16 May 2023: Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Students Aged Between 8 and 12 years
Camila Colussi Madruga-Rimoli1ABDEF, Milaine Dominici SanfinsDOI: 10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
Abstract
BACKGROUND: Learning to read and write depends on the effective functioning of various sensory systems, including the auditory system. Auditory information processing involves behavioral and electrophysiological processes. Electrophysiological procedures are used to investigate activity in the auditory pathway in response to sound stimuli, and the associated cortical activity in discrimination, integration, and attention. The study evaluated electrophysiological testing for an auditory processing disorder and reading performance in 54 school students aged between 8 and 12 years.
MATERIAL AND METHODS: The study involved 54 public school students aged between 8 and 12 years, who were divided into a study group and control group. All children underwent basic audiological assessment, rating of reading and writing ability, non-verbal intelligence, auditory brainstem response, long-latency auditory-evoked potentials (LLAEP), frequency following responses (FFR), and auditory training (AT).
RESULTS: The basic audiological evaluation showed a statistically significant difference between groups only for the frequency of 6 kHz. The LLAEP response had a statistically significant difference between groups for N1 latency, P300 latency, and amplitude. Finally, there was a statistically significant difference between pre-AT and post-AT to LLAEP for latencies of P2, N2, and P300 and amplitudes of N2 and P300, and to FFR for latency of wave C.
CONCLUSIONS: This study showed that electrophysiological tests are sensitive tools for identifying deficits in the auditory pathway. Moreover, latency measures can detect improvements from an auditory training program. In this way, an auditory intervention program might help children with reading and writing difficulties.
Keywords: Auditory Cortex, Cognition, Evoked Potentials, Simulation Training, Speech Perception, Child, Humans, Auditory Perceptual Disorders, Reading, Evoked Potentials, Auditory, Evoked Potentials, Auditory, Brain Stem, Acoustic Stimulation, Students
Background
Learning to read and write depends on the effective functioning of various sensory systems, including the auditory system. In recent years, there has been a growing interest in studying the development of children’s auditory skills, as there is evidence that children who have alterations in these abilities are more susceptible to disorders of language and learning [1–4].
Auditory stimuli reach the cerebral cortex via ipsilateral and contralateral pathways, carrying information about the timing and intensity of acoustic signals. Auditory information is conducted to the auditory area of the temporal lobe via the cochlear nuclei, superior olivary complex, lateral lemniscus, inferior colliculus, and medial geniculate body [5]. The latter structures are responsible for analyzing and interpreting auditory stimuli, that is, performing central auditory processing. Central auditory processing involves a range of behavioral and electrophysiological processes. Two ways of gauging auditory information processing involve behavioral and electrophysiological tests. In the present study, electrophysiological procedures were used to investigate activity in the auditory pathway in response to sound stimuli and the associated cortical activity in discrimination, integration, and attention [6].
According to the American Academy of Audiology, the auditory electrophysiology assessment can have an effective clinical value in screening children for auditory processing disorder [7]. The use of electrophysiological tests allows children who have difficulties and/or do not cooperate in carrying out behavioral tests to have a form of investigation and, mainly, allows for more reliable results regardless of the child’s cognitive, attentional, and language levels [8].
Electrophysiological testing can use verbal or non-verbal sound stimuli. The auditory brainstem response (ABR) allows the trajectory from the auditory nerve to the lateral lemniscus to be studied in terms of its response to a click, allowing the integrity of the auditory pathway to be assessed all the way to the brainstem [9].
Assessment by click ABR is recommended before using other procedures to analyze higher portions of the central auditory nervous system. The click ABR can be a helpful electrophysiological tool in the detection of auditory nerve integrity; however, its use for the identification of children with auditory processing disorder has not yet been clearly demonstrated. Thus, it is important to conduct new studies using the ABR click to verify its effectiveness [10]. However, there are studies that highlight the possibility of evaluating auditory evoked potentials by investigating the binaural interaction component that might be able to determine the presence of auditory processing disorder earlier than behavioral analysis [11].
The long-latency auditory-evoked potentials (LLAEP) evaluation is useful in testing patients with possible impairment in auditory information processing. The LLAEP provides an objective response to a cognitive task that requires attention, discrimination, recognition, perception, or auditory memory [6,7]. Significant differences in the latencies of the N1, P2, and P300 components are seen when the responses of children with difficulties in processing information are compared with that of their typical peers [12,13].
The components P1 and N1 can provide a marker of auditory cortex maturity in children with auditory processing disorder [14]. The component P2 is not generated by cortical structures but by several sensory modalities [15]. The component N2 can be related to the inhibitory response and could be an important marker of a problem in children with auditory processing disorder [16,17].
In general, the LLAEP, using either verbal or non-verbal stimuli, seems to be a good indicator of alterations in sound discrimination, attention, temporal auditory processing, and memory in children with language and learning difficulties. A prime target is children around 5 years of age, who are at the pre-reading stage, since any impairment can be treated with an intervention program [18–20]. One interesting study found that there seems to be a genetic component involved in LLAEP responses, since children diagnosed with specific language impairment process auditory information more slowly, as do their parents [21].
Another electrophysiological procedure that has been gaining prominence in the evaluation of individuals with speech and learning difficulties is the frequency following response (FFR). The choice to use the FFR derives from 2 important factors: (i) it uses verbal stimuli that are used in the listener’s daily life; and (ii) it evaluates the entire auditory system, since verbalizations are processed progressively from the ear to the auditory cortex. The auditory processing of speech involves complex aspects such as intensity, duration, frequency, discrimination, recognition, melody, rhythm, intonation, and temporal resolution [22–30]. Given the complexity of this type of sound stimulus and the neuronal activation throughout the entire auditory trajectory, it is understood that children with reading and writing difficulties have failures in the codification of speech, which could then lead to issues related to school impairment [22,24–29].
The electrophysiological assessment can open a new world for children with specific language impairments and auditory processing disorders owing to the possibility of early intervention in cases of probable impairments in speech perception and auditory perception, among others [22,24,27,31].
As soon as a deficit in central auditory processing is identified, auditory training (AT) is the recommended tool for rehabilitation of auditory skills. AT can be defined as the set of conditions and/or tasks designed for activating the auditory system and its associated systems. The changes in the central auditory nervous system that occur after AT are based on the plasticity of this system [30]. According to Sharma et al (2019) [31], some children can improve their auditory abilities by working on specific phonological processing, reading, and/or other abilities. In summary, this study aimed to evaluate electrophysiological testing for an auditory processing disorder and reading performance in 54 school students aged between 8 and 12 years.
Material and Methods
ETHICS:
This was an observational, quantitative cross-sectional study. The study was approved by the Ethics in Research Committee of the State University of Campinas under protocol no. 2.041.609. Data were collected from January 2018 to August 2019 at the Laboratory of Audiology, Faculty of Medical Sciences (FCM/UNICAMP). Written informed consent was obtained from all parents or guardians after explanation of the nature, purpose, and expected outcomes of the study.
PARTICIPANTS AND SAMPLE PROCEDURES:
A total of 50 children aged from 8 to 12 years old were involved. All were typically developing Brazilian-Portuguese native speakers who were right-handed and had documented normal hearing. The children were recruited from state public schools by invitation letters sent to their parents. Children who had letters signed were screened to have investigated, at their school, reading and writing performance. As well as assessing reading and writing skills and intelligence, hearing assessments were performed in an acoustically treated booth.
INCLUSION AND EXCLUSION CRITERIA:
Inclusion criteria were pure tone thresholds within normal limits, between 0.25 and 8 kHz [32]; no history of a neurological disorder; normal middle ear immittance (type A tympanograms) and the presence of contralateral acoustic reflexes in both ears [33]; and a non-verbal intelligence score as tested by the Raven Color Progressive Matrix test [34]. All children needed to achieve level III (intellectually average, a minimum 26th percentile) or above.
We separated the children into 2 basic groups based on reading and writing scores of the School Performance Test (SPT) [35]. Children who underperformed in the SPT (below the 26th percentile) were included in the study group, while the remainder were placed in a comparison control group. Thus, the control group was made up of 30 children without reading and writing difficulties as assessed by SPT, while there were 24 children with lower performance in the reading and writing tests who made up the study group. Most of the children in the study group (20 of the 24) underwent an acoustically controlled auditory training program and were reassessed about a month after 8 weeks of intervention. We designated this group of 20 children as study group II.
Exclusion criteria were hearing loss, diagnosis of neurodevelopmental disorders (attention deficit hyperactivity disorder, dyslexia, or others], history of other neurological or psychiatric condition, use of psychoactive drugs, and performance below the 26th percentile (level III, intellectually average).
RATING OF READING AND WRITING ABILITY: Rating of reading and writing ability was done in the school environment and involved application of the SPT [35], which covers the areas of reading, writing, and arithmetic. However, to assess the children’s verbal ability, only the single-word reading and writing tests were used. Only the ability to decode a word was used, and comprehension was not considered. The gross scores were converted into upper, middle, and lower rankings according to Brazilian standards for each grade level.
NON-VERBAL INTELLIGENCE: The children underwent a non-verbal intelligence assessment using the Raven Color Progressive Matrix test conducted by a neuropsychologist, according to Brazilian standards [34]. The Raven test is designed to measure a child’s reasoning ability with minimal reliance on language. For each item, the child selected 1 of 6 options to complete the matrix pattern.
PERIPHERAL AUDITORY SYSTEM: Audiometric evaluations were performed to assess air conduction thresholds at 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz using an Interacoustics AC40 audiometer and TDH39 earphones. The normality criterion for pure tone thresholds was the World Health Organization classification for children [32], for which the average threshold for frequencies of 0.5, 1, 2, and 4 kHz is less than 15 dB.
The speech recognition threshold was as follows: using a list of disyllables, the intensity at which the child correctly scored 50% of the words presented was determined.
The speech recognition index was as follows: the test was performed 40 dB above the tonal threshold of the mean of 0.5, 1, and 2 kHz using a list of monosyllabic words. The result was considered normal if the percentage of correct answers was between 88% and 100%.
Immittance audiometry (tympanometry and acoustic reflex) was performed with an Interacoustics AT235 audiometer. Tympanometry was performed using a 226 Hz tone. Ipsilateral and contralateral acoustic reflexes were tested at frequencies of 0.5, 1, 2, and 4 kHz. The participants all had a maximum compliance near 0 daPa and an equivalent volume of 0.3 to 1.3 mL, according to the proposal of Jerger [33].
ELECTROPHYSIOLOGICAL TESTING:
Electrophysiological evaluation was conducted using an IHS SmartEP and involved non-verbal and verbal stimuli. Electrode voltages were recorded while the children were sitting passively in a reclining chair, in a comfortable position within a sound-attenuating electrically shielded room. Before placing the electrodes, the children’s skin was cleaned with abrasive paste. The electrodes were fixed with an electrolytic paste, and adhesive tape was used to ensure a low impedance contact. The skin-electrode impedance was kept below 3 kΩ, and the inter-electrode impedance was kept below 2 kΩ.
AUDITORY BRAINSTEM RESPONSES: Testing aimed to verify the integrity of the brainstem, with measurement of wave I, III, and V amplitudes and interpeak latencies of I–III, I–V, and III–V. Insert phones (ER-3A; Natus Medical) with monoaural stimulation were used, and the stimuli were presented at an intensity of 80 dBHL. Skin electrodes were placed with the active electrode (Fz) on the forehead and the reference electrodes placed on the right (M2) and left (M1) mastoid, with the ground electrode in the Fpz position [36]. During a recording session, impedance was maintained below 5 kΩ and inter-electrode impedance below 3 kΩ. Stimulus parameters were 0.1 ms rarefaction clicks delivered at 19.3 clicks/s, low/high pass filter of 0.1 to 3 kHz, analysis window of 12 ms, and 2000 stimuli. To ensure reproducibility of the tracings, 2 recordings were made on each side. Normal values were those set out in the SmartEP manual.
LONG LATENCY AUDITORY EVOKED POTENTIALS: LLAEP were recorded with the active electrode positioned in the coronal plane (Cz), the reference electrode on the right (M2) or left mastoid (M1), and the ground electrode at Fz [36]. The right and left ears were evaluated separately. Two-channel equipment with a 1 to 30 Hz filter was used. Testing was done in 2 situations: (i) using non-verbal stimuli (1 kHz rare stimuli and 2 kHz frequent stimuli); and (ii) verbal stimuli (syllable /da/ as the rare stimulus and syllable /ba/ as the frequent stimulus). In both conditions, the parameters were monaural through insert earphones (ER-3A; Natus Medical) at 70 dBnHL. The stimuli were delivered monaurally through the earphones using an oddball paradigm: the rare (infrequent) stimulus at a 20% probability and the frequent stimulus at 80% probability, for a total of 300 stimuli. The presentation rate was 1 stimulus per s, and the results were analyzed within a 500-ms window. Participants were instructed to mentally count the rare stimulus, and the examiner checked their performance by asking at the end of the test how many had been counted. The components N1, P2, and N2 were marked on the frequent stimulus trace and P300 on the rare stimulus trace and were identified as positive polarity waves occurring at a latency of about 300 ms after stimulus. The normality criteria were those proposed by McPherson [6]. The children were instructed to remain with their eyes closed during the procedure and count the number of infrequent stimuli and say the number out loud. The order of the tests was randomized across participants (ie, for 50% of the participants the recording began on the right ear and for the remaining 50%, on the left).
FREQUENCY FOLLOWING RESPONSES:
FFRs were elicited using the synthetic speech syllable /da/ (40 ms duration) provided by the IHS device. The stimulus was presented monoaurally to the right and left ears using insert earphones (ER-3A; Natus Medical) at 80 dBSPL. Two blocks of 3000 artifact-free sweeps were collected, and both trials were combined to create an average wave of 6000 sweeps. Tracings of both recordings were used to identify and measure wave amplitudes and latencies. All analyses were conducted offline, and waves were visually identified and manually marked by 2 expert audiologists who were blinded about the age, sex, and group of each participant.
The parameters used for the FFR were as follows: verbal stimuli of alternating polarity, presentation rate of 10.9/s, low/high pass filter of 0.1 to 3 kHz, analysis window of 64 ms, and 3000 stimuli. Evaluation was carried out in the time domain. To this end, 7 waves were visually identified and marked manually: V, A, C, D, E, F, and O. Latency and amplitude values were then analyzed for each identified wave. The markings were also performed by an external researcher (a blinded examiner) and compared with the original markings. Agreement was found for both the ABR and the LLAEP.
All children were initially assessed audiologically, and then went on to either the central auditory processing behavioral battery or the electroacoustic and electrophysiological assessment, which were done in random order. In addition, the order of the tested ear was randomized (ie, 50% of patients began with recording of the right ear and the other 50% on the left).
AUDITORY TRAINING:
AT involved exercises from the website “Afinando o Cérebro” (
The exercises were chosen by the lead researcher with the objective of stimulating figure-ground auditory skills. They involved a binaural integration task, a monotic listening task that tested figure-ground resolution, and temporal ordering tests. The tasks followed the American Academy of Audiology criteria for presentation of varied stimuli and tasks, were graded in difficulty, and used positive reinforcement to maintain the child’s motivation. During the series of sessions, the exercises were performed only with the researcher present.
STATISTICAL ANALYSES:
The following variables were analyzed: age, sex, ear, and comparisons between groups. For the latter,
Results
PERIPHERAL AUDITORY SYSTEM:
In the basic audiological evaluation, there was a statistically significant difference between the groups only for the frequency of 6 kHz (
ELECTROPHYSIOLOGICAL TESTS:
Table 1 shows that there was a statistically significant difference between groups only for P300 latency. Children with reading and writing difficulties had longer P300 wave latency than did individuals without such difficulties. Table 2 shows that there was a statistically significant difference between groups for N1 latency and P300 latency and amplitude. Thus, children with reading and writing difficulties showed shorter latency for N1, longer latency for P300, and lower amplitude for P300 than children without such difficulties.
Table 3 shows that, for all FFR measures, there was no statistically significant difference between the groups. That is, children with or without reading and writing difficulties were effectively the same.
ELECTROPHYSIOLOGICAL PREDICTORS OF THE BENEFIT OF AUDITORY TRAINING:
Although study group I comprised 24 children, 4 of them did not want to take part in the auditory training program, and so study group II was a subset comprised of 20 children. Table 4 shows that there were statistically significant differences between the 2 assessment moments in the non-verbal LLAEP assessment in terms of latency of P1, N1, and P300. That is, after AT, the children with reading and writing difficulties showed a reduction in latency in all these waves. Figure 1 plots these values and shows that for these 3 waves, the 95% confidence intervals are clearly separated for the pre- and post-AT groups. Table 5 shows similar results for the verbally elicited LLAEP values. There is a statistically significant difference between pre-AT and post-AT evaluations in terms of the latencies of P2, N2, and P300, as well as the amplitudes of N2 and P300. Figure 2 represents the moment of the 2 evaluations (pre-AT and post-AT) in comparison with the control group, with a 95% confidence interval. Table 6 shows that for study group II there was a statistically significant difference between the pre-AT and post-AT evaluations in terms of the latency of wave C. Figure 3 plots the moments of the 2 evaluations (pre-AT and post-AT) compared with the control group, including 95% confidence intervals.
Discussion
PERIPHERAL AUDITORY SYSTEM:
When assessing auditory function, the first thing to consider is the performance of the peripheral auditory system. Normal results in basic audiological tests indicate that the structures involved are functioning well. In our study, there was a statistically significant difference between the groups only for the single test frequency of 6 kHz. However, in terms of average threshold, both groups were within the normal standard for children (5 dB) [32].
ELECTROPHYSIOLOGICAL TESTS:
Initial analysis showed no statistically significant difference for age, sex, or cognitive level between the groups.
Electrophysiological assessment is a way of probing the central auditory nervous system and is a sensitive instrument to test the effectiveness of auditory training [10,24]. In this study, ABR click responses were used to ensure the integrity of the auditory pathway [37,38]. Later, LLAEP (with verbal and non-verbal stimuli) and FFR were used.
Descriptive values and comparisons between groups (control group vs study group I) showed that statistically significant differences were observed only for P300 latency (based on LLAEP non-verbal). These findings are in line with previous studies, which have observed that individuals with reading and writing difficulties often have longer P300 wave latencies than those without. This finding suggests that children with reading and writing difficulties have auditory perceptual deficits involving longer response times, seen as greater latencies of the P300 component [39–44].
Here, electrophysiological testing used both non-verbal and verbal stimuli. Verbal stimuli require a more active and synchronized neural network, since it is necessary to analyze voice onset time as well as frequency, duration, intensity, and fundamental frequency of the stimulus. This means that verbal stimuli create a greater linguistic load than do non-verbal stimuli [21,45]. In children with reading and writing difficulties, use of verbal stimuli is important since the difficulties are associated with the processing of highly complex sounds.
When using a speech stimulus for the LLAEP, we found that more components were affected in children with reading and writing difficulties. First, in particular, we found disruption to the N1 wave, which is linked to the ability of auditory discrimination, and frequency and intensity modulations that are primary in the perceiving speech sounds. The findings of the present study corroborate previous studies that observed that the N1 component can be considered a biomarker for the analysis of auditory cortex maturity in children with auditory processing disorder [14]. Second, we found changes to the P300 wave, a feature that is linked to attention and hence to active participation in the perception of a sound stimulus. In other words, children with speech and writing difficulties have difficulty in paying attention, especially to a complex sound stimulus that requires accurate processing. Therefore, these children may not be able to follow instructions or understand explanations given by a teacher [29,46,47].
When comparing the responses of children with reading and writing difficulties with those of their typical peers, this research did not find any changes in the time domain FFR. Here, our findings differ from those of other studies that found that in children with a language and learning disorder, such as central auditory processing disorder, the children show delays in latency for the onset portion, indicating a deficit in brainstem neural synchrony for complex sounds [22,27,28]. However, a frequency domain analysis may overcome the limitation found with the time domain approach.
ELECTROPHYSIOLOGICAL TESTS AS PREDICTORS OF THE BENEFIT OF AUDITORY TRAINING:
Good auditory development in a child depends on an interplay of biological and environmental factors. For example, social stimulation, both affective and sensory, will contribute to cognitive development, language, and auditory pathways [48]. When proper development does not happen, perhaps due to environmental factors, such as the presence of recurrent otitis media [49], the child can develop a central auditory or language processing disorder. AT is the recommended tool for activating the auditory and associated systems and rehabilitating auditory skills. The changes in the central auditory nervous system that occur after AT rely on the plasticity of the central auditory nervous system, which means the system can adapt to immediate environmental influences and improve response times [30].
The AT program used in this research involved exercises based on bottom-up and top-down training, which aim to improve auditory discrimination, attention, and memory, creating an increase in neural connections. There are various ways to monitor the benefits of an auditory training program: batteries of behavioral tests, electrophysiological tests of auditory processing, or a combination of the two [30,50]. The present study used electrophysiological methods, since it is known that well-developed auditory training programs promote neural reorganization.
According to Flexer et al (2019), hearing occurs in the brain, and the ears are only the gateway to this system [51]. This underlines the importance of evaluating the central auditory pathways electrophysiologically. Several authors also report that listening is in fact the most effective way of developing spoken or written language skills; thus, the auditory skills of children with reading and writing difficulties should be closely monitored, looking for anomalies in the auditory pathways [52,53]. For this reason, the electrophysiological assessment can open a new world for children with specific language impairment and auditory processing disorder [22,24,27,31].
Turning to non-verbal stimuli (tone bursts), the present study with LLAEP found that children with reading and writing difficulties also showed improvements in the latencies of P1, N1, and P300 after the AT program. These findings confirm work that has found that exercises can promote neuronal reorganization, perhaps due to activation of the thalamus, which is considered the generator of the P1 component [54]. In addition, the AT program promotes selective attention, a function involving the supratemporal auditory cortex, a structure associated with the generation of the N1 component [14,47]. It is also worth noting that the P1 and N1 components respond to spectral characteristics of a sound stimulus [46]. Finally, changes in the P300 component can be related to cognitive functions, such as decision-making, attention, discrimination, recognition, integration, focus, and memory [41–43]. All of this corroborates findings that AT tends to reduce the latency of the P300 component [55].
As for P2, we found that, based on the LLAEP with verbal stimuli, there was a decrease in its latency. We know that there is an increase in the amplitude values of the P2 and P300 components in children with reading and writing difficulties. According to Sussman et al [56] and Eggermont et al [57], the P2 component is generated by the primary auditory cortex and depends on the characteristics of the presented sound stimulus. We presume that, by carrying out AT, our group of children had the opportunity to be exposed to complex sound stimuli and were trained to recognize fine differences in stimuli, such as the voice onset time differences between the syllables /da/ and /ba/. These improvements may have been due to the activation of neurons responsive to complex speech. These findings are in line with the literature, which indicates that the latency of the P2 component derives from several sensory modalities [15] and could be a biological marker of learning problems [56–58].
The N2 component is commonly associated with pre-attentional processes involved in the conscious discrimination of sound stimuli. This would explain why children with mild to moderate sensorineural hearing loss have a weak N2. Research has shown that the N2 is delayed even when stimulated with electronic devices [59,60]. Thus, improvements in the amplitudes and latencies of the N2 seen in the present study confirm that the AT program was effective in improving the auditory skills of children with reading and writing difficulties.
Finally, we need to consider the P300 component, in which we saw an improvement (decrease) in latency after AT. The P300 component represents the final stage of auditory information processing, being generated in the cortex after information has passed along the entire auditory trajectory. Based on tonotopic information coming from the cochlea, especially its frequency components, the P300 emerges from activation of the primary auditory cortex. However, activation of the secondary auditory cortex plays a key role in the processing of non-verbal sounds and the extraction of meaning from verbal sounds. Also, interactions between the brain lobes need to be recognized. In the present study, the reduction in P300 latencies was presumably due to greater neural activation and more efficient connections with the frontal lobes, which are responsible for decision-making and attention to auditory stimuli [61–63]. The results of the present study are in line with those of a previous study that reported, following AT, a decrease in P300 wave latency in children with language, learning, or speech difficulties [64].
As outlined above, for an individual to be able to successfully process the auditory information derived from a verbal sound, the central auditory system needs to detect, within a very short space of time, the essential elements of the utterance [23,25–27]. The coding of speech occurs throughout the entire auditory trajectory, and studies demonstrate that individuals with difficulties or delays in the development of speech, language, or learning need to be closely assessed [22,26,27,50,65]. For this purpose, the FFR is a good choice since it is an objective test that does not need the conscious and active participation of the participant [29]. In the present study, the FFR responses showed, after AT, a decrease in latency exclusively in wave C. The sources of wave C in the FFR are still not well defined; however, it is known that wave C represents the transition between the perception of a consonant and a vowel: in the present study, in the syllable /da/ at 40 ms. That is, the results show that there was an improvement in the children’s ability to identify the transition from the consonant /d/ to the vowel /a/ [23,27]. The auditory training program appears to be an effective tool for promoting neuronal modifications that improve the perception of small changes in time, frequency, and duration. In turn, these improvements can benefit the children in how they receive auditory information and improve their ability to read and write [31].
The AT program is a promising intervention in children with reading and writing difficulties, since, over a number of weeks, significant improvements were observed in the performance of electrophysiological tests. This intervention promotes the rehabilitation of bottom-up and top-down processes that contribute significantly to the learning process. The AT program could well become a strategy of first choice in the rehabilitation process, minimizing difficulties and intervention time.
A major limitation of the present study involves the way the FFR was performed, since we found that analysis in the time domain was not able to differentiate between the groups. We believe that a frequency domain analysis might provide a better perspective on how speech sounds are encoded. Unfortunately, due to equipment limitations, it was not possible to perform such an analysis here.
Conclusions
The results of the present study showed that electrophysiological tests appear to be sensitive tools for identifying deficits in the auditory pathway. Moreover, latency measures are able to detect improvements brought about by an AT program. Improvements in central auditory processing skills can help minimize reading and writing difficulties. Electrophysiological tests are ideally suited to identifying alterations in the system and can measure the progress of intervention programs. In this way, it is possible to say that an auditory intervention program such as that used in this research can be effective in overcoming problems in children with reading and writing difficulties.
Figures
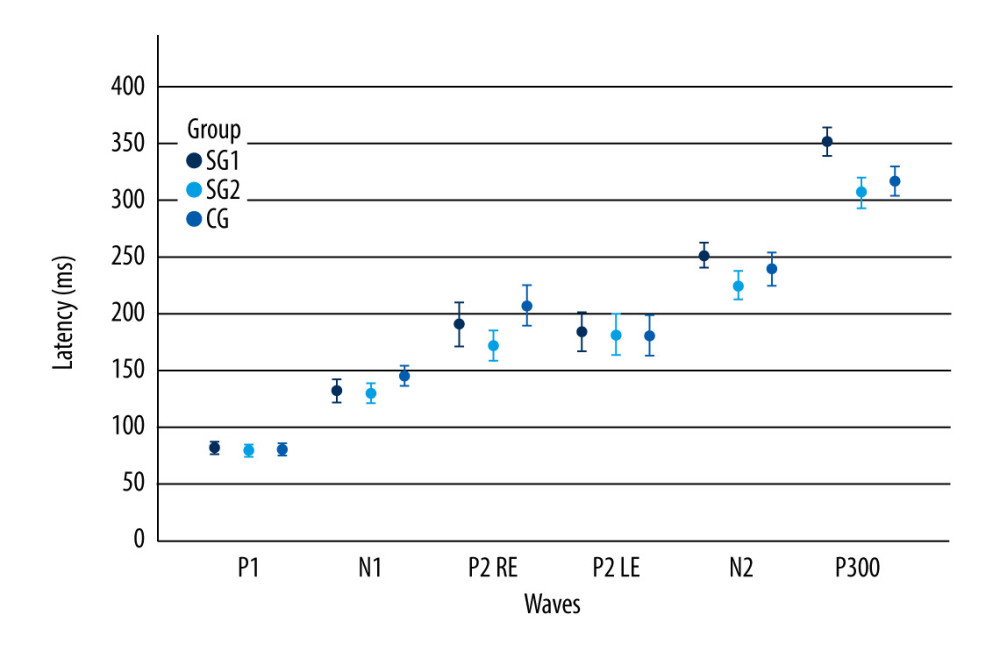 Figure 1. Long-latency auditory-evoked potentials non-verbal latency values according to the moment of the evaluation and component. The error bars represent the 95% confidence interval. SGI – study group I; SGII – study group II; CG – control group; RE – right ear; LE – left ear.
Figure 1. Long-latency auditory-evoked potentials non-verbal latency values according to the moment of the evaluation and component. The error bars represent the 95% confidence interval. SGI – study group I; SGII – study group II; CG – control group; RE – right ear; LE – left ear. 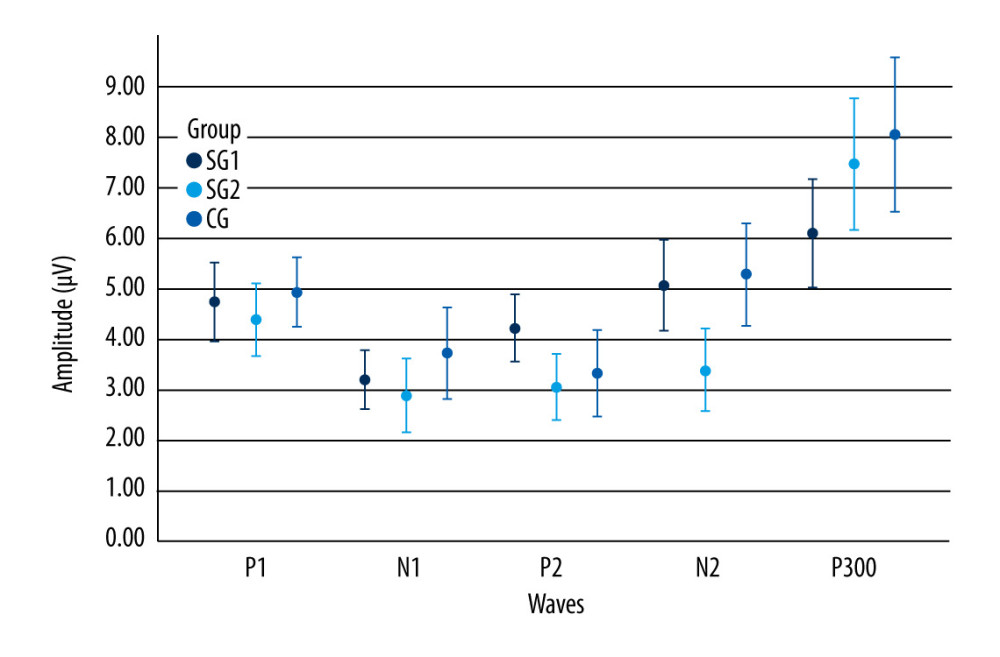 Figure 2. Long-latency auditory-evoked potentials verbal latency values according to wave component and moment of evaluation. Error bars show 95% confidence intervals. SGI – study group I; SGII – study group II; CG – control group.
Figure 2. Long-latency auditory-evoked potentials verbal latency values according to wave component and moment of evaluation. Error bars show 95% confidence intervals. SGI – study group I; SGII – study group II; CG – control group. 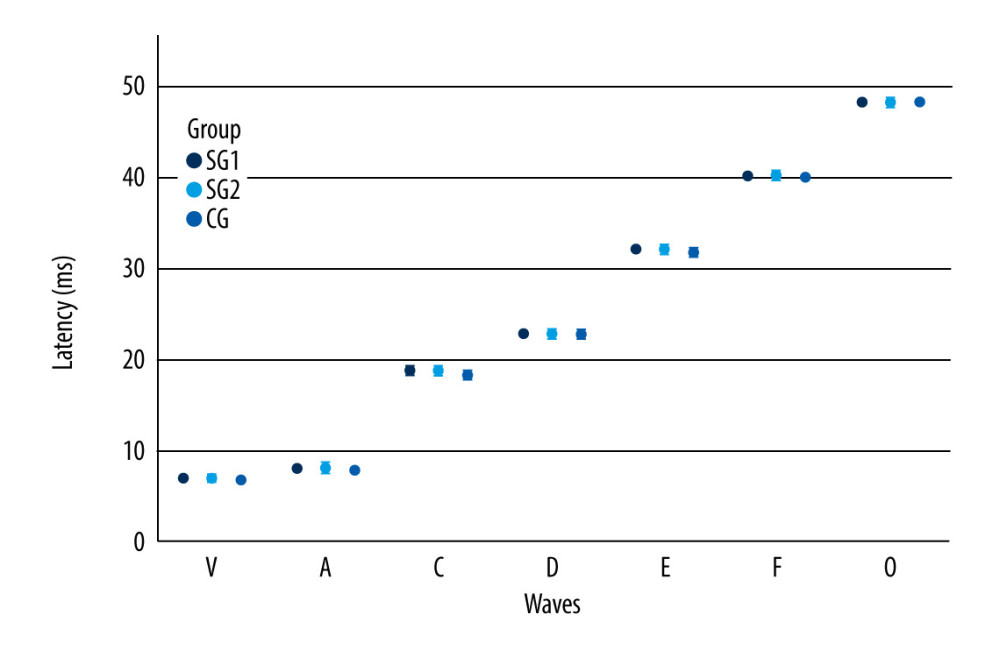 Figure 3. Frequency following responses values according to the moment of the evaluation and component. Error bars show 95% confidence intervals. SGI – study group I; SGII – study group II; CG – control group.
Figure 3. Frequency following responses values according to the moment of the evaluation and component. Error bars show 95% confidence intervals. SGI – study group I; SGII – study group II; CG – control group. Tables
Table 1. Descriptive values and comparison of groups for long-latency auditory-evoked non-verbal.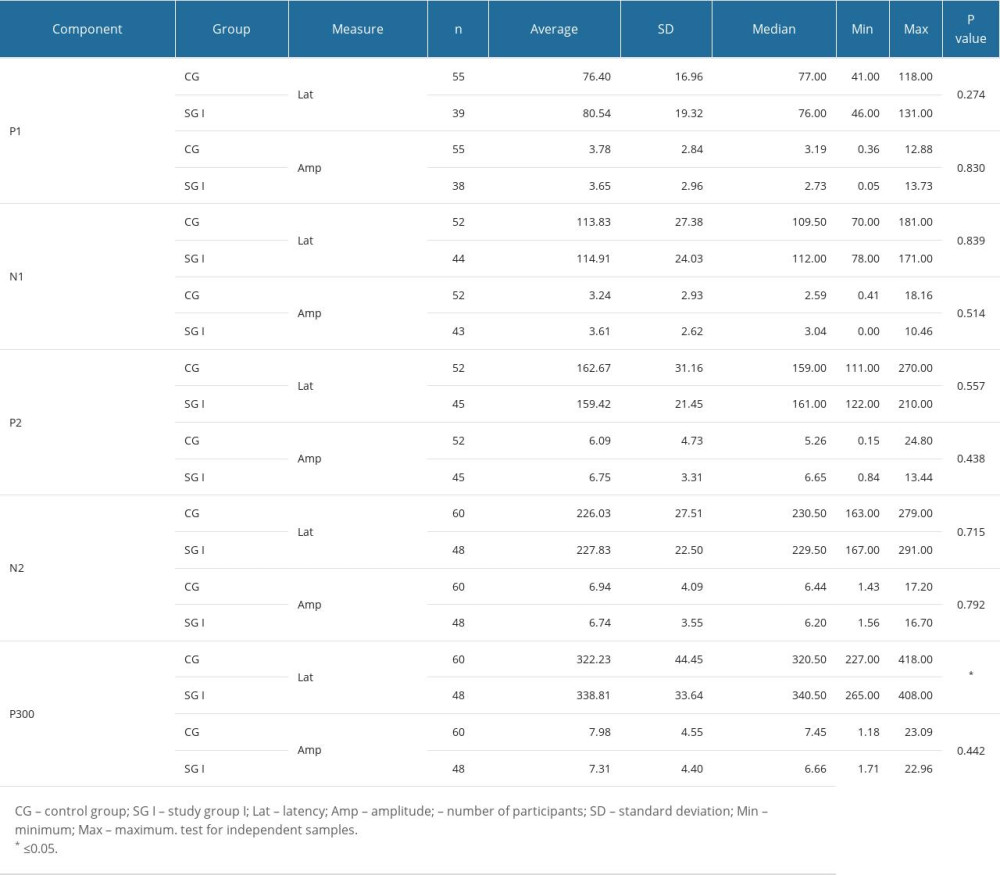 Table 2. Descriptive values and comparison of groups for long-latency auditory-evoked verbal stimuli.
Table 2. Descriptive values and comparison of groups for long-latency auditory-evoked verbal stimuli.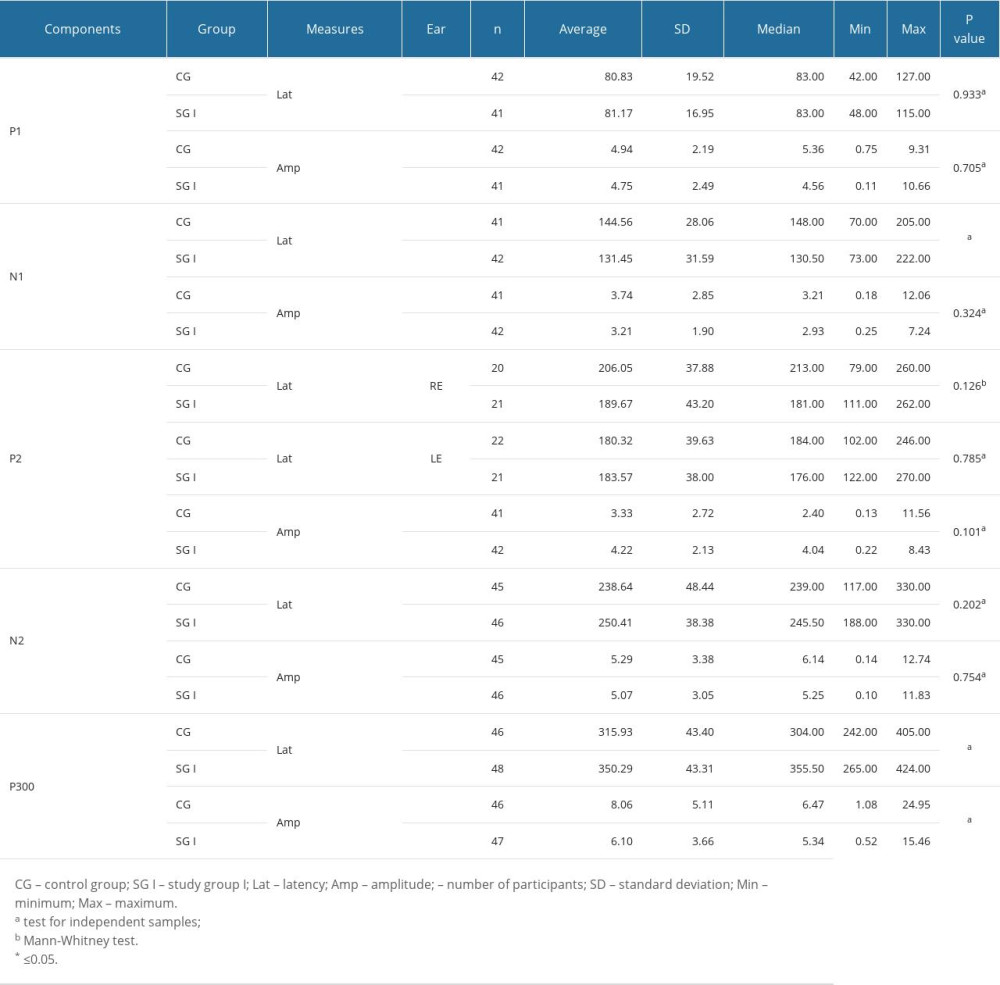 Table 3. Comparison of the groups for frequency following responses measurements.
Table 3. Comparison of the groups for frequency following responses measurements.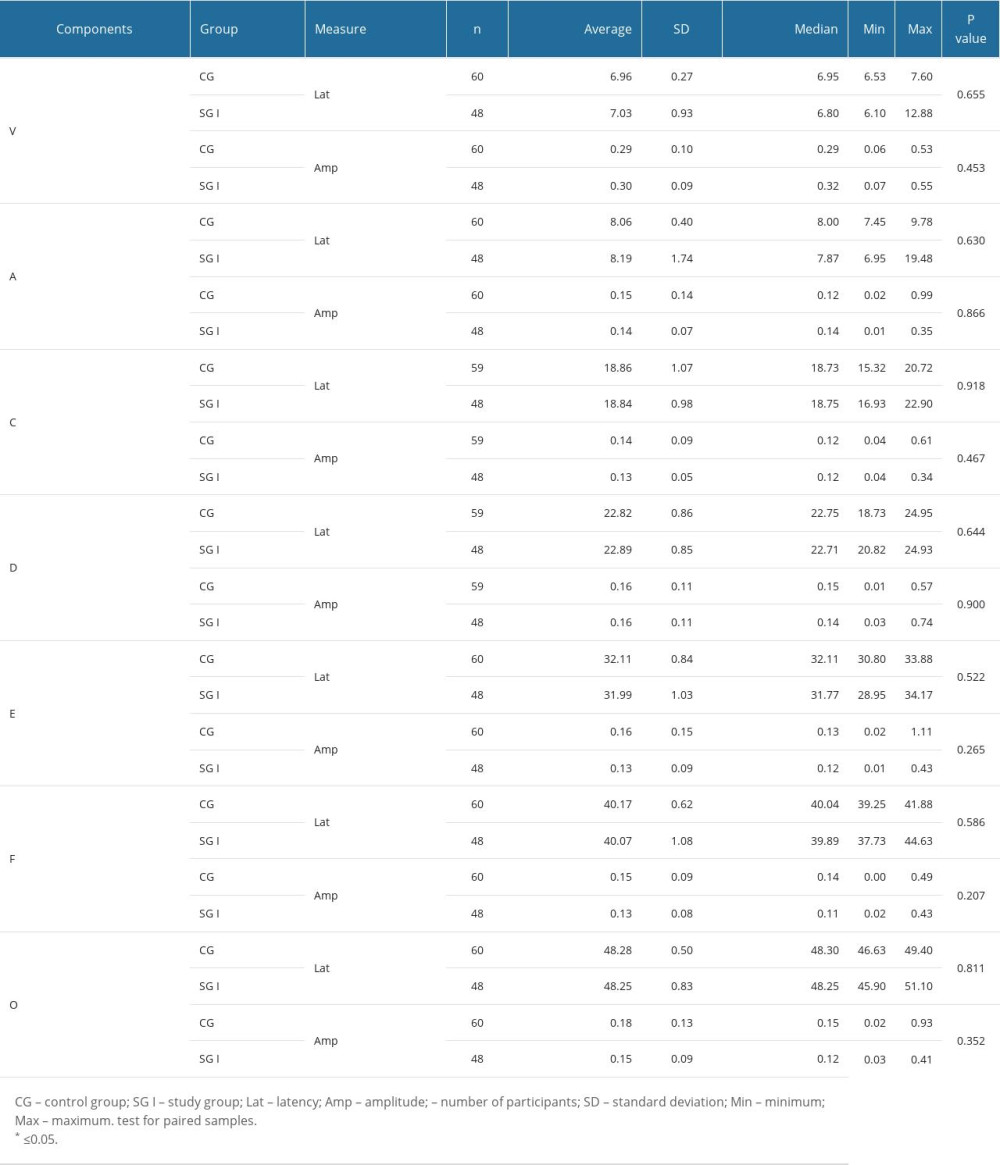 Table 4. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked non-verbal measurements.
Table 4. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked non-verbal measurements.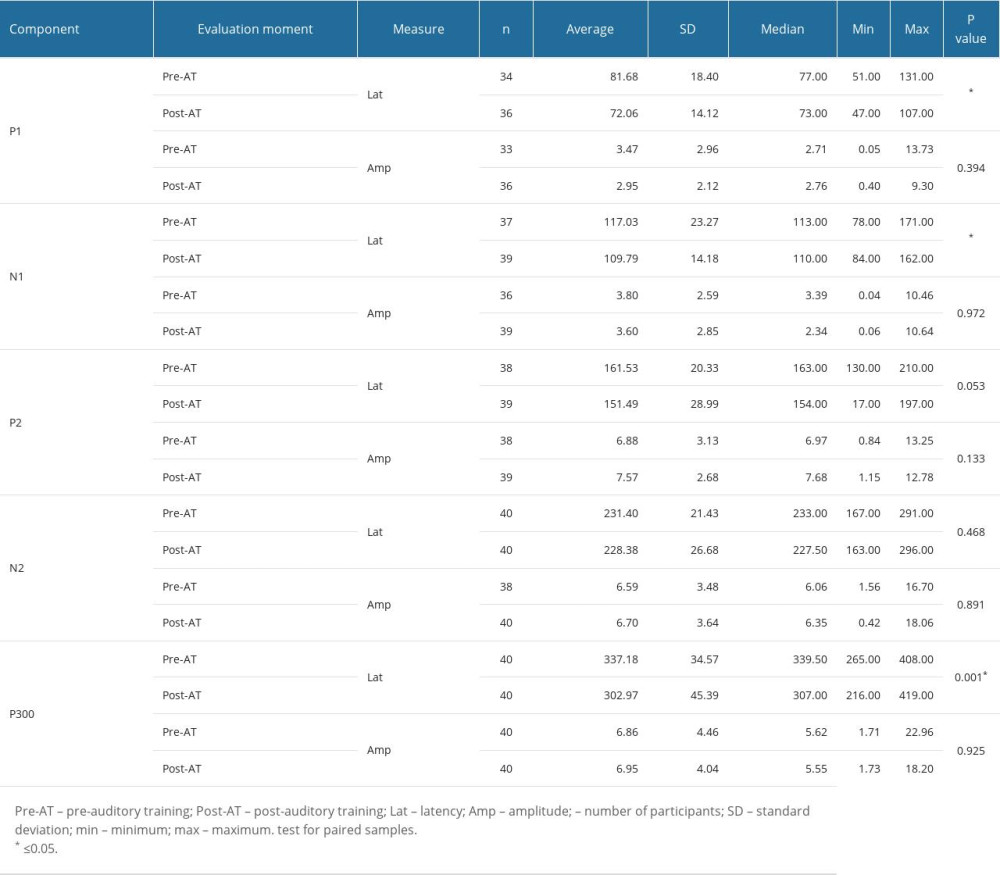 Table 5. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked verbal measurements.
Table 5. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked verbal measurements.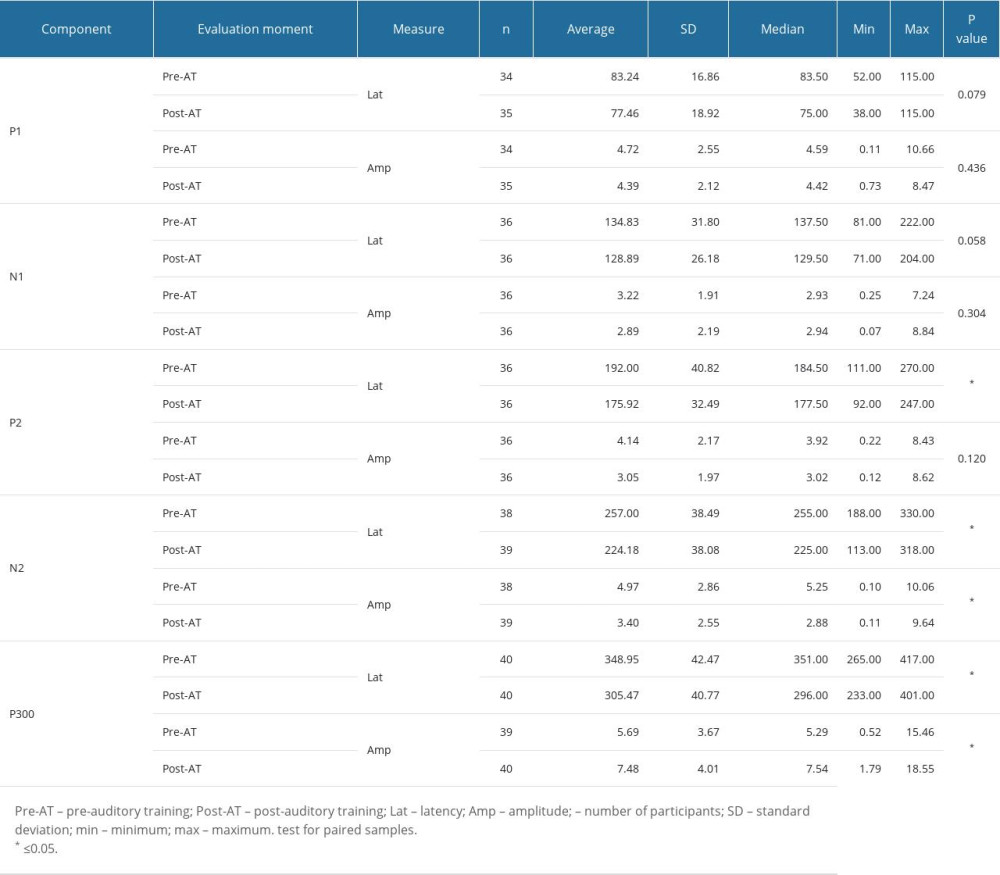 Table 6. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-training) for frequency following responses measurements.
Table 6. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-training) for frequency following responses measurements.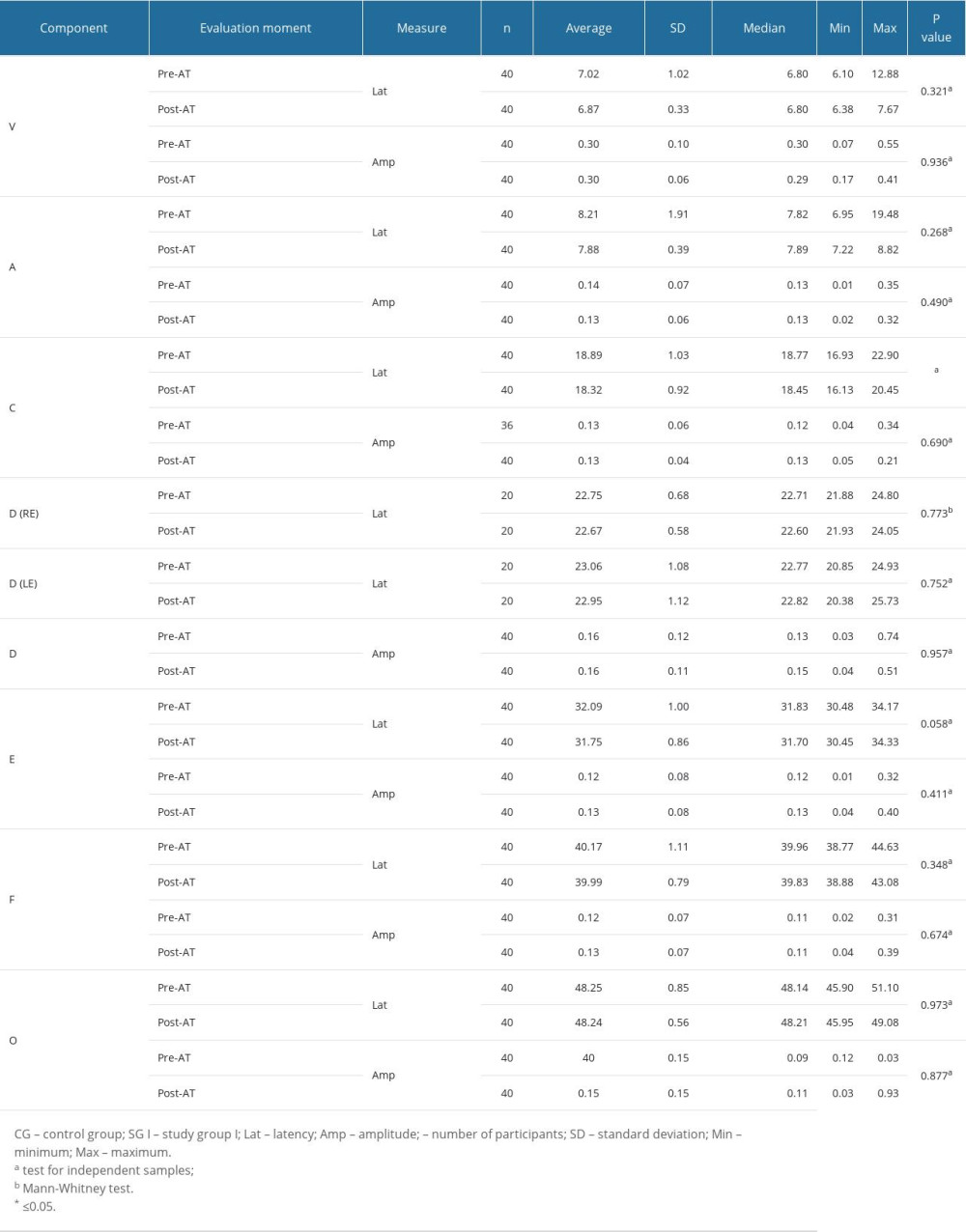
References
1. Boscariol M, Guimarães CA, Hage SR, Auditory processing disorder in patients with language-learning impairment and correlation with malformation of cortical development: Brain Dev, 2011; 33(10); 824-31
2. Tallal P, Auditory temporal perception, phonics, and reading disabilities in children: Brain Lang, 1980; 9(2); 182-98
3. Mengler ED, Hogben JH, Michie P, Bishop DV, Poor frequency discrimination is related to oral language disorder in children: A psychoacoustic study: Dyslexia, 2005; 11(3); 155-73
4. Rawool VW, Temporal processing in the auditory system: Auditory processing disorders: Assessment, management and treatment, 2007; 227-49, San Diego, Plural Publishing
5. Guinan JJ, Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans: Ear and Hearing, 2006; 27(6); 589-607
6. McPherson DL: Late potentials of the auditory system, 1996, San Diego, Singular Publishing Group
7. American Academy of Audiology: Clinical practice guidelines: Diagnosis, treatment, and management of children and adults with central auditory processing disorders August, 2010 Available from: https//audiology-web.s3.amazonaws.com/migrated/CAPD%20Guidelines%208-2010.pdf_539952af956c79.73897613.pdf
8. Sininger YS, Doyle KJ, Moore JK, The case for early identification of hearing loss in children: auditory system development, experimental auditory deprivation, and development of speech perception and hearing: Pediatr Clin North Am, 1999; 46; 1-14
9. Burkard R, Don M, Eggermont J: Auditory evoked potentials: Basic principles and clinical application, 2007, Lippincott Williams & Wilkins
10. Liu P, Zhu H, Chen M, Electrophysiological screening for children with suspected auditory processing disorder: A systematic review: Front Neurol, 2021; 12; 692840
11. Abdollahi FZ, Lotfi Y, Moosavi A, Bakhshi E, Binaural interaction component of middle latency response in children suspected to central auditory processing disorder: Indian J Otolaryngol Head Neck Surg, 2019; 71(2); 182-85
12. Jirsa RE, The clinical utility of the P3 AERP in children with auditory processing disorders: J Speech Hear Res, 1992; 35(4); 903-12
13. Jirsa RE, Clontz KB, Long latency auditory event-related potentials from children with auditory processing disorders: Ear Hear, 1990; 11(3); 222-32
14. Tomlin D, Rance G, Maturation of the central auditory nervous system in children with auditory processing disorder: Semin Hear, 2016; 37(1); 74-83
15. Ponton CW, Eggermont JJ, Kwong B, Don M, Maturation of human central auditory system activity: Evidence from multi-channel evoked potentials: Clin Neurophysiol, 2000; 111; 220-36
16. Stothart G, Kazanina N, Auditory perception in the aging brain: The roleof inhibition and facilitation in early processing: Neurobiol Aging, 2016; 47; 23-34
17. Koravand A, Jutras B, Lassonde M, Abnormalities in cortical auditory responses in children with central auditory processing disorder: Neuroscience, 2017; 346; 135-48
18. Gilley PM, Sharma A, Dorman M, Martin K, Abnormalities in central auditory maturation in children with language-based learning problems: Clin Neurophysiol, 2006; 117(9); 1949-56
19. Vanwooren S, Poelmans H, Hofmann M, Hemispheric asymmetry in auditory processing of speech envelope modulations in prereading children: J Neurosci, 2014; 22; 1523-29
20. Davies PL, Chang WP, Gavin WJ, Middle and late latency ERP components discriminate between adults, typical children, and children with sensory processing disorders: Front Integr Neurosci, 2010; 4; 16
21. Ors M, Lindgren M, Blennow G, Rosén I, Auditory event-related brain potentials in parents of children with specific language impairment: Eur J Paediatr Neurol, 2002; 6(5); 249-60
22. Ferreira L, Gubiani MB, Keske-Soares M, Analysis of the components of frequency-following response in phonological disorders: Int J Pediatr Otorhinolaryngol, 2019; 122; 47-51
23. Skoe E, Kraus N, Auditory brainstem response to complex sounds: A tutorial: Ear Hear, 2010; 31(3); 302-24
24. Sanfins MD, Donadon C, Skarzynski PH, Colella-Santos MF, Electrophysiology and Auditory Training: Auditory system – function and disorders [Internet], 2021, London, Intech Open, doi: 10.5772/intechopen.101826 Available from: https//www.intechopen.com/chapters/79860
25. Johnson KL, Nicol TG, Kraus N, Brain stem response to speech: A biological marker of auditory processing: Ear Hear, 2005; 26(5); 424-34
26. Wible B, Nicol T, Kraus N, Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems: Biol Psychol, 2004; 67(3); 299-317
27. Sanfins MD, Borges LR, Ubiali T, Colella-Santos MF, Speech auditory brainstem response (speech ABR) in the differential diagnosis of scholastic difficulties: Braz J Otorhinolaryngol, 2017; 83(1); 112-16
28. Kouni SN, Giannopoulos S, Ziavra N, Koutsojannis C, Brainstem auditory evoked potentials with the use of acoustic clicks and complex verbal sounds in young adults with learning disabilities: Am J Otolaryngol, 2013; 34(6); 646-51
29. Sanfins MD, Colella-Santos MF, Frequency following response: Tratado de eletrofisiologia para a audiologia, 2018; 97-116, Ribeirão Preto, Book Toy
30. Musiek FE, Chermak GD, Weihing J, Auditory training: Handbook of central auditory processing disorder – comprehensive intervention, 2014; 157-200, San Diego, Plural Publishing
31. Sharma M, Purdy SC, Humburg P, Cluster analyses reveals subgroups of children with suspected auditory processing disorders: Front Psychol, 2019; 10 article 2481
32. World Health Organization: Grades of hearing impairment, 2014 Available from: https://www.who.int/pbd/deafness/hearing_impairment_grades/en/
33. Jerger J, Clinical experience with impedance audiometry: Arch Otolaryngol, 1970; 92(4); 311-24
34. Raven J, Raven JC, Court JH: Matrizes progressivas coloridas de raven : Manual, 2018; 144, São Paulo, Pearson Clinical Brasil [in Portuguese]
35. Stein LM: TDE: Teste de desempenho escolar: Manual para aplicação e interpretação, 2011, São Paulo, Casapsi Livraria e editora Ltda [in Portuguese]
36. Jasper HA, The ten-twenty system of the International Federation: Electroenceph Clin Neurophysiol, 1958; 10; 371-75
37. Jewett DL, Williston JS, Auditory evoked far fields averaged from the scalp of humans: Brain, 1971; 94(4); 681-96
38. Picton TW: Human auditory evoked potentials, 2011, San Diego (CA), Plural Publishing
39. Wiemes GR, Kozlowski L, Mocellin M, Cognitive evoked potentials and central auditory processing in children with reading and writing disorders: Braz J Otorhinolaryngol, 2012; 78(3); 91-97
40. Diniz J, Mangabeira Albernaz PL, Munhoz MSL, Fukuda Y, Cognitive potentials in children with learning disabilities: Acta Oto-Laryngologica, 1997; 117(2); 211-13
41. Ferrazoli N, Donadon C, Rezende A, The Application of P300-long-latency auditory-evoked potential in Parkinson disease: Int Arch Otorhinolaryngol, 2022; 26(1); 158-66
42. Picton TW, The P300 wave of the human event-related potential: J Clin Neurophysiol, 1992; 9(04); 456-79
43. Sanfins M, Donadon C, Borges LR, Long-term effects of unilateral and bilateral otitis media and myringotomy on long-latency verbal and non-verbal auditory-evoked potentials: Int Arch Otorhinolaryngol, 2020; 24(4); e413-e22
44. Regaçone SF, Gução ACB, Giacheti CM, Potenciais evocados auditivos de longa latência em escolares com transtornos específicos de aprendizagem: Audiol Commun Res, 2014; 19(1); 13-18 [in Portuguese]
45. Fu M, Wang L, Zhang M, A mismatch negativity study in Mandarin-speaking children with sensorineural hearing loss: Int J Pediatr Otorhinolaryngol, 2016; 91(3); 128-40
46. Massa CG, Rabelo CM, Matas CG, P300 with verbal and nonverbal stimuli in normal hearing adults: Braz J Otorhinolaryngol, 2011; 77(6); 686-90
47. Alvarenga Kde F, Vicente LC, Lopes RC, The influence of speech stimuli contrast in cortical auditory evoked potentials: Braz J Otorhinolaryngol, 2013; 79(3); 336-41
48. Chonchaiya W, Tardif T, Mai X, Developmental trends in auditory processing can provide early predictions of language acquisition in young infants: Dev Sci, 2013; 16(2); 159-72
49. Borges LR, Paschoal JR, Colella-Santos MF, (Central) auditory processing: The impact of otitis media: Clinics (Sao Paulo), 2013; 68(7); 954-59
50. Donadon C, Sanfins MD, Borges LR, Colella-Santos MF, Auditory training: Effects on auditory abilities in children with history of otitis media: Int J Pediatr Otorhinolaryngol, 2019; 118; 177-80
51. Flexer C, Madell JR, Wolfe J, Schafer EC, Hearing Loss: Essential information: Pediatric audiology: Diagnosis, technology and management, 2019, New York, Thieme
52. Ching TYC, Dillon H, Major findings of the LOCHI study on children at 3 years of age and implications for audiologic management: Int J Audiol, 2013; 52(Suppl 2); 65S-68S
53. Dettman SJ, Dowell RC, Choo D, Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicenter Study: Otol Neurotol, 2016; 37(2); 82-95
54. Holschneider DP, Yang J, Guo Y, Maarek JM, Reorganization of functional brain maps after exercise training: Importance of cerebellar-thalamic-cortical pathway: Brain Res, 2007; 1184; 96-107
55. Tremblay KL, Training-related changes in the brain: Evidence from human auditory-evoked potentials: Semin Hear, 2007; 28; 120-32
56. Sussman E, Steinschneider M, Gumenyuk V, The maturation of human evoked brain potentials to sounds presented at different stimulus rates: Hear Res, 2008; 236(1–2); 61-79
57. Eggermont JJ, Ponton CW, Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: Correlations with changes in structure and speech perception: Acta Otolaryngol, 2003; 123(2); 249-52
58. Sharma A, Campbell J, Cardon G, Developmental and cross-modal plasticity in deafness: evidence from the P1 and N1 event related potentials in cochlear implanted children: Int J Psychophysiol, 2015; 95(2); 135-44
59. Hassaan MR, Aided evoked cortical potential: An objective validation tool for hearing aid benefit: Egypt J Ear Nose Throat Allied Sci, 2011(12); 155-61
60. Koravand A, Jutras B, Lassonde M, Cortical auditory evoked potentials in children with a hearing loss: A pilot study: Int J Pediatr, 2012; 2012; 250254
61. Bao S, Chang EF, Woods J, Merzenich MM, Temporal plasticity in the primary auditory cortex induced by operant perceptual learning: Nat Neurosci, 2004; 7(9); 974-81
62. Sanfins MD, Matas CG, Potencial evocado auditivo de longa latência (PEALL): Potencial cognitivo (P300): Manual de eletrofisiologia e eletroacústica, 2022; 251-63, Ribeirão Preto, Booktoy [in Portuguese]
63. Squires K, Hillyard S, Lindsay P, Cortical potentials evoked by feedback confirming and disconfirming an auditory discrimination: Percept Psychophysiol, 1973; 13; 25-31
64. Hecox K, Hogan K, Auditory evoked potentials in the language impairment: Event-related potentials in children, 1982; 99-116, Amsterdam, Elsevier Biomedical
65. Cordeiro FP, Weber MPVM, Quintino C, Assessment of learning disorder using the frequency following response: Systematic review: J Hear Sci, 2020; 10(3); 9-18
Figures
 Figure 1. Long-latency auditory-evoked potentials non-verbal latency values according to the moment of the evaluation and component. The error bars represent the 95% confidence interval. SGI – study group I; SGII – study group II; CG – control group; RE – right ear; LE – left ear.
Figure 1. Long-latency auditory-evoked potentials non-verbal latency values according to the moment of the evaluation and component. The error bars represent the 95% confidence interval. SGI – study group I; SGII – study group II; CG – control group; RE – right ear; LE – left ear. Figure 2. Long-latency auditory-evoked potentials verbal latency values according to wave component and moment of evaluation. Error bars show 95% confidence intervals. SGI – study group I; SGII – study group II; CG – control group.
Figure 2. Long-latency auditory-evoked potentials verbal latency values according to wave component and moment of evaluation. Error bars show 95% confidence intervals. SGI – study group I; SGII – study group II; CG – control group. Figure 3. Frequency following responses values according to the moment of the evaluation and component. Error bars show 95% confidence intervals. SGI – study group I; SGII – study group II; CG – control group.
Figure 3. Frequency following responses values according to the moment of the evaluation and component. Error bars show 95% confidence intervals. SGI – study group I; SGII – study group II; CG – control group. Tables
 Table 1. Descriptive values and comparison of groups for long-latency auditory-evoked non-verbal.
Table 1. Descriptive values and comparison of groups for long-latency auditory-evoked non-verbal. Table 2. Descriptive values and comparison of groups for long-latency auditory-evoked verbal stimuli.
Table 2. Descriptive values and comparison of groups for long-latency auditory-evoked verbal stimuli. Table 3. Comparison of the groups for frequency following responses measurements.
Table 3. Comparison of the groups for frequency following responses measurements. Table 4. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked non-verbal measurements.
Table 4. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked non-verbal measurements. Table 5. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked verbal measurements.
Table 5. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked verbal measurements. Table 6. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-training) for frequency following responses measurements.
Table 6. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-training) for frequency following responses measurements. Table 1. Descriptive values and comparison of groups for long-latency auditory-evoked non-verbal.
Table 1. Descriptive values and comparison of groups for long-latency auditory-evoked non-verbal. Table 2. Descriptive values and comparison of groups for long-latency auditory-evoked verbal stimuli.
Table 2. Descriptive values and comparison of groups for long-latency auditory-evoked verbal stimuli. Table 3. Comparison of the groups for frequency following responses measurements.
Table 3. Comparison of the groups for frequency following responses measurements. Table 4. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked non-verbal measurements.
Table 4. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked non-verbal measurements. Table 5. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked verbal measurements.
Table 5. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-auditory training) for long-latency auditory-evoked verbal measurements. Table 6. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-training) for frequency following responses measurements.
Table 6. Comparison of study group II in terms of 2 evaluation moments (pre-auditory training and post-training) for frequency following responses measurements. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








