16 June 2023: Clinical Research
Relationship Between D-Dimer Levels and Neutrophil-to-Lymphocyte Ratio (NLR) in Preeclamptic Pregnant Women with COVID-19: A Cohort Study
Dian Tjahyadi1ABCDEFG*, Bayu Indrayana IrsyadDOI: 10.12659/MSM.940130
Med Sci Monit 2023; 29:e940130
Abstract
BACKGROUND: Preeclampsia involves an inflammatory response and vascular endothelial dysfunction. In COVID-19, there is also tissue damage and an inflammatory response that stimulates the formation of D-dimers and an increase in the neutrophil-to-lymphocyte ratio (NLR). These 2 parameters have become laboratory tests carried out both in preeclampsia and COVID-19. This study aimed to determine the relationship between D-dimer levels and NLR in patients with both COVID-19 and preeclampsia.
MATERIAL AND METHODS: This was an observational analytic study with a retrospective approach. The subjects were pregnant women with gestational age >20 weeks diagnosed with severe preeclampsia and had D-dimer and neutrophil-to-lymphocyte ratio (NLR) laboratory results at Hasan Sadikin Hospital Bandung during the period April 2020 to July 2021. We enrolled 31 COVID-19 patients with preeclampsia and 113 COVID-19 patients without preeclampsia.
RESULTS: The mean level of D-dimer in COVID-19 patients with preeclampsia was 3.66±3.15 and in those with COVID-19 without preeclampsia it was 3.03±3.15 (P<0.05). The mean NLR value in COVID-19 patients with preeclampsia was 7.22±4.30 and in COVID-19 patients without preeclampsia it was 5.47±2.20 (p<0.05). In the Spearman correlation test, the correlation coefficient was 0.159. Area under curve (AUC) D-dimer level was 64.9% (p<0.05) and NLR was 61.7% (p<0.05).
CONCLUSIONS: There was a significant difference (P<0.05) in D-dimer and NLR between COVID-19 patients with preeclampsia and those without preeclampsia. There was also a weak positive relationship between D-dimer and NLR levels in COVID-19 patients with preeclampsia, which means that the higher the D-dimer level, the higher the NLR value in COVID-19 patients with preeclampsia.
Keywords: COVID-19, fibrin fragment D, Preeclampsia Eclampsia 2, Humans, Female, Pregnancy, Infant, COVID-19, Neutrophils, Cohort Studies, Retrospective Studies, Pregnant Women, Pre-Eclampsia, Lymphocyte Count, Lymphocytes
Background
Coronavirus disease (COVID-19) is highly contagious; in Indonesia it has infected >6.3 million people and killed 157 000 [1]. Pregnant women are at a higher risk of severe COVID-19 due to physiological changes in pregnancy, including secondary immunosuppression due to the immune response, impaired lung function, and hypercoagulability states [2].
Maternal mortality is still a problem in various parts of the world, including Indonesia. In 2015 the maternal mortality rate in Indonesia was 305/100 000 live births. The main causes of death are preeclampsia, bleeding, and infection. Preeclampsia ranks first as a cause of maternal death [3]. Preeclampsia is also still a problem for pregnant women around the world. Worldwide, there are more than 50 000 deaths from preeclampsia and eclampsia every year. The preeclampsia mortality rate is 9–26% in low-income countries and 16% in high-income countries [4].
Nearly 15% of infected pregnant women have severe COVID-19 requiring intensive care [5]. A systematic review (December 1st 2019 to May 31st 2021) showed that women with SARS-CoV-2 infection during pregnancy had a much higher chance (62%) of developing preeclampsia than did controls [6].
To reproduce and replicate, the SARS-CoV-2 virus requires a host cell. The virus will use the apoptotic pathway in the host cell to leave and invade other host cells. Apoptosis leads to a series of biochemical processes that increase levels of D-dimer and NLR. It is suspected that there is a corelation between SARS-CoV-2 infection and preeclampsia due to the mechanism of the virus and its replication in host cells [7]. Viruses require attachment to the angiotensin-converting enzyme 2 (ACE2) receptor, which primarily infects type II alveolar epithelial cells of the lungs, and it is also widely expressed in different extrapulmonary tissues such as endothelial cells in the placenta [8].
The ACE2 receptor is an important component of the renin-angiotensin system, which also plays an important role in placenta formation. ACE2 activation will result in Ang-(-1-7), which binds to Mas and triggers vasodilation, as well as having antioxidant, anti-inflammatory, anti-remodeling, and anti-proliferative effects. However, the binding of the ACE2 receptor with SARS-CoV-2 in placental tissue causes a decrease in plasma ACE2 levels, which leads to decreased levels of Ang-(-1-7) [9]. The decreasing Ang (1-7) levels contribute to remodeling abnormal maternal spiral arteries, which leads to reduction of oxygen availability and triggers inflammatory processes and hemostasis [8]. Patberg et al found that histopathological abnormalities of the placenta (fetal vascular malperfusion and vitis) were more common in people with COVID-19 than in controls [10].
Preeclampsia is one of the main causes of morbidity and mortality in pregnant women and neonates worldwide [11]. Its pathogenesis involves the inflammatory response and vascular endothelial dysfunction. These 2 processes trigger the formation of D-dimers and increase the neutrophil-to-lymphocyte ratio (NLR) [12].
D-dimers increase physiologically in normal pregnancy and more so in preeclampsia [13]. COVID-19 further increases D-dimers [14]. Damaged cells and/or tissues release endothelial cell products, stimulating D-dimer production [15]. Healthy endothelial cells can release prostacyclin and nitric oxide, which is an ectoADPase that hydrolyzes ATP to prevent platelet activation and aggregation. Under inflammation and hypoxia conditions, endothelial cells are activated to express cell adhesion molecules that facilitate the attachment of platelets, leukocytes, and other microparticles [16]. This endothelial cell activation will lead to the formation of fibrin clot formation. Fibrin deposits circulating in the blood reduce the viscosity of the blood flow, causing a decrease in erythrotic motility, which causes hemolysis [17]. This causes hypercoagulability and increased D-dimer levels of preeclamptic women [18].
The neutrophil-to-lymphocyte ratio (NLR) is a biomarker of the systemic inflammatory response (SIRS). This inflammation is often followed by the activation of neutrophils and lymphocytes and endothelial dysfunction [19]. The increase in NLR is higher in preeclampsia [20]. Inflammatory processes that occur in preeclampsia are caused by abnormal immune responses, and the vascular endothelial dysfunction is associated with increasing white blood cell counts, especially in severe preeclampsia. The activation of neutrophils and lymphocytes will release several kinds of cytokines to activate inflammatory cells and immune responses, which lead to endothelial dysfunction [20]. NLR parameters can be a useful laboratory predictor in detecting and evaluating preeclampsia associated with mortality [21]. NLR is also increased in COVID-19 [22]. It is suspected there is a relationship between D-dimer levels and NLR in COVID-19 patients with preeclampsia [20]. The purpose of this study was to determine the relationship between D-dimer levels and neutrophil-to-lymphocyte ratio (NLR) in pregnant women with preeclampsia and COVID-19.
Material and Methods
DATA COLLECTION AND STUDY POPULATION:
This was an observational analytic study with retrospective cohort method. The population in this study were all pregnant COVID-19 patients at our hospital between April 1st, 2020 and July 31st, 2021. The inclusion criteria in this study were pregnant women with gestational age >20 weeks, with or without severe preeclampsia or chronic hypertension exacerbated by preeclampsia, confirmed COVID-19 from the results of the SARS-CoV2 PCR examination, and had laboratory tests for D-dimer and NLR. The exclusion criteria were patients with blood disorders that are known before pregnancy and routinely receive therapy/transfusion (eg, thalassemia), history of using anticoagulants before pregnancy, history of deep vein thrombosis, medical record data incomplete and were not checked for D-dimer and NLR. From these results we obtained 144 samples that met the inclusion criteria, which were divided into 31 samples of COVID-19 with preeclampsia and 114 samples of COVID-19 without preeclampsia.
Confirmation of the diagnosis of COVID-19 was obtained from the presence of viral nucleic acids using real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR), which detects the presence of the E gene (envelope), the N gene (nucleocapsid), and the Orf gene (open reading frame). Clinical data in the form of demographic characteristics (patient age, gestational age, type of treatment, pulmonary involvement, and severity of COVID-19) and laboratory test results (D-dimer and NLR) were recorded.
Preterm labor was labor that occurred before 37 weeks of gestation. Patients who had respiratory problems such as shortness of breath, supported by an objective assessment of oxygen saturation <95%, or physical and radiological examinations that showed lung abnormalities were classified in the group with lung involvement.
Patients with COVID-19 have various clinical manifestations, from asymptomatic to critical illness. The degree of COVID-19 can be categorized as asymptomatic, mild, moderate, severe, and critical. Patients with one of the symptoms of COVID-19 (fever, headache, nausea, vomit, anorexia, cough, sore throat, diarrhea, loss of sense of smell and taste, malaise, and headache) are categorized at a mild degree. Patients with fever, respiratory symptoms, and pneumonia imaging were categorized as moderate degree. Patients with oxygen levels <93%, respiratory rate 30 times/min, and pulmonary infiltrates > 0% were classified as severe. Patients with one or more of the following conditions – respiratory failure (acute respiratory distress syndrome), septic shock, with or without multiple organ dysfunction – were categorized in the critical category.
Data were taken directly from medical records. This retrospective cohort study used total sampling. We collected data on all the pregnant women with COVID-19 confirmed around April 2020 until July 2021, then we checked the availability of their laboratory test results (D-dimer and NLR), and divided them into 2 groups: Preeclampsia and non-preeclampsia. We analyzed D-dimer in both groups, NLR in both groups, and D-dimer+NLR in preeclampsia groups.
STATISTICAL ANALYSIS:
Statistical analysis for the characteristics of research subjects was performed using the Mann-Whitney test and chi-square test. The Mann-Whitney test was used in analysis of differences in D-dimer levels in COVID-19 patients with and without preeclampsia. Analysis of differences in NLR values in COVID-19 patients with and without preeclampsia used the Mann-Whitney test. Analysis of the correlation between D-dimer and NLR values in COVID-19 and preeclampsia used the Spearman correlation test. Receiver operating characteristic (ROC) and area under curve (AUC) curves were used to determine the cut-off point for D-dimer levels and NLR values.
This study was registered in Research Registry under the following identification number: researchregistry8367. This paper was written using the STROCSS 2021 guidelines [23].
Results
This study obtained data on 31 subjects and 113 controls. Characteristics of the study subjects are described in Table 1.
Based on Table 1, the average age of COVID-19 patients with preeclampsia was not significantly higher than for those without preeclampsia. There was a significant difference in delivery methods among COVID-19 patients. The majority of patients did not have pulmonary involvement, and mild cases of COVID-19 accounted for a high proportion of patients.
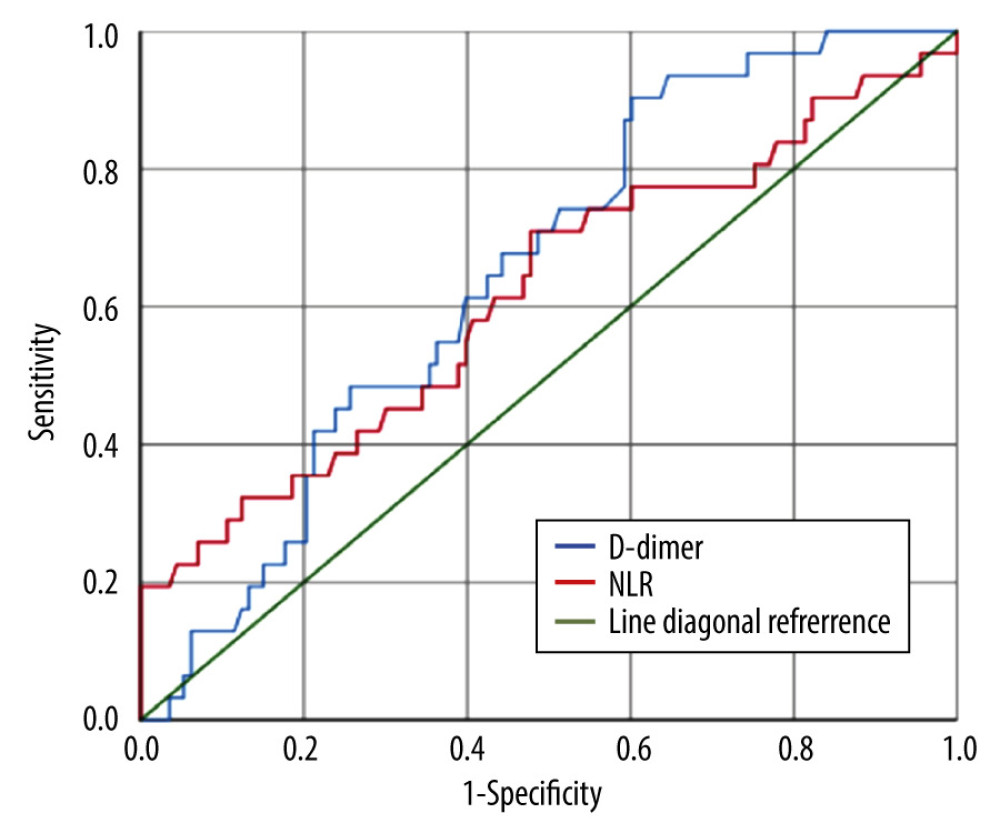
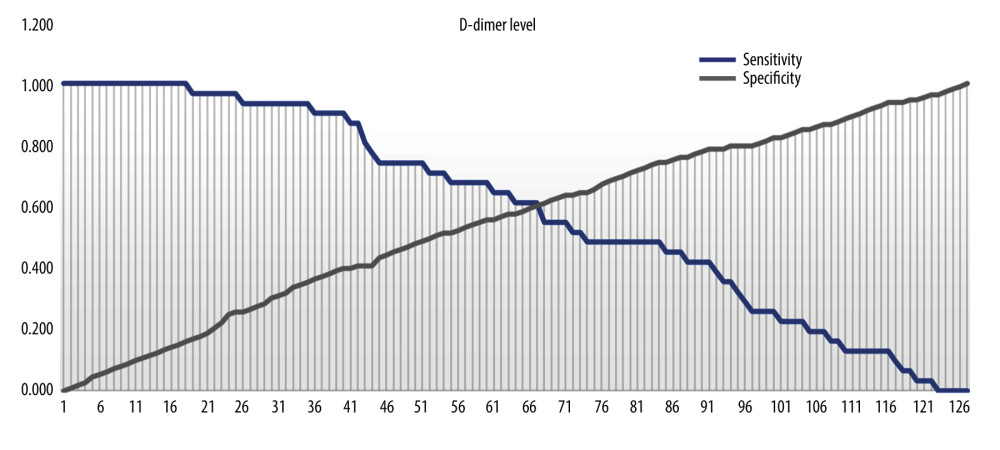
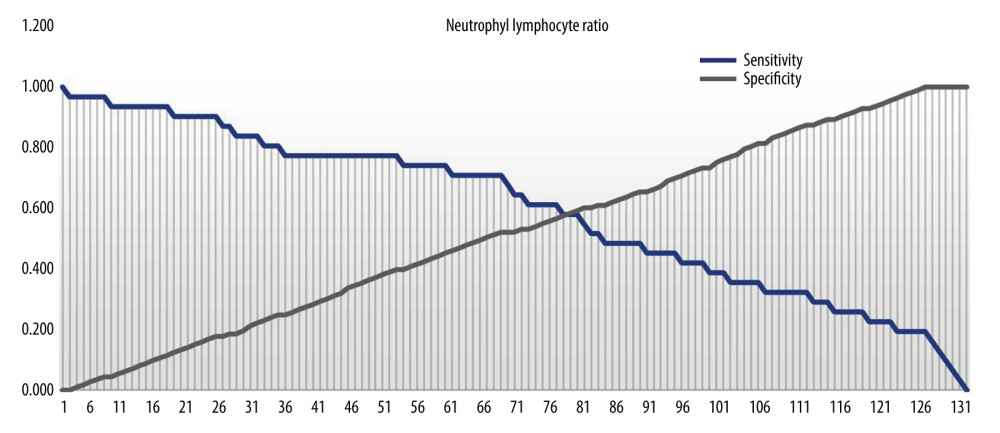
D-dimers and NLR were both significantly higher among COVID-19 patients without preeclampsia compared to those with preeclampsia (Table 2). The diagnostic value of D-dimer and NLR for predicting preeclampsia in COVID-19 patients was 64.9% (p=0.011) and 61.7% (p=0.045) respectively (Figure 1). The optimal cut-off point for D-dimers was 2.165 (sensitivity 61.3%; specificity 60.2%). The optimal cut-off point for NLR was 5.785 (sensitivity 58.1%; specificity 58.4%) (Figures 2, 3).
Discussion
Advanced maternal age is associated with higher maternal and fetal morbidity [24]. Age has a close relationship with the severity of the disease, complications, and deaths due to COVID-19 [25]. Studies found that COVID-19 in pregnancy can increase the risk of stillbirth, premature birth, or stunted fetal growth [26]. In the present study, there was no significant difference between patient age and gestational age.
The choice of delivery in COVID-19 is still controversial as studies are beginning to find that there is no association between the risk of transmission between mother and baby, either vaginally or abdominally. In the beginning, COVID-19 was the indication of most caesarean sections [27]. In the present study, there was a significant difference in delivery method between the COVID-19 group with preeclampsia and the group without preeclampsia.
The finding of pneumonia is characteristic of COVID-19, and radiological examination has diagnostic value [28]. In the present study, there was no significant difference in lung involvement between the 2 groups.
In pregnancy with preeclampsia, due to endothelial dysfunction, microvascular fibrin deposits occur, which then leads to formation of fibrin clots, which leads to formation of D-dimer. D-dimer levels are known to be higher in pregnant women with preeclampsia compared to those without preeclampsia [29]. In the present study, it was found that the level of D-dimer in COVID-19 patients with preeclampsia was higher than in COVID-19 patients without preeclampsia. This is in line with previous COVID-19 research showing a coagulation process that occurs due to an apoptotic response, an imbalance in the inflammatory response, and alveolar endothelial dysfunction, which ultimately creates a thrombus [14]. The majority of studies have found that D-dimer can be a predictor of severity in COVID-19 [30]. The cut-off values obtained are different for each study, ranging from the D-dimer cut-off value of 1.5 ng/ml [31] up to >2 ng/ml [32]. However, the examination of D-dimer in pregnant women is still controversial because hypercoagulation in pregnant women can occur physiologically [33].
The neutrophil-to-lymphocyte ratio (NLR) is a biomarker of systemic inflammatory response that is easy to perform and inexpensive, especially during the COVID-19 pandemic [34]. In preeclampsia, there is stimulation of inflammation caused by an abnormal immune response that leads to hypertension. This imbalance in the immune response will result in an increase in the NLR value in severe preeclampsia. In COVID-19, the severity often involves an inflammatory response in the host’s body, with more severe cases resulting from an exaggerated immune response called cytokine storm. The response due to viral infection will increase several inflammatory factors such as interleukin-6, interleukin-8, and granulocyte colony-stimulating factor, which all stimulate neutrophil production. In contrast to neutrophils, lymphopenia often occurs as the severity of COVID-19 increases [35]. Therefore, an increase in the number of neutrophils and a decrease in the number of lymphocytes in COVID-19 will increase the ratio of NLR values [36]. In this study, it was found that the NLR value in COVID-19 patients with preeclampsia was greater than in COVID-19 patients without preeclampsia. This is in accordance with the findings of previous studies, which have found that the NLR value increases both in the state of preeclampsia and in COVID-19. The NLR cut-off value in preeclampsia reported to be 5.6 [12]. However, there is no consensus on the NLR cut-off value, especially in COVID-19, although a meta-analysis found that NLR can predict the severity and mortality of COVID-19 patients [37], with a cut-off value of 3.3–5.9 to predict severity [36] and a cut-off of 7.9–11.8 to predict mortality [38].
Recent studies on and theories about pregnant women indicate a link between COVID-19 and the occurrence of preeclampsia. Levels of a receptor called angiotensin-converting enzyme 2 (ACE2) increase early in the development of the placenta in pregnancy. Activation of ACE2 receptors will convert Angiotensin II (Ang II) to Ang-(-1-7) to trigger vasodilating, antioxidant, anti-inflammatory, anti-remodeling, and anti-proliferative effects. At the time of placenta formation, both ACE2 and Ang II receptors can be found on the syncytiotrophoblast, cytotrophoblast, endothelial, and decidua cells [39]. Ang II can also induce apoptosis when the SARS-CoV-2 virus invades lung parenchyma cells. This continues in the activation of the intrinsic pathway of apoptosis caused by release of cytochrome-C from mitochondria and activation of caspase-9. This can be a factor in the relationship between Covid-19 and preeclampsia [40,41]. A large amount of Ang-(-1-7) is needed in normal pregnancy to create good placental development, so a low Ang-(-1-7) level can cause poor placental development and lead to preeclampsia [42]. In COVID-19, the SARS-CoV-2 virus also directly binds to ACE2 receptors throughout the body, including placental tissue if the patient is pregnant; as a result, plasma ACE2 levels will decrease followed by a decrease in Ang-(-1-7) levels and trigger preeclampsia [43]. SARS-CoV-2 infection can have signs and symptoms like preeclampsia. Women who simultaneously have COVID-19 and preeclampsia tend to have worse outcomes [44].
The findings above prove the relationship between hematological parameters (D-dimer) and inflammatory factors (NLR) that occur in the conditions of COVID-19 and preeclampsia. One of the relationships that exist in these 2 parameters is that the higher the D-dimer level and the NLR value, the higher the severity and risk of mortality from COVID-19 [45,46]. Previous research found a strong positive relationship between D-dimer levels and NLR values with length of treatment time and prognosis of intubation of COVID-19 patients undergoing intensive care [47]. However, in the state of COVID-19 and preeclampsia, there was an increase in D-dimer levels and NLR values, but the direct relationship between these has not been widely studied.
This study has several limitations. First, this study was carried out retrospectively based on medical record data during the period during the COVID-19 pandemic that was running simultaneously in Indonesia; as a result, it was difficult to know the progress of COVID-19 infection, which would require prospective research. Second, we did not assess or separate the heterogeneity associated with comorbidities and stratification of early-onset preeclampsia (early-onset preeclampsia/late-onset preeclampsia), which may have affected study outcomes. Further research is necessary to investigate the comorbidities to rule out increases in D-dimer or NLR parameters due to other inflammatory and coagulation factors.
Conclusions
There was a significant difference between D-dimer levels and NLR in COVID-19 patients with preeclampsia and without preeclampsia. There was a weak positive correlation between D-dimer levels and neutrophil-to-lymphocyte ratio (NLR) values in COVID-19 patients with preeclampsia.
References
1. Satuan Tugas Penanganan COVID-19: Asesmen Situasi Pandemi COVID-19 di Indonesia Tersedia di. Diakses pada 20 Agustus 2022 [in Indonesian]https://covid19.go.id/situasi
2. Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A, Physiological changes in pregnancy: Cardiovasc J Afr, 2016; 27(2); 89-94
3. Statistik BP: Profil Penduduk Indonesia Hasil Supas 2015, 2015 [in Indonesian]
4. Karrar SA, Hong PL, Preeclampsia: StatPearls [Internet], 2022, StatPearls Publishing
5. Allotey J, Stallings E, Bonet M, Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis: BMJ, 2020; 370; m3320
6. Conde-Agudelo A, Romero R, SARS-CoV-2 infection during pregnancy and risk of preeclampsia: A systematic review and meta-analysis: Am J Obstet Gynecol, 2022; 226(1); 68-89e3
7. Guler N, Siddiqui F, Fareed J, Is the reason of increased D-dimer levels in COVID-19 because of ACE-2-induced apoptosis in endothelium?: Clin Appl Thromb Hemost, 2020; 26; 1076029620935526
8. Beys-da-Silva WO, da Rosa RL, Santi L, The risk of COVID-19 for pregnant women: Evidences of molecular alterations associated with preeclampsia in SARS-CoV-2 infection: Biochim Biophys Acta Mol Basis Dis, 2021; 1867(3); 165999
9. Cevik M, Kuppalli K, Kindrachuk J, Peiris M, Virology, transmission, and pathogenesis of SARS-CoV-2: BMJ, 2020; 371; m3862
10. Patberg ET, Adams T, Rekawek P, Coronavirus disease 2019 infection and placental histopathology in women delivering at term: Am J Obstet Gynecol, 2021; 224(4); 382.e1-e18
11. Khader YS, Batieha A, Al-Njadat RA, Hijazi SS, Preeclampsia in Jordan: Incidence, risk factors, and its associated maternal and neonatal outcomes: J Matern Fetal Neonatal Med, 2018; 31(6); 770-76
12. Panwar M, Kumari A, Hp A, Raised neutrophil lymphocyte ratio and serum beta hCG level in early second trimester of pregnancy as predictors for development and severity of preeclampsia: Drug Discov Ther, 2019; 13(1); 34-37
13. de Pinheiro MB, Junqueira DR, Coelho FF, D-dimer in preeclampsia: Systematic review and meta-analysis: Clin Chim Acta, 2012; 414; 166-70
14. Guler N, Siddiqui F, Fareed J, Is the reason of increased D-dimer levels in COVID-19 because of ACE-2-induced apoptosis in endothelium?: Clin Appl Thromb Hemost, 2020; 26; 1076029620935526
15. Yu B, Li X, Chen J, Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: A retrospective analysis: J Thromb Thrombolysis, 2020; 50(3); 548-57
16. Egan K, Kevane B, Áinle FN, Elevated venous thromboembolism risk in preeclampsia: Molecular mechanisms and clinical impact: Biochemical Society Transactions, 2015; 43(4); 696-701
17. Dusse LM, Alpoim PN, Lwaleed BA, Is there a link between endothelial dysfunction, coagulation activation and nitric oxide synthesis in preeclampsia?: Clinica Chimica Acta, 2013; 415; 226-29
18. Heilmann L, Rath W, Pollow K, Hemostatic abnormalities in patients with severe preeclampsia: Clin Appl Thromb Hemost, 2007; 13(3); 285-91
19. Ramma W, Buhimschi IA, Zhao G, The elevation in circulating anti-angiogenic factors is independent of markers of neutrophil activation in preeclampsia: Angiogenesis, 2012; 15(3); 333-40
20. Kang Q, Li W, Yu N, Predictive role of neutrophil-to-lymphocyte ratio in preeclampsia: A meta-analysis including 3982 patients: Pregnancy Hypertens, 2020; 20; 111-18
21. Martins EC, Silveira LdF, Viegas K, Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: A case-control study: Rev Bras Ter Intensiva, 2019; 31; 64-70
22. Coperchini F, Chiovato L, Croce L, The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system: Cytokine Growth Factor Rev, 2020; 53; 25-32
23. Mathew G, Agha R, Albrecht J, Strocss 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery: Int J Surg Open, 2021; 37; 100430
24. Pinheiro RL, Areia AL, Mota Pinto A, Donato H, Advanced maternal age: Adverse outcomes of pregnancy, a meta-analysis: Acta Med Port, 2019; 32(3); 219-26
25. Guan WJ, Ni ZY, Hu Y, Clinical characteristics of coronavirus disease 2019 in China: N Engl J Med, 2020; 382(18); 1708-20
26. Piekos SN, Roper RT, Hwang YM, The effect of maternal SARS-CoV-2 infection timing on birth outcomes: A retrospective multicentre cohort study: Lancet Digit Health, 2022; 4(2); e95-e104
27. Sarastry R, Layarta C, Aladini U, Pramono BA, Delivery routes in pregnancy with COVID-19 and the risk of intrapartum vertical transmission: A meta-analysis: Medical J Indones, 2021; 30; 116-22
28. Shi H, Han X, Jiang N, Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study: Lancet Infect Dis, 2020; 20(4); 425-34
29. Bellart J, Gilabert R, Fontcuberta J, Coagulation and fibrinolysis parameters in normal pregnancy and in gestational diabetes: Am J Perinatol, 1998; 15(8); 479-86
30. Zhou F, Yu T, Du R, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study: Lancet, 2020; 395(10229); 1054-62
31. Poudel A, Poudel Y, Adhikari A, D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19: PLoS One, 2021; 16(8); e0256744
32. Zhang L, Yan X, Fan Q, D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19: J Thromb Haemost, 2020; 18(6); 1324-29
33. Hunt B, Retter A, McClintock C: Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19, 2020, Thrombosis UK, Llanwrda
34. de Jager CP, Wever PC, Gemen EF, The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia: PLoS One, 2012; 7(10); e46561
35. Di Mascio D, Khalil A, Saccone G, Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis: Am J Obstet Gynecol MFM, 2020; 2(2); 100107
36. Yang AP, Liu JP, Tao WQ, Li HM, The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients: Int Immunopharmacol, 2020; 84; 106504
37. Simadibrata DM, Calvin J, Wijaya AD, Ibrahim NAA, Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis: Am J Emerg Med, 2021; 42; 60-69
38. Yan X, Li F, Wang X, Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: A retrospective cross-sectional study: J Med Virol, 2020; 92(11); 2573-81
39. Tamanna S, Clifton VL, Rae K, Angiotensin converting enzyme 2 (ACE2) in pregnancy: Preeclampsia and small for gestational age: Front Physiol, 2020; 11; 590787
40. Li W, Moore MJ, Vasilieva N, Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus: Nature, 2003; 426(6965); 450-54
41. Lee YH, Mungunsukh O, Tutino RL, Angiotensin-II-induced apoptosis requires regulation of nucleolin and Bcl-xL by SHP-2 in primary lung endothelial cells: J Cell Sci, 2010; 123(10); 1634-43
42. Ashary N, Bhide A, Chakraborty P, Single-Cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2: Front Cell Dev Biol, 2020; 8; 783
43. Sathiya R, Rajendran J, Sumathi S, COVID-19 and preeclampsia: Overlapping features in pregnancy: Rambam Maimonides Med J, 2022; 13(1); e0007
44. Algarroba GN, Hanna NN, Rekawek P, Confirmatory evidence of the visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy: Am J Obstet Gynecol, 2020; 223(6); 953-54
45. Lombardi A, Duiella S, Piani LL, Inflammatory biomarkers in pregnant women with COVID-19: A retrospective cohort study: Sci Rep, 2021; 11(1); 13350
46. Fu J, Kong J, Wang W, The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China: Thromb Res, 2020; 192; 3-8
47. Ye W, Chen G, Li X, Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19: Respir Res, 2020; 21(1); 169
Figures
Tables
 Table 1. Characteristics of the study subjects.
Table 1. Characteristics of the study subjects.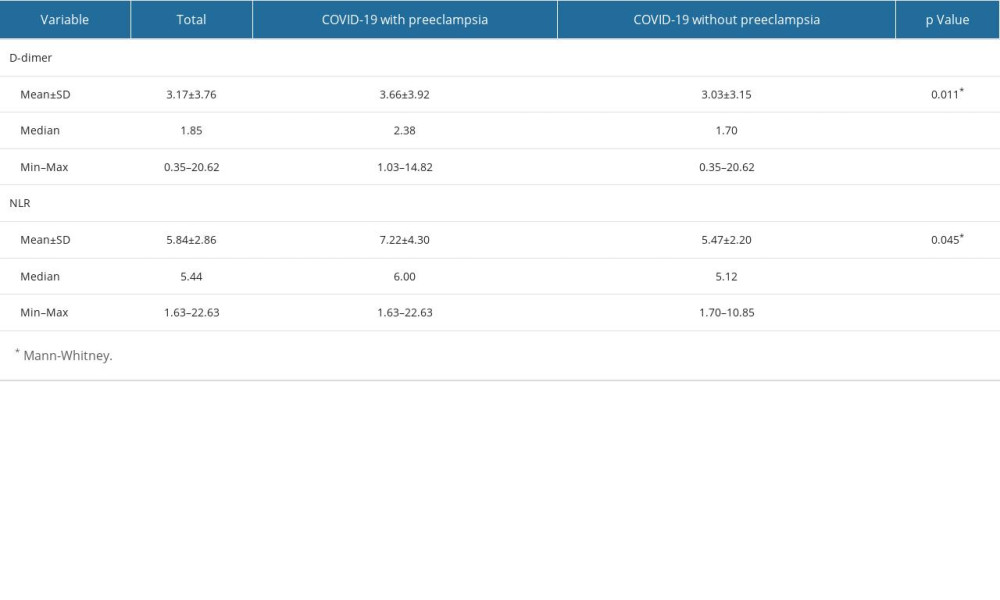 Table 2. Differences in D-dimer levels and NLR values in COVID-19 patients with and without preeclampsia.
Table 2. Differences in D-dimer levels and NLR values in COVID-19 patients with and without preeclampsia. Table 1. Characteristics of the study subjects.
Table 1. Characteristics of the study subjects. Table 2. Differences in D-dimer levels and NLR values in COVID-19 patients with and without preeclampsia.
Table 2. Differences in D-dimer levels and NLR values in COVID-19 patients with and without preeclampsia. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952