27 May 2023: Clinical Research
Brief Test of Olfactory Dysfunction Based on Diagnostic Features of Specific Odors in Early-Stage Alzheimer Disease
Egle AudronyteDOI: 10.12659/MSM.940363
Med Sci Monit 2023; 29:e940363
Abstract
BACKGROUND: Olfactory impairment is an early symptom of Alzheimer disease (AD). However, it is rarely assessed in clinical practice. This study aimed to assess the identification and discrimination of specific odors in patients with early-stage AD using the Sniffin’ Sticks test and determine the items that would be most valuable in the diagnosis of early-stage AD in order to create a brief test of olfactory dysfunction.
MATERIAL AND METHODS: Three groups of participants were enrolled, including 30 patients with mild cognitive impairment due to AD (MCI-AD group), 30 with mild dementia due to AD (MD-AD group), and 30 older participants with normal cognition (NC group). All participants underwent cognitive (Clinical Dementia Rating, Mini-Mental State Examination, Alzheimer’s Disease Assessment Scale-Cognitive Subscale, and verbal fluency tests) and olfactory (Burghart Sniffin’ Sticks odor identification and odor discrimination tests) assessments.
RESULTS: The MD-AD group scored significantly lower than the MCI-AD group and the MCI-AD group scored significantly lower than the NC group in both the odor identification (P<0.001) and discrimination (P<0.05) tasks. The shortened versions of the odor identification and discrimination tasks showed good diagnostic properties in differentiating patients with AD from the NC participants (receiver operating characteristic [ROC] area under the curve [AUC]=0.912 and 0.954, respectively) and differentiating patients with MCI-AD from the NC participants (ROC AUC=0.871 and 0.959, respectively).
CONCLUSIONS: The brief versions of olfactory tests, containing selected items that were found to differ the most between cognitively normal participants and early-stage AD patients, have good diagnostic qualities and can aid clinicians in screening for early-stage AD.
Keywords: Alzheimer Disease, cognitive dysfunction, Olfaction Disorders, Humans, odorants, Smell, Neuropsychological Tests
Background
Despite recent advances in neuroimaging, cerebrospinal fluid, and blood biomarkers, diagnosing early-stage Alzheimer disease (AD) remains challenging [1–3]. This is especially true in primary care, as these biomarkers are often expensive and not available in community settings. Currently available biomarkers for diagnosing AD measure brain amyloid-beta (Aβ) protein deposition and neuronal degeneration and require cerebrospinal fluid analysis or advanced neuroimaging techniques [1,2].
According to a survey by the Alzheimer’s Association, the lack of specialists and facilities to perform diagnostic testing was the most commonly cited challenge faced by primary care physicians in the United States when diagnosing mild cognitive impairment (MCI) due to AD [4]. The situation is even worse in low- and middle-income countries, where up to 90% of dementia cases are not diagnosed, according to estimations by Alzheimer’s Disease International [5]. Additionally, the survey by the Alzheimer’s Association showed that cognitive testing was often not sufficient to accurately identify patients with early changes, with 72% of primary care physicians stating that they found it challenging to differentiate MCI from normal aging deficits [4]. Therefore, additional objective measures that would be sensitive, affordable, and widely accessible for predicting early-stage AD are needed.
Olfactory impairment is an early and common symptom of AD. According to various studies, olfactory impairment manifests prior to cognitive decline and is present in up to 90% of patients with AD [6–9]. Odor identification tests have been the most studied olfactory assessment methods, while other olfactory functions have rarely been analyzed in patients with AD. Odor identification testing was recognized as an inexpensive and short method that can serve as an excellent screening tool by helping to accurately identify early changes and prevent a delay in diagnosis [10]. However, olfactory dysfunction assessment is rarely used in clinical practice for diagnosing AD.
One possible limitation of olfactory testing in clinical practice is the relatively long duration of assessment. The University of Pennsylvania Smell Identification Test (UPSIT) is the most commonly used odor identification test and consists of 40 odors [11,12]. The Sniffin’ Sticks test, which is particularly popular in Europe, consists of 16 odors [11,13]. However, intervals of at least 30 s are recommended between the presentation of odors to prevent olfactory desensitization [13].
Various shortened versions of the UPSIT, such as the Brief Smell Identification Test, Quick Smell Identification Test, and Pocket Smell Test, were created to provide a more convenient method for screening patients [11,14]. Some of these shortened versions have even been tested in patients with AD and have shown encouraging results [15,16]. Attempts have also been made to select UPSIT items that would be the most specific for detecting AD [10,17,18]. A shortened version of the Sniffin’ Sticks odor identification test was also created [19]. However, it was not tested in patients with AD.
Since odor discrimination has rarely been tested in previous studies, information regarding the discrimination of specific odorants and shortened versions of the tests are lacking.
The objective of this study was to explore the processing of specific odors in patients with early-stage AD using Sniffin’ Sticks odor identification and odor discrimination tasks. We aimed to assess the identification and discrimination of specific odors in patients with early-stage AD using the Sniffin’ Sticks test and determine the items that would be most valuable in the diagnosis of early-stage AD in order to create a brief test of olfactory dysfunction and explore its diagnostic qualities.
Material and Methods
PARTICIPANTS:
The study was approved by the Vilnius Regional Bioethics Committee (approval number: 2021/6-1355-830). The study was conducted according to the principles of the Declaration of Helsinki. All participants were informed of the study procedures. All participants agreed to participate in the study and provided written informed consent by signing relevant written informed consent forms.
Three groups of participants were enrolled in the study. The first group included patients diagnosed with mild dementia (MD) due to AD: MD-AD group, based on the National Institute on Aging-Alzheimer’s Association (NIA/AA) criteria for probable AD and Clinical Dementia Rating (CDR) total score of 1 [2]. The second group consisted of patients diagnosed with MCI due to AD: MCI-AD group, according to the NIA/AA criteria for MCI due to AD and CDR total score of 0.5 [1]. The third group comprised older participants with normal cognition: NC group. Each group included 30 participants.
Participants were enrolled only if they had no other central nervous system disorders except MCI due to AD or AD and no significant cerebrovascular pathology (Hachinski Ischemic Score <4).
Other exclusion criteria were history of severe brain trauma, significant psychiatric conditions (psychosis, depression [Geriatric Depression Scale score > 9], substance abuse, or psychoactive medications), history of nasal trauma or surgery, smoking, and recent viral infections potentially affecting olfaction.
ASSESSMENT OF COGNITIVE FUNCTIONS:
Demographic information (age, sex, duration of AD symptoms, and medical history) was obtained from each participant.
For the assessment of global cognitive functioning, the Mini-Mental State Examination (MMSE) and CDR were performed [20,21]. Further evaluation was performed using the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) [20]. Phonemic (PAS) and categorical (animals) verbal fluency were also evaluated.
ASSESSMENT OF OLFACTORY FUNCTION:
The Sniffin’ Sticks odor identification test and odor discrimination test were performed (Burghart®, Wedel, Germany).
The Sniffin’ Sticks odor identification test consists of 16 odors: orange, leather, cinnamon, peppermint, banana, lemon, licorice, turpentine, garlic, coffee, apple, clove, pineapple, rose, anise, and fish. Each odor was presented using a felt tip pen. The cap was removed, and the odor was presented only once for 3 to 4 s. The participants were asked to choose which of the 4 items in the answering card best described the odor. They were prompted to choose an item, even if they were uncertain. A time interval of 30 s was maintained between the odor presentations. The odor identification score is the number of correct responses out of 16 [22].
The Sniffin’ Sticks odor discrimination test consists of 16 triplets of odors, which are presented using felt tip pens. The participant was asked to identify which item had a different odor from the other two in each triplet. The odors were presented in the order provided by the test instructions. Each odor was presented only once, for 3 to 4 s. A time interval of 3 s was maintained between odors in the same triplet. A time interval of 30 s was maintained between the sets of triplets. The odor discrimination score is the number of correct responses out of 16 [22].
The examiner used odorless gloves during the olfactory testing. The participants were asked not to drink or eat anything for at least 15 min prior to testing [22].
DATA ANALYSIS:
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp, Armonk, NY, USA).
The Shapiro-Wilk test was used to determine the normality of data distribution.
Differences in categorical variables between groups were analyzed using a 2-tailed chi-square test (for determining differences between groups in the sex of the participants) and Fisher’s exact test (for determining differences between groups in the frequency of correct identification and discrimination of specific odors).
Differences between 2 groups of non-normally distributed numerical variables were analyzed using the Mann-Whitney U test. The Mann-Whitney test was used for determining the difference between MCI-AD and MD-AD groups in the duration of AD symptoms.
Comparisons between 3 groups of numerical variables were performed using one-way analysis of variance (ANOVA) for normally distributed variables (overall odor identification score) and the Kruskal-Wallis test for non-normally distributed variables (age of participants, years of education, Hachinski Ischemic Score, results of the Geriatric Depression Scale, results of the MMSE, CDR sum of boxes, results of the ADAS-Cog, results of fluency tests, and overall odor discrimination score).
Items that were found to differ the most between cognitively normal participants and early-stage AD patients were selected for the 4-odor identification score and the 4-odor discrimination score. The multiple linear regression models with age, sex, education, duration of symptoms, ADAS-Cog score, and composite verbal fluency test score (fluency PAS + fluency animals) as independent variables, and the 4-odor identification score or the 4-odor discrimination score as dependent variables were tested to determine whether these independent variables significantly predicted the 4-odor identification score and the 4-odor discrimination score.
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the accuracy of the 4-odor identification score and the 4-odor discrimination score.
Statistical significance was set at
Results
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS:
Patients in the MD-AD group were significantly older than those in the MCI-AD and NC groups. However, the MCI-AD and NC groups did not show any significant differences in age. The median age [age range] was 78 [65–85], 72 [60–84], and 74 [63–89] years in the MD-AD, MCI-AD, and NC groups, respectively (Kruskal-Wallis test,
The 3 groups did not differ significantly according to years of education, Hachinski Ischemic Score, or Geriatric Depression Scale results (Kruskal-Wallis test, P>0.05). They also did not differ significantly according to sex (2-tailed chi-square test, P>0.05; Table 1).
The median duration [range of duration] of AD symptoms was 4 [2–6] years in the MD-AD group and 3 [1–5] years in the MCI-AD group. The difference between the groups was significant (Mann-Whitney U test, P<0.001).
The results of the cognitive tests differed significantly among the 3 groups. In all cases, the Kruskal-Wallis test and post hoc analysis revealed significant differences among the 3 groups (P<0.05). The median and interquartile range of the MMSE, CDR sum of boxes, ADAS-Cog scores, and results of fluency tests are provided in Table 2.
ODOR IDENTIFICATION:
Overall odor identification scores differed significantly among the 3 groups. Mean (standard deviation [SD]) scores were 12.77 (1.43) in the NC group, 9.3 (2.23) in the MCI-AD group, and 7.0 (2.13) in the MD-AD group (one-way ANOVA,
Five odors (leather, lemon, licorice, apple, and pineapple) were excluded from further analysis because of poor identification (<70% correct responses) in the NC group.
The identification scores for the remaining odors are presented in Table 3.
Nine of the remaining 11 odors (all except anis and turpentine) had significantly worse identification scores in the MD-AD group than in the NC group (Fisher’s exact test,
Four odors that had the greatest differences in identification scores between the MD-AD and NC groups were selected (Fisher’s exact test, P<0.001). The identification score for these 4 odors (clove, garlic, cinnamon, and banana) was calculated. ROC curve analysis was performed to evaluate the performance of the 4-odor identification score in differentiating the NC participants from patients with AD (MCI-AD or MD-AD), MCI-AD, and MD-AD and the patients with MCI-AD from those with MD-AD. The ROC curves with the area under the curve (AUC) are shown in Figure 1.
A cut-off score of ≤3 for detecting AD was chosen. Using this cut-off score for differentiating patients with AD (MCI-AD or MD-AD) from NC participants, the 4-odor identification score had a sensitivity and specificity of 91.67% (95% confidence interval [CI]: 81.61–97.24%) and 76.67% (95% CI: 57.72–90.07%), respectively. The negative and positive predictive values were 82.14% (95% CI: 66.01–91.59%) and 88.71% (95% CI: 80.35–93.79%), respectively. The overall diagnostic accuracy was 86.67% (95% CI: 77.87–92.92%).
The diagnostic characteristics remained good when differentiating patients with MCI-AD from NC participants. Using the same cut-off score of ≤3, the 4-odor identification score had a sensitivity and specificity of 86.67% (95% CI: 69.28–96.24%) and 76.67% (95% CI: 57.72–90.07%), respectively. The negative and positive predictive values were 85.19% (95% CI: 69.33–93.60%) and 78.79% (95% CI: 65.67–87.82%), respectively. The overall diagnostic accuracy was 81.67% (95% CI: 69.56–90.48%).
A multiple linear regression model with age, sex, education, duration of symptoms, ADAS-Cog score, and composite verbal fluency test score (fluency PAS + fluency animals) as independent variables was tested to determine whether these variables significantly predicted the 4-odor identification score.
The overall regression was statistically significant (R2=0.471, F=14.205,
ODOR DISCRIMINATION:
Overall odor discrimination scores differed significantly among the 3 groups. Median [interquartile range] scores were 12.5 [11–14] in the NC group, 9 [7–10] in the MCI-AD group, and 6 [5–7.25] in the MD-AD group (Kruskal-Wallis test, P<0.05; post hoc analysis revealed significant differences between all 3 groups).
Five triplets were excluded from further analysis because of poor identification (<70% of correct responses) in the NC group. The discrimination scores of the remaining odors are presented in Table 4.
Odor discrimination scores in 9 of the remaining 11 triplets were significantly worse in the MD-AD group than in the NC group (Fisher’s exact test,
The 3 triplets that differed the most between the MCI-AD and NC groups and the triplet with 2-phenylethanol as the target odor and isoamyl acetate as the non-target odor that differed significantly between the MCI-AD and MD-AD groups were selected for the 4-odor discrimination score. ROC curve analysis was performed to evaluate the performance of the 4-odor discrimination score in differentiating the NC participants from patients with AD (MCI-AD or MD-AD), MCI-AD, and MD-AD and the patients with MCI-AD from those with MD-AD. The ROC curves with AUC are shown in Figure 2.
A cut-off score of ≤3 for detecting AD was chosen. Using this cut-off score for differentiating patients with AD (MCI-AD or MD-AD) from NC participants, the 4-odor discrimination score had a sensitivity and specificity of 98.33% (95% CI: 91.06–99.96%) and 76.67% (95% CI: 57.72–90.07%), respectively. The negative and positive predictive values were 95.83% (95% CI: 76.53–99.39%) and 89.39% (95% CI: 81.49–94.16%), respectively. The overall diagnostic accuracy was 91.11% (95% CI: 83.23–96.08%).
The diagnostic characteristics remained good when differentiating patients with MCI-AD from NC participants. Using the same cut-off score of ≤3, the 4-odor discrimination score had a sensitivity and specificity of 100% (95% CI: 88.43–100%) and 76.67% (95% CI: 57.72–90.07%), respectively. The negative and positive predictive values were 100% and 81.08% (95% CI: 69.14–89.13%), respectively. The overall diagnostic accuracy was 88.33% (95% CI: 77.43–95.18%).
A multiple linear regression model with age, sex, education, duration of symptoms, ADAS-Cog score, and composite verbal fluency test score (fluency PAS + fluency animals) as independent variables was used to determine whether these variables significantly predicted the 4-odor discrimination score.
The overall regression was statistically significant (R2=0.552, F=17.056,
Discussion
In this study, odor identification and odor discrimination were impaired in patients with prodromal AD (MCI due to AD). This impairment was even more pronounced in patients with MD due to AD. These findings are in accordance with previous research in which olfactory dysfunction was demonstrated in patients with early-stage AD [6–9,23]. In previous studies, the changes were also found to be already present in MCI due to AD and further worsen in the dementia stage [24].
The duration of AD symptoms was the only significant predictor of odor identification and odor discrimination scores. This result confirms that olfactory dysfunction in AD is associated with the disease processes and cannot be explained by cognitive deficits that are observed in patients with AD or by other factors that influence olfaction in the general population, such as age and sex [6,7].
The identification scores of clove, garlic, cinnamon, and banana odors differed the most between the patients with AD and healthy participants in the present study. Although the results of previous studies are not completely uniform, they also indicate that clove [10,17], banana [25,26], and garlic [26–28] are among the most sensitive odors for testing patients with AD. However, cinnamon was not found to be sensitive for detecting olfactory dysfunction in patients with AD in previous studies. Furthermore, it was not included in the sets of 10 odors that were determined to be the most suitable for testing patients with AD [10,17].
In previous studies, identification of the rose odor was most consistently found to be impaired in patients with AD [10,18,27–30]. In our study, the identification of the rose odor was also significantly impaired in the MD-AD group; however, it was not among the odors that differed the most between MD-AD and NC groups. Thus, it was not included in the shortened version of the odor identification score.
The shortened odor identification score, consisting of 4 odors (clove, garlic, cinnamon, and banana), had good diagnostic qualities for detecting AD and even MCI due to AD in the present study. Previous studies had determined that the 3-item Pocket Smell Test had acceptable, albeit slightly worse, diagnostic accuracy [15,31–33]. However, in previous studies, standard tests containing lemon/lilac/smoke or apple/gas/rose odorants were used, and these combinations were not chosen specifically for patients with AD. Considering these results of previous studies, our findings indicate that short versions (3–4 items) of the odor identification test have good diagnostic properties and could be useful in diagnosing early-stage AD, particularly if specific items that are the most sensitive for predicting AD are chosen.
The short versions of olfactory tests could improve their applicability in clinical practice because they are less time consuming. In the present study, the Sniffin’ Sticks test was used and proved to have good diagnostic qualities. Gathering data on different olfactory tests is crucial because the cost of the most commonly used tests could be a factor limiting their wider application. The UPSIT and all other tests derived from it are single-use “scratch-and-sniff” tests that must be purchased separately for each patient [11,12]. The Sniffin’ Sticks test was designed to be used repetitively over a period of several months [13].
Odor discrimination demonstrated a different pattern of impairment than odor identification. In the case of odor identification, the identification scores for most odors were significantly worse in patients with MD-AD than in the healthy participants. However, the identification scores of only 3 odors were significantly worse in patients with MCI-AD than in the healthy participants. In contrast, the scores of the odor discrimination task for most odors were significantly worse in patients with MCI-AD than in the healthy participants, indicating pronounced impairment in odor discrimination during the early stage of the disease. Accordingly, the shortened version of the odor discrimination task had better diagnostic qualities for prodromal AD than the shortened version of odor identification task. Early changes in the performance of the odor discrimination task might be related to the different nature of the tests. Both odor identification and discrimination reflect higher processing of odors; however, olfactory short-term memory is involved to a greater extent in odor discrimination tasks [34].
Additionally, both olfactory tasks had poor abilities to differentiate between the different stages of AD (MCI-MD vs MD-AD; ROC AUC=0.698 for the 4-odor identification score and ROC AUC=0.633 for the 4-odor discrimination score). Therefore, since changes in olfactory abilities appear early in the disease course, olfactory testing may be extremely helpful for diagnosing early-stage AD and less reliable for monitoring disease progression. Our findings were similar to those of previous studies, in which odor identification was determined to have better qualities for differentiating healthy participants from patients with AD than differentiating between patients with different stages of AD [35].
The present study had several limitations. First, although the study results were significant, the sample size was rather small. Thus, the results should be confirmed in studies with larger sample sizes. Second, the cross-sectional design of the study limits the accuracy of the conclusions with respect to the longitudinal changes during the disease course. Finally, cerebrospinal fluid and positron emission tomography biomarkers were not tested in this study. The use of these factors could help exclude the possibility of other neurodegenerative conditions and analyze the relationship between olfactory changes, amyloid beta deposition in the brain, and neuronal degeneration.
Conclusions
In conclusion, the present study confirmed that odor identification and discrimination are impaired in the prodromal stage of AD and that these changes progress during the course of AD. Furthermore, we demonstrated that the brief versions of olfactory tests, containing selected items that were found to differ the most between cognitively normal participants and early-stage AD patients, have good diagnostic qualities and can aid clinicians in screening for early-stage AD, facilitating the accurate and timely identification of patients requiring further assessment and treatment.
Figures
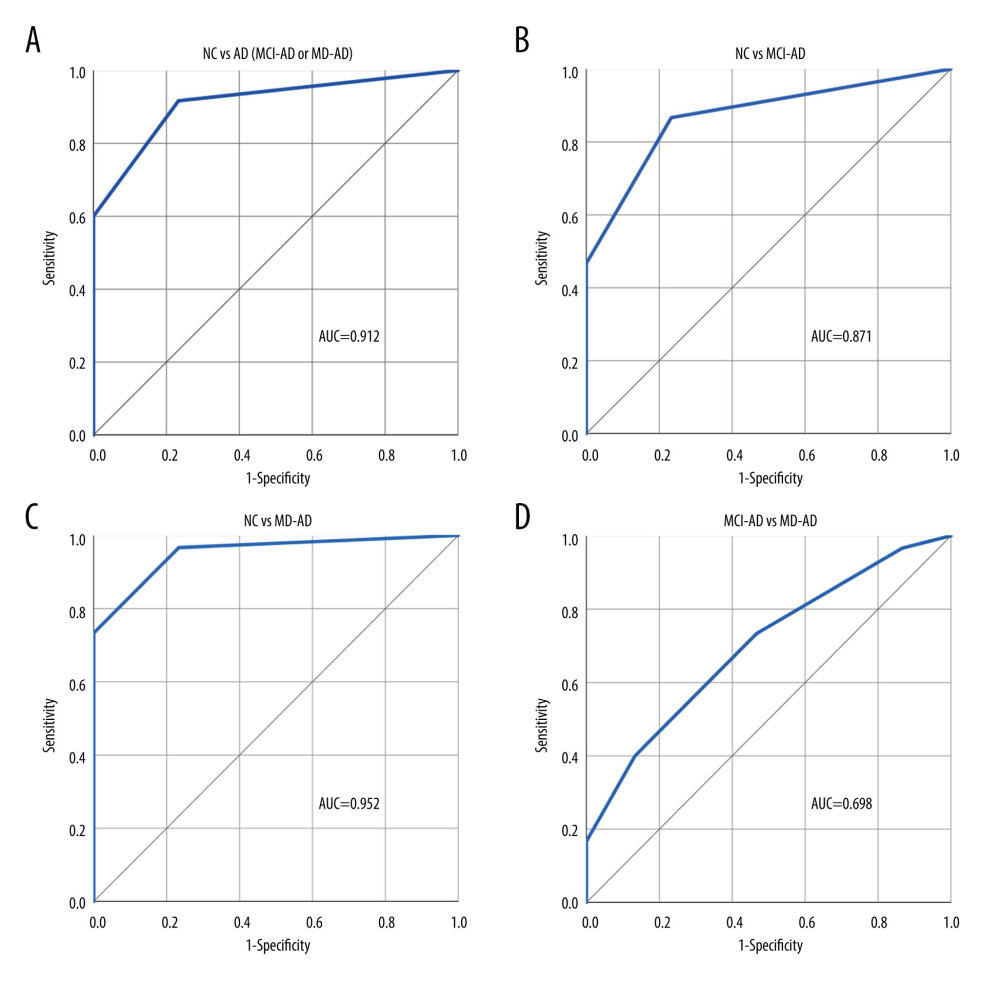 Figure 1. (A–D) Performance of the 4-odor identification score in differentiating participants of the various groups. NC – normal cognition; AD – Alzheimer disease; MCI-AD – mild cognitive impairment due to Alzheimer disease; MD-AD – mild dementia due to Alzheimer disease; AUC – receiver operating characteristic (ROC) area under the curve. Created using IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.
Figure 1. (A–D) Performance of the 4-odor identification score in differentiating participants of the various groups. NC – normal cognition; AD – Alzheimer disease; MCI-AD – mild cognitive impairment due to Alzheimer disease; MD-AD – mild dementia due to Alzheimer disease; AUC – receiver operating characteristic (ROC) area under the curve. Created using IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp. 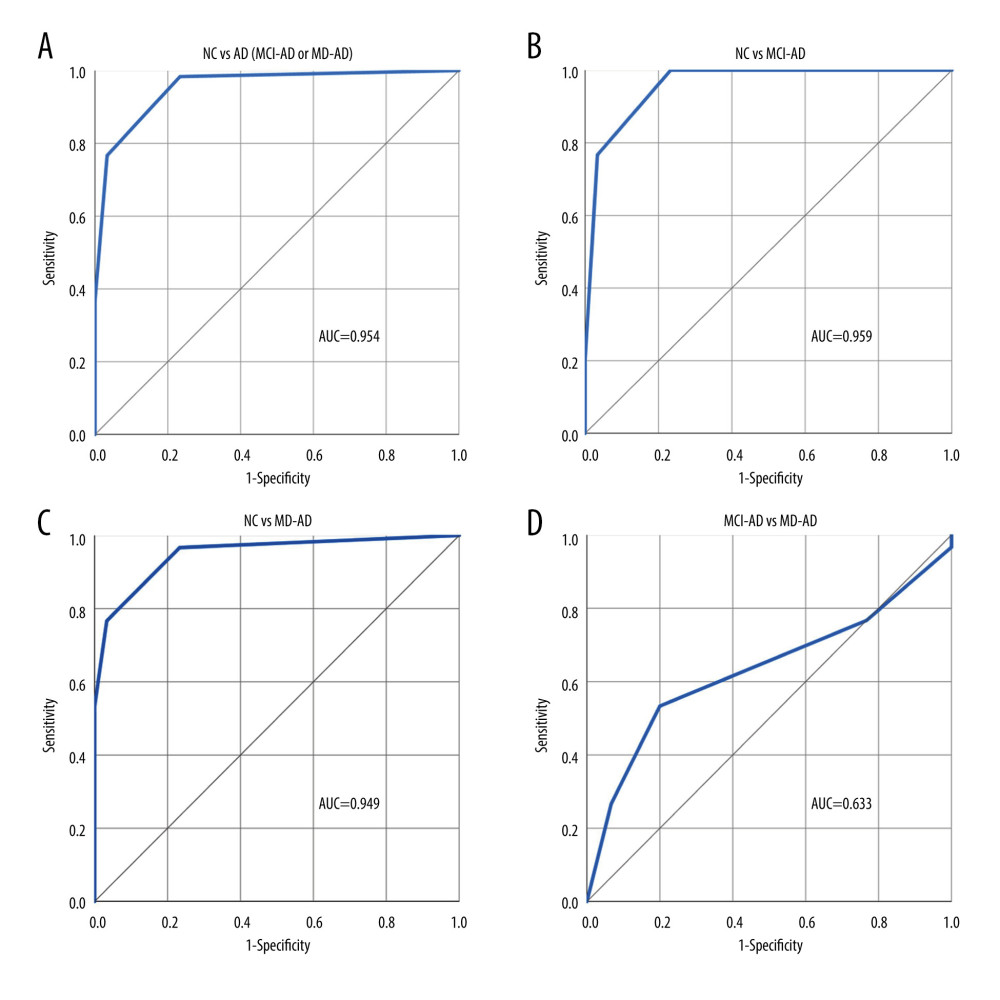 Figure 2. (A–D) Performance of the 4-odor discrimination score in differentiating participants of the various groups. NC – normal cognition; AD – Alzheimer disease; MCI-AD – mild cognitive impairment due to Alzheimer disease; MD-AD – mild dementia due to Alzheimer disease; AUC – receiver operating characteristic (ROC) area under the curve. Created using IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.
Figure 2. (A–D) Performance of the 4-odor discrimination score in differentiating participants of the various groups. NC – normal cognition; AD – Alzheimer disease; MCI-AD – mild cognitive impairment due to Alzheimer disease; MD-AD – mild dementia due to Alzheimer disease; AUC – receiver operating characteristic (ROC) area under the curve. Created using IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp. References
1. Albert MS, DeKosky ST, Dickson D, The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease: Alzheimers Dement, 2011; 7(3); 270-79
2. McKhann GM, Knopman DS, Chertkow H, The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease: Alzheimers Dement, 2011; 7(3); 263-69
3. Park JE, Gunasekaran TI, Cho YH, Diagnostic blood biomarkers in Alzheimer’s disease: Biomedicines, 2022; 10(1); 169
4. , 2022 Alzheimer’s disease facts and figures: Alzheimers Dement, 2022; 18(4); 700-89
5. Gauthier S, Rosa-Neto P, Morais JA, Webster C: World Alzheimer Report 2021: Journey through the diagnosis of dementia, 2021, London, England, Alzheimer’s Disease International
6. Jobin B, Zahal R, Bussières EL, Olfactory identification in subjective cognitive decline: A meta-analysis: J Alzheimers Dis, 2021; 79(4); 1497-507
7. Wang Q, Chen B, Zhong X, Olfactory dysfunction is already present with subjective cognitive decline and deepens with disease severity in the Alzheimer’s disease spectrum: J Alzheimers Dis, 2021; 79(2); 585-95
8. Jung HJ, Shin IS, Lee JE, Olfactory function in mild cognitive impairment and Alzheimer’s disease: A meta-analysis: Laryngoscope, 2019; 129(2); 362-69
9. Marine N, Boriana A, Olfactory markers of depression and Alzheimer’s disease: Neurosci Biobehav Rev, 2014; 45; 262-70
10. Woodward MR, Amrutkar CV, Shah HC, Validation of olfactory deficit as a biomarker of Alzheimer disease: Neurol Clin Pract, 2017; 7(1); 5-14
11. Doty RL, Psychophysical testing of smell and taste function: Handb Clin Neurol, 2019; 164; 229-46
12. Doty RL, Shaman P, Dann M, Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function: Physiol Behav, 1984; 32(3); 489-502
13. Kobal G, Hummel T, Sekinger B, “Sniffin’ sticks”: Screening of olfactory performance: Rhinology, 1996; 34(4); 222-26
14. Joseph T, Auger SD, Peress L, Screening performance of abbreviated versions of the UPSIT smell test: J Neurol, 2019; 266(8); 1897-906
15. Duff K, McCaffrey RJ, Solomon GS, The Pocket Smell Test: Successfully discriminating probable Alzheimer’s dementia from vascular dementia and major depression: J Neuropsychiatry Clin Neurosci, 2002; 14(2); 197-201
16. Kjelvik G, Sando SB, Aasly J, Use of the Brief Smell Identification Test for olfactory deficit in a Norwegian population with Alzheimer’s disease: Int J Geriatr Psychiatry, 2007; 22(10); 1020-24
17. Tabert MH, Liu X, Doty RL, A 10-item smell identification scale related to risk for Alzheimer’s disease: Ann Neurol, 2005; 58(1); 155-60
18. Woodward MR, Hafeez MU, Qi Q, Odorant item specific olfactory identification deficit may differentiate Alzheimer disease from aging: Am J Geriatr Psychiatry, 2018; 26(8); 835-46
19. Mueller C, Renner B, A new procedure for the short screening of olfactory function using five items from the “Sniffin’ Sticks” identification test kit: Am J Rhinol, 2006; 20(1); 113-16
20. , Demencijų diagnostiniai kriterijai ir vertinimo skalės: Neurologijos seminarai, 2000; 2(10); 18-38 [in Lithuanian]
21. Hughes CP, Berg L, Danziger WL, A new clinical scale for the staging of dementia: Br J Psychiatry, 1982; 140; 566-72
22. Rumeau C, Nguyen DT, Jankowski R, How to assess olfactory performance with the Sniffin’ Sticks test(®): Eur Ann Otorhinolaryngol Head Neck Dis, 2016; 133(3); 203-6
23. Kim SM, Kim HR, Min HJ, A novel olfactory threshold test for screening cognitive decline among elderly people: PLoS One, 2021; 16(7); e0254357
24. Yu Q, Guo P, Li D, Olfactory dysfunction and its relationship with clinical symptoms of Alzheimer disease: Aging Dis, 2018; 9(6); 1084-95
25. Velayudhan L, Gasper A, Pritchard M, Pattern of smell identification impairment in Alzheimer’s disease: J Alzheimers Dis, 2015; 46(2); 381-87
26. Zendehbad AS, Noroozian M, Shakiba A, Validation of Iranian Smell Identification Test for screening of mild cognitive impairment and Alzheimer’s disease: Appl Neuropsychol Adult, 2022; 29(1); 77-82
27. Yoshitake M, Maeshima E, Maeshima S, Olfactory identification ability in patients with mild cognitive impairment and Alzheimer’s disease: J Phys Ther Sci, 2022; 34(11); 710-14
28. Jimbo D, Inoue M, Taniguchi M, Urakami K, Specific feature of olfactory dysfunction with Alzheimer’s disease inspected by the Odor Stick Identification Test: Psychogeriatrics, 2011; 11(4); 196-204
29. Umeda-Kameyama Y, Ishii S, Kameyama M, Heterogeneity of odorant identification impairment in patients with Alzheimer’s Disease: Sci Rep, 2017; 7(1); 4798
30. Hori Y, Matsuda O, Ichikawa S, Olfactory function in elderly people and patients with Alzheimer’s disease: Psychogeriatrics, 2015; 15(3); 179-85
31. Makowska I, Kloszewska I, Grabowska A, Olfactory deficits in normal aging and Alzheimer’s disease in the polish elderly population: Arch Clin Neuropsychol, 2011; 26(3); 270-79
32. Solomon GS, Petrie WM, Hart JR, Brackin HB, Olfactory dysfunction discriminates Alzheimer’s dementia from major depression: J Neuropsychiatry Clin Neurosci, 1998; 10(1); 64-67
33. McCaffrey RJ, Duff K, Solomon GS, Olfactory dysfunction discriminates probable Alzheimer’s dementia from major depression: A cross-validation and extension: J Neuropsychiatry Clin Neurosci, 2000; 12(1); 29-33
34. Zucco GM, Hummel T, Tomaiuolo F, Stevenson RJ, The influence of short-term memory on standard discrimination and cued identification olfactory tasks: J Neurosci Methods, 2014; 222; 138-41
35. Quarmley M, Moberg PJ, Mechanic-Hamilton D, Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease: J Alzheimers Dis, 2017; 55(4); 1497-507
Figures
 Figure 1. (A–D) Performance of the 4-odor identification score in differentiating participants of the various groups. NC – normal cognition; AD – Alzheimer disease; MCI-AD – mild cognitive impairment due to Alzheimer disease; MD-AD – mild dementia due to Alzheimer disease; AUC – receiver operating characteristic (ROC) area under the curve. Created using IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.
Figure 1. (A–D) Performance of the 4-odor identification score in differentiating participants of the various groups. NC – normal cognition; AD – Alzheimer disease; MCI-AD – mild cognitive impairment due to Alzheimer disease; MD-AD – mild dementia due to Alzheimer disease; AUC – receiver operating characteristic (ROC) area under the curve. Created using IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp. Figure 2. (A–D) Performance of the 4-odor discrimination score in differentiating participants of the various groups. NC – normal cognition; AD – Alzheimer disease; MCI-AD – mild cognitive impairment due to Alzheimer disease; MD-AD – mild dementia due to Alzheimer disease; AUC – receiver operating characteristic (ROC) area under the curve. Created using IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.
Figure 2. (A–D) Performance of the 4-odor discrimination score in differentiating participants of the various groups. NC – normal cognition; AD – Alzheimer disease; MCI-AD – mild cognitive impairment due to Alzheimer disease; MD-AD – mild dementia due to Alzheimer disease; AUC – receiver operating characteristic (ROC) area under the curve. Created using IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp. Tables
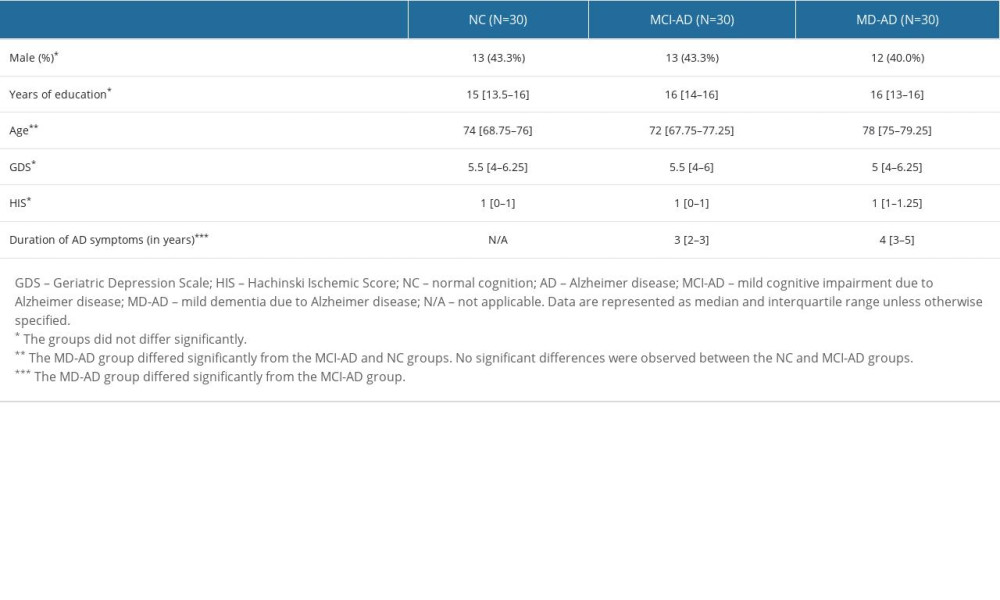 Table 1. Demographic and clinical characteristics of the participants.
Table 1. Demographic and clinical characteristics of the participants.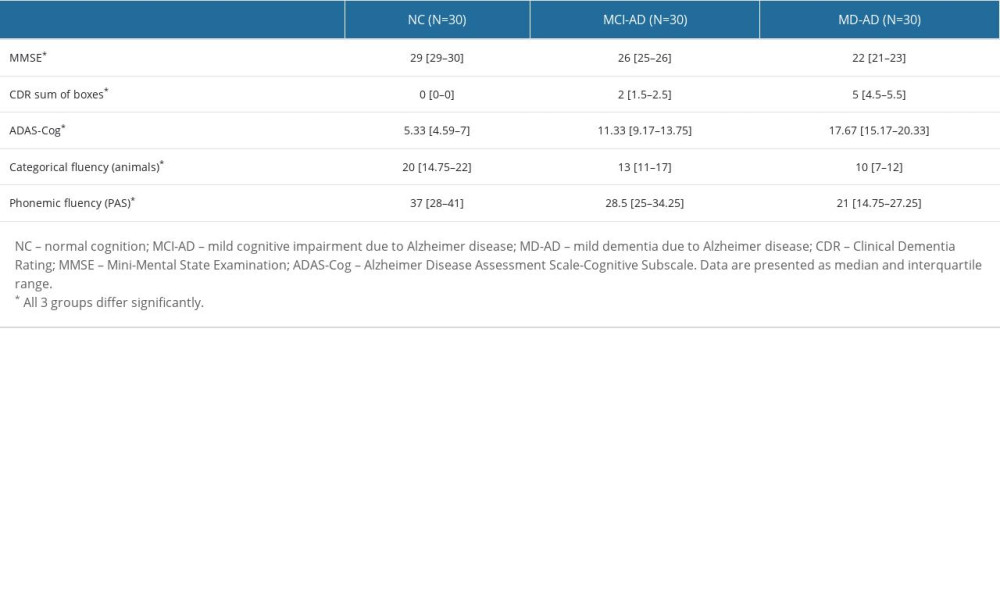 Table 2. Results of the cognitive assessments.
Table 2. Results of the cognitive assessments.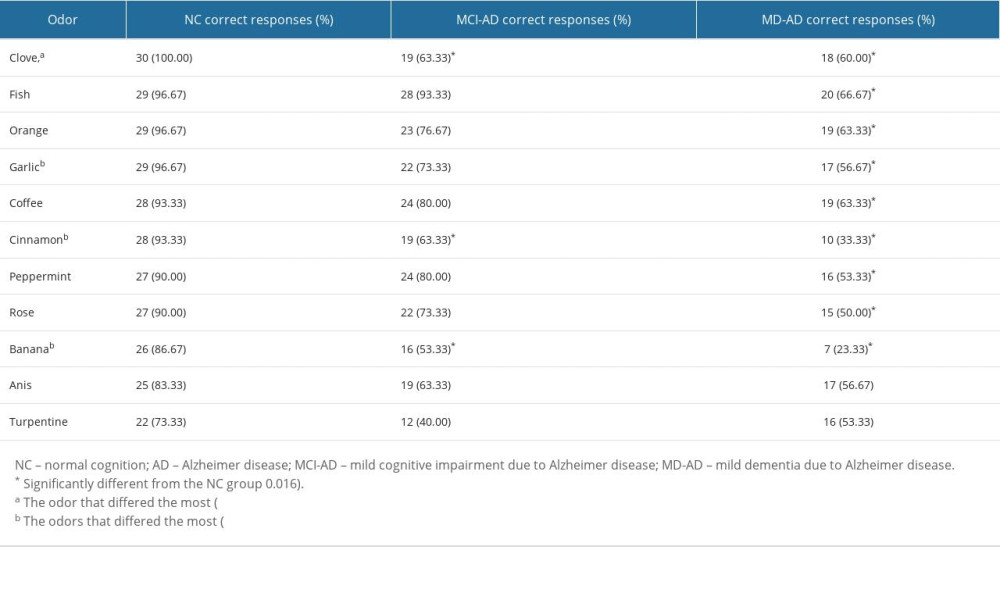 Table 3. Results of the odor identification test.
Table 3. Results of the odor identification test.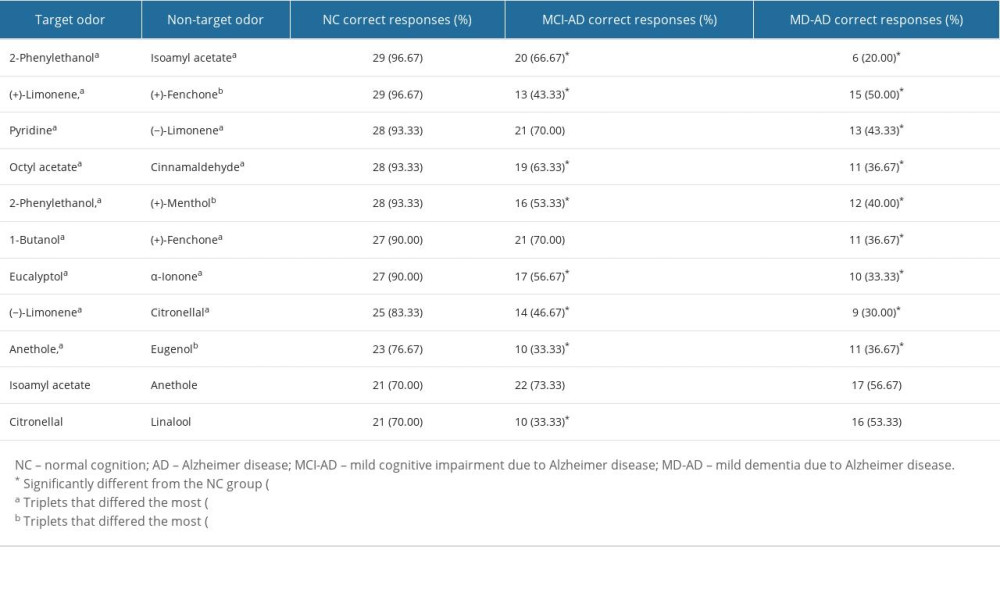 Table 4. Results of the odor discrimination test.
Table 4. Results of the odor discrimination test. Table 1. Demographic and clinical characteristics of the participants.
Table 1. Demographic and clinical characteristics of the participants. Table 2. Results of the cognitive assessments.
Table 2. Results of the cognitive assessments. Table 3. Results of the odor identification test.
Table 3. Results of the odor identification test. Table 4. Results of the odor discrimination test.
Table 4. Results of the odor discrimination test. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








