04 October 2023: Clinical Research
Efficacy of Pneumatic Retinopexy Preceding Pars Plana Vitrectomy in Managing Combined Retinal and Choroidal Detachment: A Retrospective Study of 64 Patients
Qiang XuDOI: 10.12659/MSM.940746
Med Sci Monit 2023; 29:e940746
Abstract
BACKGROUND: Combined retinal and choroidal detachment (RD-CD) poses significant challenges in ophthalmic treatment, often requiring surgical intervention for optimal outcomes. The selection of the appropriate surgical procedure is crucial for ensuring visual restoration and overall eye health. This study delves into the therapeutic benefits and outcomes of two distinct surgical approaches for RD-CD: pneumatic retinopexy (PR) followed by pars plana vitrectomy (PPV) and PPV alone, in an attempt to guide optimal clinical decision-making.
MATERIAL AND METHODS: We retrospectively analyzed data from 64 consecutive patients diagnosed with RD-CD. They were categorized into two groups: Group A consisted of 34 patients (34 eyes) who underwent PR as an initial treatment and subsequently received PPV, while Group B, serving as a control, comprised 30 patients (30 eyes) treated solely with PPV.
RESULTS: The application of PR in Group A notably accelerated intraocular pressure (IOP) increase (P<0.001) and effectively restored the CD degree (P<0.001) compared to Group B. Distinct variations were observed between the groups concerning the need for suprachoroidal drainage (P<0.001) and the duration of the vitrectomy procedure (P=0.025). However, after a 6-month follow-up period, visual acuity (VA) enhancement rates (P=0.496) and overall retinal re-attachment rates (P=0.635) showed no significant disparities between the two groups.
CONCLUSIONS: Our findings highlight that initiating treatment for RD-CD patients with PR before PPV offers advantages, including a pronounced IOP increase, CD prevention, shortened surgical time, diminished necessity for suprachoroidal drainage, and overall enhancement of subsequent PPV surgical outcomes.
Keywords: Choroidal Effusions, Intravitreal Injections, Retinal Detachment, Vitrectomy, Humans, Retrospective Studies, Visual Acuity, Glaucoma, Treatment Outcome
Background
Combined retinal and choroidal detachment (RD-CD) is a complicated type of RD frequently associated with severely low intraocular pressure (IOP), low vision, and uveitis. This type of RD is almost always accompanied by retinal hiatus, so it is also called rhegmatogenous retinal detachment with choroidal detachment (RRD-CD) [1–3]. Recently, pars plana vitrectomy (PPV) has achieved stable clinical results in patients with complicated retinal detachments [4]. However, CD and severely low IOP in patients with RD-CD pose significant surgical challenges, such as difficulty placing the infusion port and potentially exacerbating CD by infusing the suprachoroidal cavity [2]. There is also a higher risk of suprachoroidal hemorrhage caused by fluid discharge into the suprachoroidal cavity [5].
The degree of CD before PPV influences the rate of retinal re-attachment after PPV [6]. It is recommended to raise IOP and reduce CD before proceeding with PPV in patients with RD-CD [7].
Hormones, such as topical and systemic corticosteroids, are frequently used pre-operatively to attempt to increase IOP, reduce CD size, and inhibit uveal inflammation [7,8]. However, preoperative hormone therapy can worsen outcomes by delaying PPV [1]. Moreover, underlying systemic diseases can limit hormone use in middle-aged and older adults, the most common populations affected by RD-CD [6].
Intravitreal injection of expansile gas (SF6 or C3F8) can also significantly increase IOP and eliminate CD [9]. Prompt PPV in patients with RD-CD can improve postoperative visual acuity by 2 or more lines [7]. Several cases of PR followed by PPV in treating RD-CD have been reported in recent years, with some not requiring subsequent PPV due to successful re-attachment after PR alone [9].
The efficacy and safety of PR before PPV in the treatment of RD-CD has not yet been systematically evaluated. Therefore, this retrospective study of 64 patients with RD-CD aimed to compare outcomes from PR followed PPV with those of PPV alone.
Material and Methods
PATIENTS:
In this retrospective study, we enrolled consecutive patients diagnosed with RD-CD who underwent PPV at the Lixiang Eye Hospital of Soochow University between June 2018 and March 2021. The Ethics Committee of Lixiang Eye Hospital of Soochow University approved this study (approval number: SLER 2018024). All patients were informed of the procedure and provided informed consent.
We reviewed the medical records of all enrolled patients. The exclusion criteria were as follows: (1) secondary RD-CD after vitreoretinal surgery, (2) severe eye trauma, and (3) follow-up of less than 6 months. We divided the patients into 2 groups depending on whether PR was performed prior to PPV: group A (PR pretreatment, 34 patients, 34 eyes); and group B (no PR pretreatment, 30 patients, 30 eyes).
DATA COLLECTION:
Patient data, including sex, age, disease onset time, best-corrected visual acuity (BCVA), IOP, axial length, and lens status, number, and size were collected and compared between the 2 groups. The location of retinal breaks, presence of concurrent macular hole, degree of RD, degree of CD, and proliferative vitreoretinopathy grade were measured before surgery. B-ultrasonography and ultrasound bio-microscopy (UBM) were performed to diagnose CD and determine its severity. Patients were divided into mild, moderate, and severe detachment groups according to the angle and range of detachment [10]. Proliferative vitreoretinopathy severity was graded according to the standards established by the American Retinal Academic Committee in 1991 [11].
Operative data collected included changes in IOP after PR, intraoperative suprachoroidal fluid discharge, use of heavy water, and operative time. Postoperative data collected included retinal and choroidal status, BCVA, and IOP. A minimum of 6 months of follow-up was required for inclusion in the study.
PROCEDURE OF PR:
The pupil was dilated preoperatively using a mixture of tropicamide and phenylephrine eye drops. Topical anesthesia was given using 0.5% promecaine hydrochloride eye drops. Then, 100% C3F8 was injected into the vitreous cavity with a 30-gauge (G) injection needle 4 mm posterior to the corneal limbus, and the anterior chamber was punctured to lower IOP. If necessary, a paracentesis was repeated to reduce IOP. Patients were instructed to maintain an appropriate position for 2 weeks, and changes in IOP were monitored.
PROCEDURE OF PPV:
Vitreoretinal surgery was performed after retrobulbar anesthesia. Three 25G or 27G channels were used to enter the vitreous cavity through the flat part of the ciliary muscle, and silicone oil or gas was used as the vitreous cavity filling material. In patients with obvious lens opacity, phacoemulsification was performed. The intraoperative conditions determined the use of heavy water, laser, and cryotherapy. Some patients with severe CD underwent suprachoroidal drainage procedures, such as scleral puncture to release fluid or withdrawing of a portion of the cannula after injecting heavy water.
STATISTICAL ANALYSIS:
IBM SPSS 23.0 statistical software was used for the analyses. An independent
Results
COMPARISON OF BASIC CHARACTERISTICS BETWEEN THE 2 GROUPS:
A total of 64 patients (64 eyes) with RD-CD were included in this study, including 39 (60.9%) men and an overall mean age of 55.70±13.50 years. Group A consisted of 34 patients, whereas group B consisted of 30 patients. The only significant baseline differences were that group A had lower IOP (P=0.012) and more severe CD (P=0.018) than group B. The baseline data for the 2 groups are shown in Table 1.
CHANGES OF IOP AND CD AFTER PR INTERVENTION:
The IOP significantly rose after the first gas injection (6.27±0.42 pre-injection vs 15.32±0.80 mmHg post-injection, T=10.409, P<0.001). IOP is routinely measured 24 h after initial PR. Only the IOP of 2 of 34 (5.9%) of the patients remained low despite injection, and the IOP of 2 patients was 5.0 mmHg and 7.0 mmHg, respectively. The second PR treatment was performed after 24 h of observation (32/34, 94.1%). Both IOP and CD were significantly improved after PR. The ocular B-ultrasound of RD-CD patients before PR intervention is shown in Figure 1A, and the ocular B-ultrasound after PR is shown in Figure 1B. UBM measured the changes of CD in RD-CD patients before and after PR intervention. Figure 2A shows the CD results of UBM examination before PR, and Figure 2B shows the CD results of UBM examination after PR.
Three patients (3/34, 8.8%) had IOP >30 mmHg 24 h after the first gas injection, and the IOP of the 3 patients was 31.0 mmHg, 32.0 mmHg, and 34.0 mmHg, respectively. In 1 case, the IOP was as high as 41.0 mmHg 2 h after PR operation, and the IOP was relieved after temporary mannitol treatment. After the IOP was lowered with medical therapy in these cases, it remained well controlled. CDs disappeared completely or partially after PR in all group A patients, although 2 required a second injection. The average interval between gas injection and PPV was 3.24±1.21 days (range 1–6 days). The corresponding changes of IOP and CD before and after PR operation are shown in Table 2.
PPV SURGICAL CHARACTERISTICS:
Of all the 64 eyes that underwent PPV, 48 patients underwent 25G, and 16 underwent 27G PPV. The fillers after PPV were either silicone oil (viscosity index 1000–5000) or C3F8 (8%–18%). The differences in the number of suprachoroidal drainage cases (8.8 vs 40%, P=0.003) and operative time (70.7±18.85 vs 87.7±38.08, P=0.025) were statistically significant between the 2 groups. Other characteristics were not significantly different. The corresponding results are shown in Table 3.
CHANGES OF IOP AFTER PPV:
At follow-up at 1 and 6 months after PPV, there was no significant difference between the groups in terms of retinal re-attachment rate (82.4 vs 86.7% and 91.2% vs 93.3%, respectively), percentage of BCVA improvement (76.5% vs 83.3%), or IOP control (P=0.304). The corresponding results are shown in Table 4.
Discussion
In this study, pretreating RD-CD patients with PR before PPV significantly resolved CD, rapidly increased IOP, reduced suprachoroidal drainage requirements, and reduced PPV operative time. Moreover, when compared with patients undergoing PPV alone, there was no significant difference in long-term retinal re-attachment rate or degree of BCVA improvement. Compared with the traditional corticosteroid hormone method, PR has the greatest advantage of shortening the time for patients with RRD-CD to receive PPV, and this method has no harsh requirements for patients’ systemic conditions [12].
The precise mechanism underlying the development of RD-CD remains unknown. Some studies have suggested that retinal hole formation allows liquefied vitreous fluid to enter the subretinal detachment, lowering IOP, and causing increased choroidal permeability through vasodilation. Choroidal edema and detachment result, ciliary body edema reduces aqueous humor secretion further lowering IOP, and the cycle continues [1,7,8].
A significant relationship has been observed between low IOP and CD, with severe CD associated with severely low IOP, as low as 0 mmHg. PPV surgery in the context of CD and a low IOP has a higher rate of complications, such as inadvertent suprachoroidal cavity entry, intraoperative drainage of the suprachoroidal cavity, and insufficient filling of the vitreous substitute at the end of the operation [1,13].
In our study, IOP rapidly increased after intravitreal C3F8 injection. PR was first described in 1986, when Hilton et al achieved a 90% retinal re-attachment rate by injecting SF6 or C3F8 gas into the vitreous cavity for patients with simple RD [14]. Holz et al extended the indications for PR to include more complex RD types by showing a similar therapeutic effect as scleral compression [15].
PR for the uniform repair of all RD-CD cases is controversial. Giant retinal holes or inferior retinal holes are unsuitable for pneumatic retinopexy [9,16]. Extra vitreoretinal traction is produced by the bubble entering the vitreous cavity, especially when it enters between the retina and vitreous body. Moreover, when the retinal hole is larger, the contact arc width of the bubble cannot fill the entire hole. Despite these limitations, failed pneumatic retinopexy has not been shown to affect PPV outcomes [12,17,18].
PPV surgery has become the mainstay treatment method for RD [4]. In recent years, there has been an increasing number of studies on surgical methods of PR, which are currently used in patients with RRD. Previous literature has reported that PR is proposed as an alternative to PPV in certain circumstances [12]. Relatively few studies have been conducted on PR efficacy in RD-CD cases. A recent study of 9 RD-CD cases treated with PR has been reported, with 3 patients achieving re-attachment after laser photocoagulation without need for PPV. No recurrence was observed after 3 months [9]. Previously, we also reported a successful RD-CD case with this method [19].
In this present comprehensive study, more than 90% of CD cases achieved either total or partial choroidal re-attachment after a single gas injection. Two cases had IOP elevation only after the second gas injection. PPV was performed after choroidal reduction, which could be due to gas leakage or severe CD. Three patients in group A had an IOP
In this study, despite group A having more severe CD and lower IOP at baseline, the retinal re-attachment rate and BCVA improvement were similar between groups 6 months after PPV. CD severity and low IOP have previously been associated with lower retinal re-attachment rates after PPV alone [20]. Pneumatic retinopexy can significantly improve CD, increase IOP, facilitate cannula placement before PPV, and reduce the risk of mistakenly entering the suprachoroidal space. Since this was a retrospective case study, we could not directly compare the rate of catheterization complications between the 2 groups. Suprachoroidal drainage was required in 3 patients in group A and 12 patients in group B. This difference was statistically significant. Similarly, PPV operative time differed between the 2 groups, with shorter operative times in group A. Therefore, we suggest that pneumatic retinopexy pre-conditioning can improve PPV success rates in patients with RD-CD.
Patients with RD-CD underwent PPV surgery using the 25G and 27G models. There is a trend toward smaller-caliber vitrectomy systems [21–23]. A smaller caliber vitrectomy system can improve patient comfort, reduce conjunctival and scleral injuries, reduce scar formation, and improve postoperative visual acuity [24]. Only 2 cases of RD-CD required scleral sutures after PPV in this study, both of which occurred in 25G cases. Electrocoagulation was used in the rest of the cases. Statistical analysis showed that neither group experienced a low IOP due to abnormal incision closure. The 25G and 27G vitrectomy systems produced consistent clinical results in patients with RD-CD.
This study was a single-center retrospective design with a limited number of cases and methodological limitations. Multicenter prospective studies are needed to confirm the indication, safety, and efficacy of PR for RD-CD.
Conclusions
In conclusion, this study showed that for patients with RD-CD, management with PR before PPV resulted in an improved increase in IOP, prevented CD, reduced operative time, reduced the need for suprachoroidal drainage, and facilitated subsequent PPV surgery. PR is expected to be a feasible method for RRD-CD to improve CD before PPV.
Figures
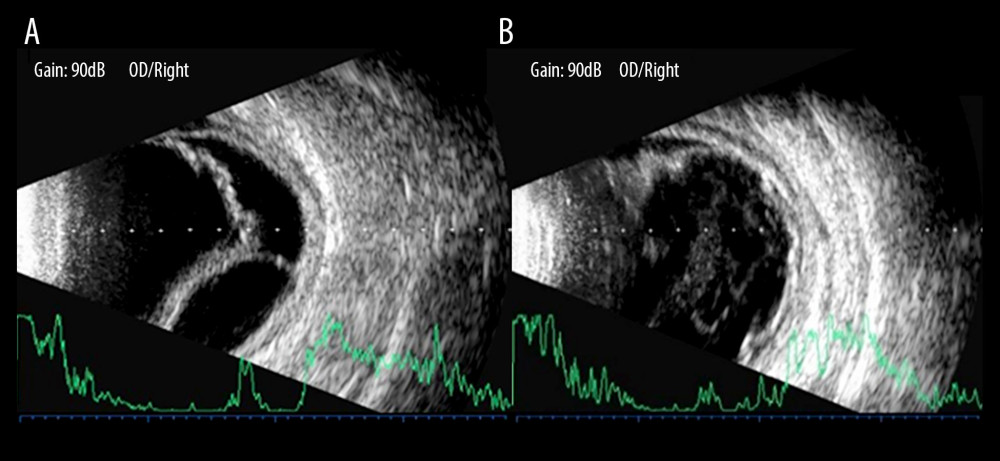 Figure 1. B-ultrasonography showed the changes of pneumatic retinopexy before and after treatment in patients with combined retinal and choroidal detachment. (A) Shows retinal and choroid detachment, and (B) shows the retinal and choroid detachment after PR treatment.
Figure 1. B-ultrasonography showed the changes of pneumatic retinopexy before and after treatment in patients with combined retinal and choroidal detachment. (A) Shows retinal and choroid detachment, and (B) shows the retinal and choroid detachment after PR treatment. 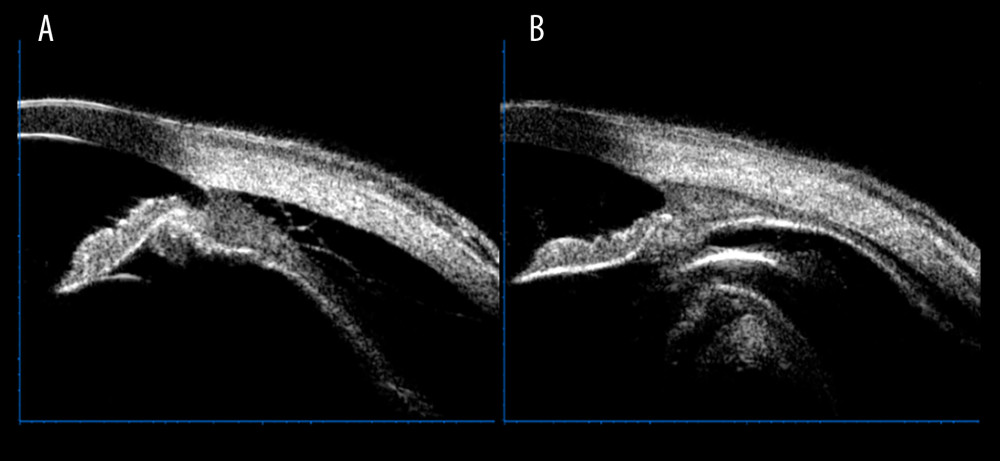 Figure 2. Ultrasound bio-microscopy examination showed the change of pneumatic retinopexy before and after treatment in patients with combined retinal and choroidal detachment. (A) Shows a marked detachment of the ciliary body from the sclera, and (B) shows a basic reduction of the detached ciliary body after pneumatic retinopexy treatment.
Figure 2. Ultrasound bio-microscopy examination showed the change of pneumatic retinopexy before and after treatment in patients with combined retinal and choroidal detachment. (A) Shows a marked detachment of the ciliary body from the sclera, and (B) shows a basic reduction of the detached ciliary body after pneumatic retinopexy treatment. Tables
Table 1. Basic characteristics of 2 groups of patients with combined retinal and choroidal detachment.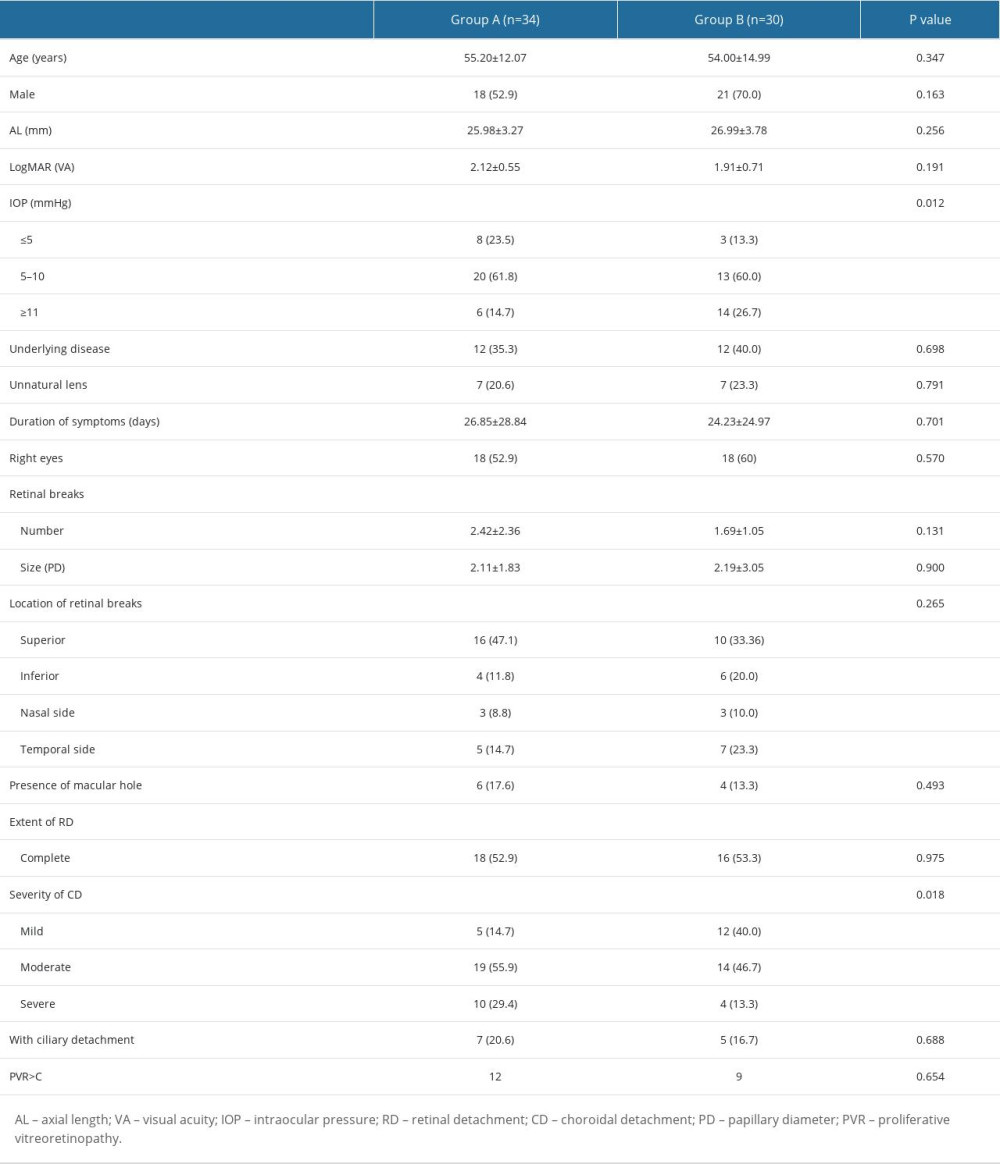 Table 2. Characteristic changes of patients with combined retinal and choroidal detachment before and after pneumatic retinopexy (PR).
Table 2. Characteristic changes of patients with combined retinal and choroidal detachment before and after pneumatic retinopexy (PR).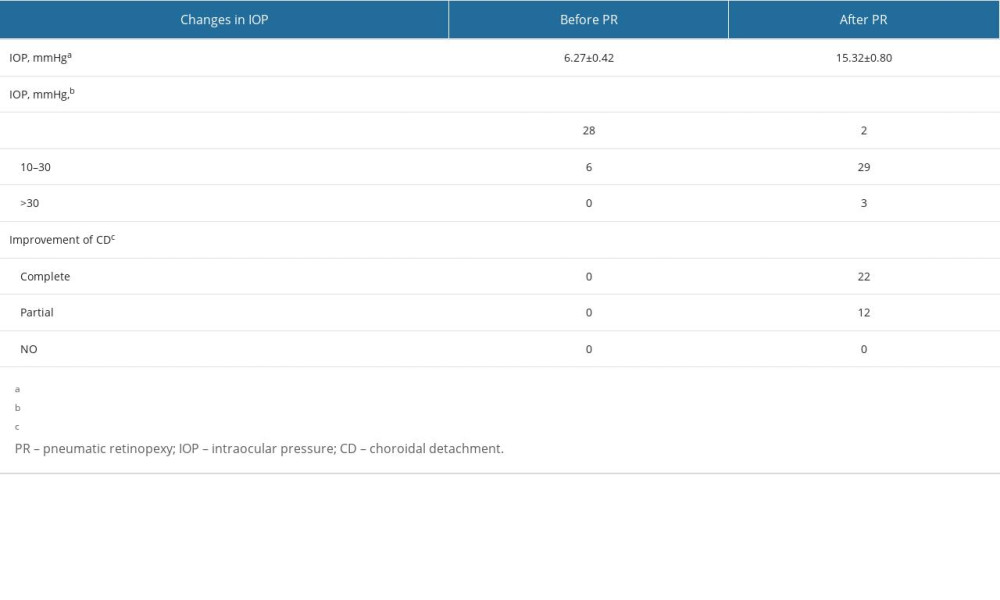 Table 3. Pars plana vitrectomy (PPV) surgery for patients with combined retinal and choroidal detachment.
Table 3. Pars plana vitrectomy (PPV) surgery for patients with combined retinal and choroidal detachment.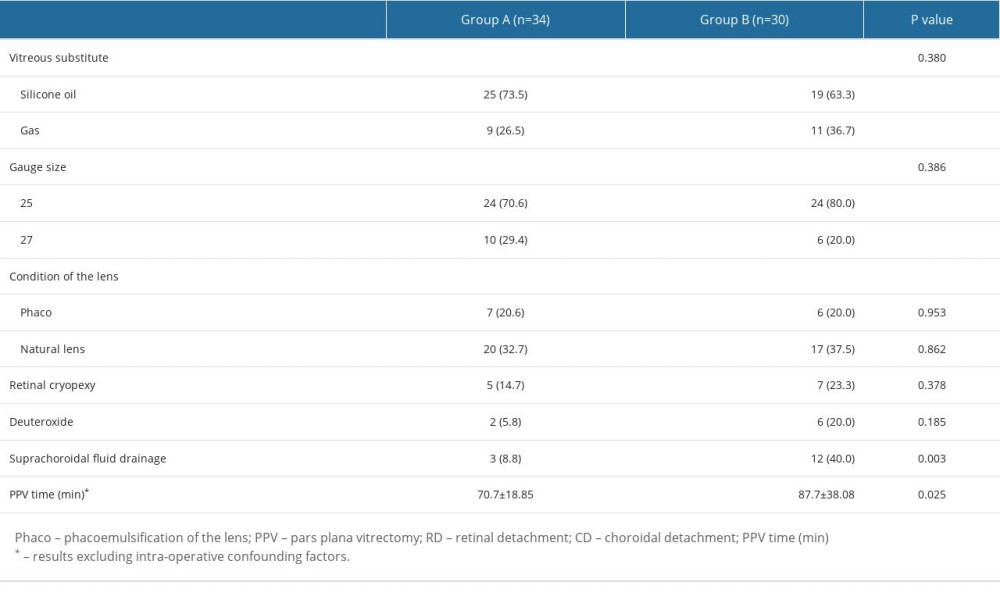 Table 4. Characteristics of follow-up information of patients with combined retinal and choroidal detachment after pars plana vitrectomy (PPV).
Table 4. Characteristics of follow-up information of patients with combined retinal and choroidal detachment after pars plana vitrectomy (PPV).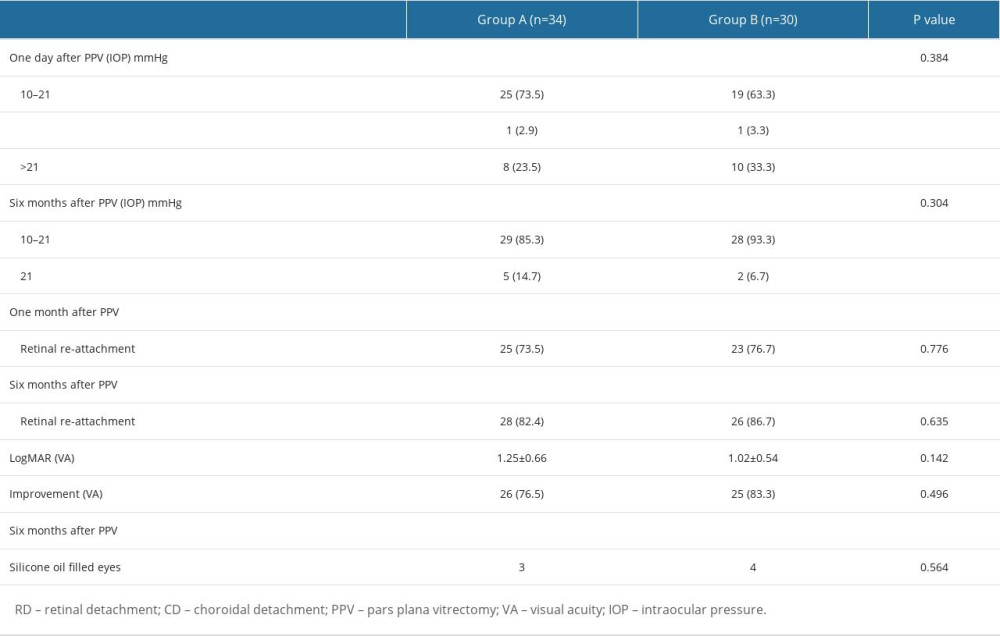
References
1. Yu Y, Yue Y, Tong N, Anatomic outcomes and prognostic factors of vitrectomy in patients with primary rhegmatogenous retinal detachment associated with choroidal detachment: Curr Eye Res, 2019; 44; 329-33
2. Zhang Z, Fang D, Peng M, Systemic approach to prevent inadvertent perfusion in eyes with extensive choroidal detachment, suprachoroidal fluid, and hypotony during pars plana vitrectomy: Adv Ther, 2019; 36; 257-64
3. Blair K, Czyz CN, Retinal detachment: StatPearls [Internet] Dec 26, 2022, Treasure Island (FL), StatPearls Publishing
4. Kohli P, Tripathy K, Agents for vitreous tamponade: StatPearls [Internet] Feb 22, 2023, Treasure Island (FL), StatPearls Publishing
5. Hu ZZ, Liu QH, Ding YZ, A modified method for suprachoroidal fluid drainage in kissing choroidal detachment: Int J Ophthalmol, 2020; 13(2); 346-48
6. Wu J, Zhang R, He J, Pre-operative steroid use in rhegmatogenous retinal detachment associated with choroidal detachment after 23-gauge vitrectomy: J Ophthalmol, 2020; 2020; 6707239
7. Doyle F, Keegan D, Anatomical and functional outcomes in combined rhegmatogenous retinal and choroidal detachment pre-treated with systemic corticosteroid: A case series: Ir J Med Sci, 2022; 191; 1937-40
8. Wei Y, Zhou R, Wang X, The effect of single periocular injection of methylprednisolone and drainage of suprachoroidal fluid in the treatment of rhegmatogenous retinal detachment combined with choroidal detachment: Eye (Lond), 2019; 33; 1387-92
9. Yeung L, Kokame GT, Brod RD, Pneumatic retinopexy for retinal detachment associated with severe choroidal detachment: Retina, 2011; 31; 87-92
10. Li Z, Li Y, Huang X, Quantitative analysis of rhegmatogenous retinal detachment associated with choroidal detachment in Chinese using UBM: Retina, 2012; 32(10); 2020-25
11. Machemer R, Aaberg TM, Freeman HM, An updated classification of retinal detachment with proliferative vitreoretinopathy: Am J Ophthalmol, 1991; 112; 159-65
12. Roshanshad A, Shirzadi S, Binder S, Pneumatic retinopexy versus pars plana vitrectomy for the management of retinal detachment: A systematic review and meta-analysis: Ophthalmol Ther, 2023; 12(2); 705-19
13. Rizzo S, Barca F, Vitreous substitute and tamponade substances for microincision vitreoretinal surgery: Dev Ophthalmol, 2014; 54; 92-101
14. Hilton GF, Grizzard WS, Pneumatic retinopexy. A two-step outpatient operation without conjunctival incision: Ophthalmology, 1986; 93; 626-41
15. Holz ER, Mieler WF, View 3: The case for pneumatic retinopexy: Br J Ophthalmol, 2003; 87; 787-89
16. Stewart S, Chan W, Pneumatic retinopexy: patient selection and specific factors: Clin Ophthalmol, 2018; 12; 493-502
17. Dhami A, Shah KK, Ratra D, Pneumatic retinopexy outcomes as primary or secondary surgical option for treating rhegmatogenous retinal detachment: Indian J Ophthalmol, 2018; 66(3); 420-25
18. Narula R, Pneumatic retinopexy: A cost-effective alternative: Indian J Ophthalmol, 2018; 66(3); 426-27
19. Chen X, Zha Y, Du S, Timely use of conventional vitrectomy and endoscope-assisted vitrectomy for endophthalmitis following open ocular trauma: A retrospective study of 18 patients: Med Sci Monit, 2019; 25; 8628-36
20. Kohli GM, Shenoy P, Halim D, Safety and efficacy of suprachoroidal triamcinolone acetonide for the management of serous choroidal detachment prior to rhegmatogenous retinal detachment surgery: A Pilot study: Indian J Ophthalmol, 2022; 70; 1302-6
21. Ma J, Wang Q, Niu H, Comparison of 27-gauge and 25-gauge microincision vitrectomy surgery for the treatment of vitreoretinal disease: A systematic review and meta-analysis: J Ophthalmol, 2020; 2020; 6149692
22. Adamiec-Mroczek J, 27-gauge sutureless vitrectomy under topical anesthesia: A pilot study: Adv Clin Exp Med, 2021; 30; 1099-103
23. Charles S, Ho AC, Dugel PU, Clinical comparison of 27-gauge and 23-gauge instruments on the outcomes of pars plana vitrectomy surgery for the treatment of vitreoretinal diseases: Curr Opin Ophthalmol, 2020; 31; 185-91
24. Recchia FM, Scott IU, Brown GC, Small-gauge pars plana vitrectomy: A report by the American Academy of Ophthalmology: Ophthalmology, 2010; 117(9); 1851-57
Figures
 Figure 1. B-ultrasonography showed the changes of pneumatic retinopexy before and after treatment in patients with combined retinal and choroidal detachment. (A) Shows retinal and choroid detachment, and (B) shows the retinal and choroid detachment after PR treatment.
Figure 1. B-ultrasonography showed the changes of pneumatic retinopexy before and after treatment in patients with combined retinal and choroidal detachment. (A) Shows retinal and choroid detachment, and (B) shows the retinal and choroid detachment after PR treatment. Figure 2. Ultrasound bio-microscopy examination showed the change of pneumatic retinopexy before and after treatment in patients with combined retinal and choroidal detachment. (A) Shows a marked detachment of the ciliary body from the sclera, and (B) shows a basic reduction of the detached ciliary body after pneumatic retinopexy treatment.
Figure 2. Ultrasound bio-microscopy examination showed the change of pneumatic retinopexy before and after treatment in patients with combined retinal and choroidal detachment. (A) Shows a marked detachment of the ciliary body from the sclera, and (B) shows a basic reduction of the detached ciliary body after pneumatic retinopexy treatment. Tables
 Table 1. Basic characteristics of 2 groups of patients with combined retinal and choroidal detachment.
Table 1. Basic characteristics of 2 groups of patients with combined retinal and choroidal detachment. Table 2. Characteristic changes of patients with combined retinal and choroidal detachment before and after pneumatic retinopexy (PR).
Table 2. Characteristic changes of patients with combined retinal and choroidal detachment before and after pneumatic retinopexy (PR). Table 3. Pars plana vitrectomy (PPV) surgery for patients with combined retinal and choroidal detachment.
Table 3. Pars plana vitrectomy (PPV) surgery for patients with combined retinal and choroidal detachment. Table 4. Characteristics of follow-up information of patients with combined retinal and choroidal detachment after pars plana vitrectomy (PPV).
Table 4. Characteristics of follow-up information of patients with combined retinal and choroidal detachment after pars plana vitrectomy (PPV). Table 1. Basic characteristics of 2 groups of patients with combined retinal and choroidal detachment.
Table 1. Basic characteristics of 2 groups of patients with combined retinal and choroidal detachment. Table 2. Characteristic changes of patients with combined retinal and choroidal detachment before and after pneumatic retinopexy (PR).
Table 2. Characteristic changes of patients with combined retinal and choroidal detachment before and after pneumatic retinopexy (PR). Table 3. Pars plana vitrectomy (PPV) surgery for patients with combined retinal and choroidal detachment.
Table 3. Pars plana vitrectomy (PPV) surgery for patients with combined retinal and choroidal detachment. Table 4. Characteristics of follow-up information of patients with combined retinal and choroidal detachment after pars plana vitrectomy (PPV).
Table 4. Characteristics of follow-up information of patients with combined retinal and choroidal detachment after pars plana vitrectomy (PPV). In Press
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








