06 July 2023: Clinical Research
Analgesic Efficacy of Combined Thoracic Paravertebral Block and Erector Spinae Plane Block for Video-Assisted Thoracic Surgery: A Prospective Randomized Clinical Trial
Lili Zhang1ABCE, Yang Hu1BEF, Hong Liu2AE, Xue Qi1CD, Hong Chen1B, Wei Cao3B, Longsheng Wang4BD, Ye ZhangDOI: 10.12659/MSM.940247
Med Sci Monit 2023; 29:e940247
Abstract
BACKGROUND: Thoracic paravertebral block (TPVB) and erector spinae plane block (ESPB) are widely used in video-assisted thoracic surgery (VATS). However, they have corresponding adverse effects, including hypotension for TPVB and unpredictable injectate spread in ESPB. An optimal perioperative analgesic strategy remains controversial. We investigated the effect of ultrasound-guided combined TPVB and ESPB (CTEB) for VATS.
MATERIAL AND METHODS: A total of 120 patients scheduled for thoracic surgery were randomized to receive either ultrasound-guided TPVB, ESPB, or CTEB preoperatively. Postoperative analgesia was achieved with sufentanil patient-controlled intravenous analgesia. The primary outcome was the static pain score at 2 h after surgery.
RESULTS: The static pain score 2 h postoperatively was significantly different among the 3 groups. This difference was statistically significant for Group ESPB vs Group TPVB (P=0.004), but not for Group ESPB vs Group CTEB (P=0.767), or Group TPVB vs Group CTEB (P=0.117). Group TPVB exhibited the highest incidence of hypotension among the 3 groups. More patients experienced a sensory loss in Groups TPVB and CTEB 30 min after the block performance. Patients receiving CTEB exhibited a lower incidence of chronic pain 6 months postoperatively than those in Group ESPB.
CONCLUSIONS: CTEB does not enhance the analgesic effect of ESPB in patients undergoing VATS; however, it may induce a faster sensory loss after nerve block and reduce the incidence of postoperative chronic pain compared with ESPB. CTEB may also help to reduce the incidence of intraoperative hypotension compared with TPVB.
Keywords: Anesthesia and Analgesia, Nerve Block, Pain, Postoperative, Thoracic Surgery, Video-Assisted, Humans, chronic pain, Prospective Studies, Analgesics, Hypotension
Background
Video-assisted thoracic surgery (VATS) has been used as a preferred alternative to thoracotomy because of its lower invasiveness, better quality of life, and shorter hospital stay [1,2]. However, patients still experience moderate-to-severe pain in the immediate postoperative period, which limits the respiratory effects and increases the risk of respiratory complications. Regional anesthesia techniques play an important role in multimodal analgesia in VATS. However, the optimal perioperative analgesic strategy remains controversial [3,4].
Thoracic paravertebral block (TPVB) is a regional anesthetic technique in which local anesthetic (LA) is administered inside the thoracic paravertebral space (TPVS), which contains the intercostal spinal nerves, spinal dorsal rami, rami communicants, sympathetic chain, intercostal vessels, and fatty tissue [5]. It has been reported that a single injection of TPVB can cover 3 to 6 dermatomal levels [6,7] and provide adequate analgesia for thoracic surgery. However, injection at a single site usually results in a large volume of LA at one vertebral level. TPVS is continuous with the epidural and intercostal spaces. A large volume of LA in the TPVS can increase the risk of epidural spread of LA, leading to sympathetic blockade complications, specifically hypotension [8–10]. Recently, the erector spinae plane block (ESPB), in which the LA is injected below the erector spinae muscle, has been reported as a valuable method for postoperative thoracic analgesia, with a better safety margin [11–13]. It can achieve blockade of the thoracic nerve roots in the TPVS without actual injection of LA directly into this space [11]. Nevertheless, the mechanism of pain relief is not completely understood, and the analgesia exhibits wide variation [11,14].
Combined regional block techniques have been demonstrated to be effective and safe in cardiothoracic anesthesia, such as the combined serratus anterior plane block and transverse thoracic muscle plane block for coronary artery bypass surgery [15] and the combined deep and superficial serratus anterior plane block for VATS [16]. Accordingly, we hypothesized that if TPVB and ESPB are combined, the needle path to the TPVS could potentiate the diffusion of LA reserved in the erector spinae plane (ESP), and the diffusion of LA in the TPVS and ESP would enhance the certainty of analgesia and decrease the adverse effect of nerve block. Therefore, we designed a randomized controlled double-blind trial to determine the postoperative analgesic efficacy of combined TPVB and ESPB (CTEB) in comparison to single TPVB or ESPB in patients undergoing unilateral VATS. Also, the local anesthetic distribution was evaluated by magnetic resonance imaging (MRI). The primary outcome was the static pain intensity assessed using the numeric rating scale (NRS) at 2 h after surgery. We hypothesized that CTEB would reduce the postoperative pain intensity compared with the ESPB, with lower sympathetic blockade complications in comparison with TPVB.
Material and Methods
ETHICS STATEMENT:
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (No: YX2020-070), and written informed consent was obtained from all patients participating in the trial. The trial was registered prior to patient enrollment in the Chinese Clinical Trial Register at http://www.chictr.org.cn (ChiCTR2000037688; principal investigator: Ye Zhang, date of registration: August 30, 2020). This prospective randomized controlled trial was conducted in the Second Affiliated Hospital of Anhui Medical University from October 2020 to July 2021. This article was in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines [17].
PARTICIPANTS:
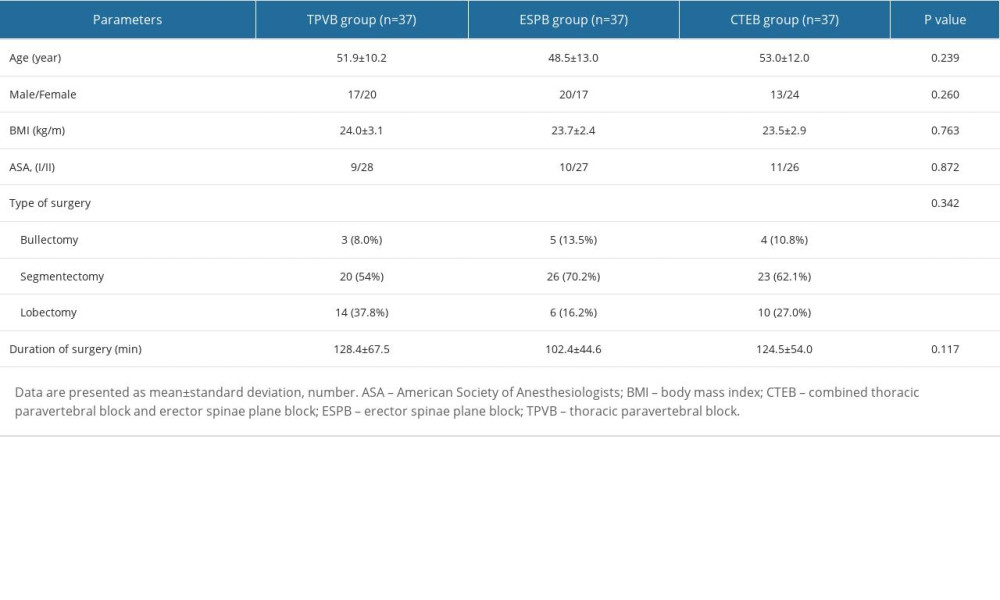
Patients undergoing elective unilateral VATS with 2 trocar ports were enrolled in this study. The ports were made at the fourth or fifth, and seventh intercostal levels. A chest drain was inserted before skin closure at the lower port. The inclusion criteria were age between 18 and 65 years and American Society of Anesthesiologists physical status I to III. The exclusion criteria were body mass index >30 kg/m2, coagulopathy, history of opiate abuse, pre-existing chronic pain, allergy to local anesthetics or analgesics, infection at the site of injection, mental or neurological disorders, and operation converted to open thoracotomy.
RANDOMIZATION AND BLINDING:
Patients were randomly allocated to receive either ultrasound-guided TPVB (TPVB group), ESPB (ESPB group), or the combined TPVB and ESPB (CTEB group) by computer software in a random group allocation of 1: 1: 1. An assistant prepared the randomization list and concealed group assignments in consecutively numbered, sealed, opaque envelopes. Thereafter, he was no longer involved in the study. A consultant anesthesiologist (Y. L.) who was unaffiliated with following management opened the envelopes shortly before the nerve block performance to reveal the group allocation and then performed the procedure according to the assignment. To ensure blinding, all patients received the block performance under midazolam-induced sedation and the ultrasound images were not shown to the patients. Therefore, patients were masked to their group allocation. Outcome assessors and clinical staff were also blinded to the intervention. Unmasking did not occur until statistical analysis was complete.
REGIONAL ANESTHESIA TECHNIQUES:
After arrival in the pre-anesthesia room, intravenous (i.v.) access was established and premedication (midazolam 0.02 mg/kg i.v.) was administered to patients. Vital parameters, including heart rate, electrocardiogram, blood pressure, and pulse oximetry, were monitored throughout the procedure. All blocks were performed with a 22-gauge block needle, using the same ultrasound machine (SonoSite Edge; SonoSite, Inc., Bothell, WA, USA) and linear ultrasound transducer (SonoSite HFL 50×; SonoSite, Inc.). All patients were placed in a lateral decubitus position, and the needle was inserted at the level of the T5 transverse process using an ultrasound-guided approach. According to a previous study in which total volume of 25 mL of 0.5% ropivacaine was injected in the TPVS [18], all patients received an equal volume and concentration of ropivacine.
TPVB and ESPB were performed as previously described [11,19], and 25 mL of 0.5% ropivacaine was injected into the TPVS and ESP, respectively. For CTEB (Video 1), the linear probe was placed on the patient in a longitudinal position at approximately 3 cm lateral to the midline. The tip of the T5 transverse process, overlying the erector spinae muscle, apex of the TPVS, and pleura were identified. A 22-gauge block needle was inserted using an out-of-plane technique. After the needle tip was advanced beyond the superior costotransverse ligament, 0.5 to 1 mL of normal saline was injected to confirm the correct needle tip position by the displacement of the pleura, and then 5 mL of 0.5% ropivacaine was injected. Subsequently, the needle was withdrawn and adjusted to come in contact with the tip of the T5 transverse process. Correct needle tip position was confirmed by hydro-dissection by visualizing the linear spread of the fluid that lifted the erector spinae muscle off the transverse process (Figure 1). Then, 20 mL of 0.5% ropivacaine was injected in a cranial-to-caudal direction into the ESP. The sensory blockade was tested using a cold test, in comparison with the contralateral dermatomes from T5 in the midclavicular line, extending over the anterolateral thoracic wall in a cranial and then in a caudal direction 30 min after the completion of all blocks.
MRI INVESTIGATION:
Thirty minutes after nerve block, MRI was performed in 9 patients (3 patients in each group). The distribution pattern of the LA was evaluated by analyses of T2-weighted, fluid-sensitive images in the axial and sagittal planes. Starting at the T5 puncture level, the axial T2-weighted sequences were analyzed in the cranial and caudal directions to evaluate the distribution of the LA in the axial planes. The sagittal plane images were analyzed in the cranial and caudal directions from C1 to L5.
GENERAL ANESTHESIA MANAGEMENT AND POSTOPERATIVE PAIN ASSESSMENT:
After nerve block, patients were transferred to the operating room and received general anesthesia with standardized monitoring. General anesthesia was induced with propofol (1–2 mg/kg), sufentanil (0.6 μg/kg), and rocuronium (0.6 mg/kg), and a double-lumen tube was placed. Anesthesia was maintained with continuous infusion of propofol and remifentanil. The patients’ heart rate and mean arterial pressure were recorded continuously. Flurbiprofen (50 mg) and dexamethasone (10 mg) were administered i.v. during skin closure to prevent postoperative pain and postoperative nausea and vomiting. After extubation in the operation room, all patients were transferred to the Anesthesia Intensive Care Unit (AICU) for recovery.
Patients received regular analgesia with flurbiprofen 50 mg per 8 h in the AICU. Additionally, i.v. patient-controlled analgesia with sufentanil (bolus dose of 0.06 μg/kg and lockout interval of 15 min, without background infusion) was initiated if the NRS (0=no pain, 10=maximum pain imaginable) was ≥4 or upon the patient’s request. An AICU nurse, blinded to the protocols, recorded postoperative pain at rest and during coughing, using the NRS at 0.5, 2, 4, 8, 12, and 24 h after surgery. The postoperative nausea and vomiting score was assessed using a 4-point scale (0=no nausea or vomiting, 1=nausea but vomiting, 2=vomiting 1 to 3 times, and 3=vomiting more than 4 times) [20]. Patients rated their overall satisfaction with pain management using the following scores: 1=very dissatisfied, 2=dissatisfied, 3=satisfied, and 4=very satisfied at discharge from the hospital [21]. Postoperative length of hospital stay and chest tube removal time were obtained from the electronic medical records.
At 6 months after thoracic surgery, the intensity of the patients’ average pain during the previous week was assessed by a telephone interview with the following questions [22,23]: (1) “Do you currently have pain related to your thoracic surgery?” and (2) “Please rate your thoracic surgery pain only by indicating the number that best describes your average pain in the last 1 week using the NRS (0 to 10; 0=no pain and 10=worst possible pain).” Chronic postoperative pain was defined as NRS pain scores ≥1.
OUTCOMES:
The primary outcome was the static NRS score at 2 h after surgery. The secondary outcomes were NRS scores and opioid consumption during the first 24 h after surgery, the number of patients who reported sensory loss 30 min after the completion of nerve block, time to first rescue opioid, postoperative nausea and vomiting score, heart rate and mean arterial pressure values during surgery, and incidence of complications such as hypotension (defined as the systolic blood pressure <90 mmHg or more than 20% decline from the baseline) [24] and postoperative pulmonary complications, which were assessed using the Melbourne Group Scale [25]. The incidence of chronic postoperative pain was recorded at 6 months after surgery.
SAMPLE SIZE CALCULATION AND STATISTICAL ANALYSIS:
Based on the results of our pilot study, in which the mean NRS score at 2 h after surgery was 1.33±0.82 for the TPVB group, 3.50±2.43 for the ESPB group, and 3.00±1.26 for the CTEB group, the sample size was calculated based on the primary outcome of NRS score at 2 h after surgery using PASS V.15.0 (PASS, NCSS, USA) for Windows. One-way analysis of variance (ANOVA) was chosen as the statistical test method in the PASS software. Group allocation ratios were equal. At a power of 0.90 and an alpha error level of 0.05, to account for 20% loss to follow-up, the required sample size for each group was calculated as 37. Additionally, since 3 patients in each group were required to undergo MRI examinations, a total of 40 patients per group were required. Thus, a total of 120 participants were needed for the study.
All statistical analyses were performed using SPSS (version 24.0; IBM, New York, USA) and GraphPad Prism 8 (GraphPad Software, La Jolla, California, USA). The Kolmogorov-Smirnov test was used to test the normality of the continuous variables. Normally distributed data are expressed as means±standard deviation, and abnormal data are expressed as medians [interquartile range]. Inter-group differences were assessed for significance using the ANOVA or Kruskal-Wallis tests, as appropriate, followed by the Bonferroni correction. Categorical variables were expressed as numbers (percentages), and inter-group differences were assessed using the chi-squared or Fisher exact tests in cases of expected frequency <5.
The repeatedly measured NRS scores were analyzed using a linear mixed model including all postoperative time points to evaluate the interaction between the NRS score over time and the intervention technique. This model included time as a repeated effect with an autoregressive 1 covariance structure. Intervention, time, and intervention-by-time interaction were set as the fixed effects, and postoperative pain scores were included as covariates. The time to first rescue analgesic administration was analyzed using Kaplan-Meier survival analysis followed by the log-rank test, with adjustment for multiple comparisons. Repeated measures ANOVA with Bonferroni correction was used for mean arterial pressure and heart rate data. The nature of the hypothesis testing was 2-tailed, and
Results
PATIENT CHARACTERISTICS:
The CONSORT flow diagram of study enrolment is presented in Figure 2. A total of 120 patients were allocated randomly into this trial. Nine patients were not analyzed for measurement, since the incision time was delayed due to the MRI scanning. There were no significant differences between the 3 groups with regard to the baseline characteristics and clinical variables (Table 1).
NRS SCORE ASSESSMENTS:
The linear mixed model showed that patients receiving ESPB had a significant higher NRS score than patients receiving TPVB at rest, at 0.5 and 2 h after surgery (mean difference=1.1, 95% confidence interval [CI] 0.4 to 1.7, P<0.001; mean difference=0.8, 95% CI 0.2 to 1.5, P=0.004, respectively). There were no significant differences in the static NRS scores 2 h after surgery between the CTEB and TPVB groups (mean difference=0.5, 95% CI −0.1 to 1.2, P=0.117), and no significant differences were observed at other time points in the first 24 h after surgery. The static NRS scores at 2 h after surgery were comparable between the ESPB and CTEB group (mean difference=0.3, 95% CI −0.2 to 1.5, P=0.767). In terms of the NRS scores on coughing, the ESPB and CTEB groups had significantly higher NRS scores than the TPVB group at 0.5 h after surgery (mean difference=1.6, 95% CI 0.8 to 2.3, P<0.001; mean difference=1.2, 95% CI 0.4 to 2.0, P=0.001, respectively), and at 2 h after surgery (mean difference=1.1, 95% CI 0.4 to 1.9, P=0.001; mean difference=1.0, 95% CI 0.3 to 1.8, P=0.004, respectively). There were no significant differences in the NRS scores among the 3 groups from 8 h to 24 h after surgery (Figure 3).
PERIOPERATIVE MEASUREMENTS AND CHRONIC PAIN ASSESSMENT:
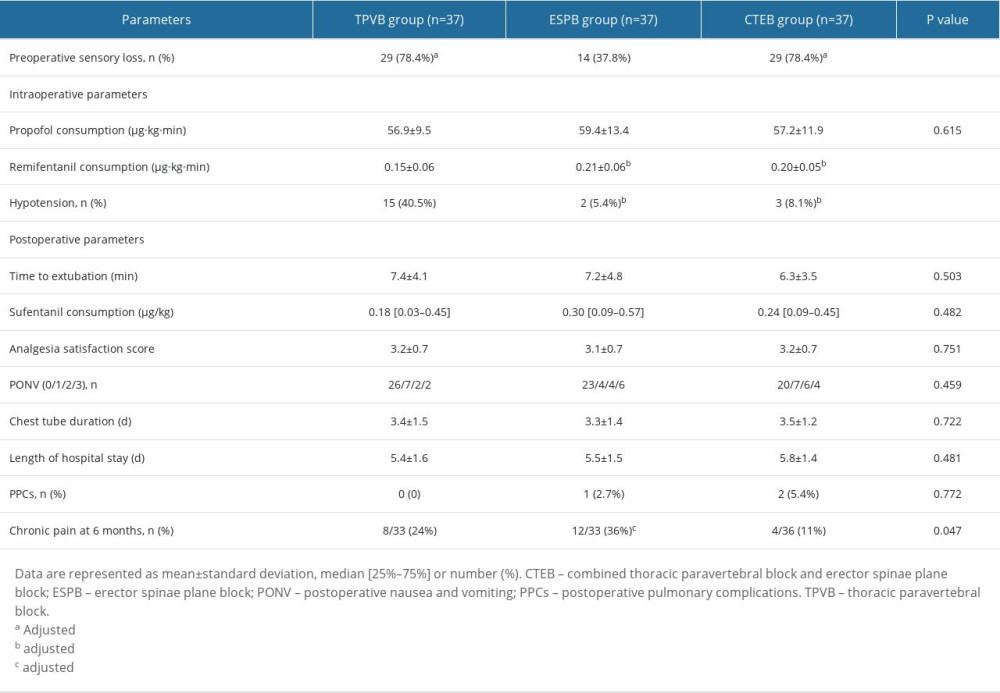
Significantly more patients in the TPVB and CTEB groups presented with a sensory loss 30 min after the completion of nerve block than did those in the ESPB group (78.4% in the TPVB group, 78.4% in the CTEB group vs 37.8% in the ESPB group, both P=0.001; Table 2). Patients receiving TPVB consumed a lower amount of remifentanil than those receiving ESPB and CTEB ([0.15±0.06] vs [0.21±0.06] μg·kg−1·min−1, P<0.001; [0.15±0.06] vs [0.20±0.05] μg·kg−1·min−1, P=0.001; Table 2), and exhibited a higher incidence of hypotension during surgery (15/37 vs 2/37 patients, P<0.001; 15/37 vs 3/37 patients, P=0.003; Table 2). Parameters of mean arterial pressure and heart rate are shown in Figure 4. The Kaplan-Meier curve for the time to the first opioid administration is shown in Figure 5. The time to first opioid administration was longer in the TPVB group than in the ESPB and CTEB group (444 [128–1437] vs 120 [23–744] min, P=0.039; 444 [128–1437] vs 114 [33–552] min, P=0.015). There were no significant differences in these parameters between the ESPB and CTEB groups. Furthermore, there were no significant differences among the 3 groups in terms of cumulative opioid consumption at 24 h after surgery, analgesia satisfaction score, postoperative nausea and vomiting score, time to chest tube removal, length of hospitalization, and incidence of pulmonary complications after surgery (Table 2). Nine patients lost contact during the 6-month follow-up. Patients in the CTEB reported a lower incidence of chronic pain than those in the ESPB group (4/36 vs 12/33, P=0.039, Table 2).
LA SPREAD PATTERN VIA MRI:
Figure 6 shows the MRI images of injectate spread of the TPVB, ESPB, and CTEB (Figure 6A–6C) and the vertebral levels of LA diffusion via the sagittal plane of MRI and the axial distribution of LA in each patient (Figure 6D). In the axial plane of the MRI, the injectate was detected inside the TPVS and intercostal space when the patients received a single TPVB (Figure 6A). The injectate was only detected inside the erector spinae muscle when the patients received a single ESPB (Figure 6B), while the injectate was detected inside the TPVS and erector spinae muscle at the level of T5 and was present in the intercostal space and neural foramen (Figure 6C) after CTEB. In the sagittal plane images, LA distribution was mainly confined to the thoracic vertebral levels when patients received TPVB or ESPB, while the LA was detected from C7 to L1 in patients receiving CTEB. For the axial distribution, the LA was detected inside the TPVS and the intercostal space in the TPVB group, inside the erector spinae muscle in the ESPB group, and inside the erector spinae muscle, TPVS, and intercostal space in the CTEB group (Figure 6D).
Discussion
The main finding of this study is that TPVB provided superior analgesia for the patients undergoing VATS compared with ESPB and CTEB. CTEB did not enhance the analgesic compared with ESPB; however, it reduced the incidence of hypotension compared with TPVB. Furthermore, our data reveal that more patients receiving TPVB and CTEB experienced a sensory loss 30 min after nerve block, and CTEB exhibited an advantage of lower incidence of postoperative chronic pain than did ESPB.
TPVB has been considered a well-established and criterion standard technique to provide analgesia for pain control and opioid sparing after thoracic surgery [26,27]. However, the TPVS communicates with the intercostal space in a lateral direction, the epidural space via the intervertebral foramina, and the contralateral TPVS via the pre-vertebral and epidural space [5]. Thus, the spread of LA solution inside the TPVS is mostly uncertain. A volunteer study has presented evidence of epidural spread of LA via MRI in 25% of TPVB. The epidural spread of LA was also found in cadaver studies, in which proportions of paravertebral administered fluid was detected in the epidural space [28]. Therefore, hypotension cannot be totally avoided in patients receiving TPVB [3,10]. ESPB has been reported as a valuable indirect TPVB method for thoracic analgesia [11,12]. The LA is injected below the erector spinae muscle and is expected to extend to the paravertebral space gradually, acting on both the ventral and dorsal rami of the spinal nerves [11,29]. Since the ultrasonographic landmark of the transverse process is the endpoint for needle advancement and distant from the pleura, ESPB can be performed more easily and safely. However, the erector spinae is a bundle of muscles and tendons, and the interspace of the transverse process is bounded by intertransverse connective tissues. Individual anatomical variations can lead to different penetration of the LA, which makes ESPB unpredictable, and sometimes only partial success is achieved [30]. In the present study, patients receiving ESPB reported significantly higher static pain scores at 0.5 h and 2 h after VATS, which is consistent with the previous study that the ESPB group demonstrated higher static pain scores than the TPVB group at 1, 2 and 24 h after VATS [31]. The combination of different regional anesthesia techniques has been investigated and applied successfully in different study settings. The combination allows LA acting in different planes, providing a synergistic action and compensating for potential failures in one of these blocks, thus enhancing pain relief after surgery [15,16,32,33]. For this reason, we performed the TPVB first and then performed the ESPB subsequently, such that the needle path could form a mini-track that potentiated the spread of the LA from the ESP to TPVS. In our MRI study, the LA distribution was limited to the ESP 30 min after ESPB. However, LA was observed inside the TPVS and ESP after CTEB, and patients in the CTEB group reported comparable static NRS scores to those in the TPVB group in the first 24 h after surgery. In contrast to our expectations, CTEB did not provide a comparable analgesia to that of TPVB at coughing immediately after surgery. Since a previous study demonstrated that continuous ESPB exhibited a similar analgesia on movement after VATS compared with TPVB [31], the CTEB catheterization may be performed to evaluate the coughing pain control in our future study.
We found that sensory loss was elicited in more patients in the TPVB and CTEB groups 30 min after nerve block performance. That may be due to the perineural infiltration in the TPVS, which leads to a quick nerve block, and accurate deposition close to targets of interest may enhance the nerve block [34]. Meanwhile, we detected an extensive distribution of LA via MRI. In terms of CTEB, we speculated that the large volume of LA in the ESP could act as a reservoir and spread to the TPVS from the needle path over time and prolong the duration of block. However, the time to the first rescue analgesic administration was not shortened in the CTEB group when compared to that in the TPVB group, although it was comparable to that in the ESPB group. Thus, the spread of the LA through the needle path may not act as fast as we expected. It is worth noting that patients receiving CTEB had a lower incidence of chronic pain 6 months after surgery than those in the ESPB group. This may be the great advantage of CTEB, since more LA may spread to the TPVS, which benefits the analgesia and prevents the chronic pain. However, in addition to postoperative acute pain, other factors like surgery-related (nerve injury) and psychological factors are also important risk factors for developing chronic pain after surgery [35]. Therefore, further studies are needed to accurately assess the effects and mechanisms of the action.
The patients in the TPVB group used the least amount of remifentanil, which may have resulted from the more potent analgesic effect of TPVB. However, 15 patients in the TPVB group were treated with an i.v. vasopressor for intraoperative hypotension, while this occurred in only 2 and 3 patients in the ESPB and CTEB groups, respectively. This could have resulted from sympathetic blockage of TPVB, which has led to investigations on an indirect TPVB approach to reduce the adverse effects, such as the ESPB, retrolaminar block [21,36], and CTEB in the present study.
This study had some limitations. First, we performed MRI in only 3 patients in each group, and this may not be enough to account for the differential diffusion of the LA. Further research with a large sample size is required to evaluate the distribution of local anesthetic via MRI after combined nerve blocks, which would further reflect the patient-based variability of LA diffusion. However, data of MRI should also be interpreted with caution, because it is a non-dynamic method of investigation and, therefore, only conclusions regarding the spread of LA at the time of MRI investigation can be drawn [5]. Second, the primary outcome, the NRS, is not an objective indicator; therefore, it can affect the efficacy of evaluation. Third, nerve blocks were used as part of a multimodal analgesia regimen, which could potentially affect the results of analgesia. However, in current clinical practice, most peripheral nerve blocks are used as part of multimodal analgesia; thus, we considered it desirable to compare these blocks in real-world clinical practice. Moreover, we did not measure the procedure time, discomfort score of patients, accurate onset time of each nerve block, or the characteristics of chronic pain.
Conclusions
In summary, we found that CTEB does not improve the analgesic effect of ESPB in patients undergoing VATS; however, it may induce a faster sensory loss after nerve block and reduce the incidence of postoperative chronic pain compared with ESPB. The CTEB may also help to reduce the incidence of intraoperative hypotension compared with TPVB. Further research is required to evaluate the efficacy and safety of CTEB in clinical practice.
Figures
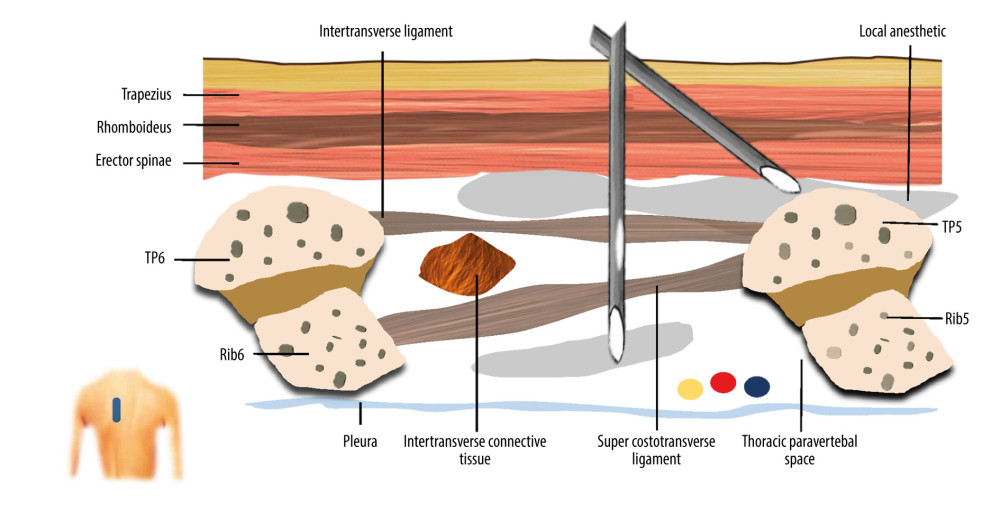 Figure 1. A schematic illustration of ultrasound guided combined thoracic paravertebral block and erector spinae plane block (CTEB). Illustration of sagittal section at level T5/T6 at approximately 3 cm lateral to the midline, depicting the trajectory of the needle and tip location. The needle path may form a mini-tunnel that would potentiate the penetration of injectate to thoracic paravertebral space from the erector spinae place, which contains a large volume of injectate, through the intertransverse ligament, intertransverse connective tissue, and super costotransverse ligament. (Microsoft PowerPoint 2019MSO, version 2303, Microsoft Corporation).
Figure 1. A schematic illustration of ultrasound guided combined thoracic paravertebral block and erector spinae plane block (CTEB). Illustration of sagittal section at level T5/T6 at approximately 3 cm lateral to the midline, depicting the trajectory of the needle and tip location. The needle path may form a mini-tunnel that would potentiate the penetration of injectate to thoracic paravertebral space from the erector spinae place, which contains a large volume of injectate, through the intertransverse ligament, intertransverse connective tissue, and super costotransverse ligament. (Microsoft PowerPoint 2019MSO, version 2303, Microsoft Corporation). 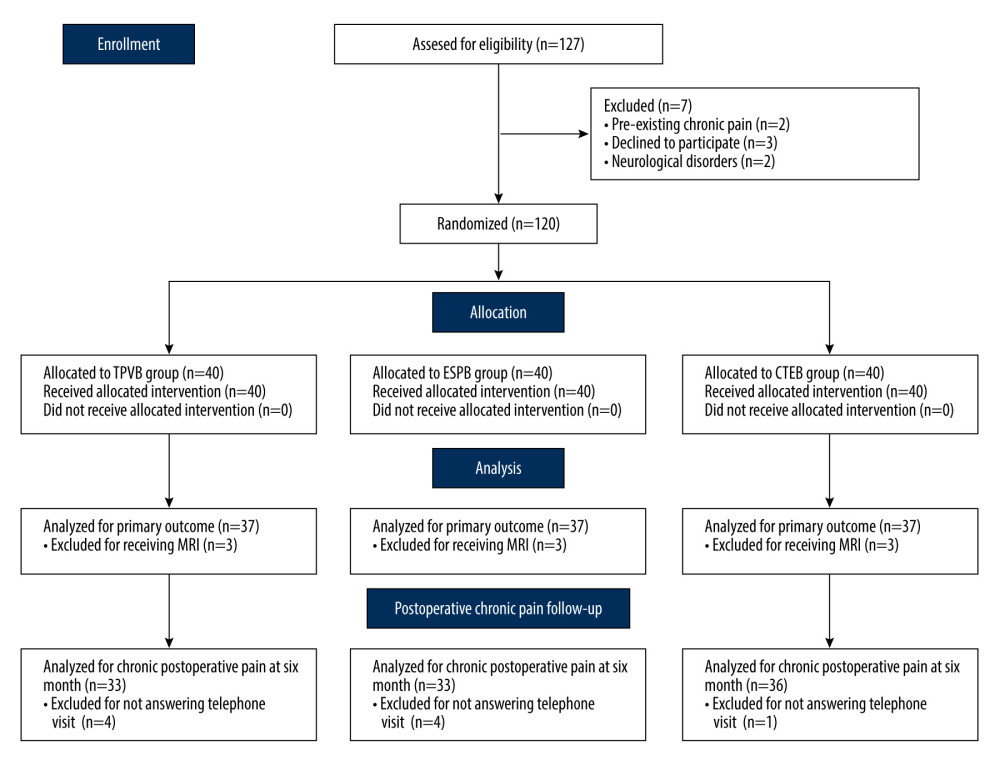 Figure 2. CONSORT flow diagram. CONSORT – Consolidated Standards of Reporting Trials; CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (Microsoft Word 2019MSO, version 2303, Microsoft Corporation).
Figure 2. CONSORT flow diagram. CONSORT – Consolidated Standards of Reporting Trials; CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (Microsoft Word 2019MSO, version 2303, Microsoft Corporation). 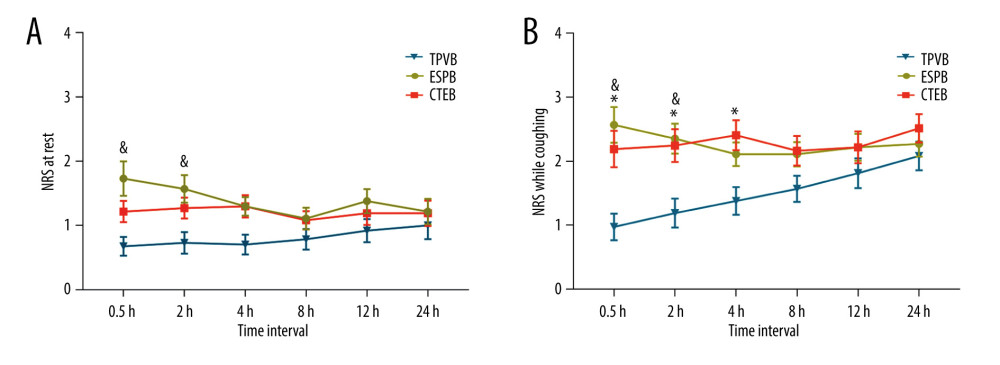 Figure 3. Mean numeric rating scale (NRS) score at rest (A) and while coughing (B) during the first 24 h after surgery. The error bars represent standard error. * P<0.05 between the CTEB group and TPVB group, & P<0.05 between the ESPB group and TPVB group. There was no significant difference between the CTEB group and ESPB group at any time points. CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA).
Figure 3. Mean numeric rating scale (NRS) score at rest (A) and while coughing (B) during the first 24 h after surgery. The error bars represent standard error. * P<0.05 between the CTEB group and TPVB group, & P<0.05 between the ESPB group and TPVB group. There was no significant difference between the CTEB group and ESPB group at any time points. CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA). 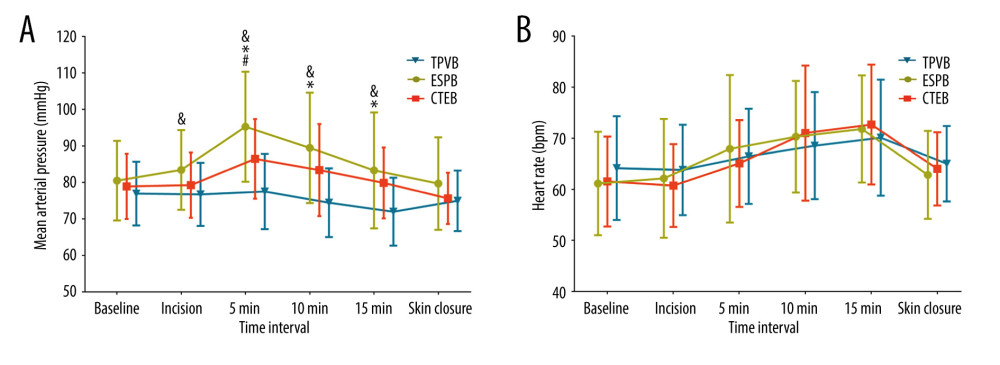 Figure 4. Mean arterial pressure (MAP) (A) and heart rate (HR) (B) at different time points during surgery in each group. Both figure parts show the mean±standard deviation at the following time points: baseline, incision, and 5 min, 10 min, and 15 min after incision, and at skin closure. Repeated measures ANOVA are used and P values are corrected using Bonferroni correction (adjusted by multiplication by number of tests). (A) The changes in MAP were relative to interaction of groups and time (P<0.001), and pairwise comparisons were performed. & P<0.05 between the ESPB and TPVB group, * P<0.05 between the CTEB and TPVB group; # P 0.05 between the TPVB and CTEB group. (B) The changes in HR showed a significant difference only in relation to time (P<0.001). ANOVA – one-way analysis of variance; CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA).
Figure 4. Mean arterial pressure (MAP) (A) and heart rate (HR) (B) at different time points during surgery in each group. Both figure parts show the mean±standard deviation at the following time points: baseline, incision, and 5 min, 10 min, and 15 min after incision, and at skin closure. Repeated measures ANOVA are used and P values are corrected using Bonferroni correction (adjusted by multiplication by number of tests). (A) The changes in MAP were relative to interaction of groups and time (P<0.001), and pairwise comparisons were performed. & P<0.05 between the ESPB and TPVB group, * P<0.05 between the CTEB and TPVB group; # P 0.05 between the TPVB and CTEB group. (B) The changes in HR showed a significant difference only in relation to time (P<0.001). ANOVA – one-way analysis of variance; CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA). 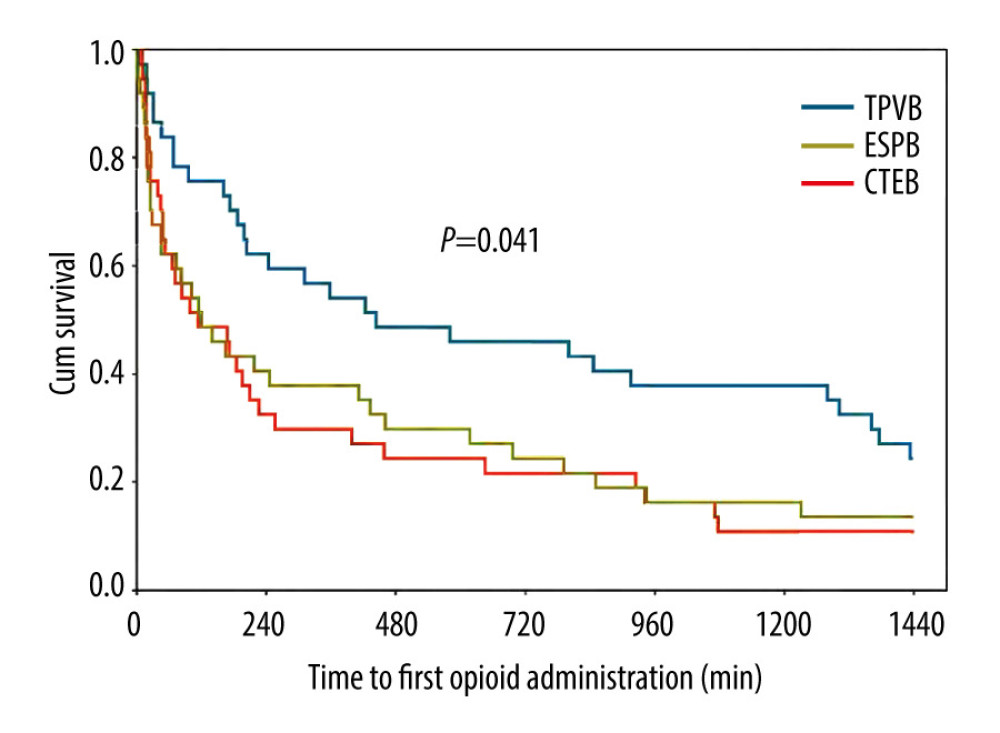 Figure 5. Kaplan-Meier survival curve representing the time to first opioid administration. P=0.041 comparing all groups using a global test; P=0.015 between the CTEB group and TPVB group; P=0.039 between the ESPB group and TPVB group. There was no significant difference between the CTEB group and ESPB group (P=0.805). CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA).
Figure 5. Kaplan-Meier survival curve representing the time to first opioid administration. P=0.041 comparing all groups using a global test; P=0.015 between the CTEB group and TPVB group; P=0.039 between the ESPB group and TPVB group. There was no significant difference between the CTEB group and ESPB group (P=0.805). CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA). 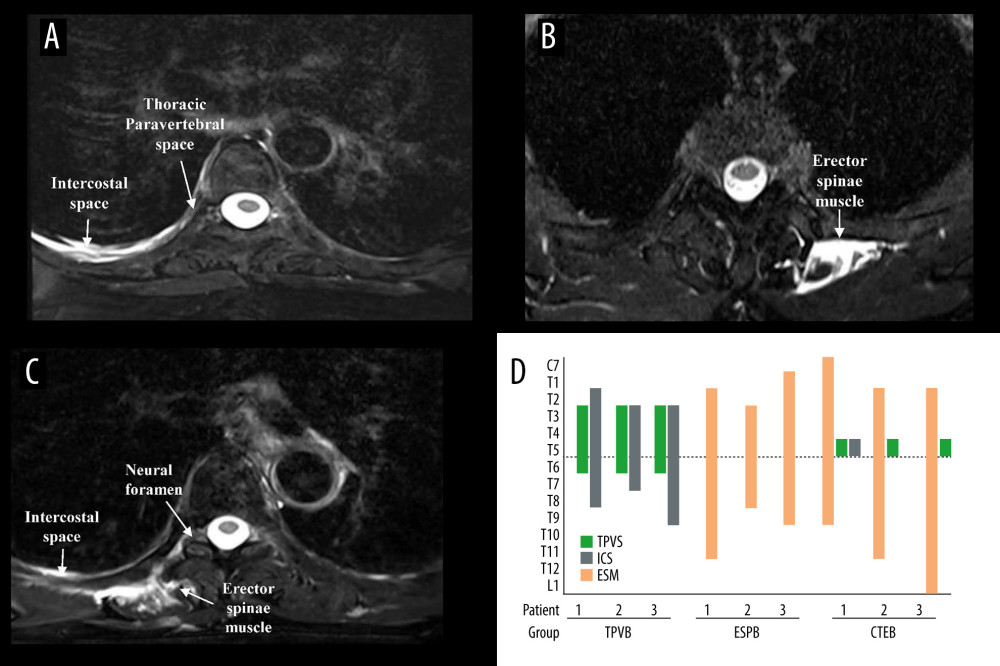 Figure 6. Axial MRI image of the spine showing the injectate spread after TPVB (A), ESPB (B), CTEB (C), and cranio-caudal spread of LA diffusion in the sagittal plane at intercostal space (ICS), thoracic paravertebral space (TPVS) and erector spinae muscle (ESM) in each patient (D). (A) Axial view of T5 vertebra, injectate dispersed into the ICS and TPVS in TPVB. (B) The injectate spread within the ESM in ESPB. (C) In CTEB, injectate was seen spreading to the ICS, the neural foramen, and the ESM at T5. (D) The LA distribution was mainly confined to the thoracic vertebral levels when patients received TPVB or ESPB. While the LA was detected from C7 to L1 in patients receiving CTEB. CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; LA – local anesthetic; TPVB – thoracic paravertebral block. (Microsoft PowerPoint 2019MSO, version 2303, Microsoft Corporation; Adobe Acrobat Pro DC, version 2015, Adobe Systems Inc.).
Figure 6. Axial MRI image of the spine showing the injectate spread after TPVB (A), ESPB (B), CTEB (C), and cranio-caudal spread of LA diffusion in the sagittal plane at intercostal space (ICS), thoracic paravertebral space (TPVS) and erector spinae muscle (ESM) in each patient (D). (A) Axial view of T5 vertebra, injectate dispersed into the ICS and TPVS in TPVB. (B) The injectate spread within the ESM in ESPB. (C) In CTEB, injectate was seen spreading to the ICS, the neural foramen, and the ESM at T5. (D) The LA distribution was mainly confined to the thoracic vertebral levels when patients received TPVB or ESPB. While the LA was detected from C7 to L1 in patients receiving CTEB. CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; LA – local anesthetic; TPVB – thoracic paravertebral block. (Microsoft PowerPoint 2019MSO, version 2303, Microsoft Corporation; Adobe Acrobat Pro DC, version 2015, Adobe Systems Inc.). References
1. Bendixen M, Jørgensen OD, Kronborg C, Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial: Lancet Oncol, 2016; 17(6); 836-44
2. McCall P, Steven M, Shelley B, Anaesthesia for video-assisted and robotic thoracic surgery: BJA Educ, 2019; 19(12); 405-11
3. Steinthorsdottir KJ, Wildgaard L, Hansen HJ, Regional analgesia for video-assisted thoracic surgery: A systematic review: Eur J Cardiothorac Surg, 2014; 45(6); 959-66
4. Umari M, Carpanese V, Moro V, Postoperative analgesia after pulmonary resection with a focus on video-assisted thoracoscopic surgery: Eur J Cardiothorac Surg, 2018; 53(5); 932-38
5. Marhofer D, Marhofer P, Kettner SC, Magnetic resonance imaging analysis of the spread of local anesthetic solution after ultrasound-guided lateral thoracic paravertebral blockade: A volunteer study: Anesthesiology, 2013; 118(5); 1106-12
6. Cheema S, Richardson J, McGurgan P, Factors affecting the spread of bupivacaine in the adult thoracic paravertebral space: Anaesthesia, 2003; 58(7); 684-87
7. Naja ZM, El-Rajab M, Al-Tannir MA, Thoracic paravertebral block: Influence of the number of injections: Reg Anesth Pain Med, 2006; 31(3); 196-201
8. Terkawi AS, Tsang S, Sessler DI, Improving analgesic efficacy and safety of thoracic paravertebral block for breast surgery: A mixed-effects meta-analysis: Pain Physician, 2015; 18(5); E757-80
9. Pace MM, Sharma B, Anderson-Dam J, Ultrasound-guided thoracic paravertebral blockade: A retrospective study of the incidence of complications: Anesth Analg, 2016; 122(4); 1186-91
10. Yang J, Hao Z, Li W, The efficacy and safety of paravertebral block combined with parecoxib during video-assisted thoracic surgery: A randomized controlled trial: J Pain Res, 2020; 13; 355-66
11. Forero M, Adhikary SD, Lopez H, The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain: Reg Anesth Pain Med, 2016; 41(5); 621-27
12. Forero M, Rajarathinam M, Adhikary S, Chin KJ, Continuous erector spinae plane block for rescue analgesia in thoracotomy after epidural failure: A case report: A A Case Rep, 2017; 8(10); 254-56
13. Scimia P, Basso Ricci E, Droghetti A, Fusco P, The ultrasound-guided continuous erector spinae plane block for postoperative analgesia in video-assisted thoracoscopic lobectomy: Reg Anesth Pain Med, 2017; 42(4); 537
14. Ivanusic J, Konishi Y, Barrington MJ, A cadaveric study investigating the mechanism of action of erector spinae blockade: Reg Anesth Pain Med, 2018; 43(6); 567-71
15. Yasar OC, Batcik S, Kazdal H, Comparison of the efficacy of two different plane blocks in isolated bypass surgery: A prospective observational study: J Cardiothorac Vasc Anesth, 2022; 36(12); 4333-40
16. Zengin M, Sazak H, Baldemir R, The effect of erector spinae plane block and combined deep and superficial serratus anterior plane block on acute pain after video-assisted thoracoscopic surgery: A randomized controlled study: J Cardiothorac Vasc Anesth, 2022; 36(8 Pt B); 2991-99
17. Schulz KF, Altman DG, Moher D, CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials: BMJ, 2010; 340; c332
18. Uppal V, Sondekoppam RV, Sodhi P, Single-injection versus multiple-injection technique of ultrasound-guided paravertebral blocks: A randomized controlled study comparing dermatomal spread: Reg Anesth Pain Med, 2017; 42(5); 575-81
19. Kulhari S, Bharti N, Bala I, Efficacy of pectoral nerve block versus thoracic paravertebral block for postoperative analgesia after radical mastectomy: A randomized controlled trial: Br J Anaesth, 2016; 117(3); 382-86
20. Boogaerts JG, Vanacker E, Seidel L, Assessment of postoperative nausea using a visual analogue scale: Acta Anaesthesiol Scand, 2000; 44(4); 470-74
21. Fang B, Wang Z, Huang X, Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: A single center randomized controlled double-blind study: Ann Transl Med, 2019; 7(8); 174
22. Bayman EO, Parekh KR, Keech J, A prospective study of chronic pain after thoracic surgery: Anesthesiology, 2017; 126(5); 938-51
23. Jiang W, Wang M, Wang X, Effects of erector spinae plane block and transmuscular quadratus lumborum block on postoperative opioid consumption in total laparoscopic hysterectomy: A randomized controlled clinical trial: Pain Ther, 2023; 12(3); 811-24
24. Zhang P, Liu S, Zhu J, Dexamethasone and dexmedetomidine as adjuvants to local anesthetic mixture in intercostal nerve block for thoracoscopic pneumonectomy: A prospective randomized study: Reg Anesth Pain Med, 2019 [Online ahead of print]
25. Agostini P, Cieslik H, Rathinam S, Postoperative pulmonary complications following thoracic surgery: Are there any modifiable risk factors: Thorax, 2010; 65(9); 815-18
26. Komatsu T, Kino A, Inoue M, Paravertebral block for video-assisted thoracoscopic surgery: Analgesic effectiveness and role in fast-track surgery: Int J Surg, 2014; 12(9); 936-39
27. Vogt A, Stieger DS, Theurillat C, Curatolo M, Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery: Br J Anaesth, 2005; 95(6); 816-21
28. Conacher ID, Resin injection of thoracic paravertebral spaces: Br J Anaesth, 1988; 61(6); 657-61
29. Chin KJ, Adhikary S, Sarwani N, Forero M, The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair: Anaesthesia, 2017; 72(4); 452-60
30. Chen N, Qiao Q, Chen R, The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: A randomized, double-blinded, clinical trial: J Clin Anesth, 2020; 59; 106-11
31. Taketa Y, Irisawa Y, Fujitani T, Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: A randomized controlled non-inferiority clinical trial: Reg Anesth Pain Med, 2019 [Online ahead of print]
32. Fu Z, Zhang Y, Zhou Y, A comparison of paravertebral block, erector spinae plane block and the combination of erector spinae plane block and paravertebral block for post-operative analgesia after video-assisted thoracoscopic surgery: A randomised controlled trial: J Minim Access Surg, 2021; 18(2); 241-47
33. Zengin M, Baldemir R, Ulger G, Postoperative analgesic efficacy of thoracic paravertebral block and erector spinae plane block combination in video-assisted thoracic surgery: Cureus, 2021; 13(6); e15614
34. Chin KJ, Lirk P, Hollmann MW, Schwarz S, Mechanisms of action of fascial plane blocks: a narrative review: Reg Anesth Pain Med, 2021; 46(7); 618-28
35. Glare P, Aubrey KR, Myles PS, Transition from acute to chronic pain after surgery: Lancet, 2019; 393(10180); 1537-46
36. Sabouri AS, Crawford L, Bick SK, Is a retrolaminar approach to the thoracic paravertebral space possible?: A human cadaveric study: Reg Anesth Pain Med, 2018; 43(8); 864-68
Figures
 Figure 1. A schematic illustration of ultrasound guided combined thoracic paravertebral block and erector spinae plane block (CTEB). Illustration of sagittal section at level T5/T6 at approximately 3 cm lateral to the midline, depicting the trajectory of the needle and tip location. The needle path may form a mini-tunnel that would potentiate the penetration of injectate to thoracic paravertebral space from the erector spinae place, which contains a large volume of injectate, through the intertransverse ligament, intertransverse connective tissue, and super costotransverse ligament. (Microsoft PowerPoint 2019MSO, version 2303, Microsoft Corporation).
Figure 1. A schematic illustration of ultrasound guided combined thoracic paravertebral block and erector spinae plane block (CTEB). Illustration of sagittal section at level T5/T6 at approximately 3 cm lateral to the midline, depicting the trajectory of the needle and tip location. The needle path may form a mini-tunnel that would potentiate the penetration of injectate to thoracic paravertebral space from the erector spinae place, which contains a large volume of injectate, through the intertransverse ligament, intertransverse connective tissue, and super costotransverse ligament. (Microsoft PowerPoint 2019MSO, version 2303, Microsoft Corporation). Figure 2. CONSORT flow diagram. CONSORT – Consolidated Standards of Reporting Trials; CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (Microsoft Word 2019MSO, version 2303, Microsoft Corporation).
Figure 2. CONSORT flow diagram. CONSORT – Consolidated Standards of Reporting Trials; CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (Microsoft Word 2019MSO, version 2303, Microsoft Corporation). Figure 3. Mean numeric rating scale (NRS) score at rest (A) and while coughing (B) during the first 24 h after surgery. The error bars represent standard error. * P<0.05 between the CTEB group and TPVB group, & P<0.05 between the ESPB group and TPVB group. There was no significant difference between the CTEB group and ESPB group at any time points. CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA).
Figure 3. Mean numeric rating scale (NRS) score at rest (A) and while coughing (B) during the first 24 h after surgery. The error bars represent standard error. * P<0.05 between the CTEB group and TPVB group, & P<0.05 between the ESPB group and TPVB group. There was no significant difference between the CTEB group and ESPB group at any time points. CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA). Figure 4. Mean arterial pressure (MAP) (A) and heart rate (HR) (B) at different time points during surgery in each group. Both figure parts show the mean±standard deviation at the following time points: baseline, incision, and 5 min, 10 min, and 15 min after incision, and at skin closure. Repeated measures ANOVA are used and P values are corrected using Bonferroni correction (adjusted by multiplication by number of tests). (A) The changes in MAP were relative to interaction of groups and time (P<0.001), and pairwise comparisons were performed. & P<0.05 between the ESPB and TPVB group, * P<0.05 between the CTEB and TPVB group; # P 0.05 between the TPVB and CTEB group. (B) The changes in HR showed a significant difference only in relation to time (P<0.001). ANOVA – one-way analysis of variance; CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA).
Figure 4. Mean arterial pressure (MAP) (A) and heart rate (HR) (B) at different time points during surgery in each group. Both figure parts show the mean±standard deviation at the following time points: baseline, incision, and 5 min, 10 min, and 15 min after incision, and at skin closure. Repeated measures ANOVA are used and P values are corrected using Bonferroni correction (adjusted by multiplication by number of tests). (A) The changes in MAP were relative to interaction of groups and time (P<0.001), and pairwise comparisons were performed. & P<0.05 between the ESPB and TPVB group, * P<0.05 between the CTEB and TPVB group; # P 0.05 between the TPVB and CTEB group. (B) The changes in HR showed a significant difference only in relation to time (P<0.001). ANOVA – one-way analysis of variance; CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA). Figure 5. Kaplan-Meier survival curve representing the time to first opioid administration. P=0.041 comparing all groups using a global test; P=0.015 between the CTEB group and TPVB group; P=0.039 between the ESPB group and TPVB group. There was no significant difference between the CTEB group and ESPB group (P=0.805). CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA).
Figure 5. Kaplan-Meier survival curve representing the time to first opioid administration. P=0.041 comparing all groups using a global test; P=0.015 between the CTEB group and TPVB group; P=0.039 between the ESPB group and TPVB group. There was no significant difference between the CTEB group and ESPB group (P=0.805). CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; TPVB – thoracic paravertebral block. (GraphPad Prism 8 software, La Jolla, California, USA). Figure 6. Axial MRI image of the spine showing the injectate spread after TPVB (A), ESPB (B), CTEB (C), and cranio-caudal spread of LA diffusion in the sagittal plane at intercostal space (ICS), thoracic paravertebral space (TPVS) and erector spinae muscle (ESM) in each patient (D). (A) Axial view of T5 vertebra, injectate dispersed into the ICS and TPVS in TPVB. (B) The injectate spread within the ESM in ESPB. (C) In CTEB, injectate was seen spreading to the ICS, the neural foramen, and the ESM at T5. (D) The LA distribution was mainly confined to the thoracic vertebral levels when patients received TPVB or ESPB. While the LA was detected from C7 to L1 in patients receiving CTEB. CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; LA – local anesthetic; TPVB – thoracic paravertebral block. (Microsoft PowerPoint 2019MSO, version 2303, Microsoft Corporation; Adobe Acrobat Pro DC, version 2015, Adobe Systems Inc.).
Figure 6. Axial MRI image of the spine showing the injectate spread after TPVB (A), ESPB (B), CTEB (C), and cranio-caudal spread of LA diffusion in the sagittal plane at intercostal space (ICS), thoracic paravertebral space (TPVS) and erector spinae muscle (ESM) in each patient (D). (A) Axial view of T5 vertebra, injectate dispersed into the ICS and TPVS in TPVB. (B) The injectate spread within the ESM in ESPB. (C) In CTEB, injectate was seen spreading to the ICS, the neural foramen, and the ESM at T5. (D) The LA distribution was mainly confined to the thoracic vertebral levels when patients received TPVB or ESPB. While the LA was detected from C7 to L1 in patients receiving CTEB. CTEB – combined thoracic paravertebral block and erector spinae plane block; ESPB – erector spinae plane block; LA – local anesthetic; TPVB – thoracic paravertebral block. (Microsoft PowerPoint 2019MSO, version 2303, Microsoft Corporation; Adobe Acrobat Pro DC, version 2015, Adobe Systems Inc.).In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952