29 May 2023: Clinical Research
Pathomorphological Changes in Mammary Glands of 16 Patients Who Underwent Surgical Aquafilling Removal: A Single-Center Study
Michał ChalcarzDOI: 10.12659/MSM.940592
Med Sci Monit 2023; 29:e940592
Abstract
BACKGROUND: Aquafilling® is a soft-tissue filler used in various procedures, including breast plastic surgery. Proponents claim it to be safe and effective without causing serious adverse effects. This study aimed to describe histological changes in breast tissue resulting from potentially harmful effects of Aquafilling®.
MATERIAL AND METHODS: Tissue samples were collected from 16 patients who underwent surgical removal of Aquafilling®. Histopathological evaluations were performed on hematoxylin and eosin-stained slides, with photographs captured using an Olympus BX 43 light microscope and an XC 30 digital camera at 40×, 100×, and 400× total magnification.
RESULTS: Inflammatory infiltrates, mainly consisting of macrophages and lymphocytes, were observed in the images. Tissue necrosis was visible in some areas. Fibrosis foci and blood vessels with thickened walls and detached endothelium were identified within mammary adipose tissue.
CONCLUSIONS: Due to the variety of clinical symptoms and presence of inflammation in all examined women, we recommend histopathological examinations for all cases of Aquafilling® surgical removal. The examination should include information on the extent of inflammation, progression of adipose and muscle tissue damage, and assessment of fibrosis severity. This will help clinicians make informed decisions about Aquafilling® use in patients and improve patient outcomes.
Keywords: Breast, Fibrosis, Histology, Surgery, Plastic, Humans, Female, Mammary Glands, Human, Mammaplasty, Inflammation
Background
Aquafilling® (BIOTRH s.r.o., Prague, Czechia) is a soft-tissue filler used in plastic surgery of the face, breasts, and buttocks [1–3]. According to the manufacturer, it consists of 98% saline and 2% polyamide [1–3]. However, there are some reports on the development of health complications as a result of using this filler, with the description of patient symptoms [1,4–6]. These include mastalgia, breast deformation, filler migration, lumps, tenderness, abscess, fistula, and inflammation of mammary glands, even if no visible symptoms or ailments are observed [1,2,7]. However, medical information, as well as advertising messages, pointed out that the main ingredient making up as much as 98% of the formulation is saline, an ingredient that is safe for humans [1–3]. Much less was said about the remaining 2%, which was polyamide, specifically polyacrylamide [1–3]. Unfortunately, these theses were not based on long-term observations [1–7]. Another preparation, “Los Deline,” with the same composition, action, and purpose, is still used in some countries [6]. From the point of view of public health, it is necessary to publicize the adverse effects of polyamide fillers [6].
Aquafilling has been used in the European Union, Malaysia, South Korea, Serbia, and Turkey [6]. As is the case with other injectable fillers, it has not been approved by the US Food and Drug Administration (FDA) for use in breast augmentation [8]. In recent years, reports have been published on the adverse effects of Aquafilling® on the health of patients in South Korea [6,9] and in Turkey [10]. Fortunately, this preparation is, in practice, no longer available in most of the countries where it has been used [6,9,10].
It is therefore concerning that Aquafilling® is still being claimed by some to be safe and provide great improvements in breast shape and volume [1–3].
In the literature on the removal of Aquafilling® from the breast, researchers have primarily focused on surgical treatment methods, and little attention has been paid to the histological changes in these patients and the possible long-term health consequences [1–3].
The available literature on the adverse effects of Aquafilling® lacks any comprehensive histopathological evaluation reports [1–10]. The purpose of this study was to describe all histological changes in the breast resulting from the harmful effects of Aquafilling on the tissues.
Material and Methods
ETHICS:
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Bioethics Committee of the Medical University of Karol Marcinkowski in Poznan, Poland (protocol code no. 682/21 from 6 October 2021). Written informed consent was obtained from all the subjects involved in the study.
POPULATION:
Out of 17 samples, 16 were included in the current analysis. One participant was excluded from the study because she withdrew her consent to participate.
The study samples consisted of tissue sections from 16 patients who underwent Aquafilling® surgical removal. Data on the patient’s age, the interval between the injection of Aquafilling and its surgical removal, and the volume of Aquafilling® injected into the left and right breasts are summarized in Table 1. Clinical data and the age of the patients studied are presented in Table 2, and inclusion and exclusion criteria are presented in Table 3.
MAGNETIC RESONANCE IMAGING:
In total, 12 out of 16 patients underwent breast MR before surgery to assess the extent of Aquafilling® deposits within the breast and chest wall structures and to exclude the presence of suspicious lesions. All examinations were performed using a 1.5 T GE SIGNA™ Creator MRI scanner with the 8-channel breast coil (1.5 T HD Breast Array, GE Healthcare Coils). The breast MR imaging protocol used is consistent with the European Society of Breast Imaging (EUSOBI) guidelines [11] and was enhanced with 2 additional sagittal T2 and IR images of each breast to better visualize the localization of Aquafilling® deposits. During each breast MR examination, a gadolinium-containing contrast agent (Gadovist, Bayer AG) was intravenously administered using a power injector to allow analyzing the morphology and kinetics of enhancing lesions. All images were read by the breast radiologist and reported according to the ACR BI-RADS® Atlas [12].
SURGICAL REMOVAL OF AQUAFILLING:
The procedure of removing Aquafilling® from the breast was performed under general anesthesia with an incision in the inframammary fold or around the nipples from which the breast was opened (Figure 1). It consisted of cleaning the individual anatomical structures of the nipple, major pectoral muscle, and chest wall to remove Aquafilling® and the inflamed tissue fragments under visual control. In the case of Aquafilling® between the layers of muscle fibers of the major pectoral muscle, the preparation was removed through delamination of the muscle fibers.
Immediately after the appearance of Aquafilling®, a swab was taken for bacteriological culture. The altered tissue fragments were collected for histopathological examination.
MORPHOLOGICAL, BIOCHEMICAL PARAMETERS, AND MICROBIOLOGICAL EXAMINATION (BEFORE OPERATION):
Morphological parameters – leucocyte, erythrocyte, monocyte counts, and hemoglobin levels – were assessed in all patients.
Biochemical parameters – C-reactive protein (CRP), urea, creatinine level, and activated partial thromboplastin time (APTT) international normalized ratio (INR) – were assessed in all patients. During the operation, a bacteriological swab was taken for culture.
HISTOLOGICAL EXAMINATION:
The collected tissue material was dehydrated by placing it in ethanol solutions of increasing concentration (dehydration series: alcohol 60%, 70%, 80%, 99.8%) and finally in xylene. The next step was embedding the examined tissue material in paraffin, first placing the tissue in a mixture of paraffin and xylene, and then in liquid paraffin (at 52°C, 18 h). Then, they were embedded inside a paraffin block, which was prepared by pouring a portion of paraffin into a metal mold and inserting a piece of tissue. The paraffin blocks were sectioned using a microtome with a section thickness of 3–4 μm. The paraffin section was placed on a glass slide. Subsequently, preparations with paraffin sections were dewaxed. The slides were placed in 2 changes of xylene, then hydrated in an alcohol series, composed similarly to the dehydration series of ethanol solutions, but in decreasing concentration, starting from absolute alcohol, up to 60% and water. They were then stained with hematoxylin and eosin (H&E) by placing slides in hematoxylin solution (4 min). Stained sections were destained in acidified alcohol (0.25 ml HCl per 100 ml 70% alcohol for 2–3 s). The preparations were rinsed under running tap water (10 min), then slides were stained in eosin solution (1 min). Slides were rinsed with distilled water. The preparations were dehydrated by passing them through the increasing concentration of ethyl alcohol to xylene. The stained and dehydrated tissue section was covered with a coverslip using Canadian balsam [10].
Photographs of histological slides were taken using an Olympus BX 43 light microscope and an XC 30 digital camera at 40×, 100×, and 400× total magnification.
STATISTICAL SAMPLE SIZE ANALYSIS:
Sample size calculations were made using a sampling calculator [13]. It was shown, assuming a confidence interval value of 95% and assuming that about 7000 women decided to change their bust size in Poland (exact statistics are not kept), that the recommended number of participants who should be included in the study is 17.
Results
:
The examined patients presented conditions such as mastalgia, breast deformity (Figure 2A), migration of Aquafilling® to other parts of the body, visible hypoechogenic nodules, breast tenderness, and inflammation of the mammary glands, sometimes accompanied by abscess formation (Figure 2B) and breast fistulas (Figure 3A, 3B).
Breast pain and deformity occurred in 3 patients. In 1 case, displacement of the filler to the anterior chest wall was observed. Two patients had pain in 1 breast during exercise. Pain in both breasts prior to the onset of each menstrual cycle was reported in 3 patients. Palpable nodules and tenderness, abscess, and fistula were observed in 3 patients. A persistent elevated body temperature of 37.2°C was reported in 1 patient. Breast pain and redness were noted in 2 patients.
However, what should be emphasized as new and unexpected is the fact that in 3 patients who did not report any symptoms or visible external symptoms, the histopathological examination showed the same changes as in patients with symptoms, although they were not as intense. The adverse effects of Aquafilling® were therefore present in tissues despite the lack of visible clinical symptoms.
EVALUATION OF THE MAGNETIC RESONANCE IMAGING:
None of the 12 patients presented suspicious lesions, and all lesions were reported as BI-RADS® category 2 (benign) based on images.
The MRI examination revealed the presence of Aquafilling® deposits located in the subcutaneous tissue, breast parenchyma, and chest wall (intramuscular and submuscular), as well as in the adipose tissue of the axilla (Figure 4).
One patient presented with Aquafilling® collection in the area surrounding both breast implants (Figure 5A, 5B).
MORPHOLOGICAL, BIOCHEMICAL PARAMETERS, AND MICROBIOLOGICAL EXAMINATION:
Before surgery, morphological and biochemical parameters were assessed in all patients and found to be within the reference ranges, and the results of microbiological tests were negative in all cases.
ANALYSIS OF THE HISTOPATHOLOGICAL BREAST TISSUE SAMPLES:
No neoplastic lesions were found in any of the breast tissue samples, but some had partially sclerotic fibrous connective tissue (Figure 6). Histological examination of a tissue section showed necrosis of adipose tissue cells, isolated macrophages, and visible fibrosis. Aquafilling® was present between the adipocytes. In addition, damage to the cell membranes of adipocytes was found in the form of Aquafilling® being integrated into the cell membranes (Figure 7A, 7B).
The tissue material also contained fragments of skeletal muscles, between which Aquafilling® was visible. Scar tissue was noted in the skeletal muscle area. In the vicinity of the skeletal muscles, there were blood vessels with an inflammatory infiltrate penetrating the muscle (Figure 8A).
In addition, fragments of the mammary gland with significant fibrosis and lymphocytic infiltration were visible in part of the examined tissue material (Figure 9). Alkaline, homogeneous Aquafilling® filler was found within the adipose, connective, and skeletal muscle tissue. On histological examination, Aquafilling® appeared as abundant masses or numerous but small foci. In some patients, it was found located within the muscle fibers, resulting in their dilation (Figure 8B).
Inflammatory granulation tissue with necrotic foci was observed in all patients. In the central part of the lesion, macrophages, multinucleated giant cells, and hemosiderin deposits were present. Extensive multinucleated giant cell infiltrates were also observed (Figure 10A). The area corresponding to Aquafilling® deposits also contained numerous macrophages (Figure 10B).
Moreover, the presence of many small blood vessels was noted, with inflammatory infiltrates often in their immediate vicinity (Figure 11A). Moreover, some vessels had thickened walls (Figure 11B) in which endothelial dissection was found in some cases. Extensive fibrosis with visible inflammatory infiltrates was also observed. Inflammation was found in these vessels. Endothelial edema and sometimes extravasation of erythrocytes were present.
The histological changes in the breasts resulting from the Aquafilling® filler reaction are summarized in Table 2.
Discussion
The clinical history of patients included symptoms such as mastalgia, palpable hypoechogenic nodules, breast tenderness, and mammary gland inflammation, sometimes accompanied by abscess formation or the presence of a fistula. Additionally, an MRI examination was performed before the removal of Aquafilling®. This enabled the exact location of the filler to be determined. MRI is also an effective initial differential diagnostic method, especially when the patient’s medical history is not precisely known. Knowledge of the radiological characteristics and migration patterns of gel fillers and related complications is useful for obtaining an accurate diagnosis [14]. In one of our patients, MRI showed the presence of Aquafilling® around silicone implants. Two years earlier, this patient underwent surgical Aquafilling® removal and simultaneous breast augmentation with silicone implants in another medical center. Kim et al, in a case report, presented a patient whose diagnosis included implant rupture in both breasts, with leakage of the injected material, which caused inflammation of the pericapsular area and the pectoral muscle. We did not find such a situation in our patient. The silicone implant showed no rupture, and the inflammatory reaction was due to the presence of Aquafilling® [6].
All patients showed chronic inflammatory changes on histopathological examination. The histological picture of the inflammation showed the presence of inflammatory infiltrates consisting mainly of macrophages and lymphocytes. In our first study on the harmful effects of Aquafilling® injected into the breasts of 4 patients, we found many immune system cells involved in the inflammatory process. Based on immunohistochemical studies, we found statistically significant changes in the surface area of the immunohistochemical reactions of the described cells. The largest surface area of the immunopositive reaction was noted for macrophages (CD68), followed by T lymphocytes (CD3) and B lymphocytes (CD20) [7].
In all patients, we observed the presence of inflammatory granulation with necrotic foci as well as extensive infiltrates from giant polynuclear cells. A similar microscopic image showed these were visible in the granulomatous lobulitis and perlobular region. Granulomas consist of epithelial cells, multinucleated giant cells, plasma cells, and lymphocytes, and necrotic foci of adipose tissue are rarely found in these cases [15,16]. Tissue necrosis was also visible in some locations. Within the adipose tissue, foci of fibrosis and the presence of blood vessels with thickened walls and a detached endothelium were observed and are morphological features indicative of chronic inflammation. The available literature does not include microscopic descriptions and does not provide comprehensive information on histopathological diagnosis [6]. A histological examination should be performed in patients who have had surgical removal of Aquafilling®, as in the case of idiopathic granulomatous mastitis, which should include an assessment of the distribution of lesions, the presence of granulomas and necrosis, and inflammatory cells (epithelial histiocytes, neutrophils, eosinophils, lymphocytes, and plasma cells), multinucleated giant cells, and atypical epithelial cells [17].
Mammary gland fibrosis, observed during the histological examination, may have future implications for normal lactation, which is especially concerning in patients of reproductive age. The presence of filler is a factor often associated with progressing fibrosis [18,19]. More than 300 proteins involved in controlling various cellular processes are expressed in this tissue. Mammary adipose tissue is also a reservoir of interstitial fluid that contains both locally secreted molecules and those assimilated from distal sites [20]. Some authors suggest that the use of Aquafilling® should be avoided in women planning childbirth and breastfeeding [5]. Based on the above data and our research results, however, we conclude that Aquafilling® should not be used at all. Furthermore, damage to the muscle fibers caused by Aquafilling® and chronic inflammation can be associated with a significant regeneration time. This process depends on various mechanisms that regulate cell–cell and cell–matrix interactions as well as extracellular secretory factors. In other studies on the same group of patients, we proved that the immunohistochemical assessment of the expression of adhesion molecules: N-cadherin and E-cadherin can be useful in assessing early changes in cellular connectivity [21].
Cellular changes during muscle recovery are multifactorial, with cells of the immune system, connective tissue, and blood vessels exhibiting distinct temporal and spatial kinetics after muscle damage. Mechanical muscle trauma at the site of Aquafilling® injection causes the formation of connective tissue, which impairs function and leads to muscle spasms and chronic pain [22,23].
Based on histological studies, breast inflammation caused by the injection of Aquafilling® can cause adverse health complications in the future. Inflammation causes damage to the adipose tissue, resulting in displacement of inflammatory contents within the breast. If left untreated, severe chronic inflammation can additionally cause fistulas by damaging not only the adipose, connective, and muscular tissue but also the skin, which can result in leakage of inflammatory content. A very similar clinical symptom is seen in idiopathic granulomatous mastitis, which is a rare form of chronic inflammation in the breast. Here, too, inflammation can lead to an abscess, fistula, or chronic suppuration [24].
A similar histological picture can also be described in the event of a spasm or the rupture of a silicone implant. Immunostimulation resulting from the impact of silicone molecules on tissues near the implant is the cause of chronic inflammation, which in turn induces the proliferation of fibroblasts. The physiological response to the presence of silicone implants or Aquafilling® filler is the proliferation of connective tissue as a response to the presence of a foreign body. Consequently, the formation of a thick, hard shell and local inflammation occurs. Aquafilling® filler, however, causes a more extensive inflammatory response than the shrinkage or rupture of a silicone implant. The extent of inflammation is the result of differences resulting not only from the greater immunogenicity of the Aquafilling® filler compared with silicone implants but also from the surgical procedure itself. Aquafilling® filler is administered to different places in the breast using a cannula, which could exacerbate inflammation [25].
Of course, our study has some limitations. Firstly, thus far, there is no histopathological classification of the inflammatory response caused by the Aquafilling® filler. However, we hope that diagnostic guidelines will be established based on tests carried out on a representative group of patients. Due to some similarities in the histopathological images, however, the consequent inflammation appears comparable to that resulting from granulomatous mastitis. Of course, other areas for improvement of the study include the relatively small number of cases, the lack of a control group, and that it was performed at a single center.
Conclusions
Due to the very different clinical symptoms and the presence of inflammation in all the examined patients, we suggest that a histopathological examination should be performed on patients in all cases where Aquafilling® has been surgically removed.
The histopathological examination should include information on both the extent of inflammation in the examined tissues and the progression of damage to adipose and muscle tissue, as well as assessment of the severity of fibrosis. This also applies to patients who have not reported any ailments or visible external symptoms.
It therefore seems right from the standpoint of public health and women’s safety to publicize the adverse effects and distant clinical consequences of the use of polyamide fillers, a component of Aquafilling®, which is used in breast augmentation procedures.
Figures
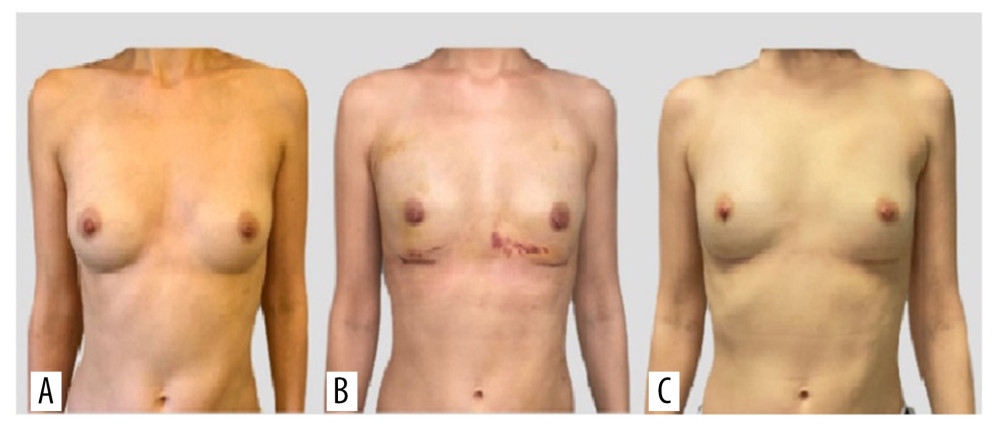 Figure 1. Photographs of the patient in the standing position: (A) preoperatively, (B) 1 week postoperatively, and (C) 6 weeks postoperatively.
Figure 1. Photographs of the patient in the standing position: (A) preoperatively, (B) 1 week postoperatively, and (C) 6 weeks postoperatively. 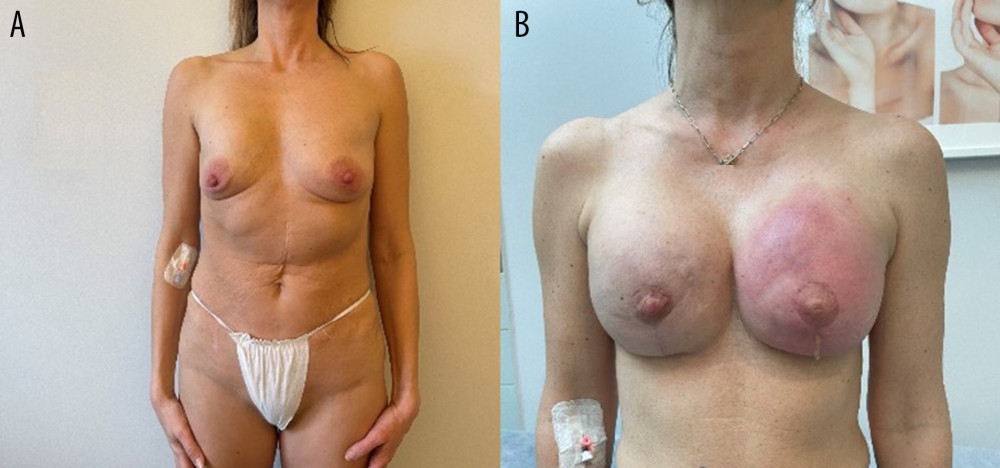 Figure 2. Images showing an example of (A) breast deformation and (B) breast inflammation.
Figure 2. Images showing an example of (A) breast deformation and (B) breast inflammation. 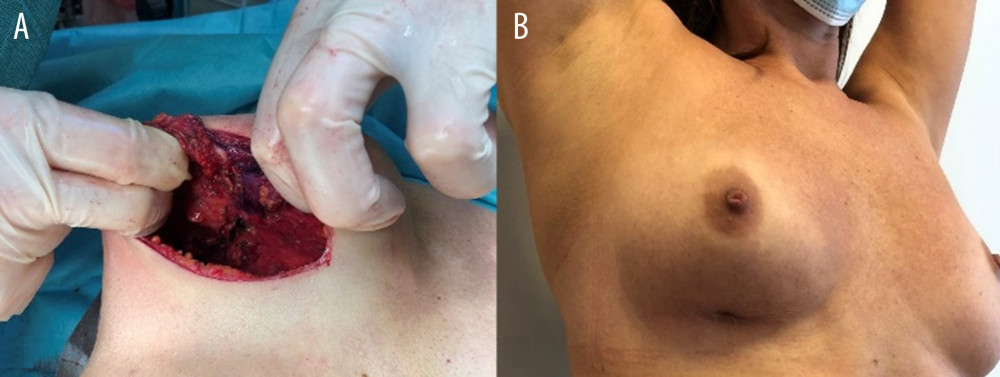 Figure 3. Images showing breast fistula (A) during and (B) before surgery.
Figure 3. Images showing breast fistula (A) during and (B) before surgery. 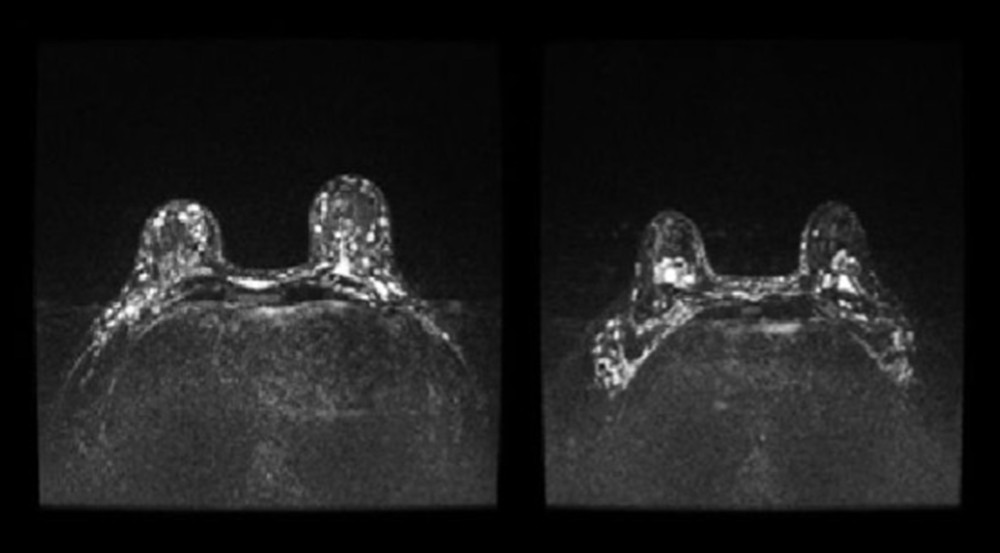 Figure 4. Patient 15, short-TI inversion recovery (STIR) axial images showing multiple Aquafilling deposits located in the subcutaneous tissue, breast parenchyma, inter-, intra-, and submuscular (both pectoral muscles), and the adipose tissue of the axilla.
Figure 4. Patient 15, short-TI inversion recovery (STIR) axial images showing multiple Aquafilling deposits located in the subcutaneous tissue, breast parenchyma, inter-, intra-, and submuscular (both pectoral muscles), and the adipose tissue of the axilla. 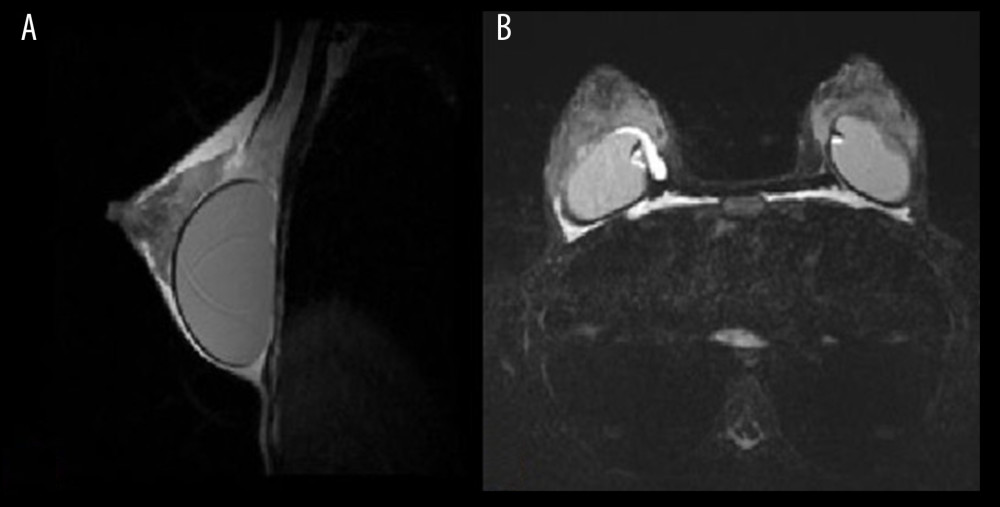 Figure 5. Patient 16. (A) T2 sagittal images, retro-pectoral silicone breast implant, Aquafilling® collection located inter- and intramuscularly (both pectoral muscles). (B) STIR axial images, collection of Aquafilling® located bilaterally subpectoral and surrounding the right breast implant.
Figure 5. Patient 16. (A) T2 sagittal images, retro-pectoral silicone breast implant, Aquafilling® collection located inter- and intramuscularly (both pectoral muscles). (B) STIR axial images, collection of Aquafilling® located bilaterally subpectoral and surrounding the right breast implant. 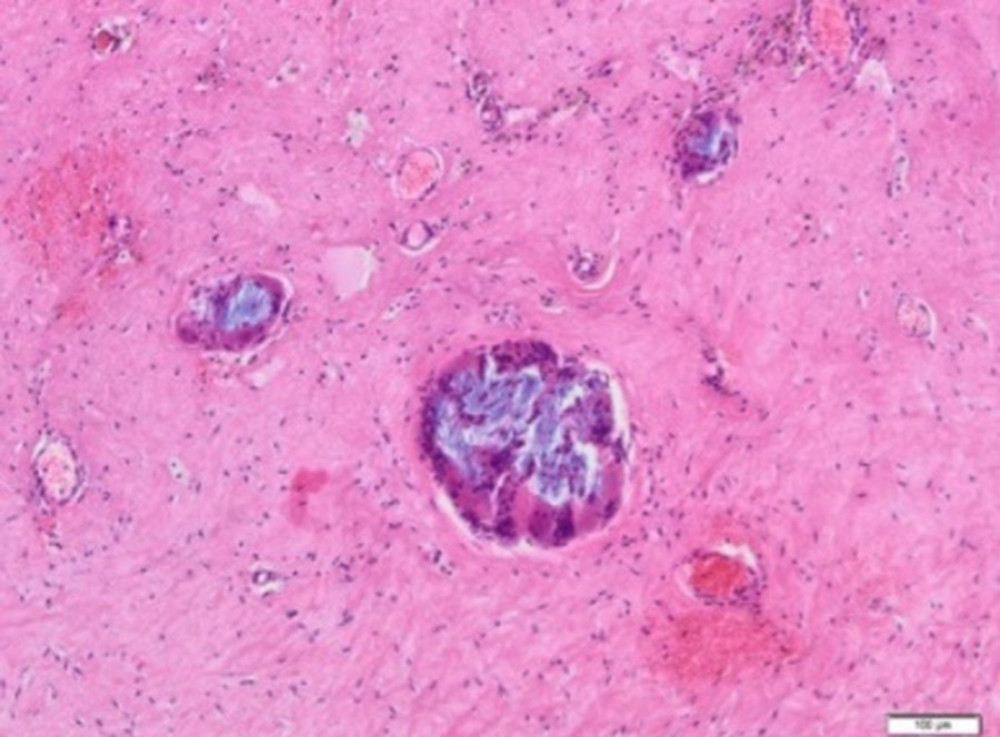 Figure 6. Aquafilling® deposits (blue) are present within the sclerotic connective tissue, with multinucleated giant cells inside (H&E, 100×).
Figure 6. Aquafilling® deposits (blue) are present within the sclerotic connective tissue, with multinucleated giant cells inside (H&E, 100×). 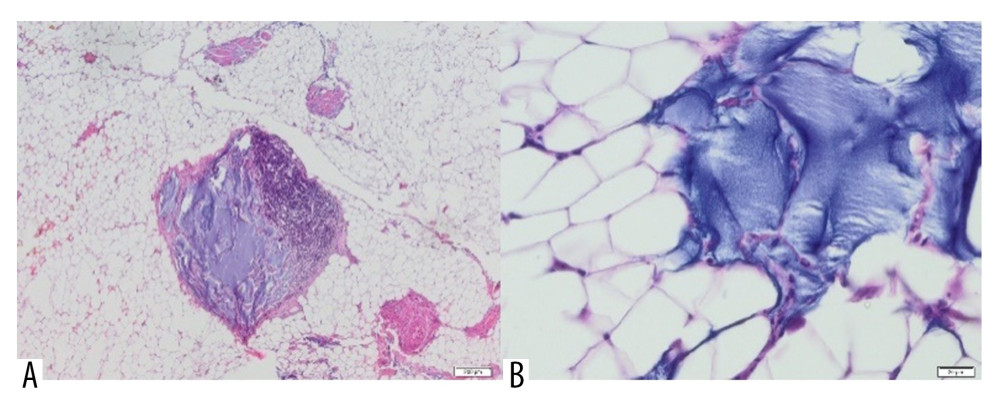 Figure 7. Adipose tissue containing Aquafilling® filler (blue) (A) with extensive inflammatory infiltrate in the area (H&E, 40×) and (B) dislodging cells of the tissue (H&E, 400×).
Figure 7. Adipose tissue containing Aquafilling® filler (blue) (A) with extensive inflammatory infiltrate in the area (H&E, 40×) and (B) dislodging cells of the tissue (H&E, 400×). 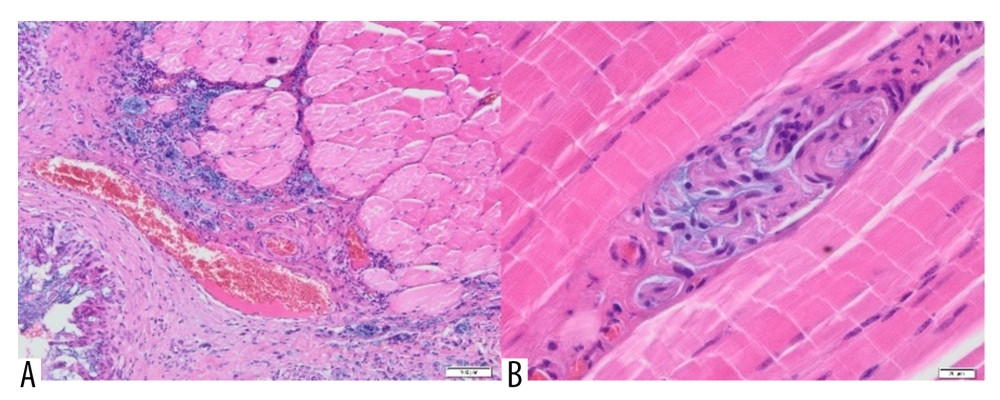 Figure 8. (A) Skeletal muscle with the presence of scar tissue, with a large blood vessel and a profuse inflammatory, infiltrate penetrating the muscle. Visible small foci containing Aquafilling® filler (blue) (H&E, 100×). (B) A small amount of Aquafilling® is visible within the skeletal muscle (blue). Lymphocytes are present within the filler (H&E, 400×).
Figure 8. (A) Skeletal muscle with the presence of scar tissue, with a large blood vessel and a profuse inflammatory, infiltrate penetrating the muscle. Visible small foci containing Aquafilling® filler (blue) (H&E, 100×). (B) A small amount of Aquafilling® is visible within the skeletal muscle (blue). Lymphocytes are present within the filler (H&E, 400×). 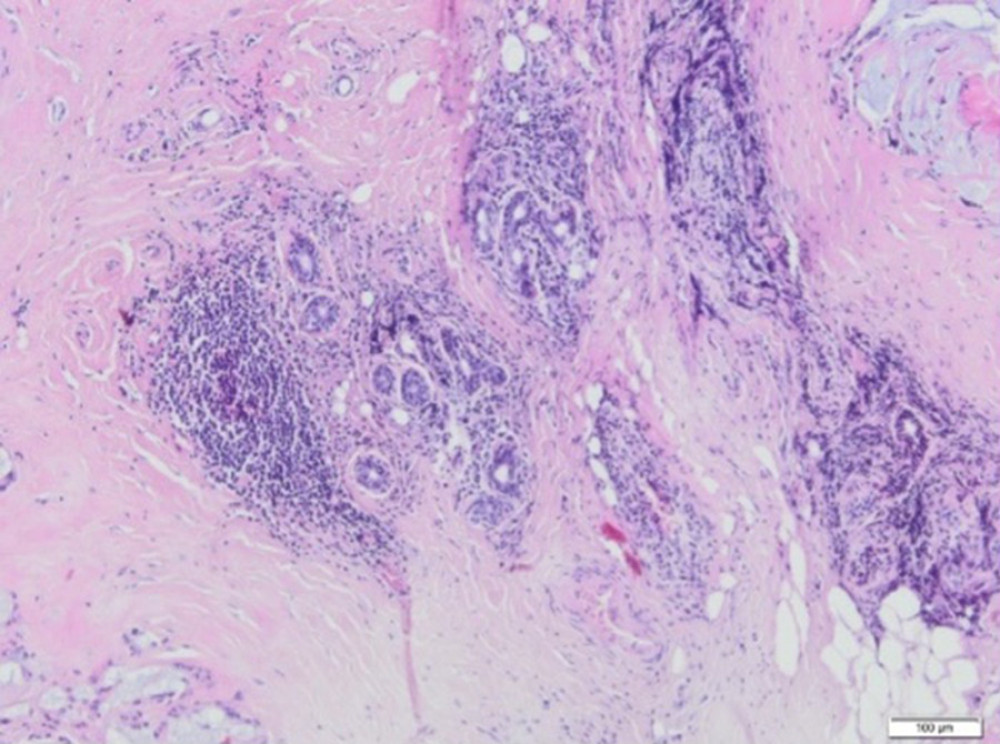 Figure 9. A fragment of the mammary gland with significant fibrosis and the presence of lymphocytic infiltrates (H&E, 100×).
Figure 9. A fragment of the mammary gland with significant fibrosis and the presence of lymphocytic infiltrates (H&E, 100×). 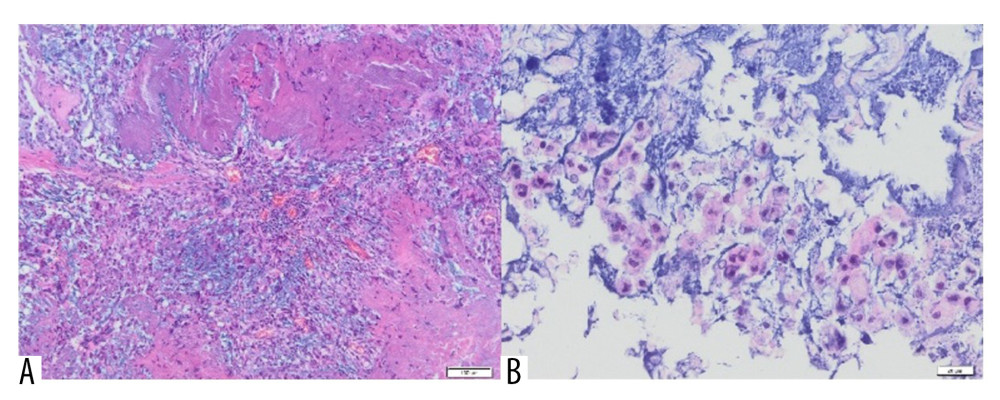 Figure 10. (A) Inflammatory granulation tissue with necrotic foci and numerous small foci of Aquafilling® deposits (blue) (H&E, 400×). (B) Visible basophilic Aquafilling® content (blue) containing numerous macrophages within (H&E, 400×).
Figure 10. (A) Inflammatory granulation tissue with necrotic foci and numerous small foci of Aquafilling® deposits (blue) (H&E, 400×). (B) Visible basophilic Aquafilling® content (blue) containing numerous macrophages within (H&E, 400×). 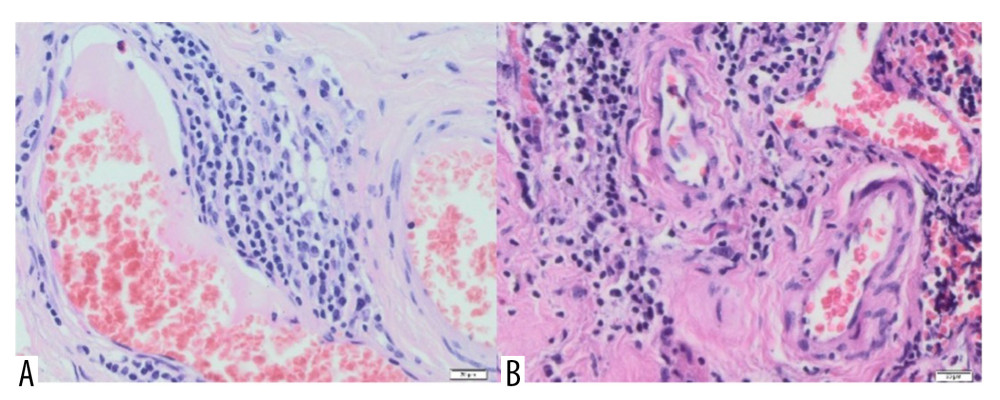 Figure 11. (A) Blood vessels are visible within the connective tissue, with lymphocyte infiltrations in the vicinity of one (H&E, 40×). (B) Vessels with thickened walls, surrounded by lymphocytic infiltration and fibrosis. H&E, 40× magnification.
Figure 11. (A) Blood vessels are visible within the connective tissue, with lymphocyte infiltrations in the vicinity of one (H&E, 40×). (B) Vessels with thickened walls, surrounded by lymphocytic infiltration and fibrosis. H&E, 40× magnification. References
1. Hee Ko K, Kyoung Jung H, Young Park A: Iran J Radiol, 2017; 14; e63468
2. Jung BK, Yun IS, Kim YS, Roh TS: Aesthetic Plast Surg, 2018; 42(5); 1252-56
3. Son MJ, Ko KH, Jung HK, Complications and radiologic features of breast augmentation via injection of Aquafilling gel: J Ultrasound Med, 2018; 37(7); 1835-39
4. Loesch JM, Eniste YS, Dedes KJ, Frauchiger-Heuer H: Eur J Plast Surg, 2022; 45; 515-20
5. Namgoong S, Kim HK, Hwang Y, Clinical experience with treatment of Aquafilling filler-associated complications: A retrospective study of 146 cases: Aesthetic Plast Surg, 2020; 44(6); 1997-2007
6. Nomoto S, Hirakawa K, Ogawa R, Safety of copolyamide filler injection for breast augmentation: Plast Reconstr Surg Glob Open, 2021; 9(2); e3296
7. Chalcarz M, Żurawski J: Aesthetic Plast Surg, 2021; 45(2); 481-90
8. Roh TS: Arch Aesthetic Plast Surg, 2016; 22(1); 45-46
9. Arslan G, Celik L, Atasoy MM, Complication of non-US guided procedure of aquafilling breast gel: Med Ultrason, 2017; 19(2); 236-37
10. Mann RM, Kuhl CK, Kinkel K, Boetes C, Breast MRI: Guidelines from the European Society of Breast Imaging: Eur Radiol, 2008; 18(7); 1307-18
11. Magny SJ, Shikhman R, Keppke AL, Breast imaging reporting and data system: StatPearls August 29, 2022, Treasure Island (FL), StatPearls Publishing
12. Feldman AT, Wolfe D, Tissue processing and hematoxylin and eosin staining: Methods Mol Biol, 2014; 1180; 31-43
13. : Kalkulator doboru próby [in Polish]https://www.naukowiec.org/dobor.html
14. Choi YJ, Lee IS, Song YS, Choi KU, Ahn HY, Distant migration of gel filler: Imaging findings following breast augmentation: Skeletal Radiol, 2022; 51(11); 2223-27
15. Benson JR, Dumitru D, Idiopathic granulomatous mastitis: Presentation, investigation and management: Future Oncol, 2016; 12(11); 1381-94
16. Steuer AB, Stern MJ, Cobos G, Clinical characteristics and medical management of idiopathic granulomatous mastitis: JAMA Dermatol, 2020; 156(4); 460-64
17. Helal TE, Shash LS, Saad El-Din SA, Saber SM, Idiopathic granulomatous mastitis: Cytologic and histologic study of 65 Egyptian patients: Acta Cytol, 2016; 60(5); 438-44
18. Bedinghaus JM, Care of the breast and support of breast-feeding: Prim Care, 1997; 24(1); 147-60
19. Kothari C, Diorio C, Durocher F, The importance of breast adipose tissue in breast cancer: Int J Mol Sci, 2020; 21(16); 5760
20. Celis JE, Moreira JM, Cabezón T, Identification of extracellular and intracellular signaling components of the mammary adipose tissue and its interstitial fluid in high risk breast cancer patients: Toward dissecting the molecular circuitry of epithelial-adipocyte stromal cell interactions: Mol Cell Proteomics, 2005; 4(4); 492-522
21. Chalcarz M, Żurawski J, The absence of early malignant changes in women subjected to Aquafilling breast augmentation on the basis of E-cadherin and N-cadherin immunohistochemical expression: Cent Eur J Immunol, 2022; 47(4); 350-56
22. Laumonier T, Menetrey J, Muscle injuries and strategies for improving their repair: J Exp Orthop, 2016; 3(1); 15
23. Yang W, Hu P, Skeletal muscle regeneration is modulated by inflammation: J Orthop Translat, 2018; 13; 25-32
24. Hashmi MU, Masood A, Yaseen S, Azam H, Idiopathic granulomatous mastitis: A rare confrontation: Cureus, 2021; 13(11); e19420
25. Lombardo GAG, Tamburino S, Magano K, The effect of Omega-3 fatty acids on capsular tissue around the breast implants: Plast Reconstr Surg, 2020; 145(3); 701-10
Figures
 Figure 1. Photographs of the patient in the standing position: (A) preoperatively, (B) 1 week postoperatively, and (C) 6 weeks postoperatively.
Figure 1. Photographs of the patient in the standing position: (A) preoperatively, (B) 1 week postoperatively, and (C) 6 weeks postoperatively. Figure 2. Images showing an example of (A) breast deformation and (B) breast inflammation.
Figure 2. Images showing an example of (A) breast deformation and (B) breast inflammation. Figure 3. Images showing breast fistula (A) during and (B) before surgery.
Figure 3. Images showing breast fistula (A) during and (B) before surgery. Figure 4. Patient 15, short-TI inversion recovery (STIR) axial images showing multiple Aquafilling deposits located in the subcutaneous tissue, breast parenchyma, inter-, intra-, and submuscular (both pectoral muscles), and the adipose tissue of the axilla.
Figure 4. Patient 15, short-TI inversion recovery (STIR) axial images showing multiple Aquafilling deposits located in the subcutaneous tissue, breast parenchyma, inter-, intra-, and submuscular (both pectoral muscles), and the adipose tissue of the axilla. Figure 5. Patient 16. (A) T2 sagittal images, retro-pectoral silicone breast implant, Aquafilling® collection located inter- and intramuscularly (both pectoral muscles). (B) STIR axial images, collection of Aquafilling® located bilaterally subpectoral and surrounding the right breast implant.
Figure 5. Patient 16. (A) T2 sagittal images, retro-pectoral silicone breast implant, Aquafilling® collection located inter- and intramuscularly (both pectoral muscles). (B) STIR axial images, collection of Aquafilling® located bilaterally subpectoral and surrounding the right breast implant. Figure 6. Aquafilling® deposits (blue) are present within the sclerotic connective tissue, with multinucleated giant cells inside (H&E, 100×).
Figure 6. Aquafilling® deposits (blue) are present within the sclerotic connective tissue, with multinucleated giant cells inside (H&E, 100×). Figure 7. Adipose tissue containing Aquafilling® filler (blue) (A) with extensive inflammatory infiltrate in the area (H&E, 40×) and (B) dislodging cells of the tissue (H&E, 400×).
Figure 7. Adipose tissue containing Aquafilling® filler (blue) (A) with extensive inflammatory infiltrate in the area (H&E, 40×) and (B) dislodging cells of the tissue (H&E, 400×). Figure 8. (A) Skeletal muscle with the presence of scar tissue, with a large blood vessel and a profuse inflammatory, infiltrate penetrating the muscle. Visible small foci containing Aquafilling® filler (blue) (H&E, 100×). (B) A small amount of Aquafilling® is visible within the skeletal muscle (blue). Lymphocytes are present within the filler (H&E, 400×).
Figure 8. (A) Skeletal muscle with the presence of scar tissue, with a large blood vessel and a profuse inflammatory, infiltrate penetrating the muscle. Visible small foci containing Aquafilling® filler (blue) (H&E, 100×). (B) A small amount of Aquafilling® is visible within the skeletal muscle (blue). Lymphocytes are present within the filler (H&E, 400×). Figure 9. A fragment of the mammary gland with significant fibrosis and the presence of lymphocytic infiltrates (H&E, 100×).
Figure 9. A fragment of the mammary gland with significant fibrosis and the presence of lymphocytic infiltrates (H&E, 100×). Figure 10. (A) Inflammatory granulation tissue with necrotic foci and numerous small foci of Aquafilling® deposits (blue) (H&E, 400×). (B) Visible basophilic Aquafilling® content (blue) containing numerous macrophages within (H&E, 400×).
Figure 10. (A) Inflammatory granulation tissue with necrotic foci and numerous small foci of Aquafilling® deposits (blue) (H&E, 400×). (B) Visible basophilic Aquafilling® content (blue) containing numerous macrophages within (H&E, 400×). Figure 11. (A) Blood vessels are visible within the connective tissue, with lymphocyte infiltrations in the vicinity of one (H&E, 40×). (B) Vessels with thickened walls, surrounded by lymphocytic infiltration and fibrosis. H&E, 40× magnification.
Figure 11. (A) Blood vessels are visible within the connective tissue, with lymphocyte infiltrations in the vicinity of one (H&E, 40×). (B) Vessels with thickened walls, surrounded by lymphocytic infiltration and fibrosis. H&E, 40× magnification. Tables
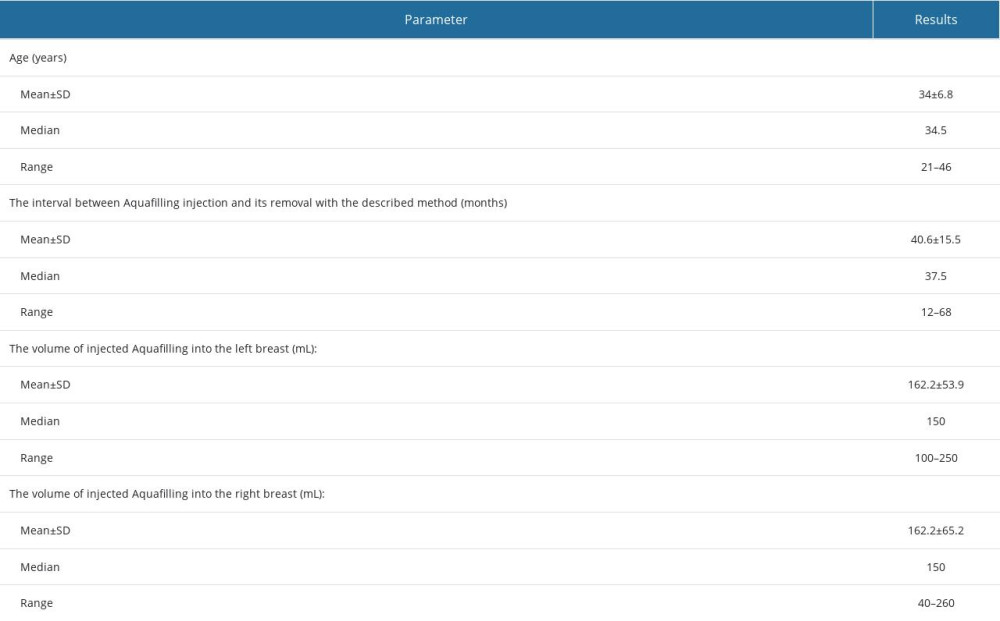 Table 1. Patients’ age, the time between Aquafilling injection and surgical removal, and the volume of injected Aquafilling.
Table 1. Patients’ age, the time between Aquafilling injection and surgical removal, and the volume of injected Aquafilling.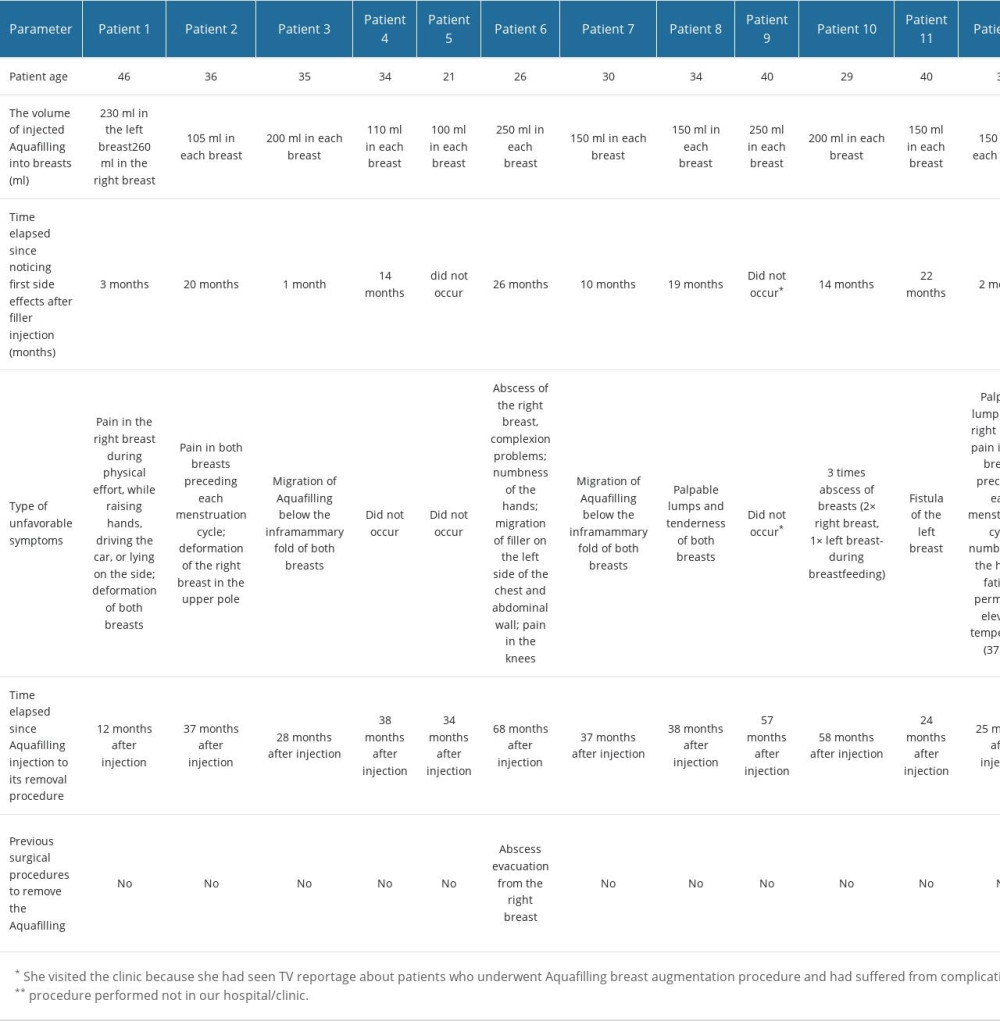 Table 2. Histological changes in the breasts resulting from the Aquafilling filler reaction.
Table 2. Histological changes in the breasts resulting from the Aquafilling filler reaction.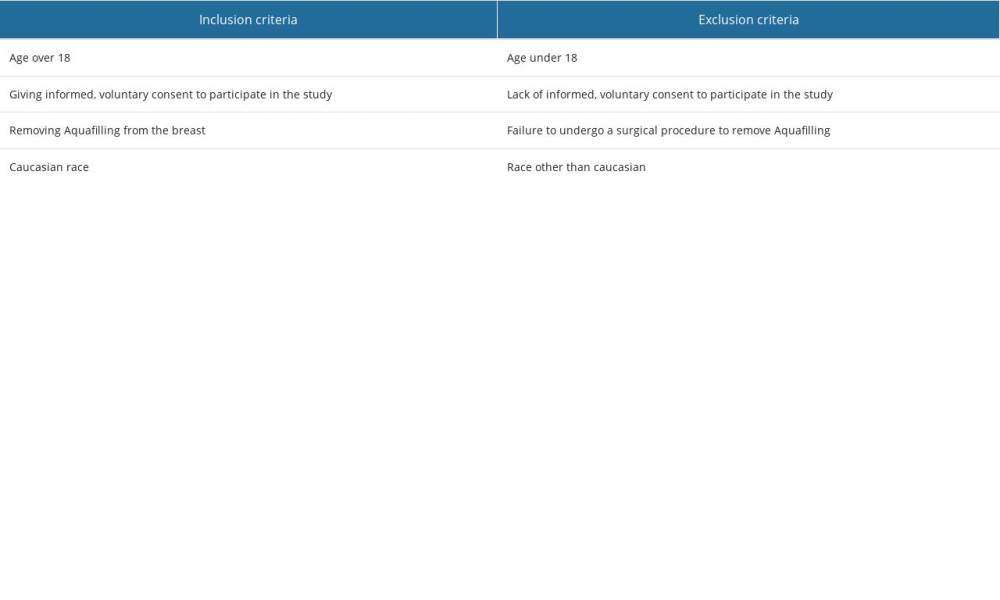 Table 3. Inclusion and exclusion criteria for the study group.
Table 3. Inclusion and exclusion criteria for the study group. Table 1. Patients’ age, the time between Aquafilling injection and surgical removal, and the volume of injected Aquafilling.
Table 1. Patients’ age, the time between Aquafilling injection and surgical removal, and the volume of injected Aquafilling. Table 2. Histological changes in the breasts resulting from the Aquafilling filler reaction.
Table 2. Histological changes in the breasts resulting from the Aquafilling filler reaction. Table 3. Inclusion and exclusion criteria for the study group.
Table 3. Inclusion and exclusion criteria for the study group. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








