25 June 2023: Review Articles
A Review of Preclinical and Clinical Studies in Support of the Role of Non-Steroidal Anti-Inflammatory Drugs in Dentistry
Anna Kotowska-Rodziewicz1ABCDEFG, Anna Zalewska1ABCDEF, Mateusz MaciejczykDOI: 10.12659/MSM.940635
Med Sci Monit 2023; 29:e940635
Abstract
ABSTRACT: Patient pain is a common problem faced by dentists and oral and maxillofacial surgeons. Craniofacial pain may be caused not only by inflammation in the teeth, but also various oral, facial, and nerve-related diseases, as well as tumors. Non-steroidal anti-inflammatory drugs (NSAIDs) constitute the basis of the analgesic ladder. According to the World Health Organisation (WHO), NSAIDs are the first-line drugs in relieving pain and inflammation of oral conditions. NSAIDs have been used in almost every field of dentistry. These drugs are applied in conservative dentistry and endodontics, dental surgery, orthodontics, periodontology, and oral mucosal diseases, as well as head and neck oncology. Some of the NSAIDs exhibit additional therapeutic effects, such as inhibition of nuclear factor kappa B (NF-κB) and inducible nitric oxide synthase (iNOS), and reduction of oxidative stress or leukocyte passage to the site of inflammation, which further reduces inflammation in tissues. The topical use of NSAIDs in dentistry is worthy of attention and further research as it will significantly reduce the adverse effects of systemic administration. This article aims to review the preclinical and clinical studies that have supported the role of NSAIDs in dentistry.
Keywords: Dentistry, Inflammation
Background
For centuries, humans have struggled with pain and its accompanying inflammation. Pain is defined as a sensation of discomfort (an unpleasant sensory and emotional experience) arising from stimulation of pain receptors (nociceptors), lowering their excitability threshold (ie, receptor pain), and damage to nervous system structures (ie, non-receptor neuropathic pain) [1]. Pain can also arise without tissue damage, which is referred to as non-receptor psychogenic pain [2]. As opposed to acute pain of a post-traumatic/postoperative nature, chronic pain lasts for at least 3 months [3]. The feeling of pain is a complex process involving 4 consecutive stages: transduction (conversion of the energy of a damaging stimulus into an electrical impulse), transmission (conduction of the impulse to the posterior horns of the spinal cord and the central nervous system), modulation (stimulation, inhibition and summation of stimuli), and perception (awareness of the existence of pain with the accompanying affective and emotional reactions [4]). The primary function of pain is a warning-protective action, which triggers a reflex response from the body that minimizes the effects of the damaging stimulus [5]. In many cases, pain is the main patients consult a doctor. A special type of pain is orofacial pain, which is mainly caused by dental infections (eg, caries, pulpitis, periodontal disease, obstructed eruption of teeth) [6], post-traumatic lesions (eg, dental injuries, injuries to the temporomandibular joint), neoplastic lesions (eg, oral cancer, tongue cancer, tumors of the mandible and the maxilla) [7], and craniofacial procedures (eg, tooth extraction, periodontal procedures, placement of implant) [8–10]. Types of pain also include phantom pain of an elusive cause and pain associated with facial nerve inflammation [11]. Moreover, pain in the area of the head and neck can originate from other organs (eg, neurological diseases, viral infections, diabetes, systemic lupus erythematosus) [12]. All of the above constitute a multidisciplinary clinical problem that requires consultation not only with a dentist but also an oral and maxillofacial surgeon, ENT specialist, neurologist, or ophthalmologist. Many studies have indicated that it is extremely important to start therapy for orofacial pain early enough. Pharmacotherapy not only eliminates discomfort but also improves the patient’s local and general condition. Indeed, pain has a negative impact on the healing process, mental health, and quality of life [13–16].
The basics of pharmacological methods of treating pain were established in ancient times [17]. Originally, only substances of plant origin were used, such as black henbane (
Pain in Dentistry
Patient pain is a common problem faced by dentists and oral and maxillofacial surgeons [42]. Dentists should be aware of the multidimensionality of pain, which, when located in the head and neck area, can be caused not only by inflammation in the teeth but also by various oral, facial, and nerve-related diseases, and tumors [43]. In dental practice, doctors should follow the “3-D” principle: 1 – diagnosis, 2 – dental treatment, 3 – drugs [44]. To make a reliable clinical assessment of pain, it is necessary to take into account, inter alia, the patient’s individual description of the pain including its location, duration, nature, and severity, as well as to assess the patient’s response to pain and its impact on physical performance [45,46]. To facilitate diagnosis, many scales taking account of various parameters have been developed, from the NRS Numeric Rating Scale based on individual feelings of a patient to the Doloplus Scale measuring changes in a patient’s behavior due to pain, as well as the NPQ (Neuropathic Pain Questionnaire), which enables differentiation of patients with neuropathic pain [47]. Knowing the nature of the pain is important for appropriate selection of the type of drug and its dose. The intensity of the feeling of pain is an individual trait and largely depends not only on the severity of the triggering factor but also on the individual’s excitability threshold.
In dentistry, various divisions of pain are recognized based on the practical aspects of this phenomenon, which allows for effective diagnosis and selection of an optimal treatment strategy. There are 9 mechanisms of pain formation: (1) provoked pain occurs under the influence of mechanical, electrical, thermal (heat, cold), and chemical stimuli acting on the tooth surface, and subsides when their action ends [48]; (2) prolonged provoked pain persists for several or more seconds longer than the duration of the provoking stimulus [49]; (3) spontaneous pain arises without the involvement of an external stimulus acting on the tooth, and is the result of an inflammatory process taking place in the tooth pulp or periapical tissues; (4) continuous pain is a type of chronic pain with periodic remissions (ie, reduced intensity of pain sensation) [50]; (5) nocturnal pain occurs with horizontal body positioning as a result of increased blood flow to the head, and is associated with inflammatory processes in the pulp [51]; (6) throbbing (pulsating) pain is characterized by pulsation that is uniform with the heart rate, and indicates the purulent nature of inflammation in the tooth area; (7) fresh pain is the first pain incident involving a particular area or tooth [52]; (8) radiating pain can involve several teeth, it difficult for the patient to locate, and it may radiate beyond the craniofacial area (eg, to the occiput, neck) [53]; (9) acute pain is severe pain with a sudden onset, and this is one of the most common reasons for a patient’s urgent visit to the dentist [51] (Figure 1).
With regard to the cause of pain, we distinguish the following types of orofacial pain: (1) dentin hypersensitivity is acute pain arising in response to a thermal or chemical stimulus, which disappears after the stimulus subsides; it also appears during restorative treatment of teeth [54]; (2) inflammation of the pulp and periapical tissues is acute or chronic, intense, radiating pain that subsides after treatment of the causative tooth [55]; (3) postoperative pain is pain arising during periodontal procedures (eg, covering gingival recessions, gingivoplasty), post-extraction, or post-implantation pain [56]; (4) pain caused by inflammation of nerves (facial nerve or trigeminal neuralgia) [11]; (5) pain arising from inflammation of the oral mucosa, which may also appear as a symptom of a general disease; this includes lesions resulting from pemphigus and syphilis [57], shingles, as well as typically located diseases of the oral mucosa, aphthous ulcers, oral lichen planus, and fungal infections; (6) pain resulting from cancer located in the area of the head and neck [58]; (7) orthodontic pain occurring in patients treated with braces [59]; (8) pain caused by inflammation of the temporomandibular joint [60]; (9) Munchausen syndrome, in which patients with psychiatric disorders present to the dentist, detailing symptoms of an illness and reporting problems that are not real [61] (Figure 2).
It is advisable that dentists and oral and maxillofacial surgeons deal with the diagnosis and treatment of craniofacial pain (especially dental pain), as only they have expertise in this area. Unfortunately, the availability of dental care is often limited. Very frequently, the patient visits a general practitioner or hospital emergency department. Therefore, clear clinical guidelines should include a management regimen for dental pain. In addition to analgesics, antibiotic therapy should be considered in such patients. In addition to pain, general symptoms such as swelling, fever, malaise, and enlarged lymph nodes are found in immunocompetent patients [27].
Effects of NSAIDs on Cyclooxygenase
NSAIDs are a diverse group of substances with anti-inflammatory, analgesic, and antipyretic effects. These drugs can be classified based on their chemical structure and selectivity of action against COX-1 and COX-2. Although a third COX isoform (COX-3) was discovered in 2002, it was eventually recognized as a variant of COX-1 [62,63]. COX-1 and COX-2 are responsible for converting arachidonic acid, a component of cell membranes, into prostaglandins found in all cells and tissues. The prostaglandins formed under the influence of COX cause, inter alia, sensibilization of nociceptors, increased platelet aggregation, increased tension of uterine muscles, and reduced gastric juice secretion [64,65]. However, the activity of individual COXs varies significantly. COX-1 is a constitutive enzyme constantly present in the endothelium of blood vessels, thrombocytes, gastric mucosa, and the kidneys. COX-1 has an anti-aggregative and vasodilatory effect on blood vessels by affecting the synthesis of prostacyclin (PGI2), regulates renal blood flow/diuresis and, by enhancing the production of prostaglandin PGE2 and PGI1, protects the mucosa of the gastrointestinal tract against injury [66]. COX-2, on the other hand, is an inducible enzyme. Although it may be present in small amounts in some tissues and organs (vascular endothelium, spinal cord, uterus), the expression of this isoenzyme significantly increases under the influence of endotoxin or proinflammatory cytokines [67]. Indeed, boosted expression of mRNA encoding COX-2 has been found during inflammation, fever, pain reactions, or during tissue injury. COX-2 increases vascular permeability and is responsible for the formation of edema and exacerbates inflammation and pain. Cytokines, through specific receptors, have been shown to induce COX-2 activity, resulting in the synthesis of PGE2, called the “final transmitter” of the febrile and inflammatory reaction. PGE2 affects neurons of the hypothalamic thermoregulatory center, leading to increased body temperature [68]. COX-2 also elevates the expression of numerous cytokines and chemokines (eg, TNF-α: tumor necrosis factor alpha, IL-1: interleukin-1, and IL-6: interleukin-6), which, in a vicious cycle, increases inflammation and fever [69]. Thus, COX-2 inhibition is the key process resulting in the anti-inflammatory effect of NSAIDs, while most of the adverse effects are due to COX-1 blockade [32,35,70,71] (Figure 3).
The specificity of NSAIDs towards COX isoforms constitutes the basis for the pharmacological division of this group of therapeutic substances. The first group of NSAIDs are preferential COX-1 inhibitors, which inhibit COX-1 more strongly than COX-2 (eg, acetylsalicylic acid, ketoprofen, ketorolac, indomethacin, acemetacin) [72,73]. The second group consists of medications that have a similar effect on both COX isoforms: ibuprofen, naproxen, diclofenac, fenoprofen, and niflumic acid [74,75]. The third group of NSAIDs are preferential coxibs that inhibit COX-2 more strongly than COX-1, including meloxicam, nimesulide, and etodolac. The fourth group is made up of selective coxibs (valdecoxib, etoricoxib, lumiracoxib), which exhibit a stronger affinity for COX-2 than COX-1. The drugs included in the first 2 groups are also referred to as first-generation NSAIDs, preferential COX-2 inhibitors are also called second-generation NSAIDs, and drugs that selectively inhibit COX-2 are called third-generation NSAIDs [76]. All NSAIDs differ in their pharmacokinetic profile (bioavailability, distribution to the site of inflammation, half-life), incidence of adverse effects, indications, and restrictions on use [17,67]
Although the primary mechanism of action of NSAIDs is based on COX blockade, these substances may demonstrate additional therapeutic properties. Lornoxicam impedes the synthesis of proinflammatory cytokines (TNF-α, IL-1, IL-6) and the expression of iNOS, reducing the cytotoxic effects of nitric oxide (NO) on cells [77,78]. Diclofenac, ASA, ketoprofen, meloxicam, and piroxicam inhibit the infiltration of leukocytes through the blood vessel wall into the site of inflammation, with meloxicam showing additional antioxidant and antiglycation properties [79–81]. Reduced release of reactive oxygen species (ROS) and limited neutrophil adhesion to the vascular endothelium were noted under the influence of nimesulide, which additionally suppresses the activity of metalloproteinases involved in extracellular matrix remodeling and transmission of pain stimuli [82,83]. Interestingly, ASA and naproxen reduce the expression of the proteolytic enzyme elastase released as a result of neutrophil degranulation [84]. In addition, these drugs inhibit the action of nuclear factor NF-κB, which induces the expression of various proinflammatory genes, including genes that encode cytokines and chemokines as well as those regulating inflammasome function. It is well known that NF-κB affects the expression of proteins associated with responses to viral and bacterial stimuli, UV radiation, oxidative stress and cell adhesion, proliferation, and apoptosis [85,86]. Thus, blockade of NF-κB by NSAIDs interrupts development of the inflammatory response.
NSAIDs in Dentistry
RESTORATIVE DENTISTRY AND ENDODONTICS:
Toothache is one of the most common reasons for patients’ unscheduled visits to the dentist. This is because the pulp-dentin complex, which is the main structure of the tooth, has a sensory function [97]. The effect of stimulation of the complex, regardless of the type of stimulus acting, is pain [98]. Nerve fibers involved in the transmission of pain stimuli are located in the pulp chamber and in the part of the dentinal tubules adjacent to the pulp. There are 2 types of nerve fibers: myelinated fibers A and unmyelinated fibers C. A-fibers (A-alpha, A-beta, A-delta) are responsible for the development of acute, short, well-localized pain associated with dentin hypersensitivity [99,100]. This pain occurs when a thermal, chemical, or mechanical stimulus is applied to exposed dentinal tubules or during dental treatment, and subsides shortly after the stimulus ceases [101]. Tooth pulp pain, on the other hand, is caused by C-fiber activation, which occurs as a result of inflammation of the pulp. It is described as dull pain and is difficult to locate [48].
Dental caries is the most widespread disease of the masticatory organ. In the initial stage of caries, the disease usually does not produce any painful symptoms; however, along with its progression, the metabolites of cariogenic bacteria successively destroy the hard tissues of the tooth [102]. Destruction of the enamel, which is the protective layer of the dentin, results in exposure of dentinal tubules and, when exposed, dentinal tubules are an “open door” to the tooth chamber, which contains the richly vascularized and innervated pulp [103,104]. Therefore, attempts have been made to use NSAIDs to treat initial pulp inflammation as well as pulp hypersensitivity. To be able to evaluate the usefulness of NSAIDs when administered topically, Chin-Pei Lin et al [105] evaluated the ability of the drugs to penetrate through dentinal tubules into the tooth. The ex vivo studies involved 30 extracted human molars cut into several 5-mm dentinal discs to evaluate drug penetration rates. Drugs from different pharmacological groups were evaluated: clindamycin (an antibiotic), betamethasone sodium phosphate (a steroid), and NSAIDs (piroxicam, lysine acetylsalicylate, and diclofenac sodium). Preliminary studies demonstrated that acetylsalicylate, compared to diclofenac sodium, piroxicam, and the other drugs, shows the best penetrability into dentin, which indicates the potential topical use of these NSAIDs. Analgesics and anti-inflammatory drugs applied to exposed dentin may penetrate deep into the tooth tissue and trigger pulp regeneration, which, in some clinical cases, could prevent root canal treatment [105]. However, this issue requires further research and clinical observations.
Untreated cavities of carious and non-carious origin can lead to inflammation of the pulp [106]. In the initial phase of inflammation, with appropriate dental treatment, the condition is reversible and the pulp is healed. However, some patients develop irreversible pulpitis, which is often associated with pain. Patients usually experience night-time pain (continuous or paroxysmal). Patients may also have increased pain when eating cold or warm foods. To eliminate the inflammation, and thus completely eliminate the pain, endodontic treatment of the causative tooth should be performed. A properly conducted process of biochemomechanical cleaning of the root canal system, tight retrograde filling of canals, and reconstruction of the coronal part is the basis of therapeutic success [107]. However, numerous studies have evaluated the possibility of using orally administered NSAIDs as a routine treatment for irreversible inflammation of the pulp and periapical tissues [108]. Despite numerous analyses and studies, the effectiveness of the preventive use of NSAIDs prior to endodontic treatment to relieve pain/inflammation after the procedure has not been confirmed [109]. The studies have mostly evaluated the effect of ibuprofen at a dose of 400–600 mg, which was recommended every 4 to 6 h to relieve pain after root canal treatment. Although the efficacy of NSAIDs has not been clinically confirmed, increasing the dose of the drug or administering another NSAID is not recommended [110]. If pain persists after NSAID, additional administration of an opioid, such as codeine, is suggested [109,111,112]. It has been proven that the combination of premedication, NSAIDs, and an increased volume of local anesthetic solution (eg, articaine and lidocaine) results in better pain control during root canal treatment of lower molars with irreversible pulpitis. The higher the dose of lidocaine or articaine (3.6 ml vs 1.8 ml), the stronger and more lasting the symptoms of anesthesia [113]. Oral administration of NSAIDs 30–60 min before injecting the anesthetic increases the effectiveness of regional anesthesia. Ibuprofen at a dose of 400–600 mg, 50 mg of diclofenac, or 10 mg of ketorolac are particularly recommended. Inflamed tooth pulp becomes less sensitive to locally administered anesthetics in the form of injections. Oral administration of NSAIDs, as recommended above, reduces the production of prostaglandins, which decreases the activity of sodium channels resistant to tetrodotoxin (TTX). It is well known that a boost in the activity of these channels is induced by prostanoids, and their activation is associated with difficulty in obtaining effective action [114,115].
The permeability of tooth tissues and the ability to absorb substances from the external environment is used in fluoridation procedures, such as the prophylactic application of fluoride gel for remineralization of tooth tissues or external bleaching [116,117]. The process of tooth bleaching occurs due to the good permeability of tooth tissues, which enables the penetration of the whitening agent (sodium perborate, urea peroxide, or hydrogen peroxide). Their mechanism of action involves the splitting of large dye molecules in oxidation or reduction reactions [118]. These phenomena are not inert to the tooth pulp and in many cases entail its temporary inflammation accompanied by pain. It is estimated that 30–80% of patients undergoing in-office whitening have postoperative sensitivity [119]. Therefore, the question was raised as to whether the use of NSAIDs prior to the bleaching procedure will alleviate inflammation and reduce pain sensations. Unfortunately, as demonstrated in clinical studies, the prophylactic NSAID administration per os prior to the procedure does not significantly alter pain after tooth whitening. This suggests limitations associated with the oral NSAID administration (eg, poor bioavailability to tooth tissues) or the involvement of inflammatory mediators insensitive to COX-1 and COX-2 blockers in pain transmission [119,120].
DENTAL SURGERY:
Dental surgery is mainly based on minor procedures such as extractions, implants, root resections, hemisections, or local bone regeneration procedures. During all of these procedures, local anesthetics are used to relieve the pain sensation for the duration of treatment. Unfortunately, after their effects have worn off, patients very often struggle with postoperative pain resulting from local inflammation [121]. Furthermore, removal of retained third molars is one of the most painful surgical procedures in the craniofacial region. Pain after this type of surgery is a useful clinical model for evaluating the effect of oral analgesics [122].
The most common cause of severe pain after tooth extraction is local alveolar osteitis, known as “dry socket”. The incidence of this complication ranges from 0.5% to 68.4% [123,124]. The preventive administration of NSAIDs before surgery in the context of dry socket prevention is controversial. For simple extractions (removal of a tooth using forceps only) that proceed without pain, the preventive administration of analgesics is controversial [125]. However, some researchers consider it reasonable to administer painkillers 1–2 h before the procedure. They emphasize that early inhibition of inflammatory processes relevant in the development of postoperative pain, such as the arachidonic acid cascade, is much more effective than antagonizing the already activated pain pathways. The most commonly recommended drugs are ibuprofen, naproxen, dexketoprofen, ketoprofen, nimesulide, and coxibs [126,127]. This is supported by a number of studies collected in the Cochrane Library systematic literature review indicating the very frequent and effective use of ibuprofen and diclofenac in post-extraction pain [28]. NSAIDs are administered 1–2 h before surgery due to the time the drug needs to reach therapeutic concentration in the bloodstream (and thus in the periodontal tissues), taking into account the duration of local anesthesia [126]. In contrast, in the case of post-extraction pain, the drugs of choice are oral NSAIDs in combination with paracetamol. These medications have a different mechanism of action, which allows for a synergistic analgesic effect [124]. The proposed regimen only works for dental surgery procedures. Toothache caused by inflammation of the pulp or periapical tissues has a different mechanism of origin and does not respond in the same manner to this treatment [128].
In contrast, the efficacy of certain orally administered NSAIDs in the prevention of trismus and postoperative edema has been demonstrated in patients who underwent tooth extraction [129]. Prostaglandins released during the inflammatory process increase vascular permeability and vasodilation associated with the formation of edema, which, in turn, leads to the compression of neuroreceptors of blood vessels, muscles, and fascia, limiting opening of the mouth. The most common surgical procedure resulting in trismus is the removal of mandibular third molars [130]. Avelar, in a study of 25 patients, demonstrated the effectiveness of administering 7.5 mg of meloxicam 1 h before surgery and every 12 h after surgery for 2 days to reduce trismus. Administration of 100 mg of nimesulide 1 h before the procedure and every 12 h after it for 2 days is also considered a more effective form of edema relief [131]. NSAIDs, by inhibiting the production of prostaglandins, reduce the permeability of blood vessels, limiting the formation of edema, and thus trismus [129,130]. However, it has been demonstrated that NSAIDs administered alone do not effectively inhibit pain. The Cochrane Collaboration, in its systematic review of studies, highlights that combining painkillers that have a distinct mechanism of action, such as combining peripherally acting analgesics with centrally acting ones, is a more effective strategy for reducing dental pain than administering NSAIDs alone [132]. It has also been proven that combining NSAIDs with drugs of another group, such as the combination of ibuprofen with paracetamol, gives better results and has fewer adverse effects than maximizing the dose of an NSAID alone. Paracetamol does not directly affect COX, but through the oxidoreductive mechanism involving the reduction of the quantity of oxidizing agents around COX (necessary for its action), it reduces activity of the enzyme [133]. Thus, the combination of paracetamol and NSAIDs provides better COX inhibition through 2 different mechanisms. The combination of diclofenac or ketoprofen with paracetamol was also positively evaluated by researchers. The dosage of analgesics from different groups should depend on the intensity of pain and the patient’s individual pain assessment. It should be remembered not to exceed the maximum daily doses: 3000 mg for paracetamol, 2400 mg for ibuprofen, 150 mg for diclofenac, and 200 mg for ketoprofen. For pain after extraction of third lower molars, the researchers proposed the following regimen: for mild pain, 200–400 mg ibuprofen every 4–6 h; for mild to moderate pain, 400–600 mg ibuprofen every 6 h for the first 24 h, then 400 mg ibuprofen every 4–6 h; for moderate to severe pain, 400–600 mg ibuprofen every 6 h for the first 24 h, then 400 mg ibuprofen with 500 mg paracetamol every 6 h; and for severe pain, 400–600 mg ibuprofen with 650 mg paracetamol and 10 mg hydrocodone every 6 h for 24–48 h is recommended, followed by 400–600 mg ibuprofen with 500 mg paracetamol every 6 h [132].
The choice of a different group of analgesics should be considered in the case of implant procedures, particularly in patients permanently taking NSAIDs. A significant amount of scientific evidence confirms their negative impact on the osseointegration process, and thus the maintenance of dental health [134–136]. Although the mechanism of bone healing disorders in patients taking NSAIDs has not been thoroughly explained [137], particular caution should be exercised when using selective COX-2 inhibitors. These drugs administered even in single doses in the postoperative period may interfere with the process of osseointegration [138]. In order to reduce the systemic side effects of NSAIDs, it is worth considering the topical application of analgesics and anti-inflammatory agents, which may improve the patient’s quality of life. Topical application of benzydamine in patients with pericoronitis was more effective than oral administration of diclofenac and flurbiprofen [139]. Surface application of the analgesics paracetamol, acetylsalicylic acid, flurbiprofen, ketorolac, and meloxicam at subtherapeutic doses showed analgesic effects comparable to orally administered agents [124]. Diclofenac rinses are also effective in relieving pain and local inflammation after minor surgical procedures [140].
ORTHODONTICS:
Bone resorption and formation are 2 components of the bone remodeling process that occurs during orthodontic treatment. The early phase of orthodontic tooth movement is associated with local inflammation of the periodontium, which is a response to biomechanical forces [141]. As a result of their action, intensive remodeling of the periodontal ligaments occurs, which is associated with a local change in vascularization and an increase in blood flow. The neurotransmitters released create a suitable environment for the bone remodeling process. Bone resorption takes place on the side of pressure and compression of periodontal fibers, while new bone formation is observed on the side of tension of periodontal fibers [142]. The inflammatory response is mediated by chemical mediators, including prostaglandin, cytokines, serotonin, and histamine [141]. Researchers point to the presence of interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) as major cytokines that promote inflammation [142]. At this stage of treatment, the patient may experience significant pain [143]. The peak of pain usually occurs within 24 h after orthodontic arch placement or replacement [141]. In such cases, it is recommended to take paracetamol, which does not interfere with orthodontic tooth movement. Its anti-inflammatory effect is negligible. It does not directly affect COX-1 and COX-2, so it does not interfere with the synthesis of prostaglandins to such a significant extent as, for example, ibuprofen or ketoprofen [142]. Among the several types of prostaglandins formed locally in dental tissues, prostaglandin E2 considerably affects vasodilation, increases vascular permeability, and participates in osteoclast activation and bone resorption, and is thus responsible for increased tooth movement. The use of NSAIDs that inhibit the formation of prostaglandin E2 may lead to a slowdown in orthodontic movement, which contributes to prolongation of orthodontic treatment and modifies the treatment plan. Therefore, patients taking NSAIDs should be carefully monitored [144–146].
NSAIDs have also found use in the treatment/relief of tension pain in the temporomandibular joint. Temporomandibular disorders (TMD), myofascial pain syndrome (MPS), and craniomandibular dysfunction (CMD) are a group of diseases accompanied by pain in the muscles, fascia, and nerves in the temporomandibular joint area [147,148]. The use of NSAIDs in these cases is first aid and should be considered as an additional tool during the treatment process. However, the primary approach in these patients is the correct diagnosis, identification of the cause of the pain, and its elimination. Very often, the dentist, the neurologist, and physiotherapist are involved in the treatment process. Standard oral doses of ibuprofen or diclofenac are most commonly recommended, and intramuscular administration may be considered if indicated [147,148].
PERIODONTAL AND ORAL MUCOSA DISEASES:
Periodontal diseases are pathological conditions of the oral cavity leading to loss of tooth attachment, destruction of soft tissues (gingiva), and loss and destruction of the alveolar bone. Although periodontal disease is mainly caused by a bacterial factor, people with metabolic diseases (such as diabetes) or compromised immunity as well as smokers are particularly predisposed to its occurrence [149–151]. The immune-inflammatory response of the host is of great importance in the development and course of the disease [151]. The inflammatory process caused by bacterial invasion can be divided into an acute immune response and a chronic phase. The acute phase is modulated by bradykinins and prostaglandins responsible for vasodilation, as well as by histamine and leukotriene, which increase vascular permeability [151]. The development of the late phase is based on the expansion of antibody-secreting plasma cells, which results in the release of excessive amounts of proinflammatory mediators, including IL-1β, IL-6, and TNF-α. It has been proven that arachidonic acid metabolites are the main mediators of the destructive inflammatory process in periodontal disease [152,153]. The results of studies on human and animal models have confirmed an outstandingly positive correlation between the concentration of PGE2 in the gingival fluid and periodontal tissues and the clinical expression of periodontal disease. It was observed that the level of PGE2 was dependent on the severity of the disease and was significantly higher in aggressive periodontitis compared to chronic inflammation [154]. Therefore, attempts have been made to use NSAIDs in patients with periodontal disease. It has been demonstrated that small doses of NSAIDs administered orally may have an inhibitory effect on inflammation and further bone destruction [154–156]. However, this effect passes immediately after discontinuation of the drug. Indeed, Jeffcoat et al [157] showed that periodontitis patients treated with flurbiprofen experienced a significant reduction in bone mass loss compared to patients receiving placebo. Unfortunately, the therapeutic effect disappears when the drug is discontinued [154]. It is suggested that this is due to the complexity of periodontal disease, the main etiologic factor of which is bacteria causing a continuous increase in the production of enterotoxins and COX-2-inducing proinflammatory factors. It is still believed that the most effective method of treating periodontitis is mechanical removal of plaque combined with chemical antimicrobial preparations to support treatment.
NSAIDs may also have an adverse effect on the oral mucosa. A number of studies have pointed to the involvement of NSAIDs in the formation of gastrointestinal mucosal ulceration, and in patients suffering from lichen planus, exacerbation of the disease symptoms and the development of the erosive form were observed [158]. Lichen planus is a chronic inflammatory disease of the mucous membranes involving not only the skin, but also the cheeks, occlusion rims, the soft palate, and even a part of the esophagus. Although the etiology of the disease is not fully understood, researchers see an interaction between genetic and environmental factors, eg, environmental pollutants or drugs, as well as diseases of civilization [159–161]. Erosive lesions in the course of lichen planus have been shown to result from an excessive immune response mediated by T lymphocytes. In addition, it has been noted that erosions occur significantly more often in patients taking NSAIDs than in those not using these drugs. A strongly positive correlation was found between the exacerbation of the erosive form of lichen planus and administration of NSAID. Discontinuation of NSAIDs led to a significant improvement in the condition of the mucosa, and exacerbation of symptoms and progression of lichen planus was observed after re-administration of NSAIDs [158]. Although the exact pathomechanism of lichen planus is still unknown, the above studies clearly show the role of NSAIDs in promoting erosive lesions. Given the incidence of numerous adverse effects after taking oral forms of NSAIDs, topical application of NSAIDs in the form of gels or rinses may be a reliable alternative. Topically administered NSAIDs are believed to produce similar therapeutic effects, minimizing the adverse effects resulting from oral administration of the drug [154]. Indeed, topical application of the active ingredient enables reduction of the dose of the drug, minimizes adverse effects, and is a good adjunct to the treatment of periodontitis and diseases of the oral mucosa [162,163]. Furthermore, the adverse effects of oral NSAIDs can be reduced by choosing COX-2-selective blockers. They act more selectively and cause fewer systemic adverse effects, which makes them particularly recommended for surgical and periodontal procedures [164].
HEAD AND NECK ONCOLOGY:
Cancer is the most important public health problem worldwide and is currently the leading cause of all deaths [165,166]. Within the oral cavity, the most frequent type of cancer is squamous cell carcinoma arising from precancerous lesions [167]. Treatment for head and neck cancer involves surgery (radical removal of lesions), chemotherapy, and radiotherapy [168–170]. A combination of topical administration of NSAIDs and systemic administration of opioids (eg, morphine, tramadol, and codeine) is also applied to relieve pain in the course of head and neck cancer [171].
Oral rinses containing NSAIDs are indicated for the prophylactic treatment of radiation-induced oral mucositis [146]. Oral and pharyngeal mucositis is a common complication of radiotherapy in the head and neck region. Damage to the oral mucosa often causes significant pain, can lead to impairment of food intake and swallowing, and is an open gateway for numerous infectious agents. According to current protocols, patients undergoing radiotherapy are advised to maintain proper oral hygiene, change their eating habits (eg, eat loose-textured foods, avoid acidic and spicy foods), as well as use analgesics and anti-inflammatory drugs. Epstein [172] unequivocally confirmed the validity of a rinse containing benzydamine in patients suffering from mucositis as a complication of radiotherapy. The diluted benzydamine solution reduced pain and burning sensation in the mouth, which significantly improved the quality of life of cancer patients.
Use of NSAIDs in cancer therapy has been assessed in numerous studies, which is not surprising, since a strong correlation has been demonstrated between inflammation and the aggressiveness and development of malignant tumors. It has been proven that NSAIDs (celecoxib and acetylsalicylic acid) have an inhibitory effect on the metastatic potential of head and neck squamous cell carcinoma [173], which accounts for 90% of cancers in the craniofacial region. However, there are few studies on the use of NSAIDs in the prevention of head and neck cancer [174]. The use of anti-inflammatory drugs as a chemopreventive agent for cancer raises many questions regarding the choice of an optimal drug, dose, and duration of therapy. In addition, the cardiovascular and gastrointestinal toxicity of NSAIDs limits their preventive use in the general population [175].
Topical Use of NSAIDs in Dentistry
Topical administration of NSAIDs enable the avoidance of the first-pass effect (degradation of the drug before it enters the systemic circulation by metabolic processes that occur in the liver), eliminates the potential breakdown of the therapeutic substance in the gastrointestinal tract, helps avoid interactions of NSAIDs with food and other drugs orally administered, and, in the case of chronic diseases, allows reducing the frequency of application of drugs with a short half-life [176–178]. It also significantly reduces the incidence of NSAID’s adverse effects [179]. Topically applied NSAIDs are most often available in the form of solution, gel, aerosol, lozenges, and chewing gums. In dentistry, their main site of action is the oral mucosa, which is a biological barrier through which NSAIDs are transported, mainly via passive diffusion. The rate of transport depends on the size of the molecule, its lipophilicity, and degree of ionization. The site of administration of the drug is also important. The permeability of NSAIDs through the oral mucosa is lowest on the hard palate and gums, and is highest in the sublingual area of the floor of the mouth and the ventral surface of the tongue [180,181]. The loss of the superficial barrier layer that occurs when lesions appear (eg, aphthous ulcers, ulcers, and mechanical trauma) results in easier and faster diffusion of NSAIDs into the blood. The drug absorbed through the jugular vein can reach the systemic circulation, thus preventing the first-pass effect [176]. However, the difficult conditions of the oral cavity, such as constant presence of saliva and mobility of the tongue, lead to dilution of some amount of the drug or even swallowing it with saliva. To increase the residence time of NSAIDs in the mouth, thereby increasing the duration of drug action, bioadhesive systems are used. In dentistry, the most common bioadhesive forms of the drug are gels and pastes. Their absorption into the circulation is then minimal and they have virtually no systemic effect. For inflammation of the oral and pharyngeal mucosa after oral surgery and for mucositis (inflammation of the oral mucosa as a consequence of radiotherapy of head and neck cancer), topical solutions and gels are the currently recommended forms of NSAID administration [172,182].
Although there are few topical NSAID preparations on the market, one can find, for instance, rinses with diclofenac [183] and gels with choline salicylate [184]. These preparations are commonly used as part of the self-treatment of aphthous ulcers and injuries to the oral mucosa. Their action consists in relieving pain and local inflammation. Aphthae and local ulceration of the oral cavity occur when the body’s defense systems are weakened (decreased immunity), in patients using orthodontic braces and dentures as a result of mechanical damage to the mucosa, as well as idiopathically without identifying a specific cause [184–186]. The NSAID most commonly used in dentistry is diclofenac, which penetrates well through the mucous membrane and accumulates in the area of inflamed tissues. It inhibits prostaglandin synthesis and has an analgesic effect. Another form of topical drug application is an aerosol containing, for example, benzydamine, which is an indole non-steroidal anti-inflammatory drug. Benzydamine has a high affinity for cell membranes and exhibits a stabilizing effect on them [187,188]. In addition to its analgesic and anti-inflammatory effects, it is also a local anesthetic. Due to the accumulation of the drug below inflamed tissues after its application, it reaches higher local concentration than when administered orally. It can also be USED in the form of lozenges for treating inflammation of the throat. However, intensive research is underway on other forms of the drug for oral administration [182,189,190].
Adverse Effects of NSAIDs Used in Dentistry
Any drug used outside the therapeutic indication or in an inappropriate dose may cause adverse effects. Due to the mechanism of action of NSAIDs (inhibition of the activity of COX-1 and COX-2), we can expect adverse effects even after taking a single dose of the drug. COX-1 is found in almost all cells of the body and performs important physiological functions; therefore, most adverse effects are due to blockade of this COX isoform. Adverse effects of NSAIDs depend on the type of therapeutic substance, the dose and time of taking the drug, as well as a person’s individual conditions. The adverse effects mainly affect the digestive system, eg, in patients with abdominal pain, heartburn, nausea, diarrhea, and vomiting. Gastric ulcers and bleeding from the gastrointestinal tract affect a much smaller group of patients [191]. To reduce the risk of ulcers, concomitant use of proton pump inhibitors (PPIs, such as omeprazole or pantoprazole) is recommended in at-risk patients [192]. With prolonged use of NSAIDs, PPI drugs should be prescribed to every patient [193].
NSAIDs increase the incidence of cardiovascular diseases [194,195]. The most severe complications affect patients with cardiovascular incidents and include hypertension, ischemic heart disease, myocardial infarction, and stroke. Acetylsalicylic acid (ASA) affects blood clotting because it irreversibly inhibits thromboxane synthesis in platelets. Moreover, blocking COX-2 increases the risk of prothrombotic effects. Therefore, selective COX-2 inhibitors should be avoided in combination therapy with ASA [196,197]. When performing surgical and periodontal procedures with tissue disruption, account should be taken of the risk of increased bleeding during the procedure as well as of the possibility of prolonged bleeding immediately afterwards. However, it is not recommended to discontinue ASA administration before minor procedures. Interestingly, administration of a single dose of ibuprofen before the procedure can also boost intraoperative bleeding [198]. Moreover, NSAIDs increase blood pressure, resulting in kidney dysfunction with sodium and water retention and accompanying edema. Increased nephrotoxicity of NSAIDs is mainly observed with long-term use of the drugs [146,199].
Another organ affected by NSAIDs is the lungs. NSAIDs inhibit the production of PGE2, thereby increasing the production of bronchoconstrictor cysteinyl leukotrienes. Thus, NSAIDs can cause breathing difficulties and asthma, which is then referred to as aspirin-induced asthma [200]. People who exhibit aspirin hypersensitivity demonstrate a group of characteristic symptoms called the aspirin triad. These include the simultaneous occurrence of bronchial asthma, urticaria, and nasal polyps [201]. NSAIDs are also hepatotoxic. Fagan et al reported that diclofenac and sulindac should be avoided by people with liver failure [202]. Kliknij lub naciśnij tutaj, aby wprowadzić tekst. On the other hand, dangerous skin reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis occur rarely under the influence of NSAIDs. Adverse effects primarily occur in the elderly [204].
From a dentist’s point of view, an important aspect of the action of NSAIDs is their effect on osteointegration and bone remodeling processes. In orthodontic treatment, this is an adverse effect, as in dental implant procedures [134]. As described earlier, prostaglandin E2 affects vasodilation, increases vascular permeability, and participates in osteoclast activation and bone resorption. On the contrary, in periodontal disease that progresses with gingivitis and bone loss, the administration of NSAIDs is beneficial, as they inhibit the inflammatory cascade. However, attention should be paid to patients permanently taking ASA, as an association has been demonstrated between taking this drug and low oral pH [205]. This contributes to the formation of erosive cavities in enamel and dentin, and promotes the development of caries. Within the oral cavity, noticeable adverse effects of NSAIDs also include reduced salivation (hyposalivation) and a feeling of dry mouth (xerostomia) [206]. Hyposalivation and xerostomia increase with the amount of medication taken and significantly worsen the patient’s quality of life. NSAIDs may also exacerbate the symptoms of an already existing disease, such as lichen planus. It has been proven that taking NSAIDs by people suffering from this disease leads to its exacerbation and the development of erosive, ie, more aggressive, form of lichen planus [158].
NSAIDs can be used in children, but attention should be paid to using the appropriate dose of the drug depending on body weight and age. For prolonged pain, it is recommended to combine ibuprofen with paracetamol. This combination demonstrates high efficiency with simultaneous good tolerance by young people [207]. However, it should be remembered that COX-2 is involved in the maturation of enamel. Therefore, long-term use of NSAIDs in early childhood may cause enamel hypomineralization [208]. In the tissues of the maturing tooth, COX-2 affects the transformation of arachidonic acid into prostaglandins responsible for increasing the vascular flow, which promotes proper function of ameloblasts. Drugs that inhibit COX-2 activity indirectly reduce the amount of calcium and magnesium ions necessary for ameloblasts in the process of enamel mineralization [209]. In addition, aspirin is one of the drugs that should not be used in children. It can result in Reye’s syndrome, which is a rare but potentially fatal liver injury accompanied by encephalopathy [146,210].
The FDA (U.S. Food and Drug Administration) classifies most NSAIDs as category B in the first and second trimester of pregnancy (animal studies have failed to demonstrate a risk to the fetus, but this has not been confirmed in pregnant women). During the last 3 months of pregnancy, NSAIDs are included in FDA’s category D, so there is a documented risk of harmful effects of these drugs on the fetus – they should be administered to pregnant women only if the mother’s life is in danger. The analgesic of choice in pregnancy is paracetamol [211].
Interactions of NSAIDs
It is not recommended to use several NSAIDs at the same time, as it amplifies adverse effects with little therapeutic gain. It is also important to bear in mind the patients taking aspirin regularly for cardioprotection, who are also using other NSAIDs on an ad hoc basis. It has been proven that ibuprofen weakens the effect of ASA because it blocks the site of COX acetylation by acetylsalicylic acid. NSAID interactions also involve drugs used for treatment of blood clotting disorders. An increased risk of bleeding has been reported in patients taking NSAIDs with oral anticoagulants [212,213]. Furthermore, upper gastrointestinal bleeding is more common in patients treated with glucocorticoids and bisphosphonates who are taking NSAIDs concomitantly [214]. High doses of NSAIDs administered orally are responsible for exacerbating the adverse effects of antidiabetic drugs, eg, intensify the hypoglycemic effect of sulfonylurea derivatives as well as cardiovascular drugs, eg, by reducing the therapeutic effectiveness of ACE inhibitors and β-blockers [180,215]. Thus, there is a need for patient education and dental awareness regarding the use of NSAIDs and associated adverse effects, as well as interactions with other drugs.
Future Directions
NSAIDs are widely used in dentistry for their analgesic, antipyretic, and anti-inflammatory effects, inhibition of the NF-κB signaling pathway, or reduction of oxidative stress. New mechanisms of action of NSAIDs are being described, which may result in the development of further NSAIDs with more selective activity and their targeted use for conditions of specific etiology. Due to the small number of reports/observations on small populations, further studies are needed to unequivocally confirm the validity of NSAID use in dentistry. Only randomized interventional studies will enable the development of clear clinical guidelines for using NSAIDs in conservative dentistry and endodontics, dental surgery, orthodontics, periodontology, and oral mucosal diseases, as well as head and neck oncology. A future focus will be topical use of NSAIDs in the oral cavity, which will negate the systemic adverse effects of this group of drugs. Although topical NSAIDs are most commonly used in the form of solution, gel, aerosol, lozenges, and chewing gum, extensive research is being conducted to develop new pharmaceutical forms of NSAIDs for oral use. These efforts aim to increase the drug’s penetration into the site of action (eg, the hard tissues of the oral cavity) or enable modified release of the drug and, minimizing adverse effects.
Conclusions
The most common reason for a patient’s urgent and unplanned visit to a dental surgeon is toothache, which is described as one of the most bothersome and most severe types of pain. The basis for the elimination of craniofacial pain is to find the cause and treat the underlying disease, of which the pain is only a symptom [28]. Accurate diagnosis and therapy should go hand in hand with rapid relief of pain symptoms so as to restore the patient’s comfort of life as soon as possible. NSAIDs have been used in almost every field of dentistry. The mechanism of action of NSAIDs involves inhibition of prostaglandin synthesis by blocking COX activity. The pharmaceutical forms of NSAIDs used in dentistry are mainly tablets and capsules. In restorative dentistry and endodontics, NSAIDs help relieve toothache caused by inflammation of the pulp or periapical tissues. In dental surgery, especially after extraction procedures, they promote the development of inflammation and trismus, and relieve postoperative pain. The most widely researched and most commonly recommended drug of this type (regardless of the cause of pain) is ibuprofen at a dose of 200–600 mg at a time (the maximum daily dose is 2400 mg) [216]. In periodontal and mucosal diseases, NSAIDs have been used via oral administration (to reduce the rate of bone destruction) and topical application. NSAID gels, rinses, and sprays are effective in the treatment of local gingivitis, mucosal lesions, aphthae, and mucositis. NSAIDs are also recommended for relieving pain in patients after radiotherapy of head and neck cancer. Only in the case of toothache during orthodontic treatment is it suggested to consider another analgesic, for instance paracetamol, since NSAIDs inhibit bone remodeling processes. While planning analgesic therapy for dental patients, it is important not to exceed the maximum and daily dose and to bear in mind the adverse effects, particularly in at-risk patients such as those with gastrointestinal and cardiovascular diseases. The goal of the dentist is not only to restore normal function of the stomatognathic system, but also to minimize pain and take care of the patient’s mental state. NSAIDs constitute the basis of the analgesic ladder, and they are the first-line drugs in relieving the pain and inflammation of oral conditions. The topical use of NSAIDs in dentistry warrants attention and further research as it will significantly reduce the adverse effects of systemic administration. Given the widespread use of NSAIDs, there is a need for patient education and dental awareness regarding the use of NSAIDs and the related adverse effects.
Figures
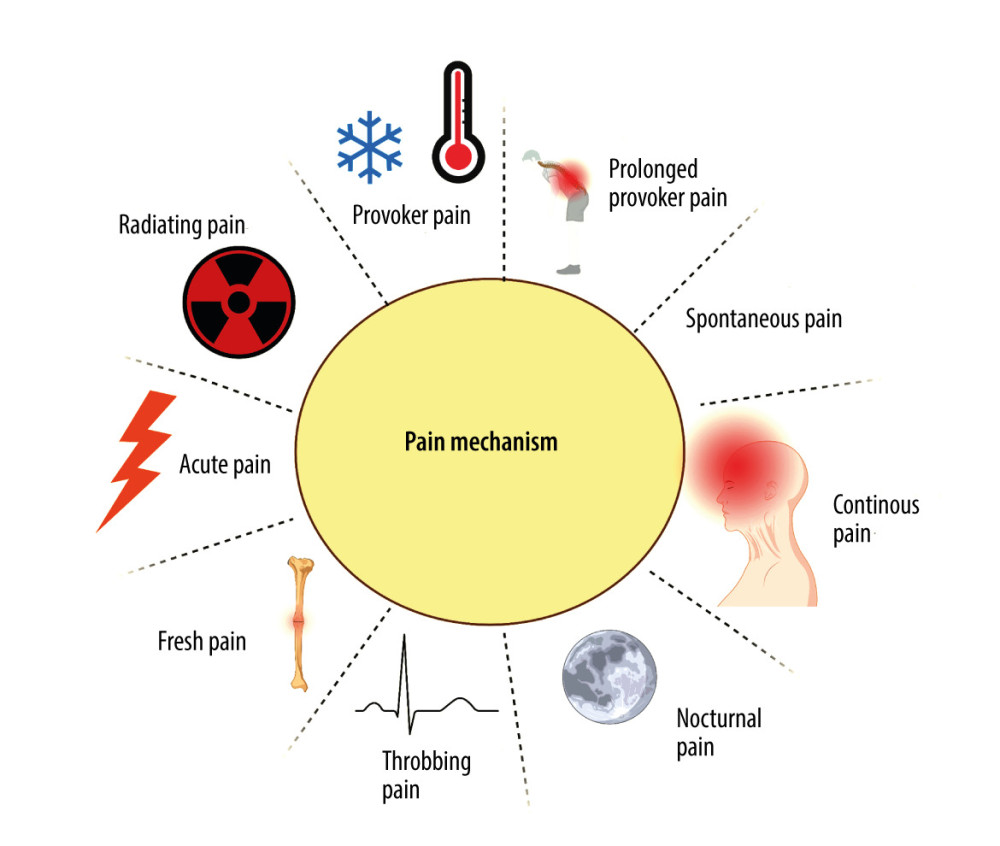 Figure 1. Mechanisms of painCreated with BioRender.com. With regard to the mechanism of pain formation in the oral cavity, a distinction is made between: provoked pain, prolonged provoked pain, spontaneous pain, continuous pain, nocturnal pain, throbbing (pulsating) pain, fresh pain, acute pain, and radiating pain.
Figure 1. Mechanisms of painCreated with BioRender.com. With regard to the mechanism of pain formation in the oral cavity, a distinction is made between: provoked pain, prolonged provoked pain, spontaneous pain, continuous pain, nocturnal pain, throbbing (pulsating) pain, fresh pain, acute pain, and radiating pain. 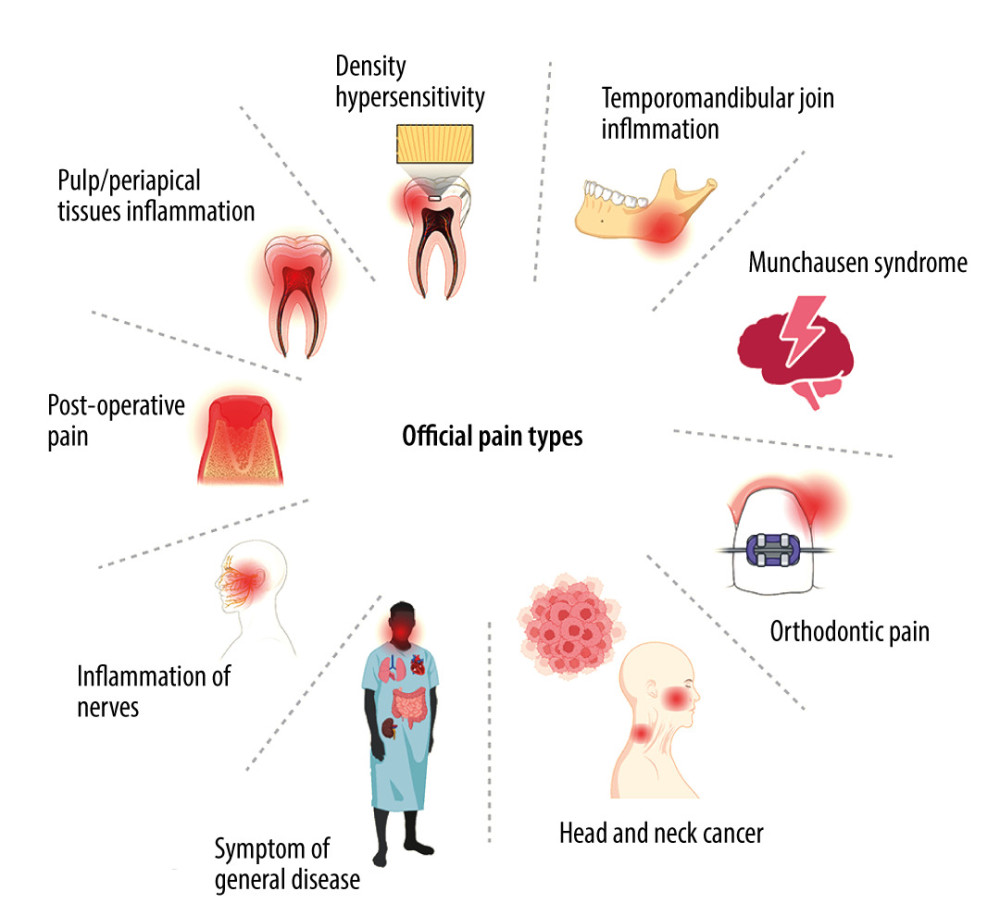 Figure 2. Types of orofacial painCreated with BioRender.com. With regard to the cause of pain in the oral cavity, we distinguish the following types of orofacial pain: dentin hypersensitivity, pain caused by inflammation of the temporomandibular joint, Munchausen syndrome, orthodontic pain, pain resulting from cancer located in the area of the head and neck, pain arising from general diseases, pain caused by inflammation of nerves, postoperative pain, inflammation of the pulp and periapical tissues, and pain arising from inflammation of the oral mucosa.
Figure 2. Types of orofacial painCreated with BioRender.com. With regard to the cause of pain in the oral cavity, we distinguish the following types of orofacial pain: dentin hypersensitivity, pain caused by inflammation of the temporomandibular joint, Munchausen syndrome, orthodontic pain, pain resulting from cancer located in the area of the head and neck, pain arising from general diseases, pain caused by inflammation of nerves, postoperative pain, inflammation of the pulp and periapical tissues, and pain arising from inflammation of the oral mucosa. 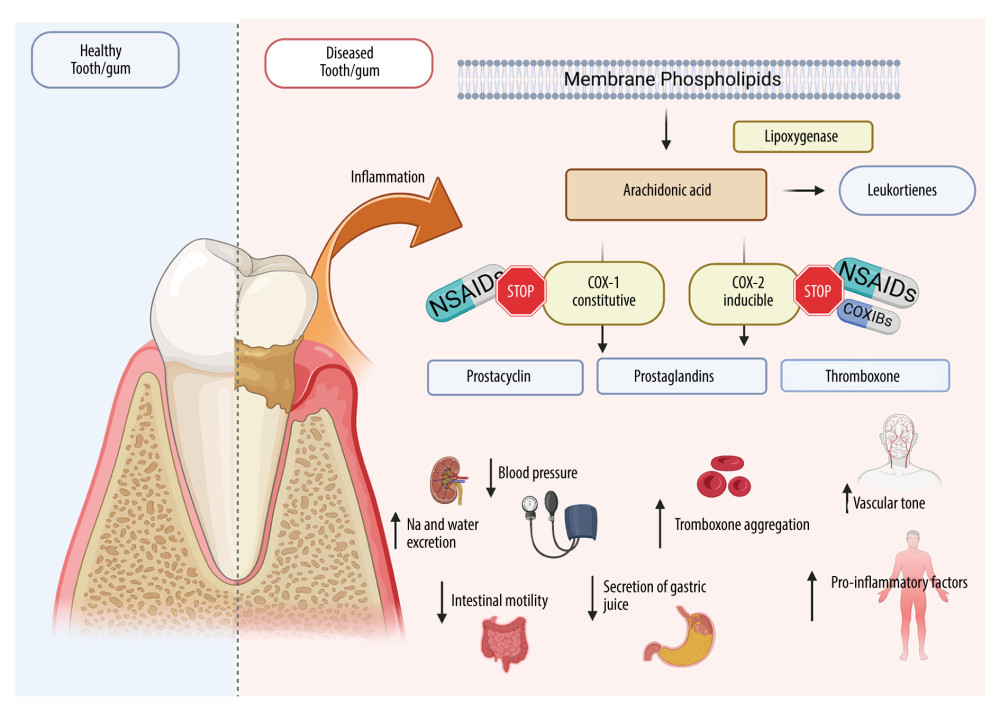 Figure 3. Effects of NSAIDs on cyclooxygenaseCreated with BioRender.com. The mechanism of action of NSAIDs is connected with the inhibition of cyclooxygenase (COX), which blocks the conversion of arachidonic acid to proinflammatory prostaglandins. NSAIDs reduce inflammation and pain in periodontitis/mucositis or after oral surgery. Although COX exists in 2 isoforms (COX-1 and COX-2), COX-2 is responsible for proinflammatory effects, while COX-1 serve mainly physiological functions. Therefore, most NSAID adverse effects are associated with COX-1 inhibition including gastrointestinal damage, liver damage or cardiovascular complications.
Figure 3. Effects of NSAIDs on cyclooxygenaseCreated with BioRender.com. The mechanism of action of NSAIDs is connected with the inhibition of cyclooxygenase (COX), which blocks the conversion of arachidonic acid to proinflammatory prostaglandins. NSAIDs reduce inflammation and pain in periodontitis/mucositis or after oral surgery. Although COX exists in 2 isoforms (COX-1 and COX-2), COX-2 is responsible for proinflammatory effects, while COX-1 serve mainly physiological functions. Therefore, most NSAID adverse effects are associated with COX-1 inhibition including gastrointestinal damage, liver damage or cardiovascular complications. References
1. Swieboda P, Filip R, Prystupa A, Drozd M, Assessment of pain: Types, mechanism and treatment: Ann Agric Environ Med, 2013(Spec no 1); 2-7
2. Isagulyan ED, Makashova ES, Myasnikova LK, Psychogenic (nociplastic) pain: Current state of diagnosis, treatment options, and potentials of neurosurgical management: Prog Brain Res, 2022; 272(1); 105-23
3. Cohen SP, Vase L, Hooten WM, Chronic pain: An update on burden, best practices, and new advances: Lancet, 2021; 397; 2082-97
4. Porreca F, Navratilova E, Reward, motivation, and emotion of pain and its relief: Pain, 2017; 158(Suppl 1); S43-S49
5. Jungquist CR, Vallerand AH, Sicoutris C, Assessing and managing acute pain: A call to action: Am J Nurs, 2017; 117; S4-S11
6. Ogle OE, Odontogenic infections: Dent Clin North Am, 2017; 61; 235-52
7. Ing JW, Head and neck cancer pain: Otolaryngol Clin North Am, 2017; 50; 793-806
8. Conti PCR, Bonjardim LR, Stuginski-Barbosa J, Pain complications of oral implants: Is that an issue?: J Oral Rehabil, 2021; 48; 195-206
9. Evans SW, McCahon RA, Management of postoperative pain in maxillofacial surgery: Br J Oral Maxillofac Surg, 2019; 57; 4-11
10. Pergolizzi JV, Magnusson P, LeQuang JA, The pharmacological management of dental pain: Expert Opin Pharmacother, 2020; 21; 591-601
11. Gambeta E, Chichorro JG, Zamponi GW, Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments: Mol Pain, 2020; 16; 1744806920901890
12. Treede R-D, Rief W, Barke A, A classification of chronic pain for ICD-11: Pain, 2015; 156; 1003-7
13. Penlington C, Urbanek M, Barker S, Psychological theories of pain: Prim Dent J, 2019; 7; 24-29
14. Ahmedzai S, Recent clinical trials of pain control: Impact on quality of life: Eur J Cancer, 1995; 31A(Suppl 6); S2-7
15. Ferrell BR, The impact of pain on quality of life. A decade of research: Nurs Clin North Am, 1995; 30; 609-24
16. Turk DC, Fillingim RB, Ohrbach R, Patel K, Assessment of psychosocial and functional impact of chronic pain: J Pain, 2016; 17; T21-49
17. Rao P, Knaus EE, Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond: J Pharm Pharm Sci, 2008; 11; 81s-110s
18. Alizadeh A, Moshiri M, Alizadeh J, Balali-Mood M, Black henbane and its toxicity – a descriptive review: Avicenna J Phytomed, 2014; 4; 297-311
19. Abrams DI, Guzman M, Cannabis in cancer care: Clin Pharmacol Ther, 2015; 97; 575-86
20. Hussain Khan Z, Poppy: The elegant flower or a lethal weapon of addiction: Acta Med Iran, 2015; 53; 594-95
21. Benítez G, Leonti M, Böck B, The rise and fall of mandrake in medicine: J Ethnopharmacol, 2022; 303; 115874
22. Montinari MR, Minelli S, de Caterina R, The First 3500 years of aspirin history from its roots – a concise summary: Vascul Pharmacol, 2019; 113; 1-8
23. Brune K, Hinz B, The discovery and development of antiinflammatory drugs: Arthritis Rheum, 2004; 50; 2391-99
24. Schug SA, Garrett WR, Gillespie G, Opioid and non-opioid analgesics: Best Pract Res Clin Anaesthesiol, 2003; 17; 91-110
25. Cherny NI, Opioid analgesics: Comparative features and prescribing guidelines: Drugs, 1996; 51; 713-37
26. Owusu Obeng A, Hamadeh I, Smith M, Review of opioid pharmacogenetics and considerations for pain management: Pharmacotherapy, 2017; 37; 1105-21
27. Timmerman A, Parashos P, Management of dental pain in primary care: Aust Prescr, 2020; 43; 39-44
28. Kim S-J, Seo JT, Selection of analgesics for the management of acute and postoperative dental pain: A mini-review: J Periodontal Implant Sci, 2020; 50; 68-73
29. Gaskell H, Derry S, Wiffen PJ, Moore RA, Single dose oral ketoprofen or dexketoprofen for acute postoperative pain in adults: Cochrane Database Syst Rev, 2017; 5; CD007355
30. Oesterling TO, Morozowich W, Roseman TJ, Prostaglandins: J Pharm Sci, 1972; 61; 1861-95
31. Dannhardt G, Kiefer W, Cyclooxygenase inhibitors – current status and future prospects: Eur J Med Chem, 2001; 36; 109-26
32. Fitzpatrick FA, Cyclooxygenase enzymes: Regulation and function: Curr Pharm Des, 2004; 10; 577-88
33. Cunningham K, Candelario DM, Angelo LB, Nonsteroidal anti-inflammatory drugs: Updates on dosage formulations and adverse effects: Orthop Nurs, 2020; 39; 408-13
34. Vane JR, Botting RM, Mechanism of action of anti-inflammatory drugs: Scand J Rheumatol Suppl, 1996; 102; 9-21
35. Vane JR, Bakhle YS, Botting RM, Cyclooxygenases 1 and 2: Annu Rev Pharmacol Toxicol, 1998; 38; 97-120
36. Fürst R, Blumenthal SB, Kiemer AK, Nuclear factor-kappa B-independent anti-inflammatory action of salicylate in human endothelial cells: Induction of heme Oxygenase-1 by the c-Jun N-terminal kinase/activator protein-1 pathway: J Pharmacol Exp Ther, 2006; 318; 389-94
37. Sclabas GM, Uwagawa T, Schmidt C, Nuclear factor kappa B activation is a potential target for preventing pancreatic carcinoma by aspirin: Cancer, 2005; 103; 2485-90
38. Berg J, Fellier H, Christoph T, Grarup J, Stimmeder D, The analgesic NSAID lornoxicam inhibits cyclooxygenase (COX)-1/-2, inducible nitric oxide synthase (INOS), and the formation of interleukin (IL)-6 in vitro: Inflamm Res, 1999; 48; 369-79
39. Stratman NC, Carter DB, Sethy VH, Ibuprofen: Effect on inducible nitric oxide synthase: Brain Res Mol Brain Res, 1997; 50; 107-12
40. Cho JY, Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses: Arch Pharm Res, 2007; 30; 64-74
41. Barrett JE, Haas DA, Perspectives and trends in pharmacological approaches to the modulation of pain: Adv Pharmacol, 2016; 75; 1-33
42. Rotpenpian N, Yakkaphan P, Review of literatures: Physiology of orofacial pain in dentistry: eNeuro, 2021; 8(2); ENEURO.0535-20.2021
43. Renton T, Tooth-related pain or not?: Headache, 2020; 60; 235-46
44. Hargreaves K, Abbott P, Drugs for pain management in dentistry: Aust Dent J, 2005; 50; S14-22
45. Fillingim RB, Loeser JD, Baron R, Edwards RR, Assessment of chronic pain: Domains, methods, and mechanisms: J Pain, 2016; 17; T10-20
46. Abbott P, Present status and future directions: Managing endodontic emergencies: Int Endod J, 2022; 55(Suppl 3); 778-803
47. Benzon HT, The neuropathic pain scales: Reg Anesth Pain Med, 2005; 30; 417-21
48. Bender IB, Pulpal pain diagnosis – a review: J Endod, 2000; 26; 175-79
49. Barnes JJ, Patel S, Contemporary endodontics – part 1: Br Dent J, 2011; 211; 463-68
50. Antonelli JR, Acute dental pain, part II: Diagnosis and emergency treatment: Compendium, 1990; 11; 526-33
51. de Laat A, Differential diagnosis of toothache to prevent erroneous and unnecessary dental treatment: J Oral Rehabil, 2020; 47; 775-81
52. Raab WHAcute and chronic toothache: Dtsch Zahnarztl Z, 1991; 46; 101-8 [in German]
53. Piattelli A, Traini T, Diagnosis and managing pulpitis: Reversible or irreversible?: Pract Proced Aesthet Dent, 2007; 19; 254-56
54. West N, Seong J, Davies M, Dentine hypersensitivity: Monogr Oral Sci, 2014; 25; 108-22
55. Galler KM, Weber M, Korkmaz Y, Inflammatory response mechanisms of the dentine-pulp complex and the periapical tissues: Int J Mol Sci, 2021; 22; 1480
56. Khan J, Zusman T, Wang Q, Eliav E, Acute and chronic pain in orofacial trauma patients: J Endod, 2019; 45; S28-S38
57. Thompson LDR, Oral syphilis: Ear Nose Throat J, 2021; 100; 538S-39S
58. Infante-Cossio P, Torres-Carranza E, Cayuela A, Quality of life in patients with oral and oropharyngeal cancer: Int J Oral Maxillofac Surg, 2009; 38; 250-55
59. Long H, Wang Y, Jian F, Current advances in orthodontic pain: Int J Oral Sci, 2016; 8; 67-75
60. Cairns BE, Pathophysiology of TMD pain – basic mechanisms and their implications for pharmacotherapy: J Oral Rehabil, 2010; 37; 391-410
61. Tatu L, Aybek S, Bogousslavsky J, Munchausen syndrome and the wide spectrum of factitious disorders: Front Neurol Neurosci, 2018; 42; 81-86
62. Burdan F, Chałas A, Szumiło JCyclooxygenase and prostanoids – biological implications: Postepy Hig Med Dosw (Online), 2006; 60; 129-41 [in Polish]
63. Berenbaum F, COX-3: Fact or fancy?: Joint Bone Spine, 2004; 71; 451-53
64. Bennett A, Prostaglandins: Their release, biological effects and relationships to pain and inflammation: Cephalalgia, 1986; 6(Suppl 4); 17-20
65. Wardle EN, Guide to the prostaglandins: Br J Hosp Med, 1985; 34; 229-32
66. Vane JR, Botting RM, Anti-inflammatory drugs and their mechanism of action: Inflamm Res, 1998; 47(Suppl 2); S78-87
67. Lipsky LP, Abramson SB, Crofford L, The classification of cyclooxygenase inhibitors: J Rheumatol, 1998; 25; 2298-303
68. Bernheim HA, Block LH, Atkins E, Fever: Pathogenesis, pathophysiology, and purpose: Ann Intern Med, 1979; 91; 261-70
69. Sharma JN, Jawad NM, Adverse effects of COX-2 inhibitors: ScientificWorldJournal, 2005; 5; 629-45
70. Mazaleuskaya LL, Ricciotti E, Druggable prostanoid pathway: Adv Exp Med Biol, 2020; 1274; 29-54
71. Pairet M, Engelhardt G, Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: Possible physiological and therapeutic implications: Fundam Clin Pharmacol, 1996; 10; 1-17
72. Perrone MG, Scilimati A, Simone L, Vitale P, Selective COX-1 inhibition: A therapeutic target to be reconsidered: Curr Med Chem, 2010; 17; 3769-805
73. Katz IM, Indomethacin: Ophthalmology, 1981; 88; 455-58
74. Hussar DA, Hodge NA, Ibuprofen: Am Pharm, 1985; NS25; 51-54
75. Small RE, Diclofenac sodium: Clin Pharm, 1989; 8; 545-58
76. Süleyman H, Demircan B, Karagöz Y, Anti-inflammatory and side effects of cyclooxygenase inhibitors: Pharmacol Rep, 2007; 59; 247-58
77. Ahmed MO, Al-Badr AA, Lornoxicam: Profiles Drug Subst Excip Relat Methodol, 2011; 36; 205-39
78. Skjodt NM, Davies NM, Clinical pharmacokinetics of lornoxicam. A short half-life oxicam: Clin Pharmacokinet, 1998; 34; 421-28
79. Davies NM, Skjodt NM, Clinical pharmacokinetics of meloxicam. A Cyclo-Oxygenase-2 preferential nonsteroidal anti-inflammatory drug: Clin Pharmacokinet, 1999; 36; 115-26
80. Khalil NY, Aldosari KF, Meloxicam: Profiles Drug Subst Excip Relat Methodol, 2020; 45; 159-97
81. Pawlukianiec C, Gryciuk ME, Mil KM, A new insight into meloxicam: Assessment of antioxidant and anti-glycating activity in in vitro studies: Pharmaceuticals (Basel), 2020; 13; 240
82. Suleyman H, Cadirci E, Albayrak A, Halici Z, Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug: Curr Med Chem, 2008; 15; 278-83
83. Singla AK, Chawla M, Singh A, Nimesulide: Some pharmaceutical and pharmacological aspects – an update: J Pharm Pharmacol, 2000; 52; 467-86
84. Segre EJ, Naproxen sodium (Anaprox): Pharmacology, pharmacokinetics and drug interactions: J Reprod Med, 1980; 25; 222-25
85. Hartung JE, Eskew O, Wong T, Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation: Brain Behav Immun, 2015; 50; 196-202
86. Lingappan K, NF-κB in oxidative stress: Curr Opin Toxicol, 2018; 7; 81-86
87. Anekar AA, Cascella M, WHO Analgesic Ladder: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing
88. Ripamonti C, Bandieri E, Pain therapy: Crit Rev Oncol Hematol, 2009; 70; 145-59
89. Brewer AR, McCarberg B, Argoff CE, Update on the use of topical NSAIDs for the treatment of soft tissue and musculoskeletal pain: A review of recent data and current treatment options: Phys Sportsmed, 2010; 38; 62-70
90. Marino M, Jamal Z, Zito PM, Pharmacodynamics: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing
91. Ong CKS, Lirk P, Tan CH, Seymour RA, An evidence-based update on nonsteroidal anti-inflammatory drugs: Clin Med Res, 2007; 5; 19-34
92. Woron J, Wordliczek J, Dobrogowski J, Porównanie niesteroidowych leków przeciwzapalnych (NLPZ): Medycyna po Dyplomie, 2011; 55-63 [in Polish]
93. Kim K-H, Seo H-J, Abdi S, Huh B, All about pain pharmacology: What pain physicians should know: Korean J Pain, 2020; 33; 108-20
94. Hersh E, Moore PA, Grosser T, Nonsteroidal anti-inflammatory drugs and opioids in postsurgical dental pain: J Dent Res, 2020; 99; 777-86
95. Kuczyńska J, Pawlak A, Nieradko-Iwanicka B, The comparison of dexketoprofen and other painkilling medications (review from 2018 to 2021): Biomed Pharmacother, 2022; 149; 112819
96. Cooper SA, Desjardins PJ, The value of the dental impaction pain model in drug development: Methods Mol Biol, 2010; 617; 175-90
97. Kleinert A, Kleinert L, Ozimirska M, Chałas R, Endodontium – together or separately?: Folia Morphol (Warsz), 2018; 77; 409-15
98. Butt K, Harris I, Making sense of sensibility: Part 1: Br Dent J, 2022; 232; 307-10
99. Aminoshariae A, Kulild JC, Current concepts of dentinal hypersensitivity: J Endod, 2021; 47; 1696-702
100. Longridge NN, Youngson CC, Dental pain: Dentine sensitivity, hypersensitivity and cracked tooth syndrome: Prim Dent J, 2019; 8; 44-51
101. Pashley DH, Dynamics of the pulpo-dentin complex: Crit Rev Oral Biol Med, 1996; 7; 104-33
102. Zero DT, Dental caries process: Dent Clin North Am, 1999; 43; 635-64
103. Tønder KJ, Vascular reactions in the dental pulp during inflammation: Acta Odontol Scand, 1983; 41; 247-56
104. van Hassel HJ, Reprint of: Physiology of the human dental pulp: J Endod, 2021; 47; 690-95
105. Lin C-P, Wang Y-L, Shen L-J, Lin C-P, The dentin permeability of anti-inflammatory and antibacterial drugs: In vitro study: J Formos Med Assoc, 2019; 118; 828-32
106. Martin FE, Carious pulpitis: Microbiological and histopathological considerations: Aust Endod J, 2003; 29; 134-37
107. Hewitt B, Coffman C, Update on endodontic, restorative, and prosthodontic therapy: Vet Clin North Am Small Anim Pract, 2022; 52; 185-20
108. Mohammadi Z, Abbott P, Shalavi S, Yazdizadeh M, Postoperative pain following treatment of teeth with irreversible pulpitis: A review: N Y State Dent J, 2017; 83; 44-53
109. Santini M, da Rosa RA, Ferreira MB, Medications used for prevention and treatment of postoperative endodontic pain: A systematic review: Eur Endod J, 2021; 6; 15-24
110. Santini M, da Rosa RA, Ferreira MB, Medications used for prevention and treatment of postoperative endodontic pain: A systematic review: Eur Endod J, 2021; 6; 15-24
111. Smith EA, Marshall JG, Selph SS, Nonsteroidal anti-inflammatory drugs for managing postoperative endodontic pain in patients who present with preoperative pain: A systematic review and meta-analysis: J Endod, 2017; 43; 7-15
112. Dionne RA, Berthold CW, Therapeutic uses of non-steroidal anti-inflammatory drugs in dentistry: Crit Rev Oral Biol Med, 2001; 12; 315-30
113. Tupyota P, Chailertvanitkul P, Laopaiboon M, Supplementary techniques for pain control during root canal treatment of lower posterior teeth with irreversible pulpitis: A systematic review and meta-analysis: Aust Endod J, 2018; 44; 14-25
114. Potocnik I, Bajrović F, Failure of inferior alveolar nerve block in endodontics: Endod Dent Traumatol, 1999; 15; 247-51
115. Pasternak M, Woron JAcute endodontic pain: Ból, 2020; 21(1); 39-45 [in Polish]
116. Joiner A, The bleaching of teeth: a review of the literature: J Dent, 2006; 34; 412-19
117. Smith GE, Fluoride, Teeth and bone: Med J Aust, 1985; 143; 283-86
118. Carey CM, Tooth whitening: What we now know: J Evid Based Dent Pract, 2014; 14(Suppl); 70-76
119. Faria-E-Silva AL, Nahsan FPS, Fernandes MTG, Martins-Filho PRS, Effect of preventive use of nonsteroidal anti-inflammatory drugs on sensitivity after dental bleaching: A systematic review and meta-analysis: J Am Dent Assoc, 2015; 146; 87-93e1
120. Markowitz K, Strickland M, The use of anti-inflammatory drugs to prevent bleaching-induced tooth sensitivity is ineffective and unnecessary: Evid Based Dent, 2020; 21; 130-31
121. Bailey E, Prevention and management of post-operative pain in oral surgery: Prim Dent J, 2018; 7; 57-63
122. Urquhart E, Analgesic agents and strategies in the dental pain model: J Dent, 1994; 22; 336-41
123. Mamoun J, Dry socket etiology, diagnosis, and clinical treatment techniques: J Korean Assoc Oral Maxillofac Surg, 2018; 44; 52-58
124. Pasternak M, Woron JPostextractional pain management: Ból, 2020; 21(1); 47-52 [in Polish]
125. Jackson DL, Moore PA, Hargreaves KM, Preoperative nonsteroidal anti-inflammatory medication for the prevention of postoperative dental pain: J Am Dent Assoc, 1989; 119; 641-47
126. Costa FWG, Esses DFS, de Barros Silva PG, Does the preemptive use of oral nonsteroidal anti-inflammatory drugs reduce postoperative pain in surgical removal of third molars? A meta-analysis of randomized clinical trials: Anesth Prog, 2015; 62; 57-63
127. Savage MG, Henry MA, Preoperative nonsteroidal anti-inflammatory agents: Review of the literature: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2004; 98; 146-52
128. Caviedes-Bucheli J, Muñoz HR, Azuero-Holguín MM, Ulate E, Neuropeptides in dental pulp: The silent protagonists: J Endod, 2008; 34; 773-88
129. Cetira Filho EL, Carvalho FSR, de Barros Silva PG, preemptive use of oral nonsteroidal anti-inflammatory drugs for the relief of inflammatory events after surgical removal of lower third molars: A systematic review with meta-analysis of placebo-controlled randomized clinical trials: J Craniomaxillofac Surg, 2020; 48; 293-307
130. Luyk NH, Steinberg B, Aetiology and diagnosis of clinically evident jaw trismus: Aust Dent J, 1990; 35; 523-29
131. Avelar RL, Primo BT, Vogt BF, Effect of partially selective cyclooxygenase-2 inhibitor in the removal of third molars: J Craniofac Surg, 2012; 23; e108-12
132. Moore PA, Hersh E, Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: Translating clinical research to dental practice: J Am Dent Assoc, 2013; 144; 898-908
133. Jóźwiak-Bebenista M, Nowak JZ, Paracetamol: Mechanism of action, applications and safety concern: Acta Pol Pharm, 2014; 71; 11-23
134. Etikala A, Tattan M, Askar H, Wang H-L, Effects of NSAIDs on periodontal and dental implant therapy: Compend Contin Educ Dent, 2019; 40; e1-e9
135. Dahners LE, Mullis BH, Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing: J Am Acad Orthop Surg, 2004; 12; 139-43
136. Luo JD, Miller C, Jirjis T, The effect of non-steroidal anti-inflammatory drugs on the osteogenic activity in osseointegration: A systematic review: Int J Implant Dent, 2018; 4; 30
137. Winnett B, Tenenbaum HC, Ganss B, Jokstad A, Perioperative use of non-steroidal anti-inflammatory drugs might impair dental implant osseointegration: Clin Oral Implants Res, 2016; 27; e1-7
138. Gomes FIF, Aragão MGB, de Paulo Teixeira Pinto V, Effects of nonsteroidal anti-inflammatory drugs on osseointegration: A review: J Oral Implantol, 2015; 41; 219-30
139. Alalwani A, Buhara O, Tüzüm MŞ, Oral health-related quality of life and the use of oral and topical nonsteroidal anti-inflammatory drugs for pericoronitis: Med Sci Monit, 2019; 25; 9200-6
140. Serafini G, Trevisan S, Saponati G, Bandettini B, Therapeutic efficacy and tolerability of the topical treatment of inflammatory conditions of the oral cavity with a mouthwash containing diclofenac epolamine : A randomized, investigator-blind, parallel-group, controlled, phase III study: Clin Drug Investig, 2012; 32; 41-49
141. Gupta M, Kandula S, Laxmikanth SM, Controlling pain during orthodontic fixed appliance therapy with non-steroidal anti-inflammatory drugs (NSAID): A randomized, double-blinded, placebo-controlled study: J Orofac Orthop, 2014; 75; 471-76
142. Corrêa AS, Almeida VL, Lopes BM, The influence of non-steroidal anti-inflammatory drugs and paracetamol used for pain control of orthodontic tooth movement: A systematic review: An Acad Bras Cienc, 2017; 89; 2851-63
143. Bergius M, Kiliaridis S, Berggren U, Pain in orthodontics. A review and discussion of the literature: J Orofac Orthop, 2000; 61; 125-37
144. Walker JB, Buring SM, NSAID impairment of orthodontic tooth movement: Ann Pharmacother, 2001; 35; 113-15
145. Knop LAH, Shintcovsk RL, Retamoso LB, Non-steroidal and steroidal anti-inflammatory use in the context of orthodontic movement: Eur J Orthod, 2012; 34; 531-35
146. Nagi R, Yashoda Devi BK, Rakesh N, Clinical implications of prescribing nonsteroidal anti-inflammatory drugs in oral health care – a review: Oral Surg Oral Med Oral Pathol Oral Radiol, 2015; 119; 264-71
147. Gangarosa LP, Mahan PE, Ciarlone AE, Pharmacologic management of temporomandibular joint disorders and chronic head and neck pain: Cranio, 1991; 9; 328-38
148. Andre A, Kang J, Dym H, Pharmacologic treatment for temporomandibular and temporomandibular joint disorders: Oral Maxillofac Surg Clin North Am, 2022; 34; 49-59
149. Kinane DF, Causation and pathogenesis of periodontal disease: Periodontol 2000, 2001; 25; 8-20
150. Kinane DF, Stathopoulou PG, Papapanou PN, Periodontal diseases: Nat Rev Dis Primers, 2017; 3; 17038
151. Hajishengallis G, Chavakis T, Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities: Nat Rev Immunol, 2021; 21; 426-40
152. Kajiya M, Kurihara H, Molecular mechanisms of periodontal disease: Int J Mol Sci, 2021; 22; 930
153. Scannapieco FA, Gershovich E, The prevention of periodontal disease – an overview: Periodontol 2000, 2020; 84; 9-13
154. Salvi GE, Lang NP, The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases: Curr Pharm Des, 2005; 11; 1757-69
155. Howell TH, Williams RC, Nonsteroidal antiinflammatory drugs as inhibitors of periodontal disease progression: Crit Rev Oral Biol Med, 1993; 4; 177-96
156. Heasman PA, The role of non-steroidal anti-inflammatory drugs in the management of periodontal disease: J Dent, 1988; 16; 247-57
157. Jeffcoat MK, Williams RC, Reddy MS, Flurbiprofen treatment of human periodontitis: Effect on alveolar bone height and metabolism: J Periodontal Res, 1988; 23; 381-85
158. Potts AJ, Hamburger J, Scully C, The medication of patients with oral lichen planus and the association of nonsteroidal anti-inflammatory drugs with erosive lesions: Oral Surg Oral Med Oral Pathol, 1987; 64; 541-43
159. Olson MA, Rogers RS, Bruce AJ, Oral lichen planus: Clin Dermatol, 2016; 34; 495-504
160. Nosratzehi T, Oral lichen planus: An overview of potential risk factors, biomarkers and treatments: Asian Pac J Cancer Prev, 2018; 19; 1161-67
161. Kurago ZB, Etiology and pathogenesis of oral lichen planus: An overview: Oral Surg Oral Med Oral Pathol Oral Radiol, 2016; 122; 72-80
162. Seymour RA, Effects of medications on the periodontal tissues in health and disease: Periodontol 2000, 2006; 40; 120-29
163. Corry D, Moran J, Assessment of acrylic bone cement as a local delivery vehicle for the application of non-steroidal anti-inflammatory drugs: Biomaterials, 1998; 19; 1295-301
164. Peres MFS, Ribeiro FV, Ruiz KGS, Steroidal and non-steroidal cyclooxygenase-2 inhibitor anti-inflammatory drugs as pre-emptive medication in patients undergoing periodontal surgery: Braz Dent J, 2012; 23; 621-28
165. Sung H, Ferlay J, Siegel RL, Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2021; 71; 209-49
166. Dyba T, Randi G, Bray F, The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers: Eur J Cancer, 2021; 157; 308-47
167. Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics, 2021: Cancer J Clin, 2021; 71; 7-33
168. Mesia R, Iglesias L, Lambea J, SEOM clinical guidelines for the treatment of head and neck cancer (2020): Clin Transl Oncol, 2021; 23; 913-21
169. Alterio D, Marvaso G, Ferrari A, Modern radiotherapy for head and neck cancer: Semin Oncol, 2019; 46; 233-45
170. Horton JD, Knochelmann HM, Day TA, Immune evasion by head and neck cancer: Foundations for combination therapy: Trends Cancer, 2019; 5; 208-32
171. Blasco MA, Cordero J, Dundar Y, Chronic pain management in head and neck oncology: Otolaryngol Clin North Am, 2020; 53; 865-75
172. Epstein JB, Stevenson-Moore P, Benzydamine hydrochloride in prevention and management of pain in oral mucositis associated with radiation therapy: Oral Surg Oral Med Oral Pathol, 1986; 62; 145-48
173. Antunes DM, Rodrigues MFSD, Guimarães DM, Nonsteroidal anti-inflammatory drugs modulate gene expression of inflammatory mediators in oral squamous cell carcinoma: Anticancer Res, 2019; 39; 2385-94
174. Saka Herrán C, Jané-Salas E, Estrugo Deves A, López-López J, Protective effects of metformin, statins and anti-inflammatory drugs on head and neck cancer: A systematic review: Oral Oncol, 2018; 85; 68-81
175. Guadagni F, Ferroni P, Palmirotta R, Non-steroidal anti-inflammatory drugs in cancer prevention and therapy: Anticancer Res, 2007; 27; 3147-62
176. Cline AE, Turrentine JE, Compounded topical analgesics for chronic pain: Dermatitis, 2016; 27; 263-71
177. Leppert W, Malec-Milewska M, Zajaczkowska R, Wordliczek J, Transdermal and topical drug administration in the treatment of pain: Molecules, 2018; 23; 681
178. McPherson ML, Cimino NM, Topical NSAID formulations: Pain Med, 2013; 14(Suppl 1); S35-39
179. Barkin RL, The pharmacology of topical analgesics: Postgrad Med, 2013; 125; 7-18
180. Verbeeck RK, Pharmacokinetic drug interactions with nonsteroidal anti-inflammatory drugs: Clin Pharmacokinet, 1990; 19; 44-66
181. Klinge SA, Sawyer GA, Effectiveness and safety of topical versus oral nonsteroidal anti-inflammatory drugs: A comprehensive review: Phys Sportsmed, 2013; 41; 64-74
182. Nicolatou-Galitis O, Bossi P, Orlandi E, Bensadoun René-Jean, The role of benzydamine in prevention and treatment of chemoradiotherapy-induced mucositis: Support Care Cancer, 2021; 29; 5701-9
183. Altman R, Bosch B, Brune K, Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology: Drugs, 2015; 75; 859-77
184. Jańczuk Z, Syryńska M, Banach J, Jarzynka WSachol-gel in the treatment of oral mucosal diseases: Czas Stomatol, 1986; 39; 61-65 [in Polish]
185. Reedy BL, A topical salicylate gel in the treatment of oral aphthous ulceration: Practitioner, 1970; 204; 846-50
186. Lum-Kam G, Pain relief with a topical choline salicylate gel in dental practice: J Can Dent Assoc (Tor), 1969; 35; 216-19
187. Nicolatou-Galitis O, Bossi P, Orlandi E, Bensadoun René-Jean, The role of benzydamine in prevention and treatment of chemoradiotherapy-induced mucositis: Support Care Cancer, 2021; 29; 5701-9
188. Quane PA, Graham GG, Ziegler JB, Pharmacology of benzydamine: inflammopharmacology, 1998; 6; 95-107
189. Chen C-Y, Kuo C-J, Lee Y-W, Benzydamine hydrochloride on postoperative sore throat: A meta-analysis of randomized controlled trials: Can J Anaesth, 2014; 61; 220-28
190. MacDonald E, Visintini S: Benzydamine for the treatment of oropharyngeal mucositis from radiation therapy: A review of clinical effectiveness and guidelines [Internet] Sep 27, 2018, Ottawa (ON), Canadian Agency for Drugs and Technologies in Health
191. Hirschowitz BI, Nonsteroidal anti-inflammatory drugs and the gut: South Med J, 1996; 89; 259-63
192. Dajani EZ, Agrawal NM, Prevention of nonsteroidal anti-inflammatory drug-induced gastroduodenal ulcers: Role of mucosal protective and gastric antisecretory drugs: Dig Dis, 1995; 13(Suppl 1); 48-61
193. Bindu S, Mazumder S, Bandyopadhyay U, Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective: Biochem Pharmacol, 2020; 180; 114147
194. Schjerning A-M, McGettigan P, Gislason G, Cardiovascular effects and safety of (non-aspirin) NSAIDs: Nat Rev Cardiol, 2020; 17; 574-84
195. Ungprasert P, Srivali N, Kittanamongkolchai W, Non-steroidal anti-inflammatory drugs and risk of heart failure exacerbation: A systematic review and meta-analysis: Eur J Intern Med, 2015; 26; 685-90
196. Alqahtani Z, Jamali F, Clinical outcomes of aspirin interaction with other non-steroidal anti-inflammatory drugs: A systematic review: J Pharm Pharm Sci, 2018; 21; 29854
197. Yokoyama H, Ito N, Soeda S, Influence of non-steroidal anti-inflammatory drugs on antiplatelet effect of aspirin: J Clin Pharm Ther, 2013; 38; 12-15
198. Braganza A, Bissada N, Hatch C, Ficara A, The effect of non-steroidal anti-inflammatory drugs on bleeding during periodontal surgery: J Periodontol, 2005; 76; 1154-60
199. Lucas GNC, Leitão ACC, Alencar RL, Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs: J Bras Nefrol, 2019; 41; 124-30
200. Morwood K, Gillis D, Smith W, Kette F, Aspirin-sensitive asthma: Intern Med J, 2005; 35; 240-46
201. Szczeklik A, Stevenson DD, Aspirin-induced asthma: Advances in pathogenesis, diagnosis, and management: J Allergy Clin Immunol, 2003; 111; 913-21 quiz 922
202. Gan TJ, Diclofenac: An update on its mechanism of action and safety profile: Curr Med Res Opin, 2010; 26; 1715-31
203. Fagan EA, Walford N, Hodgson HJ, Sulindac hepatotoxicity: Gut, 1983; 24; 1199
204. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T, Current perspectives on stevens-johnson syndrome and toxic epidermal necrolysis: Clin Rev Allergy Immunol, 2018; 54; 147-76
205. Ediriweera DS, Dilina N, Saparamadu V, Aspirin is associated with low oral PH levels and antacid helps to increase oral PH: BMC Res Notes, 2018; 11; 137
206. Sreebny LM, Schwartz SS, A reference guide to drugs and dry mouth: Gerodontology, 1997; 14; 33-47
207. Laskarides C, Update on analgesic medication for adult and pediatric dental patients: Dent Clin North Am, 2016; 60; 347-66
208. Rodd HD, Graham A, Tajmehr N, Molar incisor hypomineralisation: Current knowledge and practice: Int Dent J, 2021; 71; 285-91
209. Serna Muñoz C, Pérez Silva A, Solano F, Effect of antibiotics and NSAIDs on cyclooxygenase-2 in the enamel mineralization: Sci Rep, 2018; 8; 4132
210. Heubi JE, Partin JC, Partin JS, Schubert WK, Reye’s syndrome: Current concepts: Hepatology, 1987; 7; 155-64
211. Poveda Roda R, Bagán JV, Jiménez Soriano Y, Gallud Romero L, Use of nonsteroidal antiinflammatory drugs in dental practice. A review: Med Oral Patol Oral Cir Bucal, 2007; 12; E10-18
212. Mackenzie IS, Coughtrie MWH, MacDonald TM, Wei L, Antiplatelet drug interactions: J Intern Med, 2010; 268; 516-29
213. Furst DE, Clinically important interactions of nonsteroidal antiinflammatory drugs with other medications: J Rheumatol Suppl, 1988; 17; 58-62
214. Graham DY, What the gastroenterologist should know about the gastrointestinal safety profiles of bisphosphonates: Dig Dis Sci, 2002; 47; 1665-78
215. Brouwers JR, de Smet PA, Pharmacokinetic-pharmacodynamic drug interactions with nonsteroidal anti-inflammatory drugs: Clin Pharmacokinet, 1994; 27; 462-85
216. Rainsford KD, Ibuprofen: From invention to an OTC therapeutic mainstay: Int J Clin Pract Suppl, 2013(178); 9-20
Figures
 Figure 1. Mechanisms of painCreated with BioRender.com. With regard to the mechanism of pain formation in the oral cavity, a distinction is made between: provoked pain, prolonged provoked pain, spontaneous pain, continuous pain, nocturnal pain, throbbing (pulsating) pain, fresh pain, acute pain, and radiating pain.
Figure 1. Mechanisms of painCreated with BioRender.com. With regard to the mechanism of pain formation in the oral cavity, a distinction is made between: provoked pain, prolonged provoked pain, spontaneous pain, continuous pain, nocturnal pain, throbbing (pulsating) pain, fresh pain, acute pain, and radiating pain. Figure 2. Types of orofacial painCreated with BioRender.com. With regard to the cause of pain in the oral cavity, we distinguish the following types of orofacial pain: dentin hypersensitivity, pain caused by inflammation of the temporomandibular joint, Munchausen syndrome, orthodontic pain, pain resulting from cancer located in the area of the head and neck, pain arising from general diseases, pain caused by inflammation of nerves, postoperative pain, inflammation of the pulp and periapical tissues, and pain arising from inflammation of the oral mucosa.
Figure 2. Types of orofacial painCreated with BioRender.com. With regard to the cause of pain in the oral cavity, we distinguish the following types of orofacial pain: dentin hypersensitivity, pain caused by inflammation of the temporomandibular joint, Munchausen syndrome, orthodontic pain, pain resulting from cancer located in the area of the head and neck, pain arising from general diseases, pain caused by inflammation of nerves, postoperative pain, inflammation of the pulp and periapical tissues, and pain arising from inflammation of the oral mucosa. Figure 3. Effects of NSAIDs on cyclooxygenaseCreated with BioRender.com. The mechanism of action of NSAIDs is connected with the inhibition of cyclooxygenase (COX), which blocks the conversion of arachidonic acid to proinflammatory prostaglandins. NSAIDs reduce inflammation and pain in periodontitis/mucositis or after oral surgery. Although COX exists in 2 isoforms (COX-1 and COX-2), COX-2 is responsible for proinflammatory effects, while COX-1 serve mainly physiological functions. Therefore, most NSAID adverse effects are associated with COX-1 inhibition including gastrointestinal damage, liver damage or cardiovascular complications.
Figure 3. Effects of NSAIDs on cyclooxygenaseCreated with BioRender.com. The mechanism of action of NSAIDs is connected with the inhibition of cyclooxygenase (COX), which blocks the conversion of arachidonic acid to proinflammatory prostaglandins. NSAIDs reduce inflammation and pain in periodontitis/mucositis or after oral surgery. Although COX exists in 2 isoforms (COX-1 and COX-2), COX-2 is responsible for proinflammatory effects, while COX-1 serve mainly physiological functions. Therefore, most NSAID adverse effects are associated with COX-1 inhibition including gastrointestinal damage, liver damage or cardiovascular complications. In Press
15 Mar 2024 : Clinical Research
Impact of Cluster Nursing Intervention on ICU Patients' Psychological Well-Being and Complications Associat...Med Sci Monit In Press; DOI: 10.12659/MSM.942855
26 Mar 2024 : Clinical Research
New Computerized Planning Algorithm and Clinical Testing of Optimized Nuss Bar Design for Patients with Pec...Med Sci Monit In Press; DOI: 10.12659/MSM.943705
07 May 2024 : Clinical Research
Treatment of AVN-Induced Proximal Pole Scaphoid Nonunion Using a Fifth and Fourth Extensor Compartmental Ar...Med Sci Monit In Press; DOI: 10.12659/MSM.944553
16 Mar 2024 : Clinical Research
Diagnostic Efficiency of ACR-TIRADS Score for Differentiating Benign and Malignant Thyroid Nodules of Vario...Med Sci Monit In Press; DOI: 10.12659/MSM.943228
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952