13 June 2023: Review Articles
Review of the Pathogenesis, Diagnosis, and Management of Osteoradionecrosis of the Femoral Head
Yang Li12EF, Zhongsheng Zhou12BC, Shenghao Xu1B, Jinlan Jiang2A*, Jianlin Xiao1AEDOI: 10.12659/MSM.940264
Med Sci Monit 2023; 29:e940264
Abstract
ABSTRACT: Osteoradionecrosis (ORN) of the femoral head is an important issue for orthopedists and radiologists in clinical practice. With the rapid development of technological advances in radiation therapy and the improvement in cancer survival rates, the incidence of ORN is rising, and there is an unmet need for basic and clinical research. The pathogenesis of ORN is complex, and includes vascular injury, mesenchymal stem cell injury, bone loss, reactive oxygen species, radiation-induced fibrosis, and cell senescence. The diagnosis of ORN is challenging and requires multiple considerations, including exposure to ionizing radiation, clinical manifestations, and findings on physical examination and imaging. Differential diagnosis is essential, as clinical symptoms of ORN of the femoral head can resemble many other hip conditions. Hyperbaric oxygen therapy, total hip arthroplasty, and Girdlestone resection arthroplasty are effective treatments, each with their own advantages and disadvantages. The literature on ORN of the femoral head is incomplete and there is no criterion standard or clear consensus on management. Clinicians should gain a better and more comprehensive understanding on this disease to facilitate its early and better prevention, diagnosis, and treatment. This article aims to review the pathogenesis, diagnosis, and management of osteoradionecrosis of the femoral head.
Keywords: Diagnosis, Hip Joint, Osteoradionecrosis, Pathology, Radiotherapy, Residential Treatment, Humans, Femur Head, Diagnosis, Differential, Radiation, Ionizing, Arthroplasty, Replacement, Hip
Background
The response of normal tissues to ionizing radiation has been a major subject of research since the discovery of X-rays in the late 19th century [1]. Shortly thereafter, time-dose relationships were established for numerous normal tissue endpoints, leading to an investigation of how the magnitude of each fraction of the dose and the quality of the radiation affected it [2]. The adverse effects from radiation therapy (RT) on bone are a crucial but underappreciated issue in clinical practice [3,4]. Osteoradionecrosis (ORN), also known as radiotherapy-induced osteonecrosis, aseptic necrosis, or ischemic necrosis, was first described by Regaud in 1922 as a nonhealing osteonecrosis without residual or recurrent tumor caused by prolonged exposure to high-dose RT [5–7].
It is estimated that 150 000 to 300 000 individuals in high-income countries receive RT for pelvic diseases each year [8,9]. Ionizing radiation inhibits bone development and decreases collagen maturation and mineral deposition processes, while reducing bending strength and bone stiffness mechanical parameters [10]. However, to date, there are no effective strategies for the prevention and treatment of radiation-related bone injury. With the increasing survival and age of cancer patients, novel and effective preventive or therapeutic strategies are clinically important for the intervention of this complication [11]. Radiation therapy has been reported to have the adverse effect of reducing bone quality and density, leading to bone fragility and fractures in the long term. More than 65% of women treated for various pelvic tumors experience hip complications 5 years after RT [12].
ORN of the femoral head is a severe complication of RT for pelvic malignancies [13]. ORN of the femoral head is characterized by progressive joint pain, limited movement, and the potential for pathological fracture [5,14] and has a negative impact on patient quality of life. Current understanding of the pathogenesis and recommendations for the management of ORN are limited, and clinicians should gain a better understanding and place more focus on it. This article aims to review the pathogenesis, diagnosis, and management of osteoradionecrosis of the femoral head.
Hip Anatomy and Biomechanics
The hip joint is a ball-and-socket synovial joint, composed of the femoral head (ball) and the pelvic acetabulum (socket) [15], which is surrounded by a fibrous capsule. The femoral head is pointed in a medial, superior, and slightly anterior direction [16]. The acetabulum is formed by the ilium, ischium, and pubis, which fuse at the triradiate cartilage. The integrity of the hip is maintained by 3 capsular ligaments, the iliofemoral, ischiofemoral, and pubofemoral ligaments [17]. The hip joints facilitate weight bearing and the transmission of load and force from the axial skeleton to the lower limbs. The hip joint has the ability to create balanced strength through its range of motion, providing stability when performing daily tasks such as standing upright, maintaining a steady and balanced gait, standing up from a chair, and transitioning from squatting to lifting weights [18].
The blood supply to the femoral head is established in childhood and persists throughout adulthood. During childhood, the epiphysal and metaphyseal vessels are separated [19]. With maturity, the epiphysal and metaphyseal vessels combine to form a vascular network that supplies the proximal femur. In adults, the femoral head is primarily supplied by the deep branch of the medial femoral circumflex artery [20,21], which originates from the deep femoral artery in 65% to 81% of individuals, the common femoral artery in 4% to 34% of individuals, and the superficial femoral artery in a small minority of individuals [19,22].
Based on the unique vascular structure of the hip joint, once the main blood vessels are damaged, the remaining blood vessels have difficulty in providing the physiological needs of the femoral head, which makes it more prone to femoral head necrosis [23]. Its weight-bearing and motor functions are bound to be seriously affected, and pain and limitation of movement become its main clinical symptoms [24].
Epidemiology
With the rapid development of technology, RT has become an efficient method for treating gynecological cancers, such as cervical cancer, metastatic prostate cancer, and bladder cancer as well as pediatric hematologic malignancies [25]. RT can increase the survival rates of patients with cancer [26,27]. Treatment planning systems can precisely distribute the radiation dose and shape the treatment beam to deliver a robust dose, which can help reduce the incidence of complications [28]. However, patients still experience many adverse effects associated with RT [29]. Bone complications of radiation include vascular hyperemia, edema, hypocellularity, local hemorrhage, fat conversion, focal bone marrow changes, radiation osteitis, osteolysis of the pubic symphysis or sacroiliac joint, dysfunctional fractures, avascular necrosis, ORN, and radiation-induced tumors [30,31]. There is sparse and conflicting evidence on the prevention of radiotherapy-induced hip complications [32]. Radiation osteitis and ORN refer to radiation-induced reactions, which can be confused with primary bone tumors or bone metastases [33]. ORN of the femoral head is a severe late sequelae of RT. However, estimates of its incidence vary, as a comprehensive epidemiological study has not been performed in recent years. The incidence of ORN has decreased in the last 5 decades. Currently, the reported incidence of pelvic ORN is approximately 2.1% to 34% [13], and the prevalence of symptomatic or asymptomatic femoral head osteonecrosis in patients previously treated with chemoradiation is 4/763 (95% confidence interval 0.1%–1.3%) [34]. The extent or grade of the ORN is determined by patients’ demographic and clinical characteristics, including age, weight, skeletal comorbidities, co-medications, radiation technology, absorbed dose, the range of the radiation field, beam energy, and fractionation [13,35].
ORN mainly affects adults; however, children treated for cancer are at an increased risk for osteonecrosis, and younger children are more sensitive to the adverse effects of RT than older children [36]. RT is considered to be an important risk factor leading to osteonecrosis in children, which is usually observed in the femoral head [37]. Age is a highly significant covariate. According to the literature, the bone loss of flat pelvic bones in children younger than 5 years old is more statistically significant than in older children [38]. This may be related to radiotherapy involving endocrine organs, especially gonads, that maintain bone homeostasis, which increases the risk of osteonecrosis [37,39]. The incidence of osteonecrosis among pediatric and adolescent cancer survivors ranges from 0.43% to 1.4%. Survivors of leukemia and lymphoma have a higher risk of developing osteonecrosis, ranging from 1% to 12% in patients with acute lymphocytic leukemia, and up to 19% in children with solid tumors or lymphoma [40]. Prophylactic irradiation of the hip area is unnecessary for patients with malignancy without pathological metastases in the lymph nodes to avoid complications such as femoral head necrosis [41].
Pathogenesis
VASCULAR INJURY:
Beyond certain thresholds, radiation can damage normal tissues and cause harm. The vascular network can tolerate a lower dose of radiation (50–60 Gy) than can bone (60 Gy) [43]. Hypovascularization is considered to play a significant role in the pathogenesis of ORN. Radiation directly affects the vascular system by causing apoptosis and senescence of endothelial cells and alteration of normal homeostasis [44]. A microcomputed tomography analysis showed the vessel percentage was significantly decreased and the distance between vessels, a marker of vascular destruction, was significantly increased in irradiated bones in a rat model of ORN than in non-irradiated rats [45].
Histologically, RT affects blood vessels of all calibers, but radiation-induced vascular damage is a complex process. Veins are less sensitive to radiation than are arteries and capillaries [46]. The microvascular system is more sensitive to radiation than are the large vessels in the bone marrow. In irradiated mandibular bone marrow, a radiation dose higher than 50 Gy mainly affected smaller vessels, causing increased permeability and thrombosis, and the proportion of smaller vessels in the bone marrow decreased with increased time after exposure [47]. For large vessels, the main pathological changes of radiation-induced arterial injury include atherosclerosis, stenosis, and obstruction [48]. Radiation-induced atherosclerosis is rich in macrophages and lipids, and is characterized by plaque hemorrhage, which distinguishes it from classical atherosclerosis [49]. The severity of great vessel injury is directly proportional to the dose and duration of irradiation, for example, high-dose radiotherapy can accelerate the atherosclerotic process [48]. Since the arterial vessels of the femoral head are interconnected within the femoral head in an integrated structure, radiation-induced arterial injury causes significant damage to the blood supply to the femoral head, which in turn plays a pivotal role in the development of ORN.
Radiation–induced vascular injury begins with progressive loss of endothelial cells. In animal models, about 15% of endothelial cells are lost within 24 h of exposure to radiation doses of 5 to 200 Gy, and the loss of vascular endothelial cells continues for several months. Long-term morphological changes include endotheliocyte proliferation, basement membrane thickening, adventitia fibrosis, and hemangiectasis, which are key components of vascular remodeling [49,50]. When a relatively large dose of radiation is given, vessels can develop edema, thrombosis, or bleeding, which can cause necrosis of the vessel wall and significantly increase the risk of angiorrhexis. Although this can create a hypoxic environment for a tumor and indirectly kill cancer cells [51], it has negative effects on normal tissue. At lower doses of radiation, vascular injury may not be immediately obvious, but there can be delayed angiotelectasis and hemorrhagic infarction up to 1 to 2 years after exposure [49]. As the blood supply to the femoral head is limited, radiation-induced injury to the vasculature will interrupt blood flow, resulting in femoral head ischemia and ORN of the femoral head, as seen in Figure 2.
MESENCHYMAL STEM CELLS INJURY:
Human bone marrow mesenchymal stem cells (hBM-MSCs) are multipotent cells that have the potential to differentiate into osteoblasts, adipocytes, or chondrocytes. hBM-MSCs have high proliferative capacity and can form bone and cartilage [52–54]. Disruption in MSC-mediated bone remodeling has an important role in osteonecrosis. Radiation can induce proliferative abnormalities in MSCs, damage genomic DNA, and cause micronucleus formation [55]. MSC proliferation decreases as the radiation dose increases. The proliferation rate of MSC cell colonies treated with a radiation dose of 8 Gy and 12 Gy was significantly lower than that of colonies treated with 0 Gy [52]. mRNA expression levels of osteogenic-related markers and transcription factors, such as alkaline phosphatase and osteocalcin, are relatively low in MSCs after irradiation [55,56]. Co-culture of human umbilical cord blood MSCs with irradiated osteoblasts effectively inhibited apoptosis and promoted the differentiation, migration, and adhesion of osteoblasts [57]. The renewal and formation of bone tissue is a continuous process, whereby MSCs differentiate into osteogenic progenitor cells and mature osteoblasts. Bone defects occur when the balance between bone formation and bone absorption is disrupted.
Ionizing radiation generated during RT can induce DNA damage, chromosomal aberrations, cell cycle arrest, or cell death. Tissue injury caused by ionizing radiation results from the modification of macromolecules and the formation of superoxide anion molecules, such as reactive oxygen species (ROS) [52]. Irradiation of MSCs increases intracellular ROS levels [56]. ROS are strong oxidizing agents that can induce single-stranded and double-stranded DNA breaks [58]. The resultant direct and indirect damage to cell structure leads to abnormal cellular function and genetic mutation, which inhibits cell proliferation and differentiation of MSCs into osteoblasts [56]. hBM-MSCs have a homing/migration function and the ability for self-renewal and are highly proliferative and multipotent [59,60]; therefore, they are crucial for bone recovery after irradiation. Transplantation of human umbilical cord blood MSCs into a mouse radioactive bone injury model inhibited the differentiation of MSCs to adipocytes and reduced the number of osteoblasts in bone tissue [57]. In vivo, the homeostasis between bone formation and degradation is maintained as far as possible under pathological conditions. However, MSCs are among the cell types that are most affected by irradiation of bone tissue. The high proliferative capacity of MSCs makes them vulnerable, which may aggravate bone damage after radiation.
BONE LOSS:
Fragility fractures are a common complication following RT [61]. During RT, bony tissue absorbs 30% to 40% more radiation than surrounding tissue due to its high calcium content. Therefore, radiation-induced damage, such as hypocellularity and fibrosis, is more severe in osseous tissue than in soft tissue [42]. RT reduces the mechanical properties of bones and destroys the normal structure of the organic and inorganic bone matrix. High-dose RT can directly kill osteoblasts and osteoclasts. The ultimate force, work to failure, stiffness, elastic modulus, and Vickers hardness values of irradiated bone are lower than that of normal bone. Ionizing radiation reduces the normal anisotropy on microarchitecture of cortical bone and results in osteopsathyrosis [62]. There is an apparent deterioration of microstructure, reduced bone mineral density, bone volume fraction, and trabecular thickness, and increased trabecular spacing in trabecular bone subjected to focal irradiation compared to that of bone with no irradiation. Damage is progressive, as loss of trabecular bone is worse at 28 days after exposure than at 7 days after exposure [63].
Local irradiation can cause systemic loss of trabecular bone [64,65]. Relatively low doses of ionizing radiation can directly stimulate osteoclast differentiation. Focal irradiation of femurs with a clinically relevant dose of 2 Gy increased the activity of osteoclasts in irradiated and non-irradiated areas compared with unirradiated controls [63]. B cells and CD8+ T lymphocytes increase in blood and bone marrow after exposure to radiation, inhibit the osteogenic differentiation of MSCs, and lead to systemic bone loss [64]. Local radiation has an absolute effect on the liver, reducing the expression of hepcidin and increasing iron levels in the serum and liver, leading to increased osteoclast activation, which is a major cause of systemic bone loss [63]. After irradiation, changes in bone mass, density and structure occur locally and throughout the body [66]. When a patient has ORN of the femoral head, the contralateral hip should be examined to rule out the possibility of bilateral disease.
Ionizing radiation has differential effects on osteoblasts and osteoclasts, as seen in Figure 3. Radiation has a permanent effect on small blood vessels, causing hypoxia in bone tissue and reducing the number and activity of osteoblasts. The number of osteoblasts decreases immediately after radiation exposure. The decrease in cells is secondary to cell cycle arrest rather than to cell death induced by direct radiation [67]. If radiation doses exceed normal bone tolerance, the repair and regeneration capabilities of tissue and cells will be severely damaged. RT impairs optimal bone regeneration in a dose-dependent manner [62]. In bone remodeling, the balance of bone formation by osteoblasts and bone resorption by osteoclasts is essential for maintaining bone homeostasis. Exposure to ionizing radiation can interfere with trabecular structure by increasing osteoclast activity and decreasing osteoblast activity, resulting in an overall decrease in bone quantity and sclerotin [63,68].
REACTIVE OXYGEN SPECIES:
Inflammation can modulate many of the adverse effects of ionizing radiation in normal tissues. Chronic inflammation, a common adverse effect of radiotherapy [69], can induce genomic instability by stimulating free radicals production and inhibiting DNA repair. Cells produce endogenous ROS in mitochondria and through radiolysis of water molecules in response to ionizing radiation, which leads to oxidative damage to cells and tissue [63,67]. Impaired immune function and accelerated preclinical persistent inflammation due to aging and radiation exposure have been observed in nuclear radiation survivors more than 60 years after exposure. Increased intracellular ROS levels, especially after radiation exposure, can be associated with an enhanced inflammatory state, including elevated serum C-reactive protein levels and decreased serum iron levels [70]. The inflammatory response and free radicals generation is enhanced in the bone marrow, vessels, brain, joints, and breast cells after exposure to ionizing radiation [71]. The formation of ROS destroys the vasculature and compromises the microenvironment of MSCs [72]. Both low-dose and high-dose irradiation cause substantial oxidative damage, DNA injury, chromosomal aberrations, genomic instability, and telomere dysfunction [73].
CELL SENESCENCE:
There is increasing evidence that cell senescence plays a considerable role in ORN of the femoral head. Radiotherapy produces harmful free radicals that cause cell senescence. Radiation-induced oxidative stress, ROS production, and DNA double-strand breaks are all important causes of cell senescence [74]. In the case of radiation-induced pulmonary fibrosis, for example, cell senescence of type 2 alveolar epithelial cells plays a key role in the development and progression of the disease. In addition, removal of senescent type 2 alveolar epithelial cells and treatment with anti-aging drugs can effectively improve the symptoms of radiation-induced pulmonary fibrosis [75]. Cell cycle arrest induced by radiation or DNA damage normally activates the Ras/AKT/mTOR pathway. When the cell cycle is inhibited but mTOR is not inhibited, the cells will age [76]. The use of senescence-associated β-galactosidase as a marker of senescence showed that ionizing radiation can induce cell senescence in a time- and dose-dependent manner [77]. Senescent cells secrete a complex mix of mostly proinflammatory factors and adopt a senescence-associated secretory phenotype, which is activated in radiation-induced osteonecrosis [78]. After bone is exposed to radiation, inflammatory cytokine (IL-1α, IL-1β, IL-6, IL-8, IL-17, TNF-α, and VEGF) levels and cellular senescence are upregulated [68]. Overexpression of inflammatory cytokines triggers acute inflammation, which is characterized by increased vasopermeability, local edema, cytoclasis of endothelial cells, and thrombogenesis [68]. In human osteoblast cell line cells, irradiation increased IL-8 and IL-6 levels in a dose-dependent manner and induced human osteoblast cell line cell senescence [76]. Under conditions of stress, many cellular processes activate senescence, and to a certain extent, this plays a beneficial role in physiological and pathological processes [79]; however, accumulation of senescent cells can have adverse effects and is involved in many pathological processes, chronic diseases, and treatment-related complications.
RADIATION-INDUCED FIBROSIS:
A radiation-induced fibroatrophic (RIF) process can be involved in the pathogenesis of ORN [80]. RIF is a long-term adverse effect of external RT used to treat cancer [81]. The RIF process includes 3 clinical and histopathological stages: a prefibrotic specific inflammatory phase, a constitutive fibrotic cellular phase, and a matrix densification and remodeling phase, which can end in terminal tissue necrosis [82]. The cellular and molecular mechanisms of RIF participate in a chain-of-events that is initiated after fibroblast proliferation and extracellular matrix deposition are disrupted, and is amplified by the effects of cytokines and growth factors [80]. Numerous cytokines take part in RIF. Transforming growth factor β1 (TGF-β1) is primarily responsible for regulating the proliferation and differentiation of fibroblasts. RT induces oxidative stress and an inflammatory response, which results in the prolonged expression of TGF-β1 [83]. The expression of connective tissue growth factor is upregulated after radiation, which promotes the formation of myofibroblasts through transdifferentiation of other cells and stimulates extracellular matrix deposition [84,85]. The underlying mechanisms of ORN in bone can resemble those occurring in other low-turnover tissues, where activation and dysregulation of fibroblast activity lead to tissue shrinkage in previously irradiated areas [83]. Histopathological studies revealed that ORN was characterized by an increase in collagen [86], further confirming that fibrosis is involved in pathogenesis.
Diagnosis and Differential Diagnosis
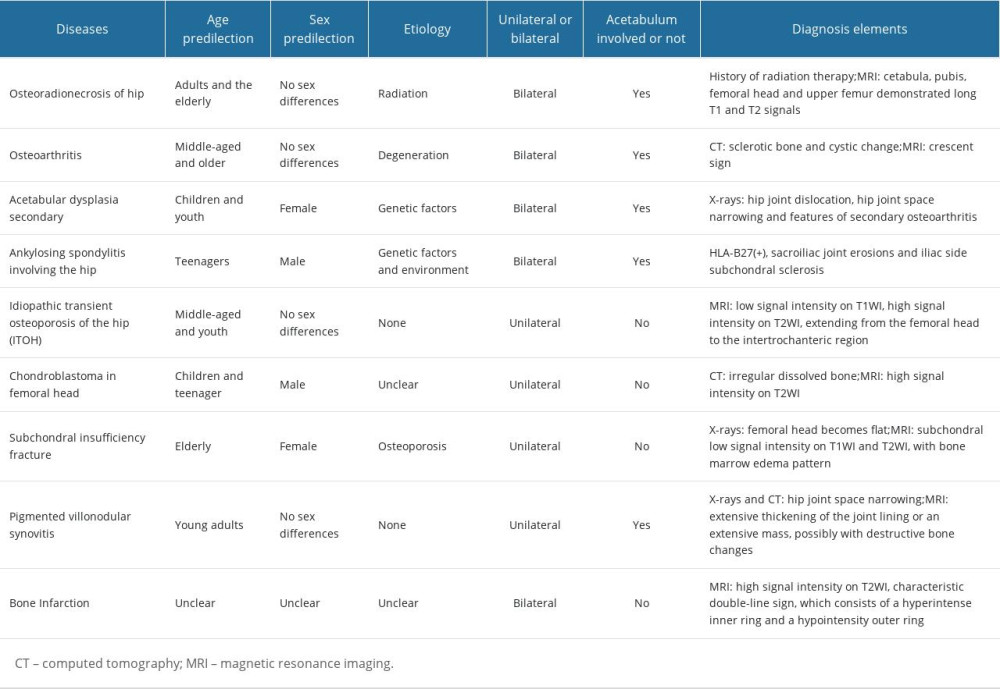
Early diagnosis of ORN of the femoral head is critical, and challenging. Early prevention, early detection, and early intervention are essential to delay or prevent the onset of more serious complications. ORN of the femoral head may not become noticeable until several years after RT, and patients may not always associate it with a previous history of radiation [5], which could lead to misdiagnosis. We have reported several cases of ORN patients in the past, and their treatment process of the disease was quite tortuous [87]. Symptomatic osteonecrosis of the femoral head is diagnosed at a relatively late stage, and its severity can remain constant or deteriorate over time [88]. Patients can have hip pain when they have to walk a short distance and have pain during the night, but conventional non-steroidal anti-inflammatory drugs (NSAIDs) do not provide significant pain relief [89]. The diagnosis of ORN requires multiple considerations, including history of RT, clinical manifestations, and findings on physical examination and imaging such as X-ray and magnetic resonance imaging (MRI) [90]. Because ORN and osseous metastasis have similar characteristics, X-ray examination alone is usually insufficient to make a diagnosis, and computed tomography (CT) and MRI are more effective as diagnostic tools [91]. Differential diagnosis is essential, as clinical symptoms of ORN of the femoral head can resemble many other hip conditions (Table 1) [92].
Treatment
HYPERBARIC OXYGEN THERAPY:
HBOT is effective as a conservative therapy for patients with early-stage femoral head necrosis [100], although its mechanism of action remains to be elucidated. When late radiation tissue injury occurs, there is a decrease in the density of small blood vessels, normal tissue is replaced with dense fibrous tissue, and tissue will progressively deteriorate until there is an insufficient oxygen supply to maintain normal function. HBOT can improve tissue quality, promote healing, and prevent breakdown of irradiated tissue [101]. HBOT has anti-inflammatory effects in the early stage of avascular necrosis of the femoral head, evidenced by a decrease in TNF-α and IL-6 expression. The reduction of inflammatory cytokines is accompanied by a reduction in edema and pain in the patient’s limb [102]. HBOT increases serum osteoprotegerin levels. Osteoprotegerin, a soluble decoy receptor used to activate receptor activator of NF-kB ligand (RANKL), can inhibit the binding of RANKL and receptor activator of NF-kB (RANK) and prevent osteoclast formation and bone resorption. Osteoprotegerin is produced by osteoblasts and other types of cells, including peripheral blood lymphocytes [103]. HBOT can promote the healing process in patients with ORN who have undergone surgery. However, HBOT has certain limitations and is associated with several complications. There is no strong scientific evidence to confirm that HBOT can be used as an adjuvant preventive treatment for ORN [104]. There is insufficient evidence to support or oppose the use of HBOT to accelerate healing or to treat established non-union fractures [105]. Complications of HBOT include tumor recurrence, visual disturbance, pressure-induced damage, and oxygen toxicity [101].
TOTAL HIP ARTHROPLASTY:
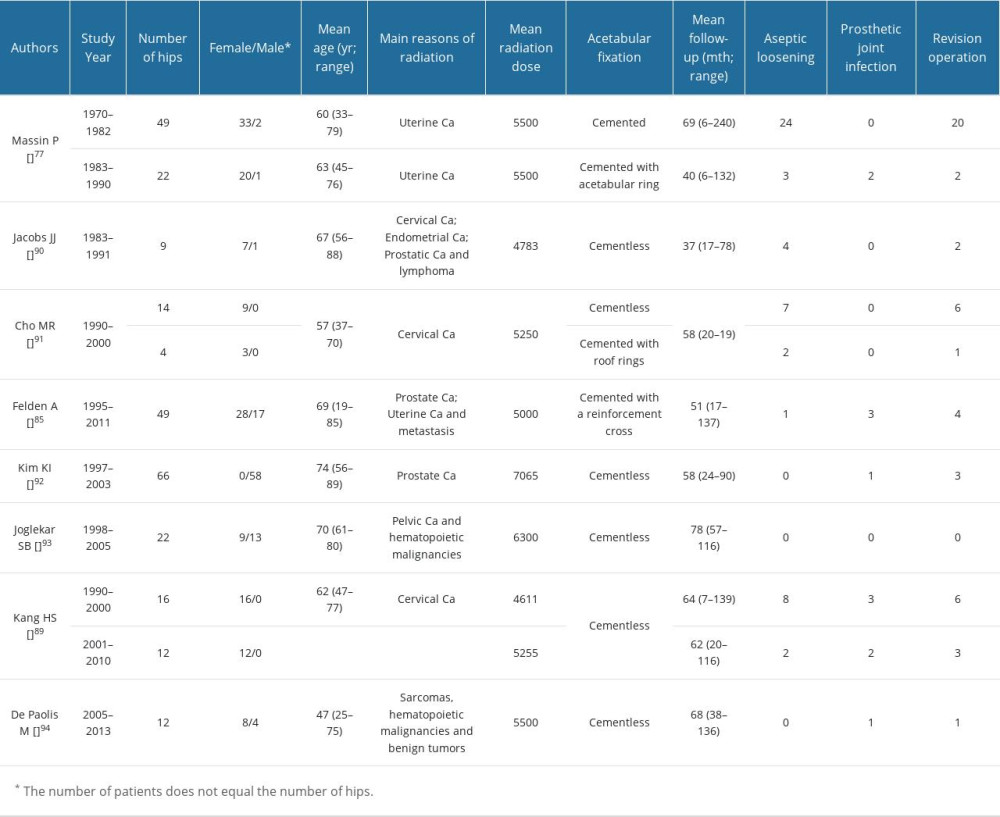
THA is a reliable and effective treatment for patients with femoral head necrosis of various etiologies. THA also has a role in the treatment of ORN of the femoral head. However, the procedure is associated with a high rate of postoperative aseptic loosening (Table 2) and subsequent acetabular failure. Loosening occurs in 44% to 52% of cemented and cementless THAs between 2 and 6 years after surgery in irradiated hips [98,106], although there is little difference in the aseptic loosening rate of cemented and cementless prostheses [107]. Radiation damages the function of osteoblasts and impairs vascular supply, which leads to a disorganized bony architecture, thickening of trabecular bone [108], inhibition of osteoblast proliferation, extracellular matrix formation, and poor osseointegration. For cementless hip prostheses, poor bone healing is particularly significant, because the bone ingrowth of osteoradionecrosis is insufficient [109]. Surrounding bone cannot adhere onto or into a cementless hip prosthesis, precluding stable fixation. Cemented prostheses provide improved stability, are relatively safe, and are associated with fewer complications, such as early periprosthetic femoral fractures, than cementless prostheses [110]. However, the cement may not effectively bond to irradiated hardened bone, cemented prostheses are prone to osteolysis, and it is difficult to remove bone cement during revision surgery [108,111–115].
Several enhanced surgical methods can be used to reduce the occurrence of aseptic loosening of the hip after THA. The risk of acetabular component failure can be mitigated by the use of an acetabular tantalum cup, which has been effective in the treatment of ORN [115]. Trabecular metal can combine with trabecular bone and provide acetabular stability. Cemented reconstruction can be performed with a Kerboull-type acetabular reinforcement device [106], which provides excellent rigidity and elasticity. The device fits the acetabulum, shares the load transmitted through the acetabulum, overcomes the loss of elasticity of the irradiated bone matrix, and achieves a good mid-term fixation effect in bone exposed to ionizing radiation.
Patients with severe bone defects of the acetabulum in the end-stage of ORN, or patients with ORN of the femoral head who have acetabular-related complications after arthroplasty, such as osteolysis or infection, require acetabular bone grafting during arthroplasty [99]. Acetabular bone graft materials include allogeneic bone, autologous iliac bone, and calcium phosphate ceramics. These can provide stable and long-lasting fixation, and restore the center of rotation and bone mass [116]. The type of bone defect can determine acetabular revision surgery. A porous hemispherical cementless cup fixed with screws can achieve good results in acetabular defects of less than 30%. The use of cement cups combined with allograft is beneficial in acetabular defects greater than 30% [117].
Female sex is closely related to aseptic loosening and THA revision, possibly because female patients have poor bone density and bone quality and can be more susceptible to the harmful effects of RT [109].
The main indications for THA are patients with severe pain and obviously restricted motion of the hip joint. Based on the underlying disease, the age requirement for THA can be relaxed for patients with ORN, in order to improve quality of life.
GIRDLESTONE RESECTION ARTHROPLASTY:
GRA is the final option for patients with end-stage ORN of the femoral head who cannot undergo hip arthroplasty. GRA can be beneficial for patients with severe clinical symptoms, unbearable pain at rest and at work, and severe damage to the acetabulum [99], in whom hip replacement surgery is challenging, even after autogenous or allogeneic bone grafting. GRA can also be applicable for patients with severe osteoporosis, as they experience a high incidence of complications, such as aseptic loosening after THA. GRA can be advantageous for patients who failed THA after repeated hip revisions, because aseptic loosening is inevitable, and reimplantation of a prosthesis is not recommended [118]. We call this the similar Girdlestone situation, which can result in an everlasting clinical situation when (1) bone quality or soft tissue coverage is not strong enough to insert the new prosthesis, (2) the clinical symptoms such as pain persist, and (3) when patients are unfit for surgery due to multiple various comorbidities [119,120].
GRA surgery has certain advantages. The technical requirements of the surgery are relatively low. After the operation, the hip joint retains a certain amount of mobility, and severe pain of the hip is significantly relieved. In most cases, patients can achieve a certain degree of functional self-care and acceptably perform activities of daily living [121]. The surgery is associated with several limitations, such as shortening of limbs, instability of the hip joint, and formation of false joints, which can impair mobility of the hip joint. In these cases, patients must use walking aids to accomplish basic functions that require the hip joint, such as walking and standing [119].
Future Directions
Clinicians should gain a better and more comprehensive understanding on ORN of the femoral head, and further research is also warranted. The goal is to prevent the occurrence of this disease and provide evidence-based treatment. With advances in tissue engineering and basic medicine, a variety of cutting-edge therapies have considerable potential for clinical application in ORN. For example, stem cell therapy may be an effective treatment for radiation-induced bone damage [57]. Biological scaffolds that promote recovery of blood vessels and bone tissue also have potential in the treatment of ORN. Further medical research, the advancement of technology, and the integration of basic science with clinical medicine should lead to novel, safer, and more effective treatment strategies for patients with ORN of the femoral head.
Conclusions
ORN of the femoral head is a severe complication, with complex treatment options. Due to the limitations of conservative treatment, current treatment methods are mainly surgical, which also have disadvantages. Adequate shielding of significant structures and organs should be recommended to reduce the total radiation dose during radiotherapy, which can help prevent the development of ORN. Moreover, patients should be followed-up regularly after pelvic radiotherapy, because early diagnosis and prompt treatment could delay disability.
Figures
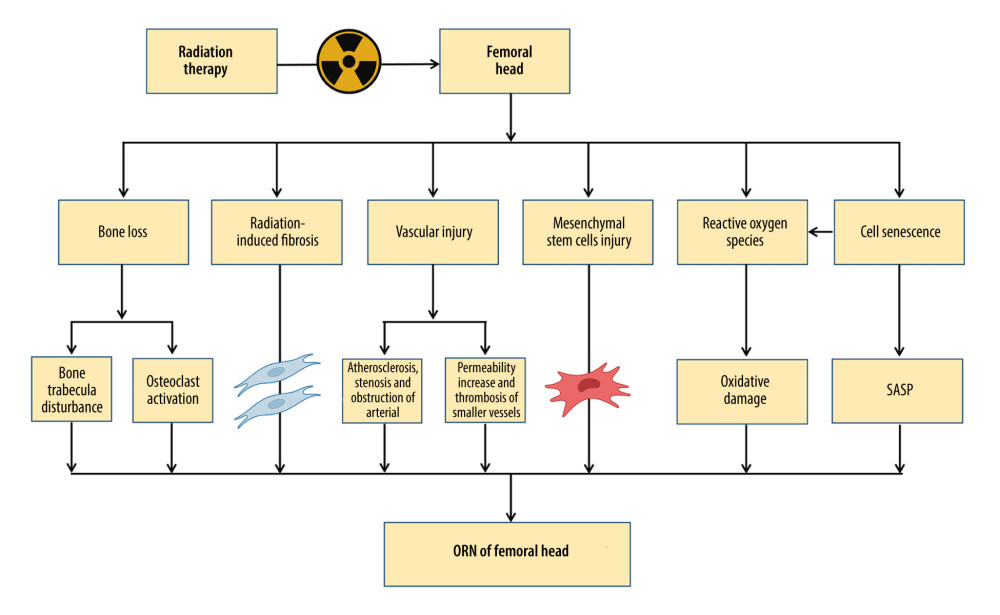 Figure 1. The pathogenesis of osteoradionecrosis (ORN). Medical research and clinical experience with radiation-induced bone injury revealed that the pathogenesis of ORN includes bone loss, radiation-induced fibrosis, vascular injury, mesenchymal stem cell injury, reactive oxygen species, and cell senescence. SASP – senescence-associated secretory phenotype. Created with BioRender.com.
Figure 1. The pathogenesis of osteoradionecrosis (ORN). Medical research and clinical experience with radiation-induced bone injury revealed that the pathogenesis of ORN includes bone loss, radiation-induced fibrosis, vascular injury, mesenchymal stem cell injury, reactive oxygen species, and cell senescence. SASP – senescence-associated secretory phenotype. Created with BioRender.com. 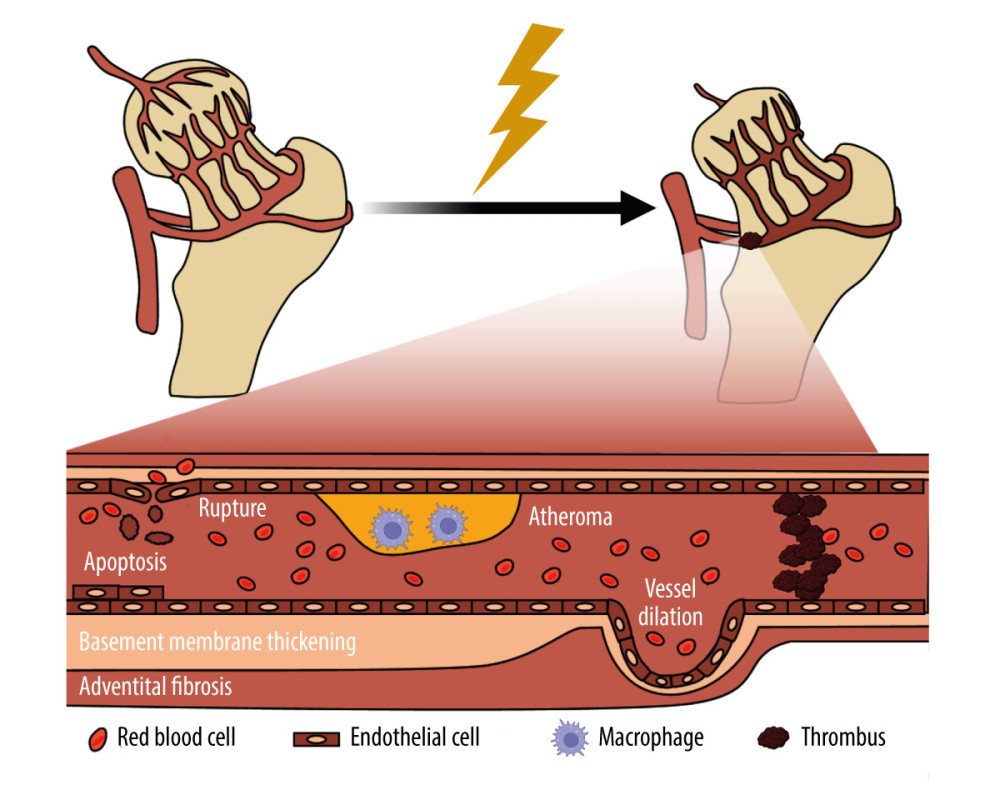 Figure 2. Schematic of the damage of radiation on blood vessel. Radiation-induced vascular injury begins with progressive endothelial loss, and followed and partially overlapped by thrombus, wall rapture, and hemorrhage. Long term morphologic changes include endothelial proliferation, basement membrane thickening, adventitial fibrosis, vessel dilatation, and atheroma, which is rich of macrophages. Created with BioRender.com.
Figure 2. Schematic of the damage of radiation on blood vessel. Radiation-induced vascular injury begins with progressive endothelial loss, and followed and partially overlapped by thrombus, wall rapture, and hemorrhage. Long term morphologic changes include endothelial proliferation, basement membrane thickening, adventitial fibrosis, vessel dilatation, and atheroma, which is rich of macrophages. Created with BioRender.com. 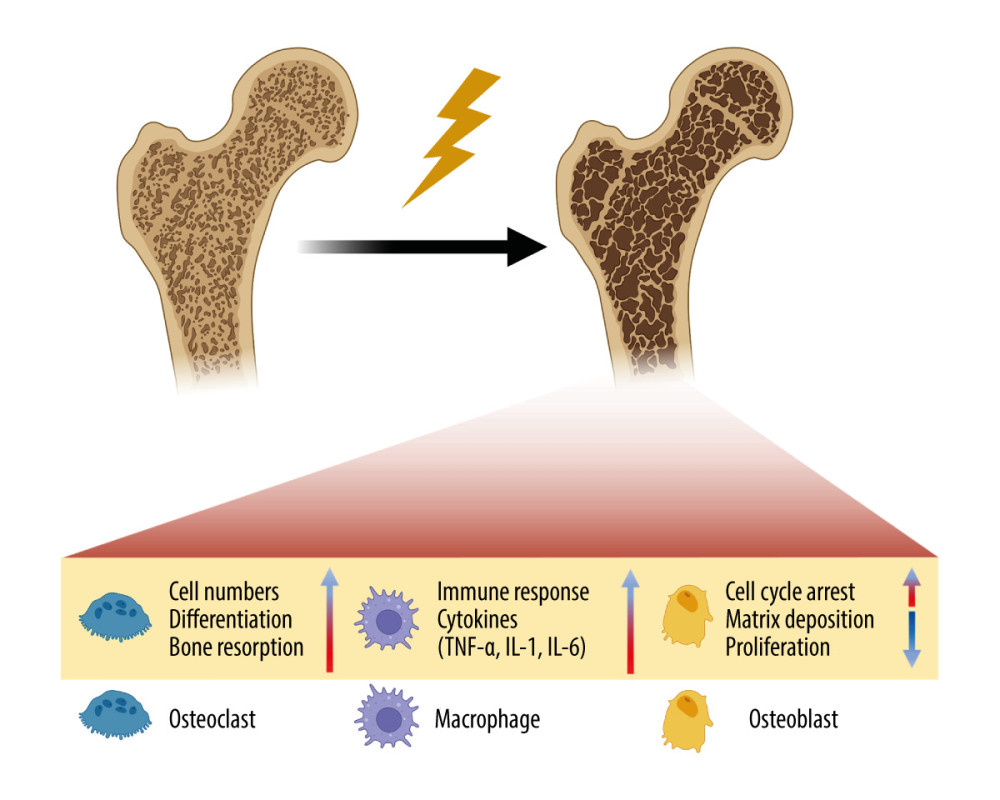 Figure 3. Schematic of the damage of radiation on bone. Radiation therapy (RT) can cause the development of osteoporosis and fragility fractures, mainly involving osteoclasts, osteoblasts and immune cells. The differentiation and number of osteoblasts are increased, making the bone resorption happen. While in osteoblasts, cell cycle arrest occurs, and the matrix deposition and proliferation rate are reduced. Meanwhile, the immune response is enhanced, and pro-inflammatory cytokines are secreted by immune cells. TNF-α – tumor necrosis factor-α; IL-1 – interleukin-1; IL-6 – interleukin-6. Created with BioRender.com.
Figure 3. Schematic of the damage of radiation on bone. Radiation therapy (RT) can cause the development of osteoporosis and fragility fractures, mainly involving osteoclasts, osteoblasts and immune cells. The differentiation and number of osteoblasts are increased, making the bone resorption happen. While in osteoblasts, cell cycle arrest occurs, and the matrix deposition and proliferation rate are reduced. Meanwhile, the immune response is enhanced, and pro-inflammatory cytokines are secreted by immune cells. TNF-α – tumor necrosis factor-α; IL-1 – interleukin-1; IL-6 – interleukin-6. Created with BioRender.com. 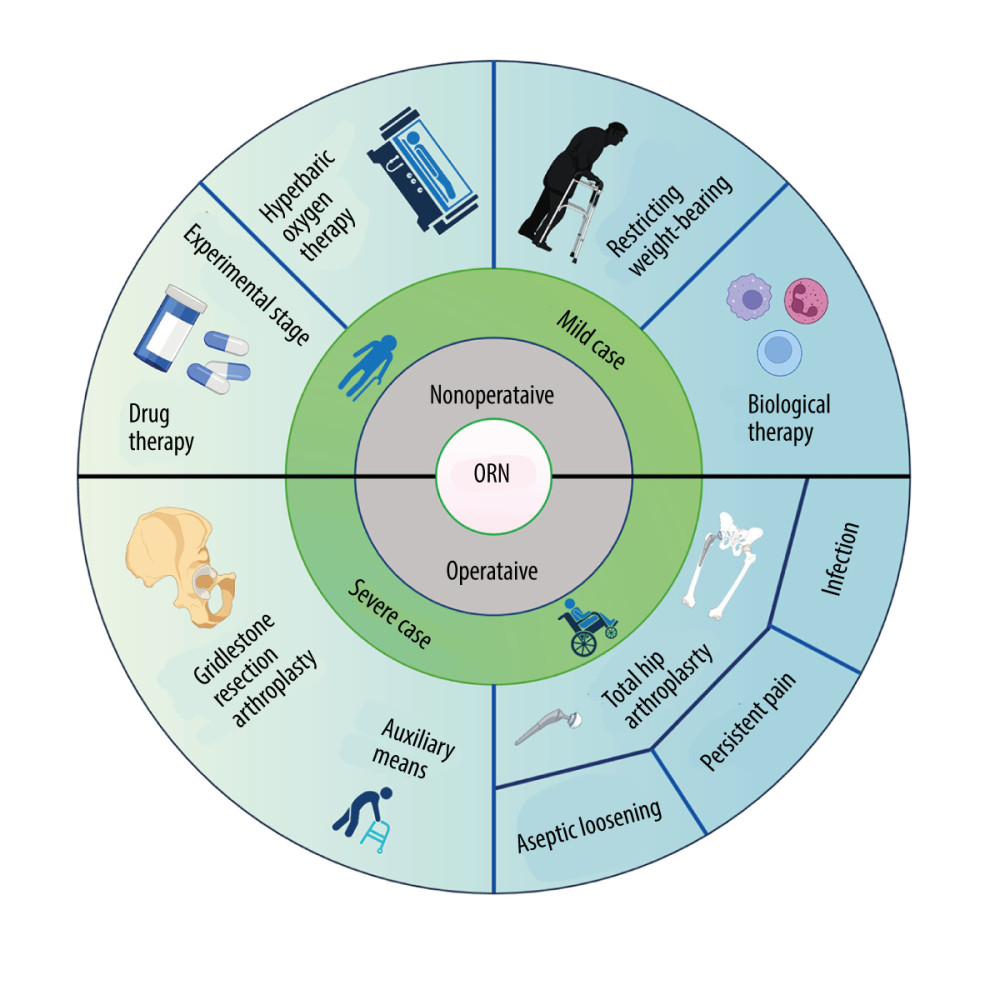 Figure 4. The treatments for osteoradionecrosis (ORN) of the femoral head. Non-surgical management includes restricting weight-bearing during exercise, hyperbaric oxygen therapy (HBOT), drugs, and biophysical therapy, which have limited effectiveness. Surgical treatments are considered for severe cases, and mainly consist of total hip arthroplasty (THA) and Girdlestone resection arthroplasty (GRA). THA has a particularly high failure rate because of the aseptic loosening, persistent pain, and infection. Created with BioRender.com.
Figure 4. The treatments for osteoradionecrosis (ORN) of the femoral head. Non-surgical management includes restricting weight-bearing during exercise, hyperbaric oxygen therapy (HBOT), drugs, and biophysical therapy, which have limited effectiveness. Surgical treatments are considered for severe cases, and mainly consist of total hip arthroplasty (THA) and Girdlestone resection arthroplasty (GRA). THA has a particularly high failure rate because of the aseptic loosening, persistent pain, and infection. Created with BioRender.com. References
1. Röntgen WK, A new form of radiation: Science, 1896; 3(72); 726-29
2. McBride WH, Schaue D, Radiation-induced tissue damage and response: J Pathol, 2020; 250(5); 647-55
3. Chargari C, Deutsch E, Blanchard P, Brachytherapy: An overview for clinicians: Cancer J Clin, 2019; 69(5); 386-401
4. Sapienza LG, Salcedo MP, Ning MS, Pelvic insufficiency fractures after external beam radiation therapy for gynecologic cancers: A meta-analysis and meta-regression of 3929 patients: Int J Radiat Oncol Biol Phys, 2020; 106(3); 475-84
5. Michalecki L, Gabryś D, Kulik R, Radiotherapy induced hip joint avascular necrosis – two cases report: Rep Pract Oncol Radiother, 2011; 16(5); 198-201
6. Win AZ, Aparici CM, Non-traumatic radiation-induced avascular necrosis of the femoral neck: Qjm, 2015; 108(3); 257-58
7. Chronopoulos A, Zarra T, Ehrenfeld M, Otto S, Osteoradionecrosis of the jaws: Definition, epidemiology, staging and clinical and radiological findings. A concise review: Int Dent J, 2018; 68(1); 22-30
8. van den Blink QU, Garcez K, Henson CC, Pharmacological interventions for the prevention of insufficiency fractures and avascular necrosis associated with pelvic radiotherapy in adults: Cochrane Database Syst Rev, 2018; 4(4); CD010604
9. Higham CE, Faithfull S, Bone health and pelvic radiotherapy: Clin Oncol (R Coll Radiol), 2015; 27(11); 668-78
10. Júnior LHF, Limirio P, Soares PBF, The effect of hyperbaric oxygen therapy on bone macroscopy, composition and biomechanical properties after ionizing radiation injury: Radiat Oncol, 2020; 15(1); 95
11. Chen X, Chen L, Tan J, Rspo1-LGR4 axis in BMSCs protects bone against radiation-induced injury through the mTOR-dependent autophagy pathway: J Cell Physiol, 2021; 236(6); 4273-89
12. Bakar AAA, Mohamad NS, Mahmud MH, Systematic review on multilevel analysis of radiation effects on bone microarchitecture: Biomed Res Int, 2022; 2022; 9890633
13. Meixel AJ, Hauswald H, Delorme S, Jobke B, From radiation osteitis to osteoradionecrosis: Incidence and MR morphology of radiation-induced sacral pathologies following pelvic radiotherapy: Eur Radiol, 2018; 28(8); 3550-59
14. Abdulkareem IH, Radiation-induced femoral head necrosis: Niger J Clin Pract, 2013; 16(1); 123-26
15. Li M, Cole PA, Anatomical considerations in adult femoral neck fractures: How anatomy influences the treatment issues?: Injury, 2015; 46(3); 453-58
16. Chang A, Breeland G, Hubbard JB, Anatomy, bony pelvis and lower limb, femur: StatPearls, 2023, Treasure Island (FL), StatPearls Publishing LLC
17. Ng KCG, Jeffers JRT, Beaulé PE, Hip joint capsular anatomy, mechanics, and surgical management: J Bone Joint Surg Am, 2019; 101(23); 2141-51
18. Glenister R, Sharma S, Anatomy, bony pelvis and lower limb, hip: StatPearls, 2023, Treasure Island (FL), StatPearls Publishing Copyright LLC
19. Seeley MA, Georgiadis AG, Sankar WN, Hip vascularity: A review of the anatomy and clinical implications: J Am Acad Orthop Surg, 2016; 24(8); 515-26
20. Dewar DC, Lazaro LE, Klinger CE, The relative contribution of the medial and lateral femoral circumflex arteries to the vascularity of the head and neck of the femur: A quantitative MRI-based assessment: Bone Joint J, 2016; 98-b(12); 1582-88
21. Zlotorowicz M, Czubak-Wrzosek M, Wrzosek P, Czubak J, The origin of the medial femoral circumflex artery, lateral femoral circumflex artery and obturator artery: Surg Radiol Anat, 2018; 40(5); 515-20
22. Vuksanović-Božarić A, Abramović M, Clinical significance of understanding lateral and medial circumflex femoral artery origin variability: Anat Sci Int, 2018; 93(4); 449-55
23. Petek D, Hannouche D, Suva D, Osteonecrosis of the femoral head: Pathophysiology and current concepts of treatment: EFORT Open Rev, 2019; 4(3); 85-97
24. Konarski W, Poboży T, Śliwczyński A, Avascular necrosis of femoral head-overview and current state of the art: Int J Environ Res Public Health, 2022; 19(12); 7348
25. Fillon M, Increased radiation intensity found to be safe for the treatment of prostate cancer: Cancer J Clin, 2020; 70(2); 73-74
26. Harrison R, Out-of-field doses in radiotherapy: Input to epidemiological studies and dose-risk models: Phys Med, 2017; 42; 239-46
27. Miller KD, Nogueira L, Mariotto AB, Cancer treatment and survivorship statistics, 2019: Cancer J Clin, 2019; 69(5); 363-85
28. Abshire D, Lang MK, The evolution of radiation therapy in treating cancer: Semin Oncol Nurs, 2018; 34(2); 151-57
29. Dilalla V, Chaput G, Williams T, Sultanem K, Radiotherapy side effects: Integrating a survivorship clinical lens to better serve patients: Curr Oncol, 2020; 27(2); 107-12
30. Ugurluer G, Akbas T, Arpaci T, Bone complications after pelvic radiation therapy: Evaluation with MRI: J Med Imaging Radiat Oncol, 2014; 58(3); 334-40
31. Ramlov A, Pedersen EM, Røhl L, Risk factors for pelvic insufficiency fractures in locally advanced cervical cancer following intensity modulated radiation therapy: Int J Radiat Oncol Biol Phys, 2017; 97(5); 1032-39
32. Galietta E, Gaiani L, Giannini C, Prophylactic radiotherapy of hip heterotopic ossification: A narrative mini review: In Vivo, 2022; 36(2); 533-42
33. Wood J, Ver Halen J, Samant S, Florendo N, Radiation-induced sarcoma masquerading as osteoradionecrosis: Case report and literature review: J Laryngol Otol, 2015; 129(3); 279-82
34. Dzik-Jurasz AS, Brooker S, Husband JE, Tait D, What is the prevalence of symptomatic or asymptomatic femoral head osteonecrosis in patients previously treated with chemoradiation? A magnetic resonance study of anal cancer patients: Clin Oncol (R Coll Radiol), 2001; 13(2); 130-34
35. Dhanda J, Pasquier D, Newman L, Shaw R, Current concepts in osteoradionecrosis after head and neck radiotherapy: Clin Oncol (R Coll Radiol), 2016; 28(7); 459-66
36. Kaste SC, Karimova EJ, Neel MD, Osteonecrosis in children after therapy for malignancy: Am J Roentgenol, 2011; 196(5); 1011-18
37. Rao SS, El Abiad JM, Puvanesarajah V, Osteonecrosis in pediatric cancer survivors: Epidemiology, risk factors, and treatment: Surg Oncol, 2019; 28; 214-21
38. Krasin MJ, Xiong X, Wu S, Merchant TE, The effects of external beam irradiation on the growth of flat bones in children: Modeling a dose-volume effect: Int J Radiat Oncol Biol Phys, 2005; 62(5); 1458-63
39. Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, Osteonecrosis in adult survivors of childhood cancer: A report from the childhood cancer survivor study: J Clin Oncol, 2008; 26(18); 3038-45
40. Liuhto N, Grönroos MH, Malila N, Diseases of renal function and bone metabolism after treatment for early onset cancer: A registry-based study: Int J Cancer, 2020; 146(5); 1324-32
41. Cheng H, Wang L, Wang L, Li Q, Discussion on the necessity of bilateral inguinal lymphatic area irradiation for cervical cancer with invasion of the lower one-third of the vagina: J Cancer Res Ther, 2023; 19(1); 20-24
42. Curi MM, Cardoso CL, de Lima HG, Histopathologic and histomorphometric analysis of irradiation injury in bone and the surrounding soft tissues of the jaws: J Oral Maxillofac Surg, 2016; 74(1); 190-99
43. Ma Y, Shen G, Distraction osteogenesis after irradiation in rabbit mandibles: Br J Oral Maxillofac Surg, 2012; 50(7); 662-67
44. Venkatesulu BP, Sanders KL, Hsieh CE, Biomarkers of radiation-induced vascular injury: Cancer Rep (Hoboken), 2019; 2(2); e1152
45. Michel G, Blery P, Pilet P, Micro-CT analysis of radiation-induced osteopenia and bone hypovascularization in rat: Calcif Tissue Int, 2015; 97(1); 62-68
46. Fajardo LF, The pathology of ionizing radiation as defined by morphologic patterns: Acta Oncol, 2005; 44(1); 13-22
47. Dekker H, Bravenboer N, van Dijk D, The irradiated human mandible: A quantitative study on bone vascularity: Oral Oncol, 2018; 87; 126-30
48. Shen Y, Jiang X, Meng L, Transplantation of bone marrow mesenchymal stem cells prevents radiation-induced artery injury by suppressing oxidative stress and inflammation: Oxid Med Cell Longev, 2018; 2018; 5942916
49. Murphy ES, Xie H, Merchant TE, Review of cranial radiotherapy-induced vasculopathy: J Neurooncol, 2015; 122(3); 421-29
50. Kralik SF, Watson GA, Shih CS, Radiation-induced large vessel cerebral vasculopathy in pediatric patients with brain tumors treated with proton radiation therapy: Int J Radiat Oncol Biol Phys, 2017; 99(4); 817-24
51. Rodríguez-Barbeito P, Díaz-Botana P, Gago-Arias A, A model of indirect cell death caused by tumor vascular damage after high-dose radiotherapy: Cancer Res, 2019; 79(23); 6044-53
52. Liu Y, Cao W, Kong X, Protective effects of α-2-macroglobulin on human bone marrow mesenchymal stem cells in radiation injury: Mol Med Rep, 2018; 18(5); 4219-28
53. Bahney CS, Zondervan RL, Allison P, Cellular biology of fracture healing: J Orthop Res, 2019; 37(1); 35-50
54. Fu X, Liu G, Halim A, Mesenchymal stem cell migration and tissue repair: Cells, 2019; 8(8); 784
55. Wang Y, Zhu G, Wang J, Chen J, Irradiation alters the differentiation potential of bone marrow mesenchymal stem cells: Mol Med Rep, 2016; 13(1); 213-23
56. Hou J, Han ZP, Jing YY, Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells: Cell Death Dis, 2013; 4(10); e844
57. Zhang Y, Deng H, Yang Z, Treatment of radiation bone injury with transplanted hUCB-MSCs via Wnt/β-Catenin: Stem Cells Int, 2021; 2021; 5660927
58. Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD, ROS and the DNA damage response in cancer: Redox Biol, 2019; 25; 101084
59. Naji A, Eitoku M, Favier B, Biological functions of mesenchymal stem cells and clinical implications: Cell Mol Life Sci, 2019; 76(17); 3323-48
60. Yin Y, Li X, He XT, Leveraging stem cell homing for therapeutic regeneration: J Dent Res, 2017; 96(6); 601-9
61. Bartlow CM, Mann KA, Damron TA, Oest ME, Limited field radiation therapy results in decreased bone fracture toughness in a murine model: PLoS One, 2018; 13(10); e0204928
62. Soares PBF, Soares CJ, Limirio P, Effect of ionizing radiation after-therapy interval on bone: Histomorphometric and biomechanical characteristics: Clin Oral Investig, 2019; 23(6); 2785-93
63. Zhang J, Zheng L, Wang Z, Lowering iron level protects against bone loss in focally irradiated and contralateral femurs through distinct mechanisms: Bone, 2019; 120; 50-60
64. Xu X, Li R, Zhou Y, Dysregulated systemic lymphocytes affect the balance of osteogenic/adipogenic differentiation of bone mesenchymal stem cells after local irradiation: Stem Cell Res Ther, 2017; 8(1); 71
65. Zhai J, He F, Wang J, Chen J, Influence of radiation exposure pattern on the bone injury and osteoclastogenesis in a rat model: Int J Mol Med, 2019; 44(6); 2265-75
66. Oest ME, Policastro CG, Mann KA, Longitudinal effects of single hindlimb radiation therapy on bone strength and morphology at local and contralateral sites: J Bone Miner Res, 2018; 33(1); 99-112
67. Pacheco R, Stock H, Effects of radiation on bone: Curr Osteoporos Rep, 2013; 11(4); 299-304
68. Costa S, Reagan MR, Therapeutic irradiation: Consequences for bone and bone marrow adipose tissue: Front Endocrinol (Lausanne), 2019; 10; 587
69. Yahyapour R, Amini P, Rezapoor S, Targeting of inflammation for radiation protection and mitigation: Curr Mol Pharmacol, 2018; 11(3); 203-10
70. Hayashi T, Furukawa K, Morishita Y, Intracellular reactive oxygen species level in blood cells of atomic bomb survivors is increased due to aging and radiation exposure: Free Radic Biol Med, 2021; 171; 126-34
71. Farhood B, Mortezaee K, Goradel NH, Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy: J Cell Physiol, 2019; 234(5); 5728-40
72. Vitzthum LK, Park H, Zakeri K, Risk of pelvic fracture with radiation therapy in older patients: Int J Radiat Oncol Biol Phys, 2020; 106(3); 485-92
73. Chandra A, Park SS, Pignolo RJ, Potential role of senescence in radiation-induced damage of the aged skeleton: Bone, 2019; 120; 423-31
74. Hernández L, Terradas M, Camps J, Aging and radiation: Bad companions: Aging Cell, 2015; 14(2); 153-61
75. Zhou S, Zhu J, Zhou PK, Gu Y, Alveolar type 2 epithelial cell senescence and radiation-induced pulmonary fibrosis: Front Cell Dev Biol, 2022; 10; 999600
76. Li XH, Ha CT, Fu D, Xiao M, REDD1 protects osteoblast cells from gamma radiation-induced premature senescence: PLoS One, 2012; 7(5); e36604
77. Hong EH, Lee SJ, Kim JS, Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase: J Biol Chem, 2010; 285(2); 1283-95
78. Herranz N, Gil J, Mechanisms and functions of cellular senescence: J Clin Invest, 2018; 128(4); 1238-46
79. He S, Sharpless NE, Senescence in health and disease: Cell, 2017; 169(6); 1000-11
80. Delanian S, Lefaix JL, The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway: Radiother Oncol, 2004; 73(2); 119-31
81. Straub JM, New J, Hamilton CD, Radiation-induced fibrosis: Mechanisms and implications for therapy: J Cancer Res Clin Oncol, 2015; 141(11); 1985-94
82. Akashi M, Hashikawa K, Wanifuchi S, Heterogeneity of necrotic changes between cortical and cancellous bone in mandibular osteoradionecrosis: A histopathological analysis of resection margin after segmental mandibulectomy: Biomed Res Int, 2017; 2017; 3125842
83. Lyons AJ, West CM, Risk JM, Osteoradionecrosis in head-and-neck cancer has a distinct genotype-dependent cause: Int J Radiat Oncol Biol Phys, 2012; 82(4); 1479-84
84. Ejaz A, Greenberger JS, Rubin PJ, Understanding the mechanism of radiation induced fibrosis and therapy options: Pharmacol Ther, 2019; 204; 107399
85. Ejaz A, Epperly MW, Hou W, Adipose-derived stem cell therapy ameliorates ionizing irradiation fibrosis via hepatocyte growth factor-mediated transforming growth factor-β downregulation and recruitment of bone marrow cells: Stem Cells, 2019; 37(6); 791-802
86. Mitsimponas KT, Moebius P, Amann K, Osteo-radio-necrosis (ORN) and bisphosphonate-related osteonecrosis of the jaws (BRONJ): The histopathological differences under the clinical similarities: Int J Clin Exp Pathol, 2014; 7(2); 496-508
87. Xu SH, Tang JS, Shen XY, Osteoradionecrosis of the hip, a troublesome complication of radiation therapy: Case series and systematic review: Front Med (Lausanne), 2022; 9; 858929
88. Niinimäki R, Suo-Palosaari M, Pokka T, The radiological and clinical follow-up of osteonecrosis in cancer patients: Acta Oncol, 2019; 58(4); 505-11
89. Quinlan JF, North J, Clarke DA, Gwynne-Jones DP, Radiation-induced osteonecrosis of the hips following genital-preserving surgery and chemoradiotherapy: N Z Med J, 2009; 122(1297); 84-86
90. Cohen-Rosenblum A, Cui Q, Osteonecrosis of the femoral head: Orthop Clin North Am, 2019; 50(2); 139-49
91. Chung KY, Chiu KH, Cheung KW, Osteoradionecrosis of the acetabulum in a total hip arthroplasty: A case report: J Orthop Surg (Hong Kong), 2010; 18(1); 110-12
92. Microsurgery Department of the Orthopedics Branch of the Chinese Medical Doctor Association; Group from the Osteonecrosis and Bone Defect Branch of the Chinese Association of Reparative and Reconstructive Surgery; Microsurgery and Reconstructive Surgery Group of the Orthopedics Branch of the Chinese Medical Association, Chinese Guideline for the Diagnosis and Treatment of Osteonecrosis of the Femoral Head in Adults: Orthop Surg, 2017; 9(1); 3-12
93. Chughtai M, Piuzzi NS, Khlopas A, An evidence-based guide to the treatment of osteonecrosis of the femoral head: Bone Joint J, 2017; 99-b(10); 1267-79
94. Massari L, Fini M, Cadossi R, Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head: J Bone Joint Surg Am, 2006; 88(Suppl 3); 56-60
95. Mont MA, Zywiel MG, Marker DR, The natural history of untreated asymptomatic osteonecrosis of the femoral head: A systematic literature review: J Bone Joint Surg Am, 2010; 92(12); 2165-70
96. Banerjee S, Issa K, Pivec R, Osteonecrosis of the hip: Treatment options and outcomes: Orthop Clin North Am, 2013; 44(4); 463-76
97. Hao Y, Guo H, Xu Z, Meta-analysis of the potential role of extracorporeal shockwave therapy in osteonecrosis of the femoral head: J Orthop Surg Res, 2018; 13(1); 166
98. Massin P, Duparc J, Total hip replacement in irradiated hips. A retrospective study of 71 cases: J Bone Joint Surg Br, 1995; 77(6); 847-52
99. Duparc J, Massin PSurgical treatment of radiation-induced lesions of the hip in adults: Bull Acad Natl Med, 1996; 180(8); 1815-36 [in French]
100. Koren L, Ginesin E, Melamed Y, Hyperbaric oxygen for stage I and II femoral head osteonecrosis: Orthopedics, 2015; 38(3); e200-5
101. Bennett MH, Feldmeier J, Hampson NB, Hyperbaric oxygen therapy for late radiation tissue injury: Cochrane Database Syst Rev, 2016; 4(4); CD005005
102. Bosco G, Vezzani G, Mrakic Sposta S, Hyperbaric oxygen therapy ameliorates osteonecrosis in patients by modulating inflammation and oxidative stress: J Enzyme Inhib Med Chem, 2018; 33(1); 1501-5
103. Vezzani G, Quartesan S, Cancellara P, Hyperbaric oxygen therapy modulates serum OPG/RANKL in femoral head necrosis patients: J Enzyme Inhib Med Chem, 2017; 32(1); 707-11
104. Jenwitheesuk K, Mahakkanukrauh A, Punjaruk W, Efficacy of adjunctive hyperbaric oxygen therapy in osteoradionecrosis: Biores Open Access, 2018; 7(1); 145-49
105. Barilaro G, Francesco Masala I, Parracchini R, The role of hyperbaric oxygen therapy in orthopedics and rheumatological diseases: Isr Med Assoc J, 2017; 19(7); 429-34
106. Felden A, Vaz G, Kreps S, A cemented acetabular component with a reinforcement cross provides excellent medium-term fixation in total hip arthroplasty after pelvic irradiation: Bone Joint J, 2015; 97-b(2); 177-84
107. Kang HS, Kim T, Chung SH, Resection arthroplasty in radiation-induced osteonecrosis of the hip: J Clin Orthop Trauma, 2019; 10(2); 364-67
108. Walters S, Prasad A, Guevel B, Systematic review of the outcome of cemented versus uncemented total hip arthroplasty following pelvic irradiation: Musculoskelet Surg, 2019; 103(3); 221-30
109. Novikov D, Cohen D, Swanson D, A meta-analysis of outcomes in total hip arthroplasty recipients following pelvic irradiation: J Arthroplasty, 2019; 34(7); 1546-52
110. Lindberg-Larsen M, Jørgensen CC, Solgaard S, Increased risk of intraoperative and early postoperative periprosthetic femoral fracture with uncemented stems: Acta Orthop, 2017; 88(4); 390-94
111. Jacobs JJ, Kull LR, Frey GA, Early failure of acetabular components inserted without cement after previous pelvic irradiation: J Bone Joint Surg Am, 1995; 77(12); 1829-35
112. Cho MR, Kwun KW, Lee DH, Latent period best predicts acetabular cup failure after total hip arthroplasties in radiated hips: Clin Orthop Relat Res, 2005; 438; 165-70
113. Kim KI, Klein GR, Sleeper J, Uncemented total hip arthroplasty in patients with a history of pelvic irradiation for prostate cancer: J Bone Joint Surg Am, 2007; 89(4); 798-805
114. Joglekar SB, Rose PS, Lewallen DG, Sim FH, Tantalum acetabular cups provide secure fixation in THA after pelvic irradiation at minimum 5-year followup: Clin Orthop Relat Res, 2012; 470(11); 3041-47
115. De Paolis M, Zucchini R, Romagnoli C, Middle term results of tantalum acetabular cups in total hip arthroplasty following pelvic irradiation: Acta Orthop Traumatol Turc, 2019; 53(3); 165-69
116. Callaghan JJ, Liu SS, Phruetthiphat OA, The revision acetabulum-allograft and bone substitutes: Vestigial organs for bone deficiency: Bone Joint J, 2014; 96-b(11 Suppl A); 70-72
117. García-Cimbrelo E, García-Rey E, Bone defect determines acetabular revision surgery: Hip Int, 2014; 24(Suppl 10); S33-36
118. Malcolm TL, Gad BV, Elsharkawy KA, Higuera CA, Complication, survival, and reoperation rates following girdlestone resection arthroplasty: J Arthroplasty, 2015; 30(7); 1183-86
119. Vincenten CM, Den Oudsten BL, Bos PK, Quality of life and health status after Girdlestone resection arthroplasty in patients with an infected total hip prosthesis: J Bone Jt Infect, 2019; 4(1); 10-15
120. Vincenten CM, Gosens T, van Susante JC, Somford MP, The Girdlestone situation: A historical essay: J Bone Jt Infect, 2019; 4(5); 203-8
121. Sawadogo M, Kafando H, Ouedraogo S, Is head and neck resection of the femur (Girdlestone’s procedure) still relevant? Indications and results about 24 cases: Open Orthop J, 2018; 12; 69-74
Figures
 Figure 1. The pathogenesis of osteoradionecrosis (ORN). Medical research and clinical experience with radiation-induced bone injury revealed that the pathogenesis of ORN includes bone loss, radiation-induced fibrosis, vascular injury, mesenchymal stem cell injury, reactive oxygen species, and cell senescence. SASP – senescence-associated secretory phenotype. Created with BioRender.com.
Figure 1. The pathogenesis of osteoradionecrosis (ORN). Medical research and clinical experience with radiation-induced bone injury revealed that the pathogenesis of ORN includes bone loss, radiation-induced fibrosis, vascular injury, mesenchymal stem cell injury, reactive oxygen species, and cell senescence. SASP – senescence-associated secretory phenotype. Created with BioRender.com. Figure 2. Schematic of the damage of radiation on blood vessel. Radiation-induced vascular injury begins with progressive endothelial loss, and followed and partially overlapped by thrombus, wall rapture, and hemorrhage. Long term morphologic changes include endothelial proliferation, basement membrane thickening, adventitial fibrosis, vessel dilatation, and atheroma, which is rich of macrophages. Created with BioRender.com.
Figure 2. Schematic of the damage of radiation on blood vessel. Radiation-induced vascular injury begins with progressive endothelial loss, and followed and partially overlapped by thrombus, wall rapture, and hemorrhage. Long term morphologic changes include endothelial proliferation, basement membrane thickening, adventitial fibrosis, vessel dilatation, and atheroma, which is rich of macrophages. Created with BioRender.com. Figure 3. Schematic of the damage of radiation on bone. Radiation therapy (RT) can cause the development of osteoporosis and fragility fractures, mainly involving osteoclasts, osteoblasts and immune cells. The differentiation and number of osteoblasts are increased, making the bone resorption happen. While in osteoblasts, cell cycle arrest occurs, and the matrix deposition and proliferation rate are reduced. Meanwhile, the immune response is enhanced, and pro-inflammatory cytokines are secreted by immune cells. TNF-α – tumor necrosis factor-α; IL-1 – interleukin-1; IL-6 – interleukin-6. Created with BioRender.com.
Figure 3. Schematic of the damage of radiation on bone. Radiation therapy (RT) can cause the development of osteoporosis and fragility fractures, mainly involving osteoclasts, osteoblasts and immune cells. The differentiation and number of osteoblasts are increased, making the bone resorption happen. While in osteoblasts, cell cycle arrest occurs, and the matrix deposition and proliferation rate are reduced. Meanwhile, the immune response is enhanced, and pro-inflammatory cytokines are secreted by immune cells. TNF-α – tumor necrosis factor-α; IL-1 – interleukin-1; IL-6 – interleukin-6. Created with BioRender.com. Figure 4. The treatments for osteoradionecrosis (ORN) of the femoral head. Non-surgical management includes restricting weight-bearing during exercise, hyperbaric oxygen therapy (HBOT), drugs, and biophysical therapy, which have limited effectiveness. Surgical treatments are considered for severe cases, and mainly consist of total hip arthroplasty (THA) and Girdlestone resection arthroplasty (GRA). THA has a particularly high failure rate because of the aseptic loosening, persistent pain, and infection. Created with BioRender.com.
Figure 4. The treatments for osteoradionecrosis (ORN) of the femoral head. Non-surgical management includes restricting weight-bearing during exercise, hyperbaric oxygen therapy (HBOT), drugs, and biophysical therapy, which have limited effectiveness. Surgical treatments are considered for severe cases, and mainly consist of total hip arthroplasty (THA) and Girdlestone resection arthroplasty (GRA). THA has a particularly high failure rate because of the aseptic loosening, persistent pain, and infection. Created with BioRender.com. Tables
In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952