25 July 2023: Clinical Research
Optimizing Sterilization Packaging through Root Cause Analysis: An Exploration into Sealing Defects of Paper-Plastic Pouches
Sixin Jiang12ABCE, Liangying Yi12ABCE*, Yanhua Chen32E, Ruixue Hu32EDOI: 10.12659/MSM.940342
Med Sci Monit 2023; 29:e940342
Abstract
BACKGROUND: Paper-plastic sterilization pouches are essential in healthcare for preventing instrument contamination. However, sealing defects in these pouches can jeopardize patient safety. To address this issue, our study uses Root Cause Analysis (RCA), aiming to identify contributing factors to these defects and propose practical solutions. Through this, we aim to enhance the overall sterilization process.
MATERIAL AND METHODS: A retrospective analysis was conducted on 35,762 instruments sterilized and packaged in paper-plastic pouches at our hospital's Central Sterile Supply Department (CSSD) across two periods: July 2020 to June 2021 (pre-RCA, 17,563 instruments) and September 2021 to August 2022 (post-RCA, 18,199 instruments). We evaluated RCA scores, packaging personnel's perceptions of sealing quality, and sealing defect rates before and after RCA implementation.
RESULTS: Root causes for sealing defects included lack of a standardized inspection procedure, inadequately sized packing table, missed inspections, incorrect distribution procedures, inadequate staff training, and insufficient lighting through the pass-through window between storage and distribution rooms. Among these, lack of a standardized inspection procedure, small packing table size, and missed inspections were statistically significant risk factors (P<0.05). The sealing defect rate decreased from 0.15% pre-RCA implementation to 0.07% post-RCA implementation.
CONCLUSIONS: Implementing RCA has been shown to effectively enhance the CSSD staff's perception of sealing quality and significantly reduce the incidence of sealing defects in paper-plastic pouches. Thus, RCA serves as an invaluable tool for quality improvement in sterilization packaging processes.
Keywords: Product Packaging, quality improvement, Root Cause Analysis, Surgical Instruments, Humans, Retrospective Studies, Health Facilities, Patient safety, Plastics, Sterilization
Background
The Central Sterile Supply Department (CSSD), a key department for nosocomial infection prevention and control, supplies a variety of sterile instruments, utensils, and articles for the hospital. The quality of CSSD work can directly affect medical treatment quality and patient safety [1,2]. The “Central Sterile Supply Department (CSSD) – Part II: Standard for operating procedure of cleaning, disinfection and sterilization” implemented in China in June 2017 [3] states that the sealing width of paper-plastic pouches should be ≥6 mm. Also, adhesive or the hot melt method could be applied to sealings to make the paper and plastic layers closely connected, ensuring the sealing integrity and avoiding contamination of sterile items in the storage process. Disposable paper-plastic pouches are a new type of packaging material that have the functions of providing a biological barrier and effectively blocking the entry of external dust. This means that sterile packaging can be stored for a long time, quality of the sterile packages and the safety of clinical use can be ensured, packaging cost can be reduced, and service life of the packaged articles can be extended [4].
The rapid developments in medical technology and increased stocktaking by surgeries in recent years have placed upon hospitals an increasing demand for instruments being packaged using paper-plastic pouches. Plastic sealing quality is one of the significant factors affecting the sterilization quality of the paper-plastic pouches, and modern hospitals must have in place effective measures for preventing quality defects in plastic sealing pouches [5,6]. In the process of using paper-plastic pouches, we found a high incidence of sealing defects, up to 0.15%. Not only does this affect their use in clinical departments, but it can affect the safety of patient lives, directly affect medical safety, and easily cause nosocomial infection [7].
Root cause analysis (RCA) is a systematic problem handling process that involves determining and analyzing the scope, frequency, and cause of a problem, finding the solutions, and formulating problem prevention and improvement measures. The process of problem handling includes defining the problem, analyzing the cause, planning the solution, implementing the solution, and tracking the feedback. Commonly used tools are the fishbone diagram and brainstorming method [8]. RCA, a retrospective error analysis tool that scientifically analyzes the occurrence of adverse events and is used to locate and correct their underlying causes, is a widely recognized scientific quality management method [9]. This approach can be used for continuous quality improvement in health systems to help hospital risk managers determine how to improve systems to reduce the number and severity of adverse events. In this study, we investigated the application of RCA in sealing defects of paper-plastic pouches.
Material and Methods
ETHICS APPROVAL:
This study was conducted in accordance with the Declaration of Helsinki. All research methods were carried out in accordance with the relevant guidelines and regulations. This study was approved by the medical ethics committee of the authors’ institution [2023 Medical Scientific research for Ethical Approval No. 008]. Verbal informed consent to participate in this study was obtained from all participants. The medical ethics committee of the authors’ institution approved the procedure of verbal informed consent of this study.
STUDY SETTING:
This was a retrospective study. A total of 35 762 instruments packaged using paper-plastic pouches by 11 packaging personnel in our hospital’s CSSD during July 2020 to June 2021 and during September 2021 to August 2022 were included in this study. Of them, 17 563 instruments packaged using paper-plastic pouches prior to RCA implementation during July 2020 to June 2021 were classified as the control group; 18 199 instruments packaged using paper-plastic pouches following the implementation of RCA and improvement measures during September 2021 to August 2022 were classified as the experimental group.
In adhering with appropriate sealing quality criteria of paper-plastic packaging [10], only the instruments packaged using paper-plastic pouches that met the following criteria were included in this study: (1) reusable instrument that could be assembled, sealed, packaged, and labeled; (2) paper-plastic pouches, the quality of which were found to conform with the criteria; and, (3) pouches could withstand the conditions during the sterilization process and were free from damage and wrinkles. Instrument packages that were larger than 30×30×50 cm or heavier than 7 kg were excluded from this study.
The packaging personnel meeting the following criteria were included in this study: (1) nurses who had worked in the CSSD for at least 3 years and had received paper-plastic packaging training; (2) workers who had worked in the CSSD for at least 5 years and had received paper-plastic packaging training; (3) those who packaged the instruments using paper-plastic pouches before and after implementation of the improvement measures. The included packaging personnel were aged 35 to 45 years. Visiting staff members, interns, CSSD workers with less than 5 years of work experience, and newly employed CSSD nurses were excluded from this study.
SEALING QUALITY CRITERIA FOR PAPER-PLASTIC POUCHES:
According to CSSD’s technical operation specification for cleaning, disinfection, and sterilization and relevant literature [10,11], the following sealing features are considered acceptable (and in this study were rated ‘pass’): sealing width ≥6 mm; the distance between the instrument inside the pouch and the seal was ≥2.5 cm; and, no bubbles, folds, crevices, or cracks in the seal.
RCA QUALITY MANAGEMENT TEAM:
A RCA Quality Management Team was established in July 2021. The team consisted of 1 head nurse (senior associate-level professional title), 4 supervising nurses (1 was a quality controller who was enrolled on a postgraduate program; the other 3 held undergraduate qualifications), 3 nurse practitioners (1 held a postgraduate qualification, and the other 2 held undergraduate qualifications), 2 packaging workers with more than 6 years of packaging experience, and 1 sterilization worker who possessed 8 years of work experience and a special equipment manipulation qualification certificate. The head nurse was the team leader. The other team members had received RCA training and passed the examinations prior to joining the team.
RISK FACTORS:
The RCA Quality Management Team collected the information concerning the sealing defects (eg, incomplete seal, seal width <6 mm, bubbles, folds, or crevices in the seal) of 17 563 paper-plastic pouches processed by our hospital’s CSSD during July 2020 to June 2021. They recalled the events and brainstormed at team meetings using the fishbone diagram (Figure 1) to analyze the risk factors for sealing defects posted by 5 components (manpower, machine, materials, environment, and methods) and identify the root causes of the sealing defects.
RCA RATING FORM:
The root causes of sealing defects in the paper-plastic pouches were analyzed to identify the true causes. A self-designed RCA rating form was used to evaluate 3 dimensions of risk: severity, occurrence, and detectability. The design of the RCA rating form was based on the failure mode quantitative evaluation form [12]. The potential root causes were the indicators in the RCA form. The scores of each of these 3 dimensions ranged from 1 to 10. The total score of an indicator is the sum of its severity, occurrence, and detectability scores. The higher the indicator’s score, the higher the risk.
For severity of defect, a score of 1–2 was considered ‘mild’; a score of 3–4 was considered ‘mild to moderate’; a score of 5–6 was considered ‘moderate’; a score of 7–8 was considered ‘severe’; and, a score of 9–10 was considered ‘extremely severe’.
For occurrence, a score of 1–2 indicated that a defect was likely to occur every year or occasionally; a score of 3–4 indicated that a defect was likely to occur at least quarterly; a score of 5–6 indicated that a defect was likely to occur monthly; a score of 7–8 indicated that a defect was likely to occur weekly; and, a score of 9–10 indicated that a defect was likely to occur every day.
For detectability: a score of 1–2 indicated a defect was likely to be detected every year or occasionally; a score of 3–4 indicated that a defect was likely to be detected at least quarterly; a score of 5–6 indicated that a defect was likely to be detected monthly; a score of 7–8 indicated that a defect was likely to be detected weekly; and, a score of 9–10 indicated that a defect was likely to be detected every day.
FORMULATION AND IMPLEMENTATION OF IMPROVEMENT MEASURES BASED ON RCA RESULTS:
The causes identified as significant in this study were as follows: lack of a standard operating procedure for inspection of plastic sealings; small size of the packing table; missed inspections by the inspector; insufficient training; and insufficient light through the pass-through window between the storage and distribution rooms. The RCA rating form was used to identify the true causes. The following improvement measures were formulated and implemented to address these influencing factors:
RCA SCORES:
The RCA scores of the sealing defects before and after RCA implementation were calculated.
COMPARISON OF RCA SCORES BEFORE AND AFTER RCA IMPLEMENTATION:
The RCA scores before and after RCA implementation was compared. An obvious reduction in RCA scores after RCA implementation compared with those before RCA implementation indicated that the improvement measures were effective.
PACKAGING PERSONNEL’S PERCEPTIONS OF SEALING QUALITY:
A self-designed questionnaire survey was conducted to investigate the same 11 packaging personnel’s perceptions of sealing quality of paper-plastic pouches before and after RCA implementation. The questions involved 4 components: plastic sealing procedures, perceptions of sealing defects of paper-plastic pouches, plastic sealing cautions, and perceptions of criteria for sealing quality of paper-plastic pouches. For each component, they had to answer 20 questions. The maximum score of each component was 100. The 5-point Likert scale was used. The higher the score, the better the packaging personnel member’s perception of sealing quality.
SEALING DEFECT RATES:
As mentioned, data relating to the sealing quality were collected by the RCA Quality Management Team members during 2 sampling periods (July 2020 to June 2021, September 2021 to August 2022) for a total of 35 762 instruments.
DATA COLLECTION:
The sealing quality of the included instruments was evaluated by the CSSD quality controller. The evaluation results were recorded in the quality control register. The following data were collected: (1) number of the instruments packaged using paper-plastic pouches; (2) number of sealing defects in the pouches before and after RCA implementation; and, (3) clinical departments’ feedback about the sealing quality control during the 2 sampling periods.
STATISTICAL ANALYSIS:
SPSS 25.0 was used for data analysis. Normally distributed measurement data are presented as mean±standard deviation and were analyzed further using the
Results
RCA SCORES:
The RCA shows the following true causes of the sealing defects: a lack of a standard operating procedure for inspection of plastic sealings; small size of the packing table; missed inspections by the inspector; insufficient training; and insufficient light through the pass-through window between the storage and distribution rooms. Any of these factors could impact the sealing quality of paper-plastic pouches. Paper-plastic pouches with sealing defects are more likely to cause nosocomial infections. Therefore, these defects could have extremely severe consequences (Table 1).
COMPARISON OF RCA SCORES BEFORE AND AFTER RCA IMPLEMENTATION:
The scores of the risk factors (lack of a standard operating procedure for inspection of plastic sealings; small size of the packing table; missed inspections by the inspector; insufficient training; and insufficient light through the pass-through window between the storage and distribution rooms) prior to RCA implementation were higher than those after RCA implementation, and statistically significant differences existed (P<0.05). No statistically significant differences were found for the incorrect distribution procedures (P>0.05). The RCA scores after RCA implementation were obviously lower than those before RCA implementation due to formulating an SOP for inspection of plastic sealings, widening the packing table, providing training for inspectors, emphasizing the significance of inspection in ensuring sealing quality, and adding lighting for the pass-through window between the storage and distribution rooms (Table 2).
PACKAGING PERSONNEL’S PERCEPTIONS OF SEALING QUALITY:
The packaging personnel’s scores relating to plastic sealing procedures, perceptions of adverse events caused by sealing defects of paper-plastic pouches, plastic sealing cautions, and the criteria for sealing quality of paper-plastic pouches following the implementation of RCA and improvement measures were significantly higher than those prior to RCA implementation (P<0.05; Table 3).
SEALING DEFECT RATES:
The sealing defect rate following the implementation of RCA and improvement measures was significantly lower than that prior to RCA implementation (P<0.05; Table 4).
Discussion
LIMITATIONS:
This study was based on the sealing defects of paper-plastic pouches in a single center. There could be some limitations in the search for human and equipment factors. In this study, the content of the previous literature should have been cited as the basis of the research, and the review of the literature could have provided a theoretical basis for the research topic. However, due to the different research topics involved, the relevant literature might be limited.
Conclusions
Through an analysis of the root cause of the sealing defects of paper-plastic pouches, we found the root cause affecting the sealing quality, developed and implemented corresponding improvement measures, and achieved good effects. RCA can effectively improve the CSSD staff’s perception of sealing quality and team execution and is beneficial in reducing the incidence of sealing defects of paper-plastic pouches. We found that there were few studies on the sealing defects of paper-plastic pouches in the world, so further research on the subject should be undertaken to improve understanding.
Figures
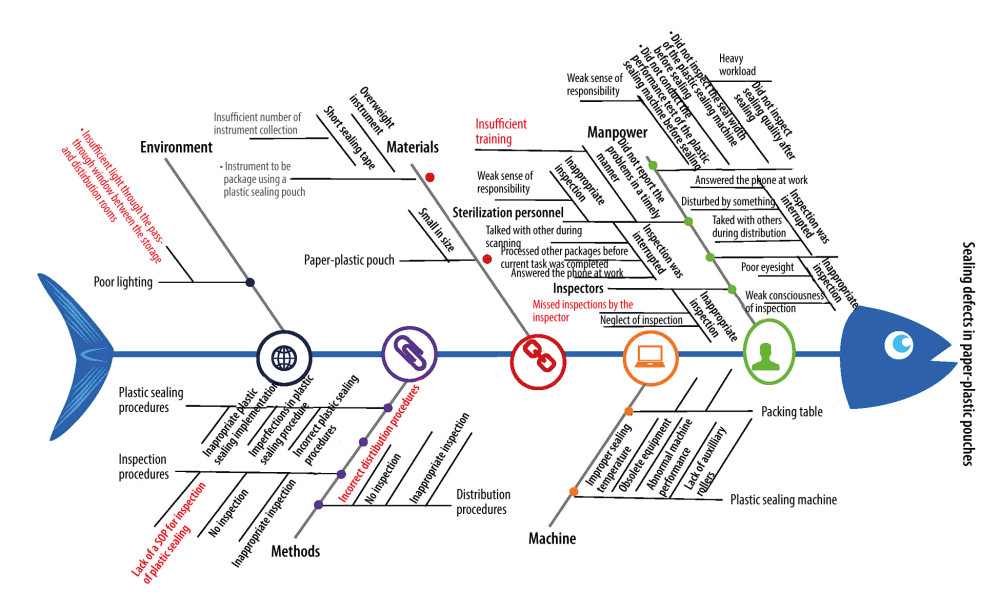 Figure 1. Risk factors for sealing defects of paper-plastic pouches (Software: Xmind-Mind Map; Version: 2020; Manufacturer: Xmind Ltd.).
Figure 1. Risk factors for sealing defects of paper-plastic pouches (Software: Xmind-Mind Map; Version: 2020; Manufacturer: Xmind Ltd.). 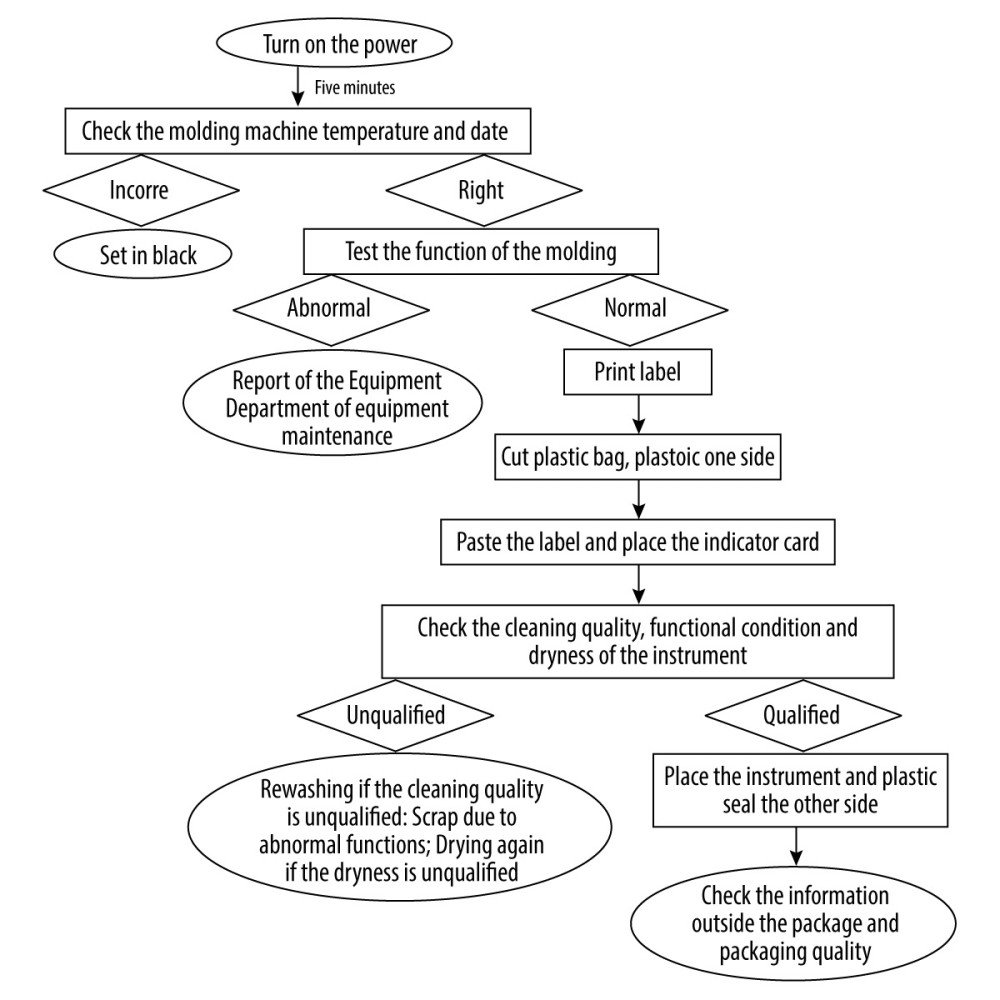 Figure 2. Plastic sealing flowchart (Software: Microsoft® Word 2019MSO; Version: 2019; Manufacturer: Microsoft Corporation).
Figure 2. Plastic sealing flowchart (Software: Microsoft® Word 2019MSO; Version: 2019; Manufacturer: Microsoft Corporation). 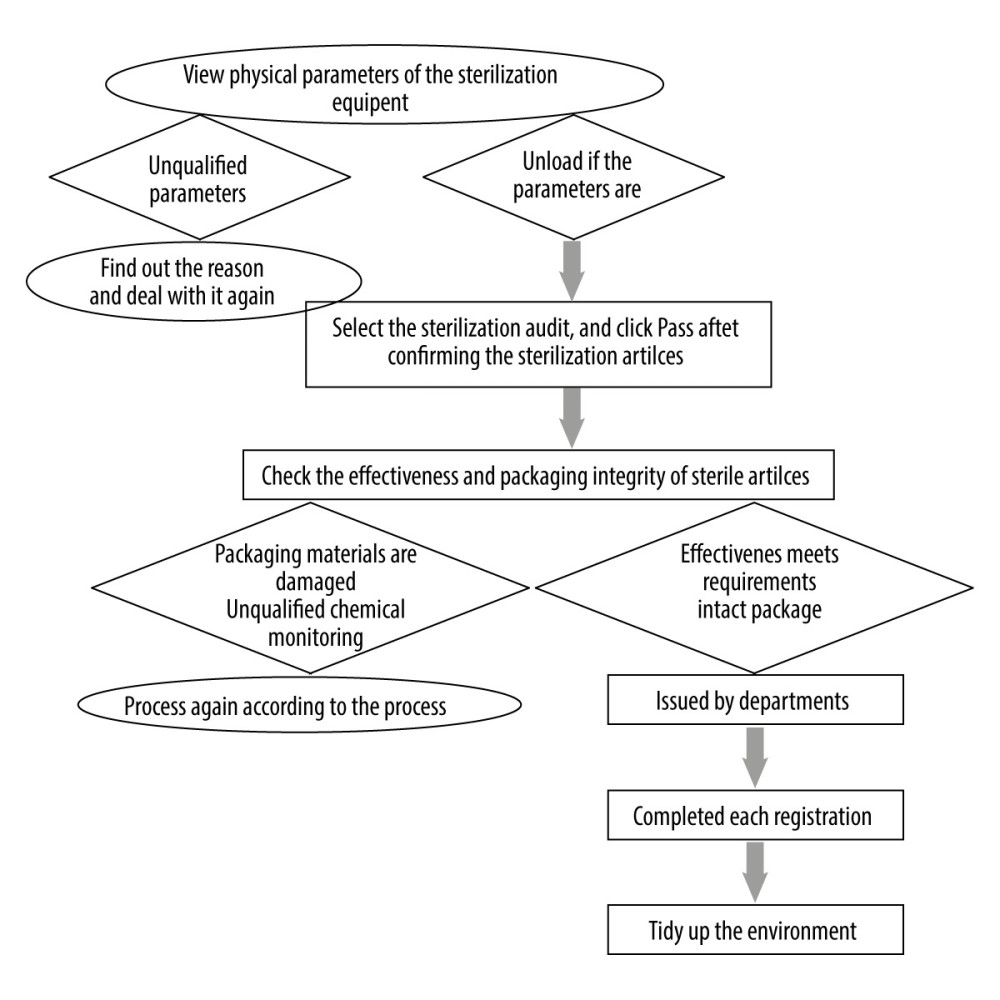 Figure 3. Distribution flowchart (Software: Microsoft® Word 2019MSO; Version: 2019; Manufacturer: Microsoft Corporation).
Figure 3. Distribution flowchart (Software: Microsoft® Word 2019MSO; Version: 2019; Manufacturer: Microsoft Corporation). Tables
Table 1. Root cause analysis of sealing defects of paper-plastic pouches.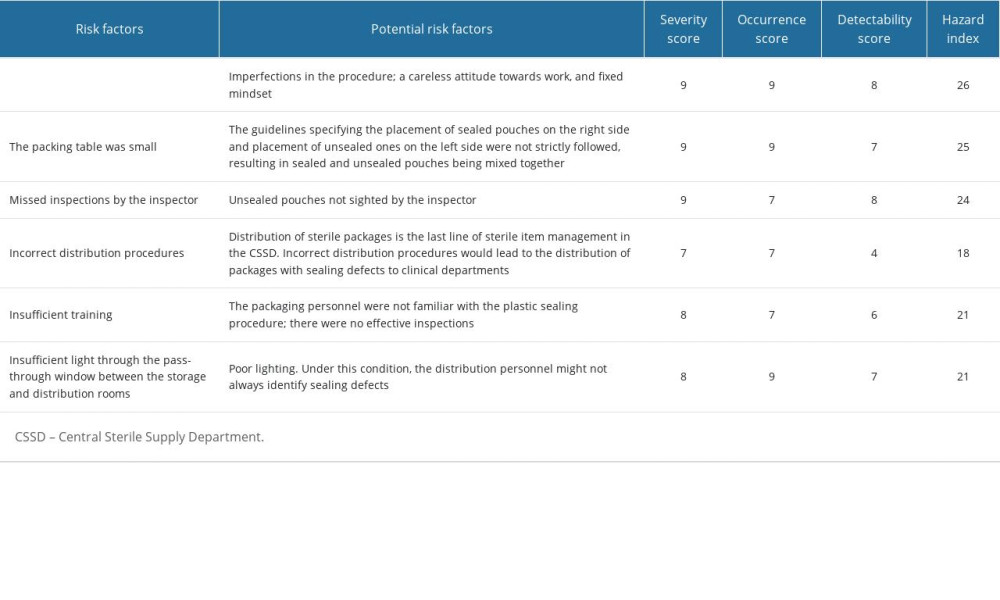 Table 2. Root cause analysis scores before and after root cause analysis implementation.
Table 2. Root cause analysis scores before and after root cause analysis implementation.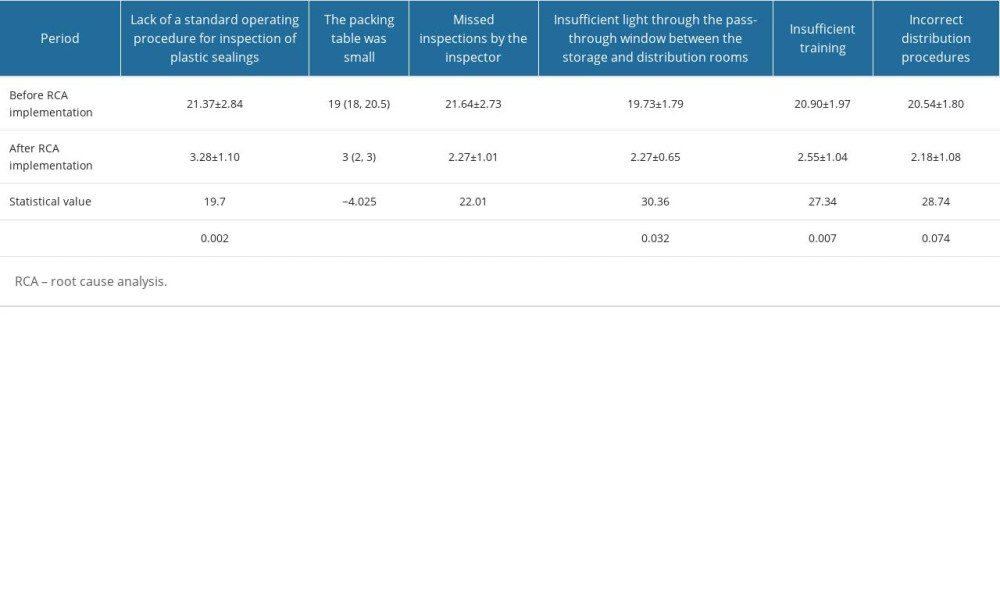 Table 3. Packaging personnel’s perceptions of sealing quality before and after root cause analysis implementation.
Table 3. Packaging personnel’s perceptions of sealing quality before and after root cause analysis implementation.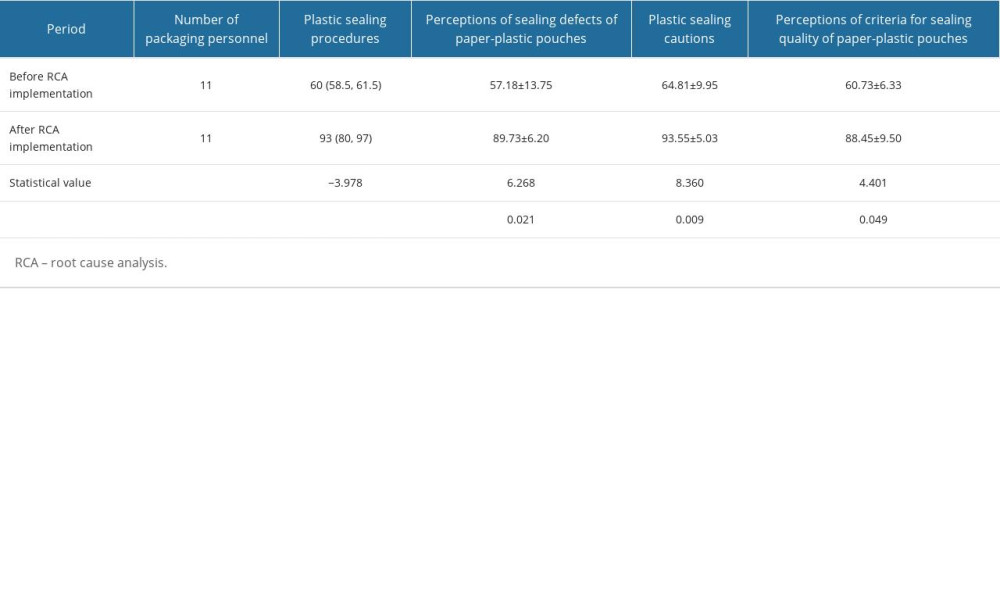 Table 4. Comparison of sealing defects rate.
Table 4. Comparison of sealing defects rate.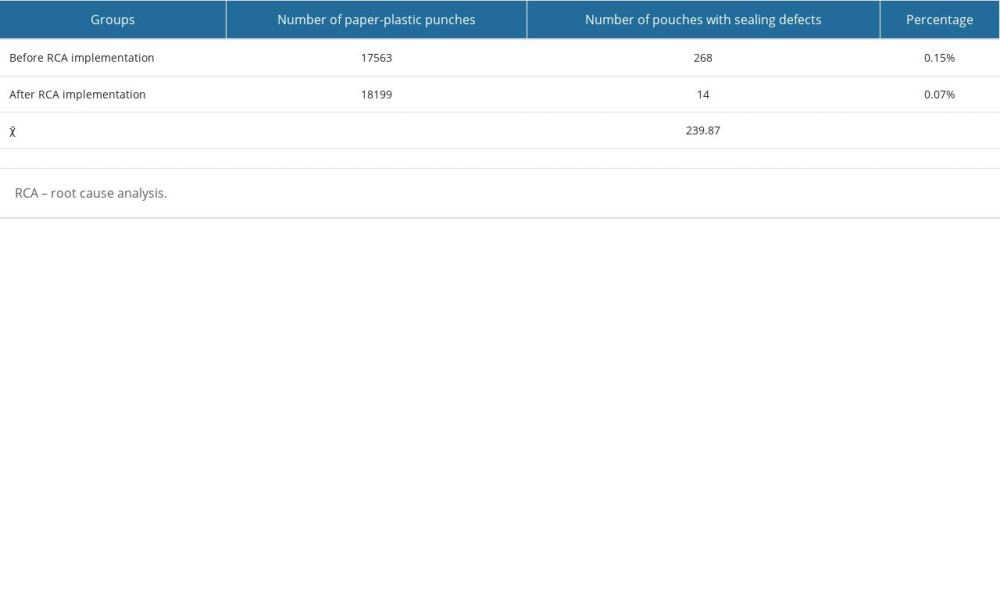
References
1. Jing W, Mu Y, Cai Y, Central sterile supply department (CSSD) management quality sensitive index constructed by management mode under the guidance of key point control theory and its effect on CSSD management quality: A retrospective study: Ann Palliat Med, 2022; 11; 2050-60
2. Ge Y, Gan L, Zheng LAnalysis of the significance of department contact system and quality traceability information system for nursing management of disinfection supply center: Chinese Journal of Disinfection, 2021; 38; 781-84 [in Chinese]
3. Hospital Disinfection Supply Center Part 2: Technical operation specification for cleaning, disinfection and sterilization WS310.2-2016: Chinese Journal of Infection Control, 2017; 16; 986-92 [in Chinese]
4. Chen L, Zheng Y, Li YDiscussion on the causes of sealing failure in paper plastic packaging: Chinese Journal of Disinfection, 2008; 25; 194-95 [in Chinese]
5. Chi S, Duan Z, Sun XQuality management of paper plastic packaging bags in disinfection supply centers and clinical use: Chinese Journal of Modern Nursing, 2011; 17; 2084-85 [in Chinese]
6. Hoefel HHK, Pozzer C, Acunã A, Bundles for the central sterile supply department: Am J Infect Control, 2019; 47; 1352-57
7. Dai C, Jin J, Nong LAnalysis of the reasons for impregnation of pulsating vacuum pressure sterilized paper-plastic bag packaging: Chinese Journal of Disinfection, 2016; 33; 1224-25 [in Chinese]
8. Lima LA, Silva LCMA, Dantas JKDS, Root cause analysis, failures and effects in pediatric total quality management: A scoping review: Rev Bras Enferm 20, 2021; 74(6); e20200954
9. Dhingra J, Farrell MB, Halkar RK, Root cause analysis in nuclear medicine for sentinel events: J Nucl Med Technol, 2022 [Online ahead of print]
10. Chen Y, Jiang J, Luo YStudy on sterilization quality of instruments with paper-plastic bag packaging: Chinese Journal of Nosocomiology, 2015(7); 1678-80 [in Chinese]
11. Rutala WA, Weber DJ, Disinfection and sterilization in health care facilities: An overview and current issues: Infect Dis Clin North Am, 2021; 35; 575-607
12. Feng C, Chao L, Li XApplication effect of root cause analysis method in safety management of electrosurgical instruments and equipment: Chinese Journal of Modern Nursing, 2022; 28; 1355-59 [in Chinese]
13. Black JM, Root cause analysis for hospital-acquired pressure injury: J Wound Ostomy Continence Nurs, 2019; 46(4); 298-304
14. Beugniez C, Sauvanet A, Sulpice L, Root-cause analysis of mortality after pancreatic resection (CARE Study): A multicenter cohort study: Ann Surg, 2021; 274; 789-96
15. Yang L, Xun Q, Xu J, Application of the defect management improvement mode under Joint Commission International standard to improve the instrument cleaning and disinfection effect and management quality in the central sterile supply department: A randomized trial: Ann Transl Med, 2022; 10; 137
16. Ying J, Wang H, Ye H, The supervision and management mode of disinfection supply center improves the standardization of sterile goods management in clinical departments: Comput Math Methods Med, 2022; 2022; 6916212
17. Xu X, Deng D, Ke DRoot cause analysis as used in China’s hospital management: Current research and application: Chinese Journal of Hospital Administration, 2017; 33; 623-26 [in Chinese]
Figures
 Figure 1. Risk factors for sealing defects of paper-plastic pouches (Software: Xmind-Mind Map; Version: 2020; Manufacturer: Xmind Ltd.).
Figure 1. Risk factors for sealing defects of paper-plastic pouches (Software: Xmind-Mind Map; Version: 2020; Manufacturer: Xmind Ltd.). Figure 2. Plastic sealing flowchart (Software: Microsoft® Word 2019MSO; Version: 2019; Manufacturer: Microsoft Corporation).
Figure 2. Plastic sealing flowchart (Software: Microsoft® Word 2019MSO; Version: 2019; Manufacturer: Microsoft Corporation). Figure 3. Distribution flowchart (Software: Microsoft® Word 2019MSO; Version: 2019; Manufacturer: Microsoft Corporation).
Figure 3. Distribution flowchart (Software: Microsoft® Word 2019MSO; Version: 2019; Manufacturer: Microsoft Corporation). Tables
 Table 1. Root cause analysis of sealing defects of paper-plastic pouches.
Table 1. Root cause analysis of sealing defects of paper-plastic pouches. Table 2. Root cause analysis scores before and after root cause analysis implementation.
Table 2. Root cause analysis scores before and after root cause analysis implementation. Table 3. Packaging personnel’s perceptions of sealing quality before and after root cause analysis implementation.
Table 3. Packaging personnel’s perceptions of sealing quality before and after root cause analysis implementation. Table 4. Comparison of sealing defects rate.
Table 4. Comparison of sealing defects rate. Table 1. Root cause analysis of sealing defects of paper-plastic pouches.
Table 1. Root cause analysis of sealing defects of paper-plastic pouches. Table 2. Root cause analysis scores before and after root cause analysis implementation.
Table 2. Root cause analysis scores before and after root cause analysis implementation. Table 3. Packaging personnel’s perceptions of sealing quality before and after root cause analysis implementation.
Table 3. Packaging personnel’s perceptions of sealing quality before and after root cause analysis implementation. Table 4. Comparison of sealing defects rate.
Table 4. Comparison of sealing defects rate. In Press
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
15 Mar 2024 : Clinical Research
Evaluation of an Optimized Workflow for the Radiofrequency Catheter Ablation of Paroxysmal Atrial FibrillationMed Sci Monit In Press; DOI: 10.12659/MSM.943526
09 May 2024 : Review article
A Review of the Current Status of Disease-Modifying Therapies and Prevention of Alzheimer’s DiseaseMed Sci Monit In Press; DOI: 10.12659/MSM.945091
09 Apr 2024 : Clinical Research
Correlation between Thalamocortical Tract and Default Mode Network with Consciousness Levels in Hypoxic-Isc...Med Sci Monit In Press; DOI: 10.12659/MSM.943802
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








