21 July 2023: Clinical Research
Ultrasound-Guided Pectoserratus Plane Block and Superficial Serratus Anterior Plane Block for Subcutaneous Implantable Cardioverter-Defibrillator Implantation: A Comparative Study
Marek SzamborskiDOI: 10.12659/MSM.940541
Med Sci Monit 2023; 29:e940541
Abstract
BACKGROUND: The combination of pectoserratus plane block (PSP) and superficial serratus anterior plane block (S-SAP) was established to reduce the risk of general anesthesia for subcutaneous implantable cardioverter-defibrillator (S-ICD) implantation in patients with high operative risk (American Society of Anesthesiologistsgrade III or IV). This study compared outcomes from ultrasound-guided PSP and S-SAP in 16 patients requiring a subcutaneous implantable cardioverter-defibrillator (S-ICD) at a single center in Poland.
MATERIAL AND METHODS: A group of 16 patients with ASA grade III and IV qualified for S-ICD implantation was included. The pain assessment using numerical rating scale (NRS), patient’s comfort using Quality of Recovery-15 (QoR-15), the operator’s satisfaction using Operator’s Comfort Scale, adverse event occurrence, and the parameters’ stability were evaluated.
RESULTS: The mean volume of the local anesthetics mixture of PSP block was 19.4 mL; S-SAP was 34.7 mL (mean total volume, 54.1 mL). The mean duration of the block was 21.3 min; the mean time of the S-ICD implantation was 108.4 min. Neither circulatory nor respiratory instability was observed. In 8 patients (50%), non-opioid analgesics were administered intraoperatively; in 11 patients (69%), fentanyl bolus ≤200 μg was administered. The intraoperative NRS score was low (max 2 points); NRS 24 h after the procedure was low (max 4 points). The mean value of QoR-15 was 133.9 points.
CONCLUSIONS: S-SAP combined with PSP is feasible and safe in providing anesthesia/analgesia during S-ICD implantation and showed good effects in a group of patients with high operative risk (ASA III or IV).
Keywords: Anesthesia, Cardiac Procedures, Anesthesia, Local, Anesthetics, Combined, cardiac resynchronization therapy, Patient safety, Humans, Defibrillators, Implantable, Nerve Block, Anesthesia, General, Anesthetics, Local, Ultrasonography, Interventional, Pain, Postoperative
Background
The first implantation of the classic cardioverter-defibrillator was conducted in 1980, and the procedure has become the standard electrotherapy for dangerous arrhythmias in thousands of patients worldwide [1–5]. The device consists of a defibrillating electrode in the right ventricle connected to a battery and an electronic system, which includes a capacitor, implanted under the skin of the left subclavicular region [6,7]. In the past decades, the world has seen a dynamic development of electrophysiological capabilities in the field of stimulation, defibrillation, and long-term electrotherapy. Among other things, the dynamic miniaturization of electronics and the improvement of electrode design have led to progress [8].
The long-term presence of electrodes in the venous system and heart chambers can be associated with numerous complications, including acute complications, such as pneumothorax, vascular bleeding, and perforation; subacute complications, such as vein thrombosis and lead dislodgement; and chronic complications, such as lead infection, endocarditis, chronic venous occlusion, mechanical lead failure, inappropriate shocks due to lead failure, lead-related tricuspid regurgitation, and need for extraction [8–20]. Therefore, the subcutaneous cardioverter-defibrillator (S-ICD) has been developed to reduce the risk associated with implanting electrodes into the venous system. It consists exclusively of batteries, an electronic system monitoring the electrical work of the heart, and a subcutaneous electrode, which does not require the implantation of electrodes into the heart chamber. Su et al, in their meta-analysis, showed lower long-term complications with S-ICD than with transvenous ICD [21–26].
In 2014, the first implantation of the S-ICD was performed in Poland; from then until 2021, approximately 450 S-ICD implantations were performed in Poland, and a few thousand worldwide [27–29]. Patients with contraindications to transvenous electrode implantation are qualified for implantation of the S-ICD. A significant limitation of S-ICD implantation is higher perioperative risk associated with general anesthesia or deep sedation. The growing population of patients with American Society of Anesthesiologists (ASA) risk classification of III or IV and who are qualified for S-ICD implantation, especially the group with reduced ejection fraction, poses a challenge to performing safe anesthesia in this group of patients [30–32].
Dynamically developing regional anesthesia techniques performed under the control of ultrasound within the chest to reduce the risk associated with general anesthesia and deep sedation for S-ICD implantation procedures encouraged anesthesiologists in the Department of Anesthesiology and Intensive Care in 4th Clinical Military Hospital to begin to anesthetize these patients using primarily regional fascial blocks. As a result, the anesthesia formula has been established in the form of a modified combination of fascial regional blocks, namely the pectoserratus plane block (PSP) and superficial serratus anterior plane block (S-SAP) [33–37].
The PSP block was formerly known as pectoral nerves plane block II (PECS II), but in 2021, the ASRA-ESRA Delphi international consensus standardized the naming of chest blocks, giving the PECS II block a new name [35,38].
The PSP block is a modification of the interpectoral plane block, formerly known as the pectoral nerves plane block I (PECS I); after the local anesthetic deposition between the pectoral muscles, an additional dose of a local anesthetic is administered to the fascial compartment between the pectoralis minor muscle and the serratus anterior muscle, blocking the lateral branches of the intercostal nerves Th2–Th6, the intercostobrachial nerve, and the long thoracic nerve. The PSP block can be used for the procedures described above and for more extensive breast surgery procedures (mastectomy, quadrantectomy, portacath implantation) [34,39,40].
The naming of the serratus anterior plane (SAP) block was unified in 2021 (ASRA-ESRA Delphi consensus) [38]. The original SAP block was divided into 2 separate blocks depending on the technique and anatomical place of anesthetic deposit: the S-SAP and the deep serratus anterior plane block. SAP blocks the lateral cutaneous branches of intercostal nerves located below the serratus anterior muscle within the region of Th3–Th9. The superficial variant of the block also includes the long thoracic and thoracodorsal nerve. Local anesthetics are administered in the fourth and fifth rib regions into the fascial space between the serratus anterior muscle and the latissimus dorsi muscle [38,40].
For S-ICD implantation, the use of other fascial blockades has been described: the deep parasternal intercostal plane block, formerly known as the transversus thoracis plane block, and the superficial parasternal intercostal plane block, formerly known as the parasternal block [41,42].
Therefore, in this study, we aimed to compare outcomes from ultrasound-guided PSP block and S-SAP block in 16 patients requiring a subcutaneous S-ICD, at a single center in Poland.
Material and Methods
ETHICAL CONSIDERATIONS:
This study was approved by the the Bioethical Committee of the Military Medical Chamber (approval No.: KB 2/21 180/21, date of approval: January 26, 2021). Written informed consent was obtained from all patients participating in the trial as a part of enrollment for research. The trial was registered prior to patient enrollment at the Australian New Zealand Clinical Trials Registry platform (ACTRN12621001386820, registration date: October 14, 2021). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and Good Clinical Practice. The study was a non-randomized, uncontrolled, open label trial and did not meet the CONSORT criteria.
DESIGN AND SELECTION CRITERIA:
This single-center, prospective, and interventional pilot study initially involved 22 patients. Finally, the analysis was conducted on consecutive procedures of a group of 16 patients (Figure 1). The study included patients of both sexes aged 23 to 71 years with ASA grade III or IV anesthesia risk assessment [43] who were qualified for S-ICD at the Cardiology Department of the 4th Clinical Military Clinical Hospital in Wrocław in the period from October 2021 to June 2022, and qualified for planned S-ICD implantation for tachyarrhythmia treatment.
The exclusion criteria were as follows: patient under 18 years, lack of patient consent, coagulation disorder, known allergy to the study drugs, inflammation at the site of the planned block, missing data in the research protocol, or technical difficulties in the implementation of the block. The study was conducted without a control group, since patients who qualified for general anesthesia or deep sedation were deemed unsuitable by the anesthesiologist due to their high-risk profile. Although patients who only required local anesthesia could have been considered, there was a potential risk of exceeding the maximum local anesthesia dosage, ultimately leading to the exclusion of such patients from the study.
REGIONAL BLOCS APPLICATION:
In all patients, the procedure was performed on the anterolateral chest wall on the left side in order to maintain a short distance between the S-ICD subcutaneous electrode and the heart.
PREPARING THE PATIENTS FOR ANESTHESIA AND SURGERY:
For premedication, patients with a body weight >50 kg received 150 mg of pregabalin orally 30 min before the procedure. For patients with a body weight <50 kg, the dose of pregabalin was reduced to 75 mg. Anesthesia was performed by an anesthesiology resident with over 3 years of experience in performing regional blocks under the expert supervision of an anesthesiology and intensive care specialist. The S-ICD implantation procedure was performed by a team of 2 cardiologists: an operator with 20 years of experience in electrophysiology implantations who had performed the S-ICD procedure independently several times, and an attending physician with 2 years of experience in electrophysiology implantations who had assisted in the S-ICD implantation procedure several times.
The anesthesia protocol for the procedure was performed immediately before the surgery. The patients were placed on their back with slight support under the left shoulder and left thoracic region achieving a 10° to 15° tilt of the torso to the right side, with the abduction of the left limb in the shoulder joint by an angle of 90o.
PSP APPLICATION:
For PSP application, after aseptic preparation of the injection site, a linear ultrasound probe (GE Vivid I with a 5–12 MHz linear probe) was placed parallel to the clavicle in the midclavicular line in the sagittal plane, locating the subclavian vessels. The probe was then moved toward the axillary fossa with a gradual rotation of approximately 90°. As the probe was moved laterally, successive ribs were counted down (the landmark being the axillary artery at the height of the second rib). After placing the probe at the height of the third rib, slightly medially from the anterior axillary line, individual anatomical structures were identified: pectoralis major and minor muscles, serratus anterior muscle, and intercostal muscles. After ultrasound identification of the anatomical structures at the level of the third rib, a guide needle (Vygon Echoplex with a diameter of 22 Ga and a length of 85 mm) was inserted using the in-plane technique, parallel to the deltoid-thoracic sulcus toward the lower pole of the axillary fossa at an angle of 30° to 45° (Figure 2A). After confirming that the intended space between the pectoralis major and pectoralis minor muscles had been reached, a local analgesic was deposited, achieving the delamination of fascial planes over a length of 6 cm and a width of at least 1 cm. The needle was then inserted deeper, and after identifying the space between the pectoralis minor muscle and the serratus anterior muscle, a local analgesic was deposited, thus anesthetizing the nerve structures located there: the lateral branches of the Th2–Th4 intercostal nerves, the medial and lateral pectoral nerve, and partially the long thoracic nerve (Figure 2C) [34,37,39–41].
S-SAP APPLICATION:
For S-SAP application, the probe was then moved caudally in a sagittal line halfway between the midclavicular line and the anterior axillary line, identifying the fifth rib. In a further step, the probe was moved in the direction of the axillary fossa in a parallel line between the mid axillary line and the posterior axillary line. The interfascial compartment between the latissimus dorsi and serratus anterior muscles was identified. The anatomical point that facilitates the identification of structures is the thoracodorsal artery, located in this space. The fourth intercostal space and the fourth and fifth ribs should be visible in the ultrasound image. After identifying the structures, a guide needle (Vygon Echoplex with a diameter of 22 Ga and a length of 85 mm) was inserted using the in-plane technique, parallel to the posterior axillary line from the axillary fossa in the caudal direction at an angle of 45° to 60° (Figure 2B), reaching the fascial compartment between the latissimus dorsi muscle and the serratus anterior muscle. A local anesthetic was deposited in the space in an amount sufficient to delaminate fascial planes over a length of 10 cm, and a width of at least 1 cm; on ultrasound images, delamination of the fascia at the level of the fourth and fifth ribs was achieved (Figure 2D). The extent of the block includes the Th3–Th9 intercostal nerves, as well as the thoracic-dorsal nerve and partially the long thoracic nerve. The maximum volume of the anesthetic mixture per both blocks was 60 mL [34,37,39,41,44–47].
In the case of insufficient skin anesthesia, before the skin incision in the mid axillary line, linear infiltration anesthesia was performed using 0.5% lidocaine with 0.005% epinephrine in a volume of 5 mL. In addition, in the case of insufficient skin anesthesia, separate local anesthesia was administered to the skin incisions in the left parasternal line and, if performed, in the region of the parallel incision to the manubrium of sternum 2/3 cm cephalad from the xiphoid process in a volume of 5 mL each of 0.5% lidocaine (Figure 3).
S-ICD IMPLANTATION:
After the start of the procedure, the patients remained in direct contact with the anesthesiologist. During this time, the implant team prepared the skin, subcutaneous tissue, and fascia, successively reaching the muscle layer at the level of the sixth intercostal space in the anterior axillary line. Then a pocket under the latissimus dorsi muscle measuring 5×5 cm was created “bluntly”. Using a bayonet guidewire, an electrode was implanted along the parasternal line, and at the level of the fifth intercostal space, a gentle subcutaneous arc tunnel was created into the pocket under the axillary fossa (Figure 4A) [24,26,44,48].
The procedure was performed using a 3-incision technique: the pocket and 2 incisions in the parasternal line. The subcutaneous ICD electrode (Q-TRAK, Boston Scientific, Marlborough, MA, USA) was then connected to the pulse generator (A209 EMBLEM model, Boston Scientific, Marlborough, MA, USA), attaching the system to the fascia (Figure 4B). The last part of the procedure was a test of the implanted device. After the device pocket was closed, defibrillation threshold testing was performed by induction of ventricular fibrillation and delivery of at least 1 shock from the S-ICD [45]. Before the test, all the patients received propofol intravenously (i.v.) at a dose of 1 to 2 mg/kg of body weight in a titrated bolus. Propofol dose for defibrillation threshold testing was not included to the list of i.v. anesthetics used for regional block before defibrillation threshold testing and is not shown in Table 1.
ANALGESIA PROTOCOL:
In the case of significant discomfort of a non-specific nature: squishing, pressure, or stretching, single doses of propofol at a dose of 0.1 to 0.5 mg/kg i.v. or ketamine at a dose of 0.1 to 0.5 mg i.v. were administered. In addition, for pain rated at more than 5 points on the NRS scale, a bolus of fentanyl at a dose of 0.5 to 1 μg/kg i.v. was administered.
OUTCOMES ASSESSMENT:
Pain was assessed during and after the procedure using the 11-point NRS scale [46]. In addition, after 24 h, the patient’s comfort was assessed using the QoR-15 questionnaire [47,49]. During the procedure, every 15 min, the patients assessed their pain comfort on the NRS scale, and the operator assessed the comfort of the procedure on the proprietary Operator’s Comfort Scale. This scale assesses 3 domains: (1) patient reaction to commands, (2) cooperation with the patient/stability of the surgical field, and (3) pain management/quality of anesthesia of deep tissues. Scoring is as follows: 3, good; 2, moderately good; and 1, unacceptable. Total score ranges are as follows: 9–8 points, indicating comfortable conditions for performing the procedure for the operator; 7–6 points, indicating difficult conditions for performing the procedure; and <6 points, indicating conditions categorically unacceptable.
POSTOPERATIVE CARE:
After the procedure, the patients were transferred to the Cardiac Intensive Care Unit for 24 h. On postoperative day 1, i.v. analgesics were routinely administered: paracetamol 1 g every 6 h, metamizole 1 g every 6 h, and ketoprofen 100 mg every 12 h, and oral pregabalin 75 mg in the evening. At discharge from the Cardiac Intensive Care Unit, the patients were evaluated with the Aldrete score, which consists of assessing 5 clinical criteria performed every 30 min. If a patient scored at least 9 points twice, no more than 30 min apart, they were discharged from the unit. On day 2 and day 3 after the procedure, oral analgesics were used: paracetamol and metamizole, at a dose of 1 g every 8 h.
STUDY ENDPOINTS:
The study protocol recorded the time of the procedure, time to the start of the procedure, duration of the procedure, volume of analgesics used in each block, total volume of local analgesics, mean arterial pressure, heart rate, respiratory rate, and total dose of anesthetics and i.v. analgesics until the device implantation was completed (Figure 5). Additionally, any adverse reactions and events within 24 h after the procedure were recorded. After the procedure, patients were asked to subjectively evaluate the technique when answering whether they would recommend it to a family member.
STATISTICAL ANALYSIS:
The statistical analysis was performed using Statistica 13 (TIBCO Software Inc., USA). The research is a preliminary report. The sample size will be estimated for future studies based on the results. Arithmetic means, medians, standard deviations, quartiles, and the range of variability (extreme values) were calculated for measurable variables. The frequency of their occurrence (percentage) was calculated for qualitative variables.
Results
PATIENT CHARACTERISTICS:
The analysis was conducted on a group of 16 patients. The basic anthropometric data and data on anesthesia, surgery, complications, and analgesic use after the procedure are shown in Table 2. The median age of the patients was 42.5 years; 81% were men.
LOCAL ANESTHESIA TYPE AND DOSAGE:
The use of local anesthesia, analgesics, anesthetics during the procedure, and adverse events during and 24 h after the procedure are shown in Table 1. Ropivacaine and lidocaine were used as the basic anesthetic mixture in the following concentrations for PSP: ropivacaine 0.25% + lidocaine 0.5% (n=16, 100%) as well as for S-SAP: ropivacaine 0.25% + lidocaine 0.5% (n=6, 37%) and ropivacaine 0.125% + lidocaine 0.25% (n=10, 63%). The mixture of ropivacaine and lidocaine in the concentrations used gave a rapid onset of anesthesia (lidocaine) and effective and long period of action (ropivacaine). In 8 patients (50%), non-opioid analgesics were additionally administered during the procedure. In 11 patients (69%), intraoperative fentanyl bolus was administered, in a dose not exceeding 200 μg. All patients received a single bolus of anesthetics in moments of significant discomfort (pocket preparation, implantation of the subcutaneous electrode). Three adverse effects were observed during the procedure: 2 episodes of moderate hypoglycemia with the patient’s agitation during the block, and 1 episode of hypertension above 180/110 mmHg.
The mean volume of the local anesthetics mixture for PSP was 19.4 mL, and the mean volume of the local anesthesia mixture for S-SAP was 34.7 mL. The mean total volume of local anesthesia for both blocks was 54.1 mL.
PERFORMANCE TIME OF ANESTHESIA AND SURGERY:
Performance time for the block for the PSP block was 21.3 min. The time interval between block completion and start of surgery was 49.7 min; the mean time of the S-ICD implantation alone (surgery) was 108.4 min. Neither circulatory nor respiratory instability was observed during the procedure. The operator’s assessment of the procedure’s comfort was high (Q3 was 9 points); 94% of patients asked whether they would recommend the technique to their family members answered yes.
The value of the Aldrete score, indicating the possibility of safe transfer of the patient from the Cardiac Intensive Care Unit to the general ward 24 h after the procedure was high, and the upper quartile was 10 (maximum value).
PATIENT EVALUATION AND ADVERSE EFFECTS:
Results of patient pain assessment on the NRS scale and patient satisfaction during the first 24 h after the surgery on the QoR-15 score, including subsequent domains, are shown in Table 3. The intraoperative NRS score was very low (Q3=1 point, max=2 points), which indicated a very good analgesic effect of the block. Similarly, the pain assessment 24 h after the procedure was good (Q3=2 points, max=4 points). The mean postoperative patient satisfaction score on the QoR-15 was 133.9 points. Two adverse events were observed 24 h after the procedure: confusion with short-term memory disorders after implantation lasting <1 h, and nausea without vomiting until 2 h after the procedure.
Discussion
STUDY LIMITATIONS:
This study had several potential limitations, such as the lack of randomization and the small sample size of the study group. The primary endpoint of the study was the pain intensity reported by the patient in the different phases of the procedure and the postoperative period, as measured on a verbal rating scale. Pain intensity is a subjective parameter, and its assessment was not blinded.
Conclusions
The results of this pilot study confirm the feasibility and safety of using a combination of S-SAP and PSP blocks to provide anesthesia and analgesia during S-ICD implantation in patients with high operative risk (ASA grade III or IV). This technique eliminates the need for general anesthesia or deep sedation and has shown good efficacy. However, further large-population randomized studies are required to validate these findings. If the results are confirmed, the combined SAP and PSP blocks may represent a valuable alternative to general anesthesia or deep sedation for optimal anesthesia and analgesia during S-ICD implantation.
Figures
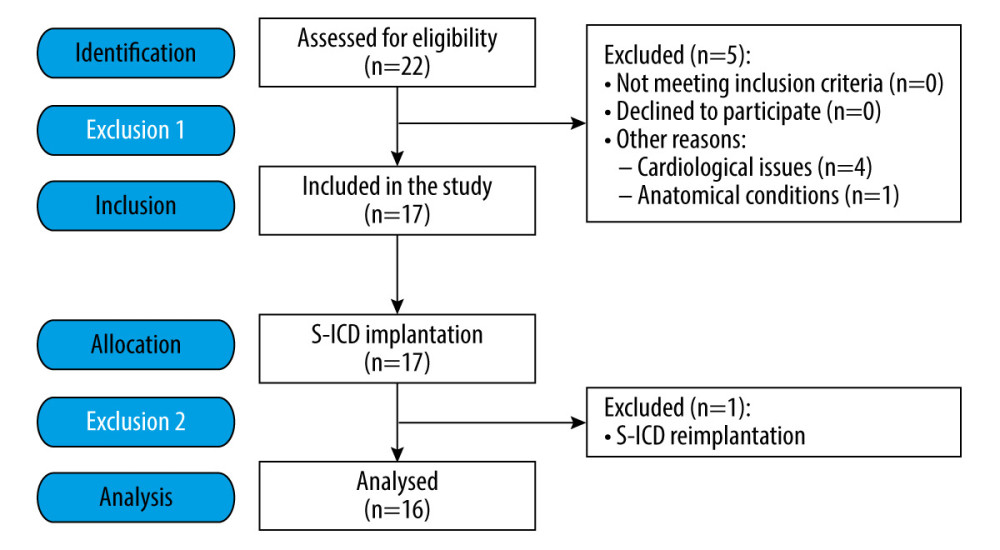 Figure 1. Flow diagram of the study participants.
Figure 1. Flow diagram of the study participants. 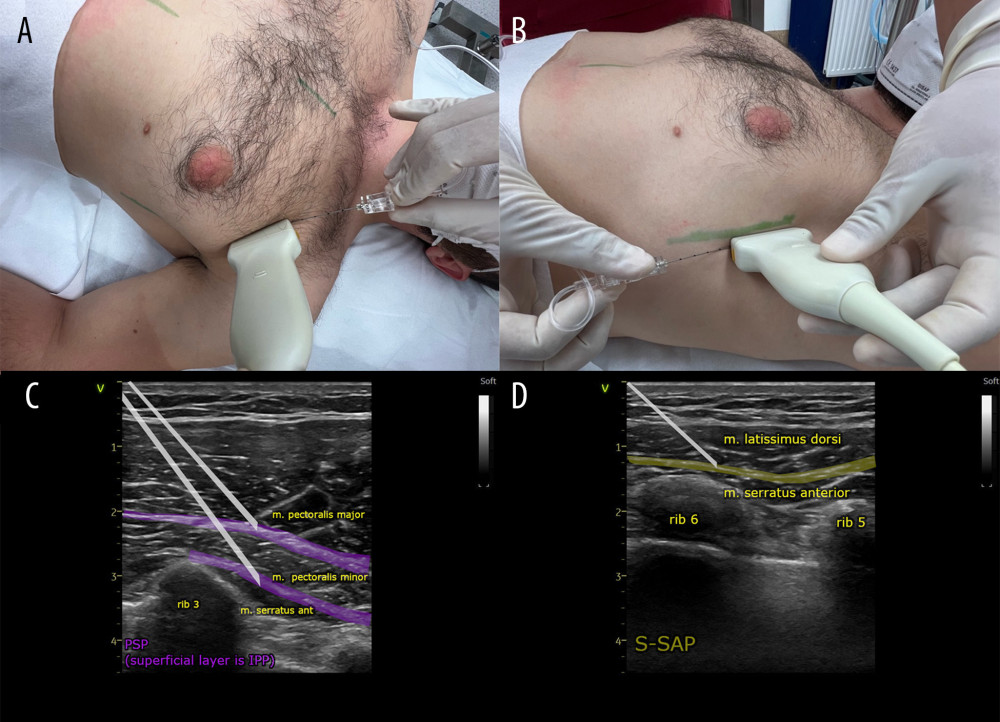 Figure 2. (A, C) Ultrasound transducer positioning and direction of the needle during the pectoserratus plane block (PSP) and (B, D) superficial serratus anterior plane block (S-SAP).
Figure 2. (A, C) Ultrasound transducer positioning and direction of the needle during the pectoserratus plane block (PSP) and (B, D) superficial serratus anterior plane block (S-SAP). 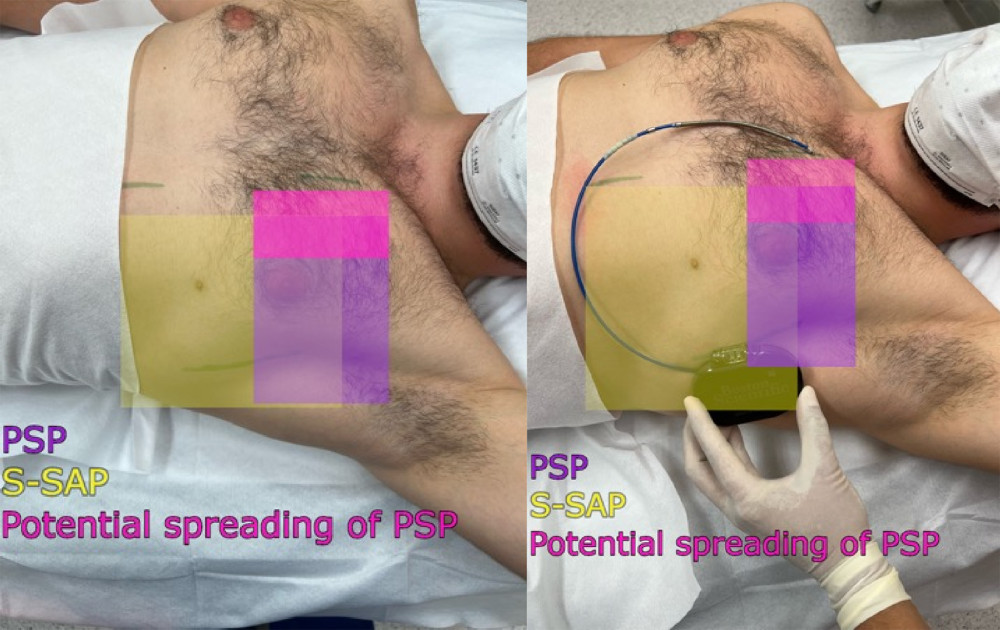 Figure 3. Area covered by the pectoserratus plane block (PSP) and superficial serratus anterior plane block (S-SAP): green lines indicate skin incision lines during subcutaneous cardioverter-defibrillator implantation.
Figure 3. Area covered by the pectoserratus plane block (PSP) and superficial serratus anterior plane block (S-SAP): green lines indicate skin incision lines during subcutaneous cardioverter-defibrillator implantation. 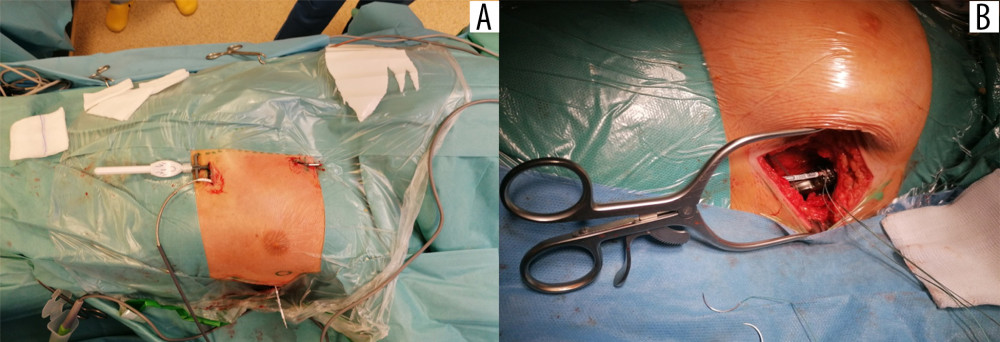 Figure 4. Implantation of the subcutaneous cardioverter-defibrillator (S-ICD). Insertion of the subcutaneous electrode and its tunnel leading into the axillary area, where the S-ICD can be implanted: (A) marked with a circle; (B) implantation of the cardioverter-defibrillator body into the pocket in the axillary line.
Figure 4. Implantation of the subcutaneous cardioverter-defibrillator (S-ICD). Insertion of the subcutaneous electrode and its tunnel leading into the axillary area, where the S-ICD can be implanted: (A) marked with a circle; (B) implantation of the cardioverter-defibrillator body into the pocket in the axillary line. 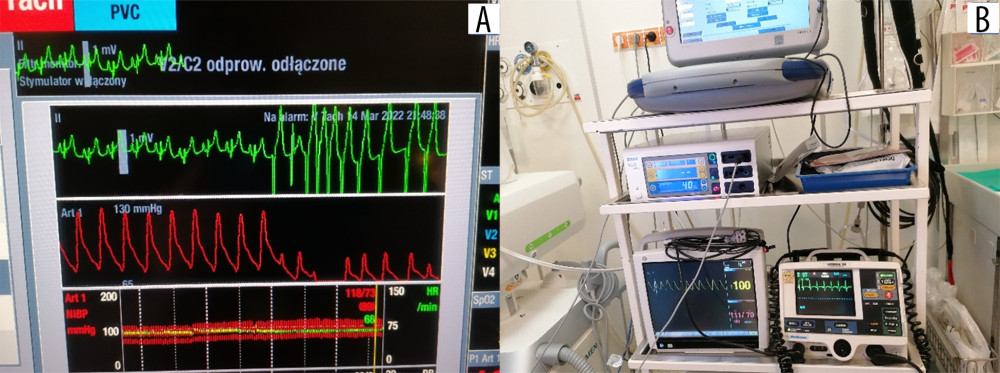 Figure 5. (A, B) Intraoperative electrocardiogram findings.
Figure 5. (A, B) Intraoperative electrocardiogram findings. Tables
Table 1. The use of local anesthesia, analgesics, and anesthetics during and after the procedure and adverse events. Table 2. The basic anthropometric data, intraoperative parameters data on anesthesia and surgery.
Table 2. The basic anthropometric data, intraoperative parameters data on anesthesia and surgery.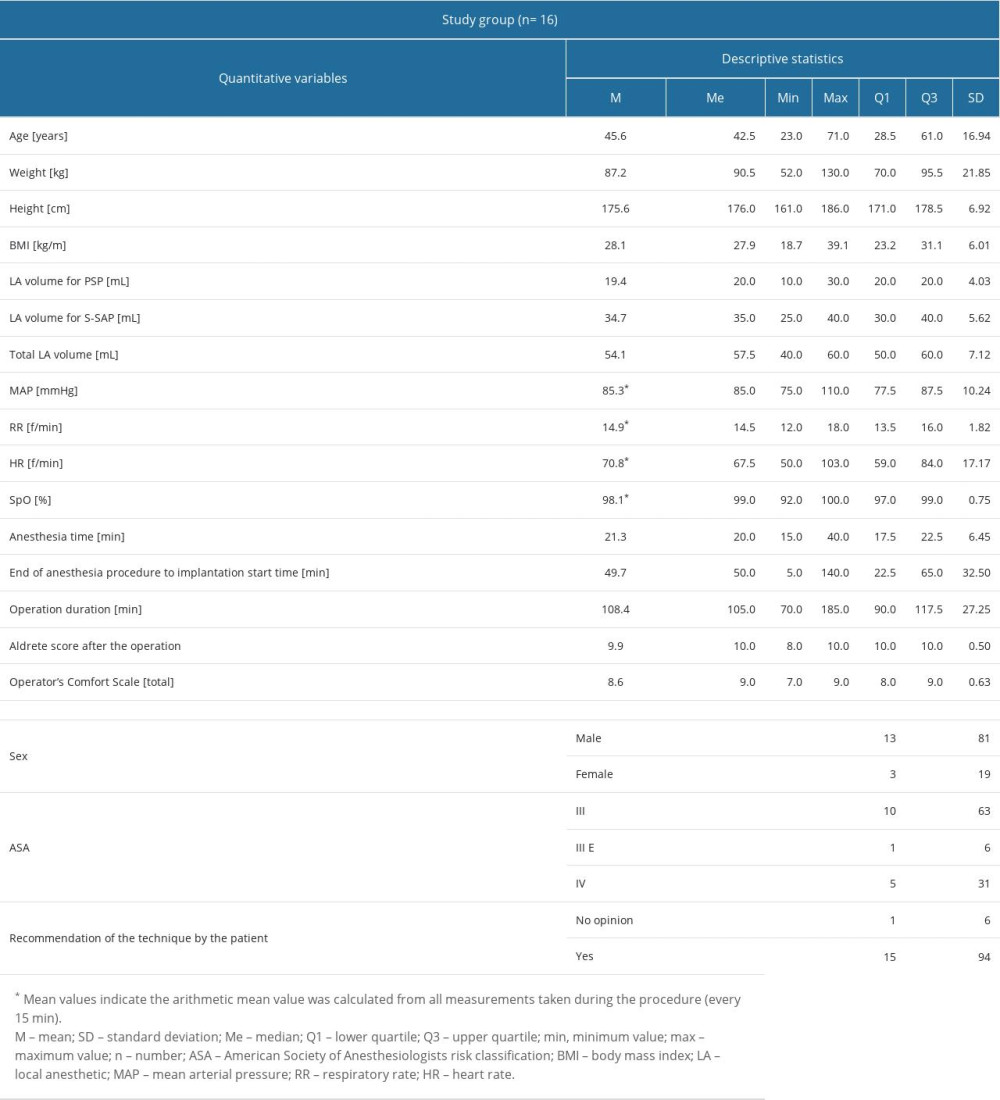 Table 3. Comparison of selected laboratory blood tests in patients with and without acute kidney injury and continuous renal replacement therapy
Table 3. Comparison of selected laboratory blood tests in patients with and without acute kidney injury and continuous renal replacement therapy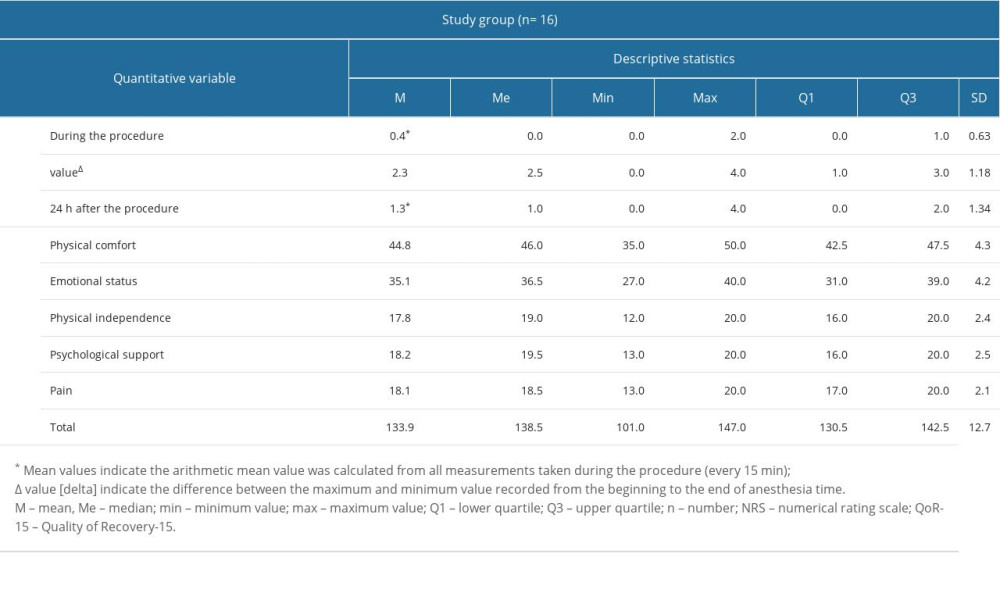
References
1. Aquilina O, A brief history of cardiac pacing: Images Paediatr Cardiol, 2006; 8(2); 17-81
2. Borne RT, Katz D, Betz J, Implantable cardioverter defibrillators for secondary prevention of sudden cardiac death: A review: J Am Heart Assoc Cardiovasc Cerebrovasc Dis, 2017; 6(3); e005515
3. Zeppenfeld K, Tfelt-Hansen J, de Riva M, 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Eur Heart J, 2022; 43(40); 3997-4126
4. Gómez-Mesa JE, Márquez-Murillo M, Figueiredo M, Inter-American Society of Cardiology (CIFACAH-ELECTROSIAC) and Latin-American Heart Rhythm Society (LAHRS): Multidisciplinary review on the appropriate use of implantable cardiodefibrillator in heart failure with reduced ejection fraction: J Interv Card Electrophysiol, 2022 [Online ahead of print]
5. Kaya E, Rassaf T, Wakili R, Subcutaneous ICD: Current standards and future perspective: Int J Cardiol Heart Vasc, 2019; 24; 100409
6. Ammannaya GKK, Implantable cardioverter defibrillators – the past, present and future: Arch Med Sci Atheroscler Dis, 2020; 5; e163-e70
7. van Welsenes GH, Borleffs CJW, van Rees JB, Improvements in 25 years of implantable cardioverter defibrillator therapy: Neth Heart J, 2011; 19(1); 24-30
8. Hauser RG, Development and industrialization of the implantable cardioverter-defibrillator: A personal and historical perspective: Card Electrophysiol Clin, 2009; 1(1); 117-27
9. Frausing MHJP, Nielsen JC, Johansen JB, Cardiac surgery in patients with cardiac implantable electronic devices and risk of device infections: A nationwide nested case-control study: J Interv Card Electrophysiol, 2023; 66(4); 897-904
10. Ząbek A, Boczar K, Ulman M, Mechanical extraction of implantable cardioverter-defibrillator leads with a dwell time of more than 10 years: Insights from a single high-volume centre: Europace, 2023; 25(3); 1100-9
11. Breeman KTN, Beurskens NEG, Driessen AHG, Timing and mid-term outcomes of using leadless pacemakers as replacement for infected cardiac implantable electronic devices: J Interv Card Electrophysiol, 2022 [Online ahead of print]
12. Breeman KTN, Peijster AJL, De Bruin-Bon HaCM, Worsening tricuspid regurgitation after ICD implantation is rather due to transvenous lead than natural progression: Int J Cardiol, 2023; 376; 76-80
13. Witt CT, Ng Kam Chuen MJ, Kronborg MB, Non-infective left ventricular lead complications requiring re-intervention following cardiac resynchronization therapy: Prevalence, causes and outcomes: J Interv Card Electrophysiol, 2022; 63(1); 69-75
14. Giacopelli D, Azzolina D, Comoretto RI, Implantable cardioverter defibrillator lead performance: A systematic review and individual patient data meta-analysis: Int J Cardiol, 2023; 373; 57-63
15. Dhruva SS, Zeitler EP, Caños D, Evaluation of cardiovascular implantable electronic device leads post implant: ElectroPhysiology Predictable And SuStainable Implementation of National Registries (EP PASSION): J Interv Card Electrophysiol, 2023; 66(4); 997-1004
16. Kusumoto FM, Schoenfeld MH, Wilkoff BL, 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction: Heart Rhythm, 2017; 14(12); e503-e51
17. Hasan F, Nedios S, Karosiene Z, Perioperative complications after pacemaker implantation: Higher complication rates with subclavian vein puncture than with cephalic vein cutdown: J Interv Card Electrophysiol, 2023; 66(4); 857-63
18. Al-Khatib SM, Stevenson WG, Ackerman MJ, 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society: Circulation, 2018; 138(13); e210-e71
19. Ezzat VA, Lee V, Ahsan S, A systematic review of ICD complications in randomised controlled trials versus registries: is our ‘real-world’ data an underestimation?: Open Heart, 2015; 2(1); e000198
20. Zeitler EP, Friedman DJ, Loring Z, Complications involving the subcutaneous implantable cardioverter defibrillator: Lessons learned from MAUDE: Heart Rhythm, 2020; 17(3); 447-54
21. Matchett M, Sears SF, Hazelton G, The implantable cardioverter defibrillator: Its history, current psychological impact and future: Expert Rev Med Devices, 2009; 6(1); 43-50
22. Willy K, Reinke F, Rath B, Pitfalls of the S-ICD therapy: Experiences from a large tertiary centre: Clin Res Cardiol, 2021; 110(6); 861-67
23. Leo M, Sharp AJ, Gala ABE, Transvenous or subcutaneous implantable cardioverter defibrillator: A review to aid decision-making: J Interv Card Electrophysiol, 2022 [Online ahead of print]
24. Giacomin E, Falzone PV, Dall’Aglio PB, Subcutaneous implantable cardioverter defibrillator after transvenous lead extraction: safety, efficacy and outcome: J Interv Card Electrophysiol, 2022 [Online ahead of print]
25. Droghetti A, Pecora D, Maffè S, “Shift and cover technique”: Conservative management of complications for the rescue of S-ICD subcutaneous implantable defibrillator systems: J Interv Card Electrophysiol, 2022 [Online ahead of print]
26. Su L, Guo J, Hao Y, Tan H, Comparing the safety of subcutaneous versus transvenous ICDs: A meta-analysis: J Interv Card Electrophysiol, 2021; 60(3); 355-63
27. Kempa M, Przybylski A, Budrejko S, Evolution of implantation technique and indications for a subcutaneous cardioverter-defibrillator: Over 7 years of experience in Poland: Kardiol Pol, 2021; 79(9); 1016-18
28. Lambiase PD, Theuns DA, Murgatroyd F, Subcutaneous implantable cardioverter-defibrillators: Long-term results of the EFFORTLESS study: Eur Heart J, 2022; 43(21); 2037-50
29. Gold MR, Lambiase PD, El-Chami MF, Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial: Circulation, 2021; 143(1); 7-17
30. Kaczmarek K, Zwolinski R, Bartczak K, A subcutaneous implantable cardioverter-defibrillator – the first implantation in Poland: Kardiol Pol, 2015; 73(1); 62
31. Chen CQ, Wang X, Zhang J, Zhu SM, Anesthetic management of patients with dilated cardiomyopathy for noncardiac surgery: Eur Rev Med Pharmacol Sci, 2017; 21(3); 627-34
32. Kristensen SD, Knuuti J, Saraste A, 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA): Eur Heart J, 2014; 35(35); 2383-431
33. Blanco R, The “pecs block”: A novel technique for providing analgesia after breast surgery: Anaesthesia, 2011; 66(9); 847-48
34. Blanco R, Fajardo M, Parras Maldonado T, Ultrasound description of Pecs II (modified Pecs I): A novel approach to breast surgery: Rev Esp Anestesiol Reanim, 2012; 59(9); 470-75
35. Blanco R, Parras T, McDonnell JG, Prats-Galino A, Serratus plane block: A novel ultrasound-guided thoracic wall nerve block: Anaesthesia, 2013; 68(11); 1107-13
36. Albrecht E, Chin KJ, Advances in regional anaesthesia and acute pain management: A narrative review: Anaesthesia, 2020; 75(Suppl 1); e101-e10
37. Smith LM, Barrington MJSt Vincent’s Hospital, Melbourne, Ultrasound-guided blocks for cardiovascular surgery: Which block for which patient?: Curr Opin Anaesthesiol, 2020; 33(1); 64-70
38. El-Boghdadly K, Wolmarans M, Stengel AD, Standardizing nomenclature in regional anesthesia: An ASRA-ESRA Delphi consensus study of abdominal wall, paraspinal, and chest wall blocks: Reg Anesth Pain Med, 2021; 46(7); 571-80
39. Calì Cassi L, Biffoli F, Francesconi D, Anesthesia and analgesia in breast surgery: The benefits of peripheral nerve block: Eur Rev Med Pharmacol Sci, 2017; 21(6); 1341-45
40. Janc J, Szamborski M, Milnerowicz A, Evaluation of the effectiveness of modified pectoral nerve blocks type II (PECS II) for vascular access port implantation using cephalic vein venesection: J Clin Med, 2021; 10(24); 5759
41. Uran C, Giojelli A, Borgogna DA, Ultrasound-guided serratus anterior plane block combined with parasternal block in subcutaneous implantable cardioverter defibrillator implantation: Results of a pilot study: Pacing Clin Electrophysiol, 2020; 43(7); 705-12
42. Abdelbaser I, Mageed NA, Safety of ultrasound-guided transversus thoracis plane block in pediatric cardiac surgery: A retrospective cohort study: J Cardiothorac Vasc Anesth, 2022; 36(8 Pt B); 2870-75
43. Doyle DJ, Goyal A, Bansal P, Garmon EHAmerican Society of Anesthesiologists Classification: StatPearls, 2021, StatPearls Publishing http://www.ncbi.nlm.nih.gov/books/NBK441940/
44. Droghetti A, Basso Ricci E, Ultrasound-guided serratus anterior plane block combined with the two-incision technique for subcutaneous ICD implantation: Pacing Clin Electrophysiol, 2018; 41(5); 517-23
45. Na JS, Sokolow M, Childress J, Han P, Recent temporal trends in hospital costs for non-surgical patients receiving implantable cardioverter defibrillators: J Interv Card Electrophysiol Int J Arrhythm Pacing, 2022; 63(2); 231-37
46. Haefeli M, Elfering A, Pain assessment: Eur Spine J, 2006; 15(Suppl 1); S17-S24
47. Stark PA, Myles PS, Burke JA, Development and psychometric evaluation of a postoperative quality of recovery score: The QoR-15: Anesthesiology, 2013; 118(6); 1332-40
48. Migliore F, De Franceschi P, De Lazzari M, Ultrasound-guided serratus anterior plane block for subcutaneous implantable cardioverter defibrillator implantation using the intermuscular two-incision technique: J Interv Card Electrophysiol, 2020; 57(2); 303-9
49. Chazapis M, Walker EMK, Rooms MA, Measuring quality of recovery – 15 after day case surgery: Br J Anaesth, 2016; 116(2); 241-48
50. Sarubbi B, Colonna D, Correra A, Subcutaneous implantable cardioverter defibrillator in children and adolescents: results from the S-ICD “Monaldi care” registry: J Interv Card Electrophysiol Int J Arrhythm Pacing, 2022; 63(2); 283-93
51. Knops RE, Olde Nordkamp LRA, de Groot JR, Wilde AAM, Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator: Heart Rhythm, 2013; 10(8); 1240-43
52. Brouwer TF, Miller MA, Quast AFBE, Implantation of the subcutaneous implantable cardioverter-defibrillator: An evaluation of 4 implantation techniques: Circ Arrhythm Electrophysiol, 2017; 10(1); e004663
53. Migliore F, Pittorru R, Giacomin E, Intermuscular two-incision technique for implantation of the subcutaneous implantable cardioverter defibrillator: A 3-year follow-up: J Interv Card Electrophysiol, 2023 [Online ahead of print]
54. Weiss R, Knight BP, Gold MR, Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator: Circulation, 2013; 128(9); 944-53
55. Lambiase PD, Barr C, Theuns DAMJ, Worldwide experience with a totally subcutaneous implantable defibrillator: Early results from the EFFORTLESS S-ICD Registry: Eur Heart J, 2014; 35(25); 1657-65
56. Essandoh MK, Mark GE, Aasbo JD, Anesthesia for subcutaneous implantable cardioverter-defibrillator implantation: Perspectives from the clinical experience of a U.S. panel of physicians: Pacing Clin Electrophysiol, 2018; 41(7); 807-16
57. Szamborski M, Janc J, Rosińczuk J, Use of ultrasound-guided interfascial plane blocks in anterior and lateral thoracic wall region as safe method for patient anesthesia and analgesia: Review of techniques and approaches during COVID-19 pandemic: Int J Environ Res Public Health, 2022; 19(14); 8696
58. Miller MA, Garg J, Salter B, Feasibility of subcutaneous implantable cardioverter-defibrillator implantation with opioid sparing truncal plane blocks and deep sedation: J Cardiovasc Electrophysiol, 2019; 30(1); 141-48
59. Ziacchi M, Bisignani G, Palmisano P, Serratus anterior plane block in subcutaneous implantable cardioverter defibrillator implantation: A case-control analysis: J Cardiovasc Electrophysiol, 2020; 31(1); 144-49
60. Shariat A, Ghia S, Gui JL, Use of serratus anterior plane and transversus thoracis plane blocks for subcutaneous implantable cardioverter-defibrillator (S-ICD) implantation decreases intraoperative opioid requirements: J Cardiothorac Vasc Anesth, 2021; 35(11); 3294-98
61. Marrone F, Paventi S, Tomei M, Bosco M, Ultrasound-guided serratus anterior plane block (US-SAP block) and ultrasound-guided parasternal block (US-PsB) for s-ICD implantation in severe dilated post-ischaemic cardiomyopathy: A case study: Reg Anesth Pain Med, 2021; 70(Suppl 1); A80-81
62. Roriz D, Brandão J, Graça R, S-ICD implantation under the serratus plane block and transversus thoracis muscle plane block. A clinical case: Rev Esp Anestesiol Reanim, 2022; 69(2); 102-4
63. Elders J, AlHashimi H, Gomes M, Subcutaneous ICD implantation under ultrasound-guided serratus anterior plane block: Single-center experience in the Netherlands: Int J Cardiol Heart Vasc, 2022; 38; 100949
64. Sepolvere G, Tognù A, Tedesco M, Avoiding the internal mammary artery during parasternal blocks: Ultrasound identification and technique considerations: J Cardiothorac Vasc Anesth, 2021; 35(6); 1594-602
Figures
 Figure 1. Flow diagram of the study participants.
Figure 1. Flow diagram of the study participants. Figure 2. (A, C) Ultrasound transducer positioning and direction of the needle during the pectoserratus plane block (PSP) and (B, D) superficial serratus anterior plane block (S-SAP).
Figure 2. (A, C) Ultrasound transducer positioning and direction of the needle during the pectoserratus plane block (PSP) and (B, D) superficial serratus anterior plane block (S-SAP). Figure 3. Area covered by the pectoserratus plane block (PSP) and superficial serratus anterior plane block (S-SAP): green lines indicate skin incision lines during subcutaneous cardioverter-defibrillator implantation.
Figure 3. Area covered by the pectoserratus plane block (PSP) and superficial serratus anterior plane block (S-SAP): green lines indicate skin incision lines during subcutaneous cardioverter-defibrillator implantation. Figure 4. Implantation of the subcutaneous cardioverter-defibrillator (S-ICD). Insertion of the subcutaneous electrode and its tunnel leading into the axillary area, where the S-ICD can be implanted: (A) marked with a circle; (B) implantation of the cardioverter-defibrillator body into the pocket in the axillary line.
Figure 4. Implantation of the subcutaneous cardioverter-defibrillator (S-ICD). Insertion of the subcutaneous electrode and its tunnel leading into the axillary area, where the S-ICD can be implanted: (A) marked with a circle; (B) implantation of the cardioverter-defibrillator body into the pocket in the axillary line. Figure 5. (A, B) Intraoperative electrocardiogram findings.
Figure 5. (A, B) Intraoperative electrocardiogram findings. Tables
 Table 1. The use of local anesthesia, analgesics, and anesthetics during and after the procedure and adverse events.
Table 1. The use of local anesthesia, analgesics, and anesthetics during and after the procedure and adverse events. Table 2. The basic anthropometric data, intraoperative parameters data on anesthesia and surgery.
Table 2. The basic anthropometric data, intraoperative parameters data on anesthesia and surgery. Table 3. Comparison of selected laboratory blood tests in patients with and without acute kidney injury and continuous renal replacement therapy
Table 3. Comparison of selected laboratory blood tests in patients with and without acute kidney injury and continuous renal replacement therapy Table 1. The use of local anesthesia, analgesics, and anesthetics during and after the procedure and adverse events.
Table 1. The use of local anesthesia, analgesics, and anesthetics during and after the procedure and adverse events. Table 2. The basic anthropometric data, intraoperative parameters data on anesthesia and surgery.
Table 2. The basic anthropometric data, intraoperative parameters data on anesthesia and surgery. Table 3. Comparison of selected laboratory blood tests in patients with and without acute kidney injury and continuous renal replacement therapy
Table 3. Comparison of selected laboratory blood tests in patients with and without acute kidney injury and continuous renal replacement therapy In Press
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
15 Mar 2024 : Clinical Research
Evaluation of an Optimized Workflow for the Radiofrequency Catheter Ablation of Paroxysmal Atrial FibrillationMed Sci Monit In Press; DOI: 10.12659/MSM.943526
09 Apr 2024 : Clinical Research
Correlation between Thalamocortical Tract and Default Mode Network with Consciousness Levels in Hypoxic-Isc...Med Sci Monit In Press; DOI: 10.12659/MSM.943802
19 Apr 2024 : Clinical Research
Comparative Analysis of Postoperative Sagittal Balance in Expansive Open-Door Laminoplasty versus Laminecto...Med Sci Monit In Press; DOI: 10.12659/MSM.943057
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








