01 December 2022: Clinical Research
Clinical Outcomes of Extracranial Carotid Artery-Related Stroke Eligible for Mechanical Reperfusion on Top of Per-Guidelines Thrombolytic Therapy: Analysis from a 6-Month Consecutive Patient Sample in 2 Centers
Karolina Dzierwa 12ABDEF* , Magdalena Knapik 234ABDEF , Łukasz Tekieli 235ABDEF , Adam Mazurek 23ABDEF , Małgorzata Urbańczyk-Zawadzka 26BCD , Artur Klecha 7ABD , Tomasz Kowalczyk 7ABD , Teresa Koźmik 7ABD , Łukasz Wiewiórka 2567ABD , Paweł Banyś 6BCD , Ewa Węglarz 25BC , Justyna Stefaniak 8CD , Rafał T. Nizankowski 9ADF , Iris Q. Grunwald 1011ADEF , Piotr Musiałek 23ABCDEF*DOI: 10.12659/MSM.938549
Med Sci Monit 2022; 28:e938549
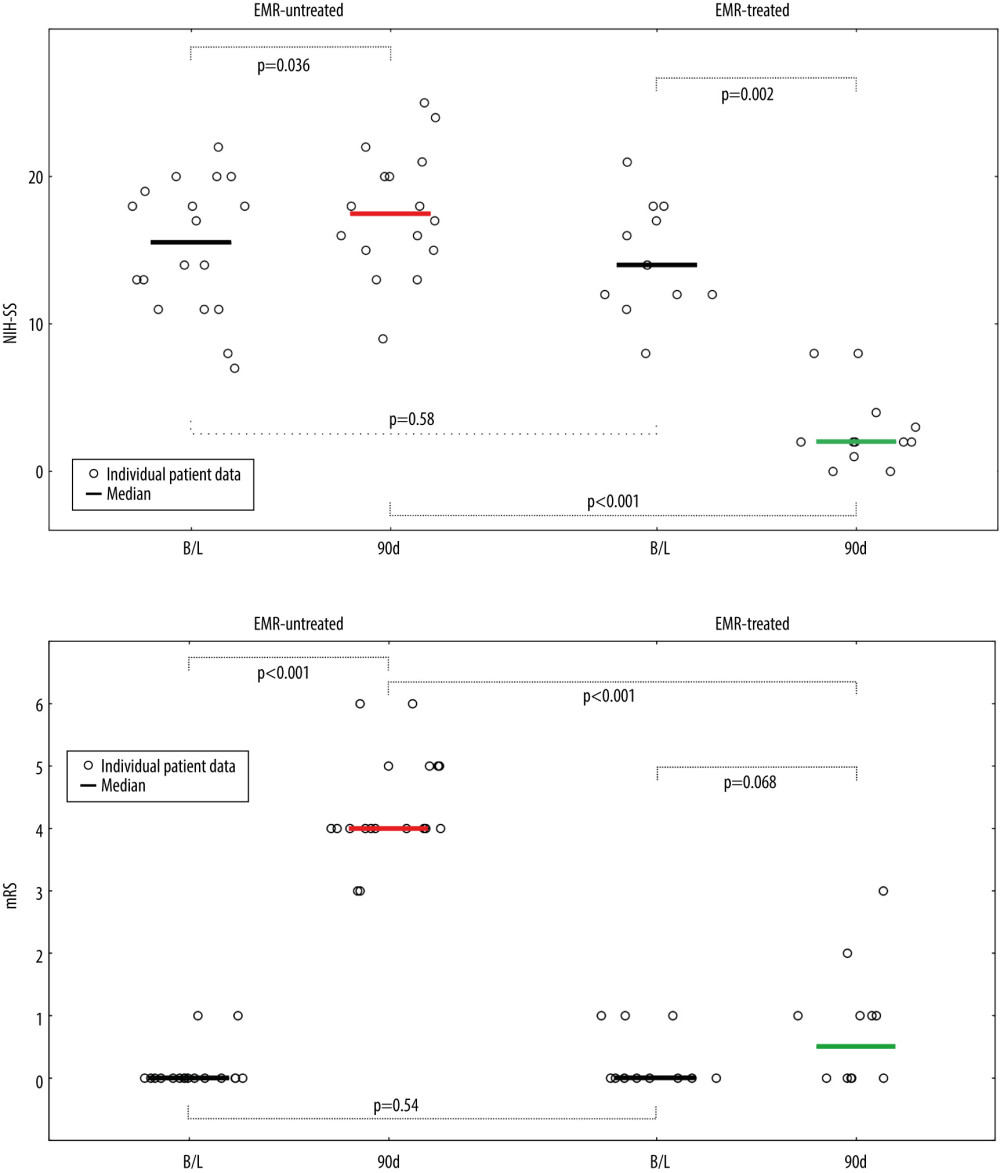
Figure 3 Evolution of National Institutes of Health Stroke Scale (NIHSS), (A) and functional status (modified Rankin Score – mRS), (B) in patients not treated and treated with EMR (Emergency Mechanical Reperfusion). Individual patient and group data on acute ischemic stroke of carotid artery origin (AIS-CA) clinical severity (NIHSS, A) and patient functional status (mRS, B) are provided at baseline at 90 days. Emergency mechanical reperfusion (EMR) treatment effect is demonstrated by comparison of EMR-untreated (natural history, left) and EMR-treated patients (right). With the particularly large volume of cerebral tissue-at-risk in AIS-CA, EMR profoundly impacts clinical outcomes. Note the striking difference in NIHSS and mRS at 90 days in EMR-treated patients versus those who did not receive mechanical reperfusion. The EMR-untreated patients would have been accepted for treatment in the cardioangiology cathlab-based Thrombectomy-Capable Stroke Center (operator team with experience in proximal-protected carotid artery stenting). The figure was created with the use of Statistica 10 (StatSoft GmBH, Hamburg, Germany).


