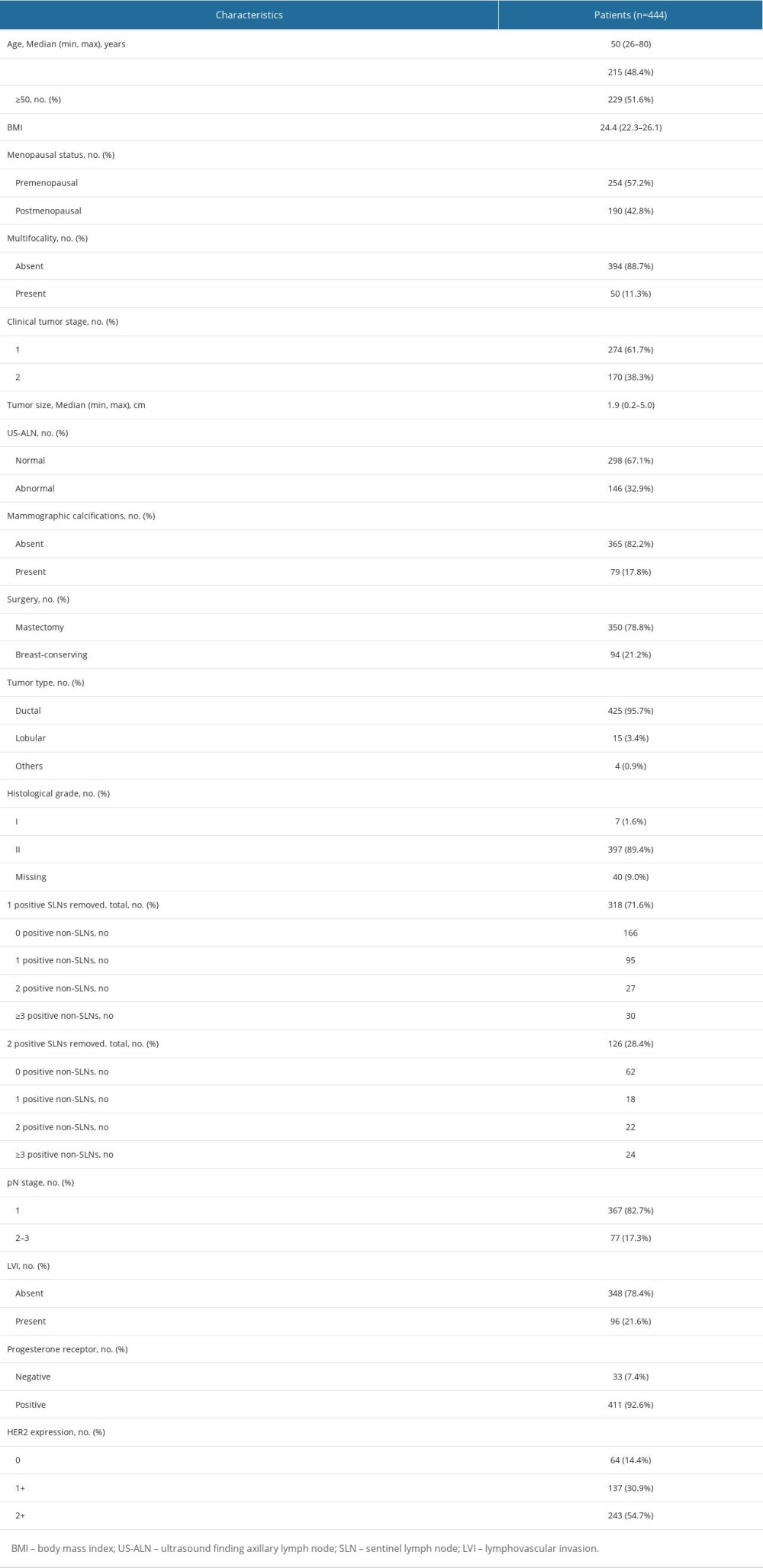
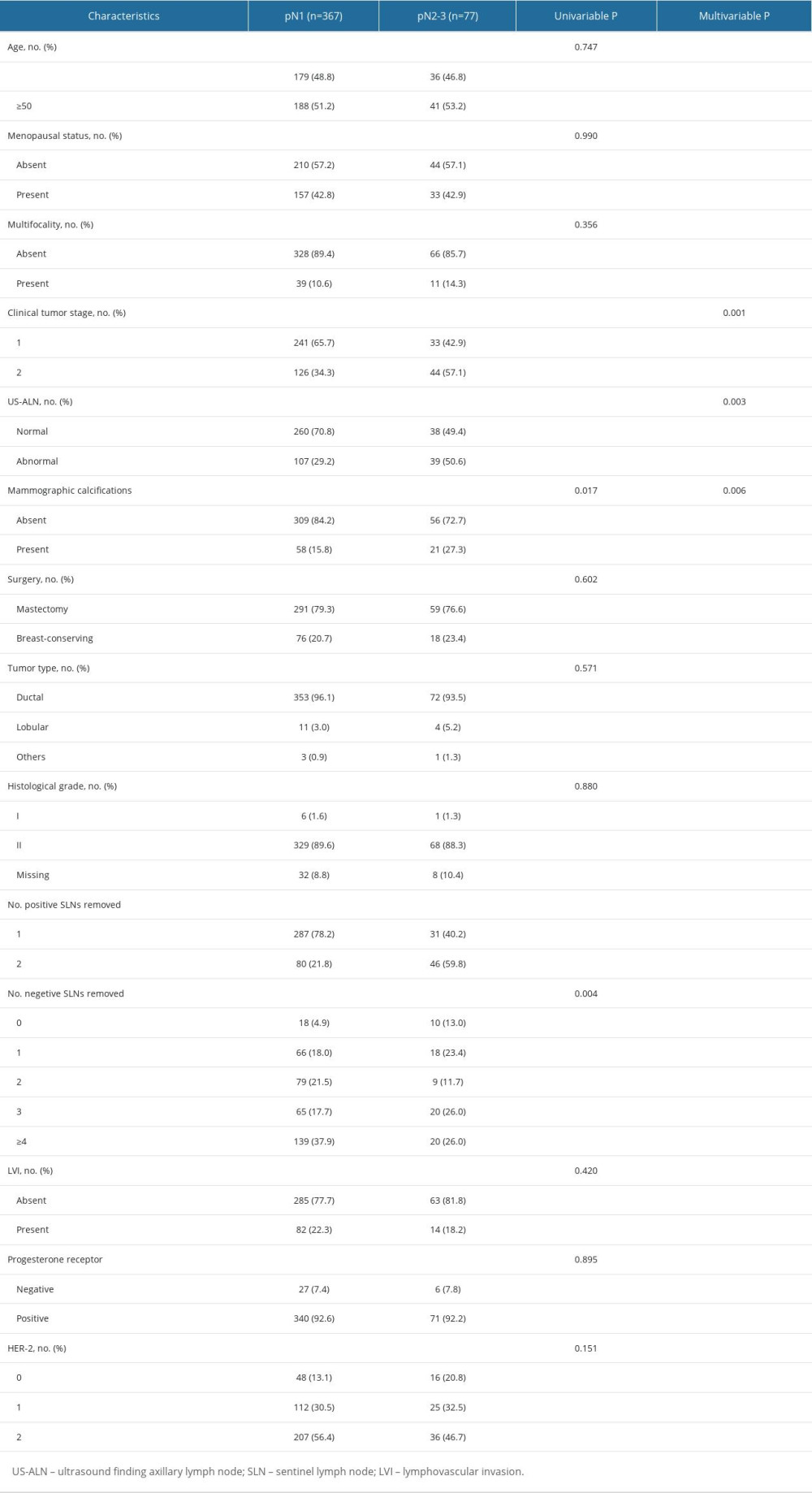
We recruited 444 breast cancer patients who fulfilled the inclusion criteria from January 2015 to June 2022. Patients’ baseline characteristics are displayed in Table 1. The median age was 50 years. There were 274 patients (61.7%) who had cT1 tumors, 318 patients (71.6%) had 1 positive SLN, and 126 patients (28.4%) had 2 positive SLNs. Among patients with 1 positive SLN, 9.43% had positive non-SLNs ≥3, and among those with 2 positive SLNs, 36.5% had positive non-SLNs ≥2. A total of 367 (82.7%) patients had 1–3 positive ALNs (pN1), and 77 (17.3%) patients had positive ALNs ≥4(pN2-3) (Table 2). Univariate and multivariate logistic regression analyses were performed to identify factors for positive ALNs ≥4. Factors with
Discussion
In this study, a nomogram was constructed using clinicopathological factors to assess the risk of positive ALNs ≥4 among cT1-2N0M0 HR+/Her2− patients with 1–2 positive SLNs. The nomogram included US-ALN, mammographic calcifications, T stage, and positive SLN number. Internal validation suggested that the nomogram had good discrimination, calibration, and clinical efficacy.
At present, the main trends in breast cancer treatment are de-escalating chemotherapy for low-risk patients and intensive systemic therapy for high-risk patients. Multiple gene detection methods focusing on HR+/Her2− breast cancer (eg, Oncotype DX [9,10] and MammaPrint [11] have been developed to devise adjuvant chemotherapy protocols for early-stage breast cancer patients with HR+ and negative lymph nodes or 1–3 positive lymph nodes. Patients with high-risk clinicopathological characteristics (eg, subjects in the MonarchE study) had mortality rates similar those of patients with triple-negative breast cancer (TNBC) [12]. The MonarchE study [5] showed that 2-year administration of abemaciclib can improve clinical benefits. Currently, most patients who fulfilled the Z0011 criteria have omitted ALND, making it impossible to acquire complete ALN metastatic data, so partial patients do not have a basis for intensive treatment. The Ki-67 index can reflect cell proliferation degree, and patients with high Ki67 levels had worse prognoses [13]. TheMonarchE study population was allowed to be treated with abemaciclib when Ki-67 ≥20% as long as there was lymph node metastasis, with the exception of only ≥4 lymph node metastases when Ki-67 was <20%. Therefore, those with Ki-67 ≥20% were excluded from this study. In the ITT and cohort 1 of the MonarchE trial, the abemaciclib treatment significantly improved iDFS in patients with Ki-67 high-expression tumors. In cohort 1, the benefit of abemaciclib was consistently observed regardless of the Ki-67 expression, indicating that Ki-67 could not predict the therapeutic benefits of abemaciclib. Notably, patients with high Ki-67 expression had higher recurrence rates than those with low Ki-67 expression. Thus, Ki-67 expression is a prognostic indicator for recurrence, but cannot predict abemaciclib’s therapeutic benefits [14]. A previous study indicated that 13.0–18.4% of patients who met the Z0011 criteria had over 4 positive ALNs [1,2,6]. Our study showed that the possibility of positive ALNs ≥4 among cT1-2N0 HR+/Her2− patients with 1–2 positive SLNs was 17.1%, 9.43% for 1 positive SLN, and 38.1% for 2 positive SLNs.
Most predictive studies have focused on non-SLN metastasis presence, while studies concerning the exact lymph node metastasis number (eg, positive lymph nodes ≥4) are scarce. In addition, variables included in those studies were primarily SLN metastatic focus size, extramembranous infiltration of lymph nodes, and LVI [15], which were all based on postoperative pathological examinations. Gilles et al [16] established a preoperative clinical model and a postoperative pathological model predicting the risk of ALN metastases based on 12572 early-stage breast cancer cases, showing that the postoperative model had better discrimination (AUC=0.780) than the preoperative model (AUC=0.717). Intraoperative models are scarce. Shimazu et al [17] constructed an intraoperative predictive model for non-SLN metastasis using the one-step nucleic acid amplification method. The model included total tumor burden and tumor size, with an AUC of 0.70. The AUC of the model in this study was 0.777, and it was as effective as the postoperative model above. It is believed that tumor size, histological grade, LVI, age at diagnosis, positive SLN number, hormone receptor expression, Her-2 status, and molecular subtype are all significant risk factors for non-SLN metastasis [18]. This study revealed that US-ALN, mammographic calcifications, T stage, and positive SLN number are independent predictors for positive ALNs ≥4.
In the Z0011 trial [1], IBCSG 23-01 trial [19], and AMAROS trial [2], clinically negative lymph nodes were defined as the absence of enlarged lymph nodes in physical examination. Nevertheless, assessing ALN status via clinical examinations is inaccurate, and ultrasound is conducive to identifying patients with high axillary lymph node burden[20,21]. In this study, 146 patients had abnormal ALN on ultrasound, of which 39 patients (26.7%) had ≥4 positive lymph nodes, 260 patients had normal ANL ultrasound, and 38 (14.6%) of them had positive lymph nodes ≥4. It was shown that patients with abnormal ALN on ultrasound had higher axillary burden (OR=2.289, 95% CI: 1.327–3.948,
The association between imaging manifestations of the primary tumor and ALN metastasis has not been adequately explored, especially with the commonly-used mammography. Zheng et al [25] found a significant association between ALN metastasis and mammographic calcifications among 7317 patients. Yan et al [26] reported that mammographic calcifications were significantly related to high ALN metastasis burden as an independent prognostic factor for breast cancer patients. Primary locus imaging manifestations were not included in studies like the Z0011 trial. However, we demonstrated a significant correlation between mammographic calcifications and positive ALNs ≥4 (OR=2.452, 95% CI: 1.297–4.638,
LVI is an independent predictor for lymph node metastasis and unfavorable outcomes [27,28]. It requires postoperative sections routinely stained by hematoxylin and eosin (HE), which is unsuitable for intraoperative models. LVI correlates significantly with age, T stage, histological grade, and hormone receptor expression [29]. In this study, LVI was found to be irrelevant to ALN metastasis (
Her2 expression is significantly correlated with the metastatic potential of breast cancer cells. Ahmed et al [30] reported that Her2 expression is an independent predictor for ALN metastasis. It is currently believed that low Her2 expression may be a novel molecular subtype. Francesco [31] revealed that patients with HR-positive diseases (65.4%) had a higher proportion of low Her2 expression than patients with TNBC (36.6%). Low Her2 expression was irrelevant to the overall survival of patients with HR-positive breast cancers. Few studies have explored the relationship between Her2 expression and ALN metastasis. Our study showed that the proportion of low Her2 expression (85.6%) was higher than Her2 0 (14.4%) among HR+/Her2− patients, consistent with the Francesco study. However, no significant correlation was observed between low Her2 expression and HER2 0 status with the lymph node metastasis number (
The nomogram in this study exhibited a C-index of 0.802 (95%CI: 0.779–0.824), demonstrating good discrimination. Regarding calibration evaluation, the calibration curve displayed a strong concordance between the apparent predicted value distribution curve of the model and the distribution curve obtained after correcting for overfitting through resampling. It closely aligned with the optimal curve (standard curve), indicating good calibration of the model. To assess clinical effectiveness, decision curve analysis (DCA) was employed to calculate the net benefit and construct the DCA curve. The DCA curve illustrated that the model’s decision curve deviated from the 2 extreme scenario curves, signifying good clinical effectiveness.
The present study has some limitations. First, it was a single-center retrospective study with potential biases. Second, the nomogram was not externally validated. Thus, prospective multi-center studies are needed to evaluate and validate the findings.
Conclusions
We established an intraoperative predictive nomogram to predict the possibility of positive ALNs ≥4 among HR+/Her2− patients with positive SLNs and omitted ALND to assist clinical decision-making. The predictive model is accurate and can help oncologists identify patients who need abemaciclib therapy. Therefore, patients can be offered better management strategies, including de-escalation surgery and systemic escalation therapy in the SLNB and precision medicine era.
Figures
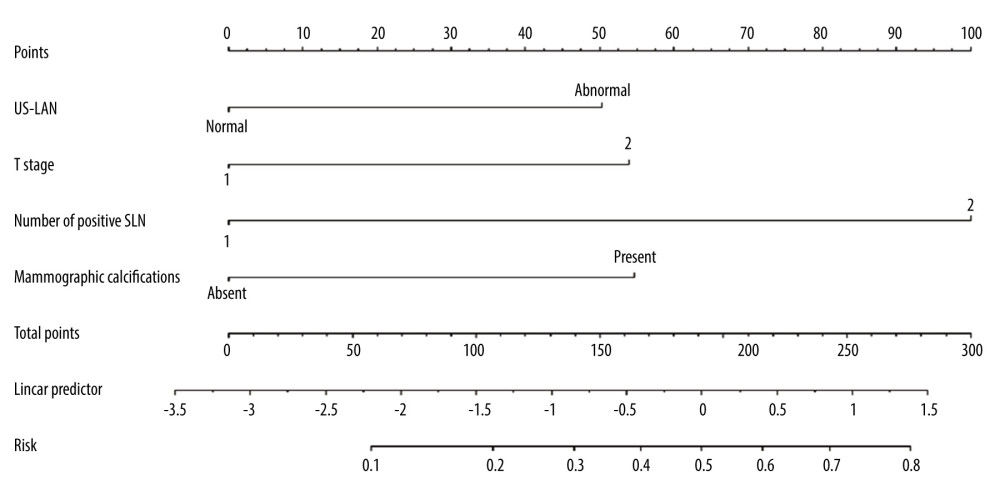 Figure 1. The nomogram to predict patients with ≥4 positive total ALNs in HR+/HER2− population with 1–2 positive SLNs. The scores for the 4 factors were summed to calculate the probability of ≥4 positive total ALNs and the total scores and bottom risk scale were referenced. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna).
Figure 1. The nomogram to predict patients with ≥4 positive total ALNs in HR+/HER2− population with 1–2 positive SLNs. The scores for the 4 factors were summed to calculate the probability of ≥4 positive total ALNs and the total scores and bottom risk scale were referenced. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna). 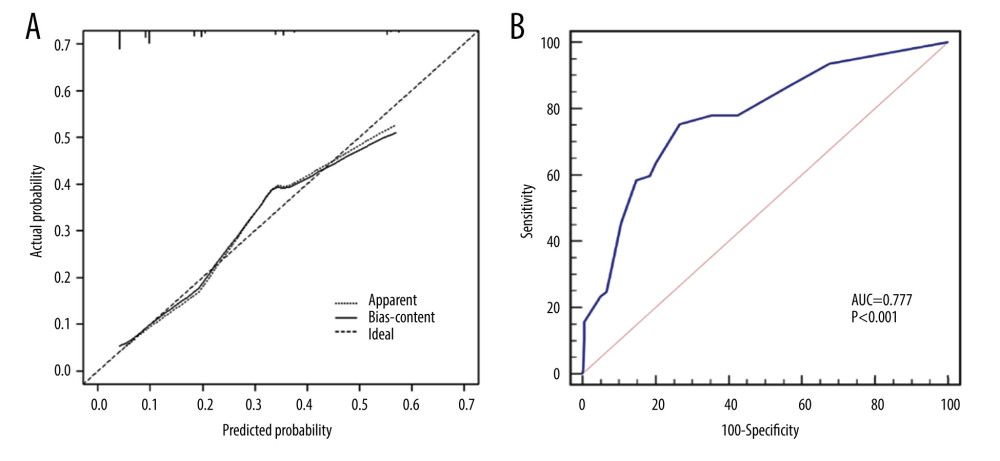 Figure 2. (A) The calibration curve showed a satisfactory fit between the predictive and actual observation. (B) The ROC curve of the nomogram. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna).
Figure 2. (A) The calibration curve showed a satisfactory fit between the predictive and actual observation. (B) The ROC curve of the nomogram. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna). 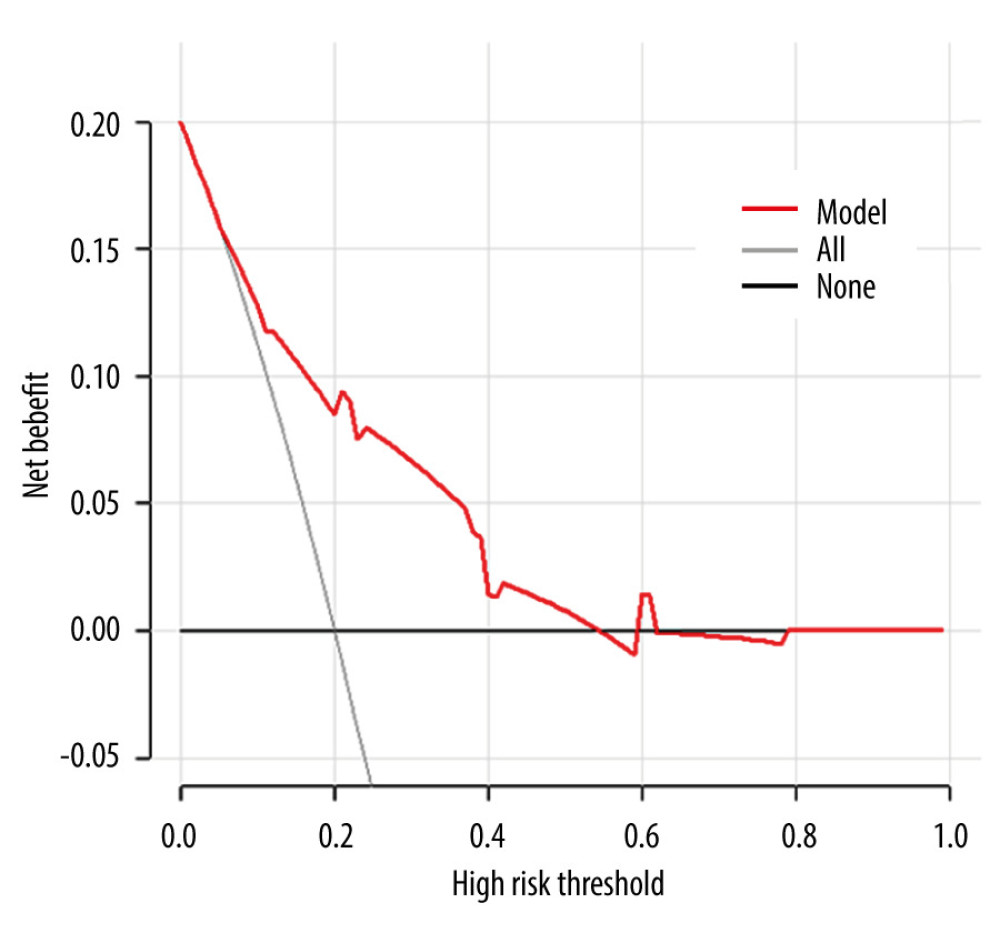 Figure 3. The DCA curve demonstrates the clinical efficacy of the nomogram. The red line is a nomogram predicting the risk of ≥4 positive nodes in patients. The grey line indicates all patients with ≥4 positive nodes, while the black line indicates no patient with ≥4 positive nodes. This DCA could provide a larger net benefit, with ranges of 5% to 54%. DCA – decision curve analysis. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna).
Figure 3. The DCA curve demonstrates the clinical efficacy of the nomogram. The red line is a nomogram predicting the risk of ≥4 positive nodes in patients. The grey line indicates all patients with ≥4 positive nodes, while the black line indicates no patient with ≥4 positive nodes. This DCA could provide a larger net benefit, with ranges of 5% to 54%. DCA – decision curve analysis. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna). References
1. Giuliano AE, Ballman KV, McCall L, Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial: JAMA, 2017; 318(10); 918
2. Donker M, van Tienhoven G, Straver ME, Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial: Lancet Oncol, 2014; 15(12); 1303-10
3. Howlader N, Altekruse SF, Li CI, US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status: J Natl Cancer Inst, 2014; 106(5); dju055
4. Pan H, Gray R, Braybrooke J, 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years: N Engl J Med, 2017; 377(19); 1836-46
5. Johnston SRD, Toi M, O’Shaughnessy J, Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial: Lancet Oncol, 2022; 24(1); 77-90
6. Katz A, Smith BL, Golshan M, Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer: J Clin Oncol, 2008; 26(13); 2093-98
7. Wolff AC, Hammond MEH, Allison KH, Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update: Arch Pathol Lab Med, 2018; 142(11); 1364-82
8. Hammond MEH, Hayes DF, Dowsett M, American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer (Unabridged Version): Arch Pathol Lab Med, 2010; 134; 25
9. Sparano JA, Gray RJ, Makower DF, Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer: N Engl J Med, 2018; 379(2); 111-21
10. Kalinsky K, Barlow WE, Gralow JR, 21-gene assay to inform chemotherapy benefit in node-positive breast cancer: N Engl J Med, 2021; 385(25); 2336-47
11. Cardoso F, van’t Veer LJ, Bogaerts J, 70-gene signature as an aid to treatment decisions in early-stage breast cancer: N Engl J Med, 2016; 375(8); 717-29
12. Nelson DR, Brown J, Morikawa A, Method M, Breast cancer-specific mortality in early breast cancer as defined by high-risk clinical and pathologic characteristics: PLoS One, 2022; 17(2); e0264637
13. Yerushalmi R, Woods R, Ravdin PM, Ki67 in breast cancer: Prognostic and predictive potential: Lancet Oncol, 2010; 11(2); 174-83
14. Harbeck N, Rastogi P, Martin M, Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study: Ann Oncol, 2021; 32(12); 1571-81
15. Kim I, Ryu JM, Kim JM, Development of a nomogram to predict N2 or N3 stage in T1-2 invasive breast cancer patients with no palpable lymphadenopathy: J Breast Cancer, 2017; 20(3); 270
16. Houvenaeghel G, Lambaudie E, Classe JM, Lymph node positivity in different early breast carcinoma phenotypes: A predictive model: BMC Cancer, 2019; 19(1); 45
17. Shimazu K, Sato N, Ogiya A, Intraoperative nomograms, based on one-step nucleic acid amplification, for prediction of non-sentinel node metastasis and four or more axillary node metastases in breast cancer patients with sentinel node metastasis: Ann Surg Oncol, 2018; 25(9); 2603-11
18. van la Parra RFD, Peer PGM, Ernst MF, Bosscha K, Meta-analysis of predictive factors for non-sentinel lymph node metastases in breast cancer patients with a positive SLN: Eur J Surg Oncol, 2011; 37(4); 290-99
19. Galimberti V, Cole BF, Zurrida S, Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): A phase 3 randomised controlled trial: Lancet Oncol, 2013; 14(4); 297-305
20. Farrell TPJ, Adams NC, Stenson M, The Z0011 Trial: Is this the end of axillary ultrasound in the pre-operative assessment of breast cancer patients?: Eur Radiol, 2015; 25(9); 2682-87
21. Ahmed M, Jozsa F, Baker R, Meta-analysis of tumour burden in pre-operative axillary ultrasound positive and negative breast cancer patients: Breast Cancer Res Treat, 2017; 166(2); 329-36
22. Lim GH, Upadhyaya VS, Acosta HA, Preoperative predictors of high and low axillary nodal burden in Z0011 eligible breast cancer patients with a positive lymph node needle biopsy result: Eur J Surg Oncol, 2018; 44(7); 945-50
23. Kuijs VJL, Moossdorff M, Schipper RJ, The role of MRI in axillary lymph node imaging in breast cancer patients: A systematic review: Insights Imaging, 2015; 6(2); 203-15
24. Schacht DV, Drukker K, Pak I, Using quantitative image analysis to classify axillary lymph nodes on breast MRI: A new application for the Z 0011 Era: Eur J Radiol, 2015; 84(3); 392-97
25. Zheng K, Tan JX, Li F, Relationship between mammographic calcifications and the clinicopathologic characteristics of breast cancer in Western China: A retrospective multi-center study of 7317 female patients: Breast Cancer Res Treat, 2017; 166(2); 569-82
26. Li Y, Cao J, Zhou Y, Mammographic casting-type calcification is an independent prognostic factor in invasive breast cancer: Sci Rep, 2019; 9(1); 10544
27. Houvenaeghel G, Lymphovascular invasion has a significant prognostic impact in patients with early breast cancer, results from a large, national, multicenter, retrospective cohort study: ESMO Open, 2021; 6(6); 10
28. Rakha EA, Martin S, Lee AH, The prognostic significance of lymphovascular invasion in invasive breast carcinoma: Cancer, 2012; 118(15); 3670-80
29. Aleskandarany MA, Sonbul SN, Mukherjee A, Rakha EA, Molecular mechanisms underlying lymphovascular invasion in invasive breast cancer: Pathobiology, 2015; 82(3–4); 113-23
30. Ahmed ARH, HER2 expression is a strong independent predictor of nodal metastasis in breast cancer: J Egypt Natl Cancer Inst, 2016; 28(4); 219-27
31. Schettini F, Chic N, Brasó-Maristany F, Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer: NPJ Breast Cancer, 2021; 7(1); 1
Figures
 Figure 1. The nomogram to predict patients with ≥4 positive total ALNs in HR+/HER2− population with 1–2 positive SLNs. The scores for the 4 factors were summed to calculate the probability of ≥4 positive total ALNs and the total scores and bottom risk scale were referenced. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna).
Figure 1. The nomogram to predict patients with ≥4 positive total ALNs in HR+/HER2− population with 1–2 positive SLNs. The scores for the 4 factors were summed to calculate the probability of ≥4 positive total ALNs and the total scores and bottom risk scale were referenced. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna). Figure 2. (A) The calibration curve showed a satisfactory fit between the predictive and actual observation. (B) The ROC curve of the nomogram. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna).
Figure 2. (A) The calibration curve showed a satisfactory fit between the predictive and actual observation. (B) The ROC curve of the nomogram. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna). Figure 3. The DCA curve demonstrates the clinical efficacy of the nomogram. The red line is a nomogram predicting the risk of ≥4 positive nodes in patients. The grey line indicates all patients with ≥4 positive nodes, while the black line indicates no patient with ≥4 positive nodes. This DCA could provide a larger net benefit, with ranges of 5% to 54%. DCA – decision curve analysis. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna).
Figure 3. The DCA curve demonstrates the clinical efficacy of the nomogram. The red line is a nomogram predicting the risk of ≥4 positive nodes in patients. The grey line indicates all patients with ≥4 positive nodes, while the black line indicates no patient with ≥4 positive nodes. This DCA could provide a larger net benefit, with ranges of 5% to 54%. DCA – decision curve analysis. R software 4.1.1 (The R Foundation for Statistical Computing, Austria, Vienna). In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952