03 November 2023: Clinical Research
Impact of Visceral Fat Area on Intraoperative Complexity and Surgical Approach Decision for Robot-Assisted Partial Nephrectomy: A Comparative Analysis with BMI
Bo CuiDOI: 10.12659/MSM.941953
Med Sci Monit 2023; 29:e941953
Abstract
BACKGROUND: Optimizing surgical approaches for robot-assisted partial nephrectomy (RAPN) is vital for better patient outcomes. This retrospective study aimed to examine how visceral fat area (VFA) and body mass index (BMI) correlate with intraoperative complexities, thereby guiding the selection of surgical techniques for RAPN.
MATERIAL AND METHODS: The study analyzed the medical records of 213 Chinese patients diagnosed with a range of benign and malignant renal neoplasms and treated with RAPN in 2020. Visceral fat area was quantified using computed tomography (CT) scans taken at the umbilical level. Various perioperative indicators, such as demographic details, clinicopathological parameters, operation time, estimated blood loss (EBL), warm ischemic time (WIT), and intraoperative complications, were assessed.
RESULTS: For the retroperitoneal approach, patients with either visceral obesity or general obesity had longer operation times (P<0.001 and P=0.004) and had a tendency for higher EBL (P=0.003 and P=0.001) compared to non-obese patients. In the transperitoneal approach, those with visceral obesity had significantly longer operation times (P=0.008) than their non-viscerally obese counterparts; however, general obesity showed no impact on operation time (P=0.251). Estimated blood loss was higher for patients with visceral obesity (P=0.004), but no significant difference was noted among those with general obesity (P=0.980).
CONCLUSIONS: VFA appears to offer predictive advantages over BMI in assessing intraoperative complexities for transperitoneal RAPN. When used in conjunction with BMI, it could serve as a valuable tool in selecting the most appropriate surgical approach for RAPN.
Keywords: Intra-Abdominal Fat, Body Mass Index
Background
Renal cell carcinoma (RCC) is one of the most common malignancies of the genitourinary system; it is the sixth most common malignancy in men and the tenth most common in women [1], and has the third-highest mortality rate of all urologic malignancies [2]. Most patients with RCC are 60–70 years old, and the male-to-female ratio is approximately 2: 1 [1,3]. Clear cell renal cell carcinomas (ccRCCs), which originate from the tubular epithelium, account for 60–80% of all RCCs. Other types of RCC include papillary (15%) and chromophobe (5%) RCC [4,5]. The incidence of RCC has increased significantly in recent years. Obesity is becoming increasingly prevalent in China, and almost one-third of the Chinese population is obese or overweight. Obesity can affect not only RCC development, but also the clinicopathological features of ccRCC [6]. Several recent studies have shown that visceral obesity increases surgical complexity and perioperative risks [7]. Although body mass index (BMI) has been traditionally used to evaluate obesity or overweight status, it cannot distinguish the weight of visceral fat from the weight of muscle [8]. In addition, Asians are at greater risk for visceral obesity than Westerners [9], and all subjects in our research were Chinese. Notably, obesity in the Chinese population is obviously different from that in the Western population, and the characteristics of the Chinese population are rarely reported [10,11]. Similar to BMI, computed tomography (CT)-detected visceral fat area (VFA) is an ideal parameter for assessing fat distribution and assessing the effect of obesity on malignant tumors [12,13]. More importantly, these factors can guide the choice of surgical approach. Therefore, this respective study aimed to investigate the association between VFA/BMI with indicators of surgical complexity, including operation time and estimated blood loss (EBL), and between VFA/BMI with surgical approach decisions in 213 patients with different types of renal neoplasms treated with robot-assisted partial nephrectomy (RAPN) at a single center in 2020.
Material and Methods
ETHICS STATEMENT:
This current retrospective study was approved by the Ethics Committee of the Chinese PLA General Hospital. All the patients signed the informed consent form prior to minimally-invasive surgery and relevant medical data collection.
PATIENTS STUDIED:
This retrospective comparative study was conducted on 213 patients with various renal neoplasms, including ccRCCs (164/213), papillary RCC (8/213), chromophobe RCC (10/213), clear cell and papillary RCC (2/213), MiT family translocation RCC (1/213), TFE3 gene fusion-related RCC (1/213), other types of RCC (6/213), renal angiomyolipoma (16/213), renal oncocytoma (4/213), and adult cystic nephroma (1/213). All patients in our study underwent CT to identify renal tumor size and location. Other medical examinations were performed, including blood tests, coagulation tests, renal function tests, liver function tests, and serum electrolyte tests. Although there were various types of benign and malignant tumors in the included cases, the surgical treatment methods were the same. All the included cases underwent unilateral RAPN between January 2020 and December 2020 in the Chinese People’s Liberation Army General Hospital, Beijing, China. Surgeries were performed by the same 3 experienced surgeons who had advanced and reliable skills, with the assistance of the third-generation Da Vinci surgical robot [14].
PATIENT EXCLUSION CRITERIA:
The exclusion criteria were: multifocal neoplasms, bilateral renal tumors, renal carcinomas with an inferior vena cava thrombus, metastatic renal cancers, and patients with clampless renal artery or hypothermic perfusion during surgery. We also excluded patients with a history of upper-abdominal surgery, cardiopulmonary insufficiency, uncontrolled diabetes, hypertension, systemic hemorrhagic disease, and unacceptable anesthesia risk.
METHODS:
The study classified all 213 patients into 2 groups based on the obesity criteria defined by VFA/BMI: obesity and non-obesity. A VFA ≥100 cm2 was used to indicate visceral obesity [15–17]. General obesity in Chinese subjects is defined as a BMI ≥28 kg/m2 [18]. The VFA was blindly measured via CT scanning at the umbilical level of the abdominal section [12,19], and the CT images were blindly analyzed using Medcare software (AnyPACS2.0). The calculation method of VFA involves circling the outline of visceral fat, after which the Medcare software automatically calculates VFA (Figure 1A, 1B). Fat-tissue cross-sectional areas (in square centimeters) were determined using the standard Hounsfield unit range (−190 to −30 HU) [20]. All preoperative measurements were performed by 2 experienced radiologists. BMI was calculated as weight in kilograms divided by height in meters squared for all included patients [21]. The clinical and pathological parameters and the perioperative outcomes were compared between obese and non-obese patients based on VFA/BMI.
STATISTICAL ANALYSIS:
Statistical analysis was performed to assess differences in perioperative parameters [sex, age, BMI, VFA, laterality, location, tumor size, American Joint Committee on Cancer (AJCC) stage, RENAL score, tumor types, Fuhrman grade, operation time, estimated blood loss (EBL), warm ischemic time (WIT), and complications]. Data were compared using the chi-squared or Fisher exact test for categorical variables, and the 2-sample
Results
GENERAL INFORMATION:
Of the 213 patients, 145 (68.1%) were male and 68 (31.9%) were female; the mean age was 52.6±13.0 years. The overall median (upper and lower quartile) VFA determined via CT was 107.1(73.1, 153.9) cm2. Of the 213 patients, 121 (56.8%) were identified as viscerally obese, and 92 (43.2%) were identified as viscerally non-obese according to VFA; 53 (24.9%) were classified as generally obese and 160 (75.1%) were classified as generally non-obese according to BMI. The obese and non-obese groups classified by VFA vs BMI had distinctive clinical and pathological features (Tables 1, 2).
DEMOGRAPHIC AND CLINICOPATHOLOGICAL CHARACTERISTICS:
Sex distribution differed significantly between the obese and non-obese groups defined by VFA and BMI (P<0.001, P<0.001, respectively). Age was significantly associated with VFA [54.0(48.0, 63.0) vs 49.0(40.0, 62.0) years, P=0.011] but not with BMI (52.2±11.0 vs 52.7±13.6 years, P=0.811). Tumor laterality differed between the patients with visceral obesity and those without visceral obesity (P=0.042), but did not differ between the patients with general obesity and those without general obesity (P=0.191). In addition, no significant difference in tumor size was observed [VFA classification: 3.0(2.5, 3.8) and 3.0(2.5, 3.8) cm, P=0.874; BMI classification: 3.0(2.5, 3.5) and 3.0(2.5, 4.0) cm, P=0.774]. The tumor types were obviously different between the viscerally obese and non-obese groups (P<0.001), but, based on the same index, there was no significant difference between the general obese and non-obese groups (P=0.114). VFA- and BMI-defined obesity were not associated with the RENAL nephrometry score (P=0.395, P=0.056) or AJCC stage of malignant renal carcinomas (P=0.158, P=0.313). In addition, the Fuhrman grade in patients with ccRCC and papillary renal cell carcinoma was not significantly correlated with obesity and non-obesity defined by VFA or BMI (P=0.662, P=0.928) (Tables 1, 2).
OPERATION TIME, ESTIMATED BLOOD LOSS, AND WARM ISCHEMIA TIME:
In the retroperitoneal approach, patients with a larger VFA or BMI tended to have longer operation times [140.0(119.8, 160.0) vs 115.0(90.0, 130.0) min, P<0.001; 142.0(125.0, 160.0) vs 121.5(100.0, 140.0) min, P=0.004] and greater EBL [50.0(20.0, 50.0) vs 20.0(20.0, 30.0) mL, P=0.003; 50.0(20.0, 100.0) vs 20.0(20.0, 50.0) mL, P=0.001] than their counterparts. In the transperitoneal approach, patients with a larger VFA also tended to have longer operation times [140.0(120.0, 165.0) vs 125.0(95.0, 150.0) min, P=0.008] and greater EBL [50.0(30.0, 100.0) vs 30.0(20.0, 50.0) mL, P =0.004]. In contrast, compared with their counterparts, patients with a higher BMI did not have a significantly different operation time or EBL [operation time: 139.0(118.0, 160.0) vs 130.0(106.8, 155.0) min, P=0.251; EBL: [50.0(20.0, 50.0) vs 50.0(20.0, 100.0) mL, P=0.980]. In our research, VFA- and BMI-defined obesity was not associated with the WIT in the retroperitoneal group [20.0(15.0, 23.3) vs 17.0(14.0, 23.0) min, P=0.431; 18.0(14.0, 24.0) vs 19.5(15.0, 23.0) min, P=0.868] or the transperitoneal group [20.0(15.0, 24.0) vs 18.0(11.5, 20.0) min, P=0.080; 20.0(14.0, 23.5) vs 19.0(14.8, 21.3) min, P=0.642] (Table 3, 4).
To investigate whether the predictive effect of BMI/VFA on intraoperative complexity depends on the chosen cut-off, we used bean plots to visualize the distribution of operative time (Figure 2) or EBL (Figure 3) for different BMI/VFA cut-off points. The results indicated that different BMI/VFA cut-offs did not essentially change the statistical significance of operation time/EBL. For patients with a larger VFA using either surgical approach, and for patients with a higher BMI using the retroperitoneal approach, the operation time and EBL were significantly longer and higher regardless of the BMI/VFA cut-off. Moreover, for patients with a higher BMI using the transperitoneal approach, the operation time/EBL was not significantly different regardless of the cut-off.
INTRAOPERATIVE AND POSTOPERATIVE COMPLICATIONS:
The overall intraoperative and postoperative complication rates were 1.4% (3/213) and 0.5% (1/213), respectively, and no deaths occurred. Intraoperative complications included transfusion, open conversion, visceral injury, and other complications. In our retrospective comparative study, 3 patients had intraoperative complications, and all 3 were treated with intraoperative blood transfusion. For postoperative complications, only 1 patient had grade I complications, and no patient had a grade II or higher Clavien-Dindo complication. Patients classified as having visceral obesity were not at greater risk for intraoperative complications (P=0.811), as the risk was not significantly different between the patients with general obesity and those without general obesity (P=0.740) (Table 5).
Discussion
In this retrospective study, we investigated the relationship between VFA/BMI and surgical complexity. We also explored the association between VFA/BMI and the decision regarding surgical approach. Our findings suggest that apart from BMI, VFA may also play a role in influencing intraoperative complexity. Importantly, we observed that VFA, along with BMI, could potentially assist in determining the appropriate surgical approach.
Surgery remains the key treatment for benign and malignant renal tumors. At present, minimally-invasive surgery mainly includes laparoscopic surgery and robotic laparoscopic surgery. The latter is more accurate and technologically advanced than the former. Therefore, we included patients with RAPN in this study [22]. Minimally-invasive RAPN is one of the criterion standard treatments for renal neoplasms. In RAPN, 2 approaches are commonly used: the retroperitoneal approach and the transperitoneal approach. The surgical approach for RAPN was selected based on factors such as tumor location, liver and spleen coverage, and previous abdominal surgery. In general, if the neoplasm is in the upper pole and dorsal to the kidney, the retroperitoneal approach should be chosen. If the tumor is in the lower pole and ventral to the kidney, the transperitoneal approach should be used. However, when the tumor location is not typical, as mentioned above, the best surgical approach is difficult to choose and is sometimes chosen based on the surgeon’s personal habits and training experience. In these cases, VFA/BMI may be helpful in choosing the surgical approach.
As a prevalent and growing problem in society, obesity is considered to be an important factor that affects the complexity of abdominal surgery, including RAPN. BMI reflects the percentage of total body fat, whereas VFA reflects the accumulation of abdominal fat [23]. General obesity is correlated with perioperative outcomes and renal function following RAPN [24]. Visceral obesity affects surgical outcomes through several possible mechanisms, including exacerbating inflammation, lowering tissue oxygen levels, and triggering microvascular dysfunction and insulin resistance [25]. Although the precise mechanisms at play remain unknown, recent investigations proved the association between visceral adiposity and carcinogenesis [26]. In patients with gastrointestinal cancer [15] and those who undergo laparoscopic radical nephrectomy [19], an increased VFA leads to high intraoperative complexity and poor surgical outcomes. However, there are no reports assessing the relationships between BMI/VFA and intraoperative complexity in RAPN, which is a complex procedure with a long operative time. There is also a lack of adiposity analysis that differentiates retroperitoneal and transperitoneal RAPN approaches. Thus, hoping to guide the choice of surgical approach for RAPN, we compared VFA and BMI as predictors of intraoperative complexity in the retroperitoneal and transperitoneal approaches, respectively.
Because Asian people tend to be different in size from European and American people, we used obesity standards for Asians instead of the WHO criteria (a BMI ≥30 kg/m2 as general obesity and a VFA ≥80 cm2 as visceral obesity) [27]. In our study, general obesity was defined as a BMI ≥28 kg/m2 according to criteria set by the Chinese National Health Commission [18], and visceral obesity was defined as a VFA ≥100 cm2 at the level of the umbilicus for Asians [16,17].
We investigated the relationships between VFA/BMI and parameters such as operation time, EBL, WIT, RENAL nephrometry score, AJCC stage, tumor size, Fuhrman grade, tumor types, and intraoperative and postoperative complications in RAPN. We found that VFA-defined visceral obesity increased the operation time and EBL in both surgical approaches, while a higher BMI increased the operation time and EBL in the retroperitoneal approach but not in the transperitoneal approach. Our results indicated that VFA may have a unique advantage over BMI in the prediction of intraoperative complexity in transperitoneal RAPN, possibly because visceral fat seriously occluded the view of the surgical field, narrowed the operative region, and limited the removal of pathological tissue due to risk of injury to adjacent organs and vessels. In addition, in the transperitoneal approach, the anatomical landmarks are clear, the surgical space is large, and multiple auxiliary holes are allowed. After successful establishment of the transperitoneal approach, subcutaneous fat may have a minimal effect on surgery. Regardless of the thickness of the visceral fat, the dissection plane of the adipose tissue is explicit. However, in the retroperitoneal approach, the anatomical landmarks are unclear, the surgical space is relatively small, and both the adhesion of visceral fat and the thickness of subcutaneous fat have an effect. According to our results, the transperitoneal approach is the optimum selection for treatment of neoplasms in an atypical position and for patients with a BMI ≥28 kg/m2, regardless of their VFA, and patients with a BMI <28 kg/m2 and a VFA ≥100 cm2. Moreover, for patients with a VFA <100 cm2 and a BMI <28 kg/m2, the retroperitoneal approach was more suitable. In addition, we further confirmed our findings under different BMI or VFA cut-offs. Moreover, VFA may be more sensitive than BMI in predicting the intraoperative complexity of the transperitoneal approach, where VFA can serve as an important preoperative marker.
Previous research has shown that BMI-defined obesity is associated with surgical complications [28]. Furthermore, visceral obesity may be a prognostic indicator of complications in patients with small renal arteries treated with minimally-invasive partial nephrectomy [29]. In contrast, several studies found that visceral obesity had no significant effect on postoperative outcomes, but BMI did [30]. In our investigation, we did not find that VFA or BMI was correlated with intraoperative complications in patients with renal tumors because few patients with intraoperative complications were included. The low complication rate is mainly due to the advanced surgical skills of surgeons, which comes from performing thousands of procedures. In a previous study, a larger VFA was strongly associated with higher Fuhrman grades [31], and a larger VFA was indicative of the invasiveness of small RCC. However, we did not find that a larger VFA or a higher BMI was associated with higher Fuhrman grades, potentially due to the limited sample size. Despite the differences in tumor types between the patients with visceral obesity and those without visceral obesity in our research, the tumor type itself did not independently influence the indication or planning for RAPN. Most patients in our research had ccRCC. The impact of tumor resection margins on surgical complexity and surgical approach management was found to be inconclusive, possibly due to the limited sample size included in the study. Linehan et al [32] recognized that ccRCC is a metabolic disease, and abnormal lipid metabolism is closely related to the development of ccRCC. The proportion of lipid components in patients with ccRCC is higher than that in patients with other benign or malignant renal tumors. Previous research has demonstrated a correlation between the MAP score and surgical complexity in laparoscopic partial nephrectomy [33]. Our investigation also found that tumor types were closely correlated with VFA-defined obesity. In terms of the potential influence of VFA on endocrine functions, there may be a correlation between VFA and ccRCC, but the correlation and mechanisms need to be further explored.
Our work has several limitations. Given the retrospective study design, unknown biases may have affected our results. Because the surgeries were performed by the same surgical team in the same year, our sample size was relatively small, thereby limiting the statistical power of the analysis. Future investigations should include other races and larger sample sizes, and may validate and strengthen our results.
Conclusions
VFA-defined visceral obesity increased the surgical complexity in both surgical approaches, while BMI predicted increased surgical complexity in the retroperitoneal approach but not in the transperitoneal approach. Thus, VFA may have unique advantages over BMI in predicting intraoperative complexity in transperitoneal RAPN. Moreover, it is crucial to recognize that VFA and BMI together may help guide the selection of surgical approach for RAPN.
Figures
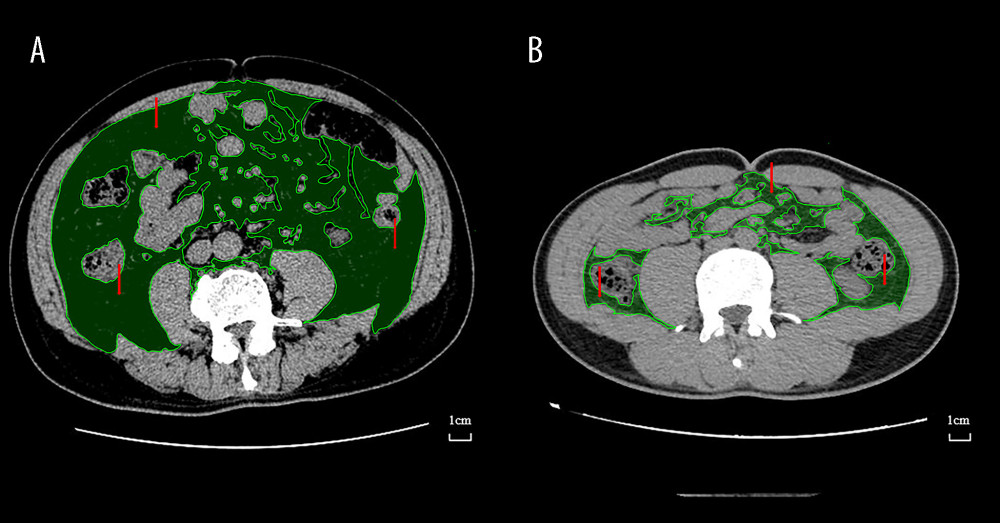 Figure 1. Preoperative Axial CT images of 2 different patients at the level of umbilicus used for evaluation of visceral obesity (represented). The green area indicated by the red arrow is the VFA. (A) Visceral obesity by VFA (B) Non-visceral obesity by VFA. CT – computed tomography; VFA – visceral fat area. Medcare software (AnyPACS2.0).
Figure 1. Preoperative Axial CT images of 2 different patients at the level of umbilicus used for evaluation of visceral obesity (represented). The green area indicated by the red arrow is the VFA. (A) Visceral obesity by VFA (B) Non-visceral obesity by VFA. CT – computed tomography; VFA – visceral fat area. Medcare software (AnyPACS2.0). 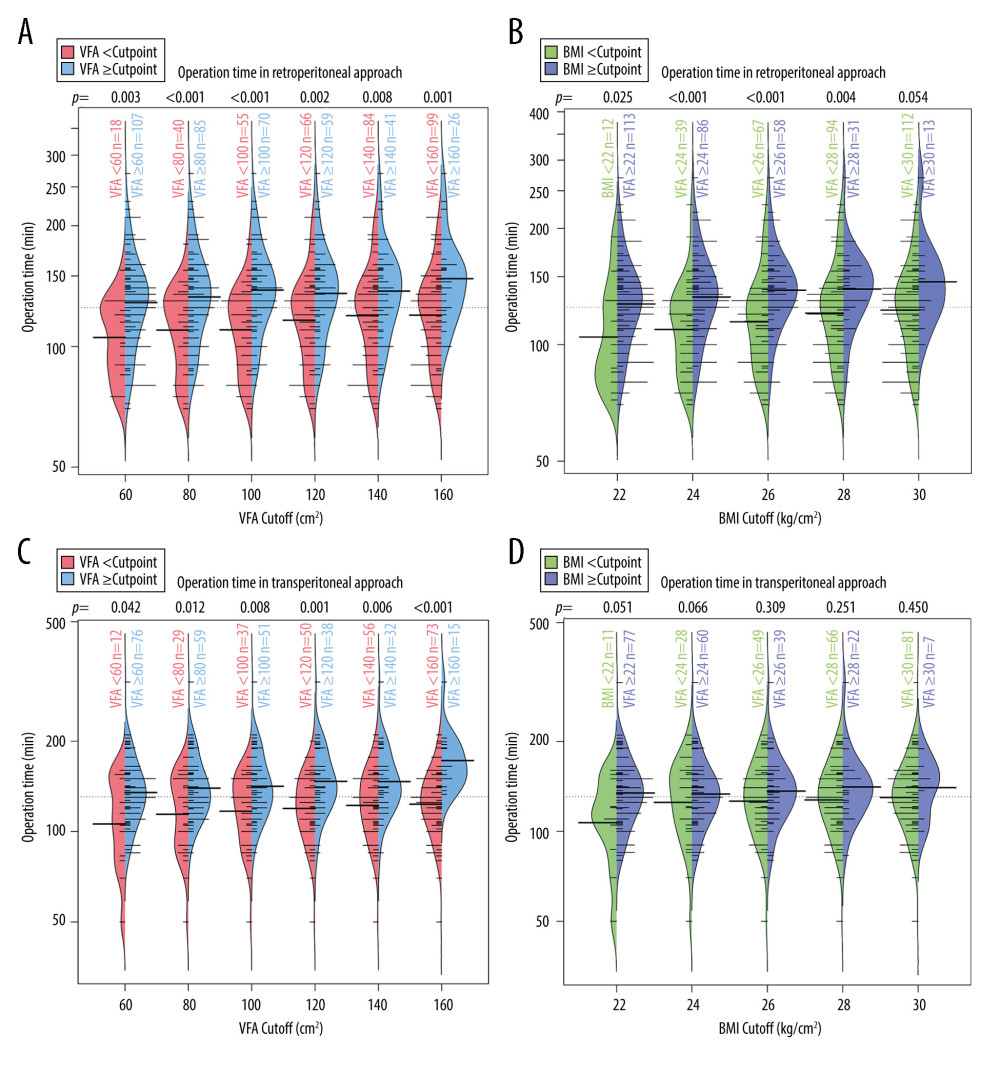 Figure 2. Bean plots of operation time under different BMI/VFA cut-offs. (A) Operation time in retroperitoneal approach at different VFA cut-offs. (B) Operation time in retroperitoneal approach at different BMI cut-offs. (C) Operation time in transperitoneal approach at different VFA cut-offs. (D) Operation time in transperitoneal approach at different BMI cut-offs. The P values at the top of each graph represent the Mann-Whitney U test results. Thick bars indicate medians of operation time and thin bars show the frequency of them. VFA – visceral fat area; BMI – body mass index. (R language, version R 4.2.3).
Figure 2. Bean plots of operation time under different BMI/VFA cut-offs. (A) Operation time in retroperitoneal approach at different VFA cut-offs. (B) Operation time in retroperitoneal approach at different BMI cut-offs. (C) Operation time in transperitoneal approach at different VFA cut-offs. (D) Operation time in transperitoneal approach at different BMI cut-offs. The P values at the top of each graph represent the Mann-Whitney U test results. Thick bars indicate medians of operation time and thin bars show the frequency of them. VFA – visceral fat area; BMI – body mass index. (R language, version R 4.2.3). 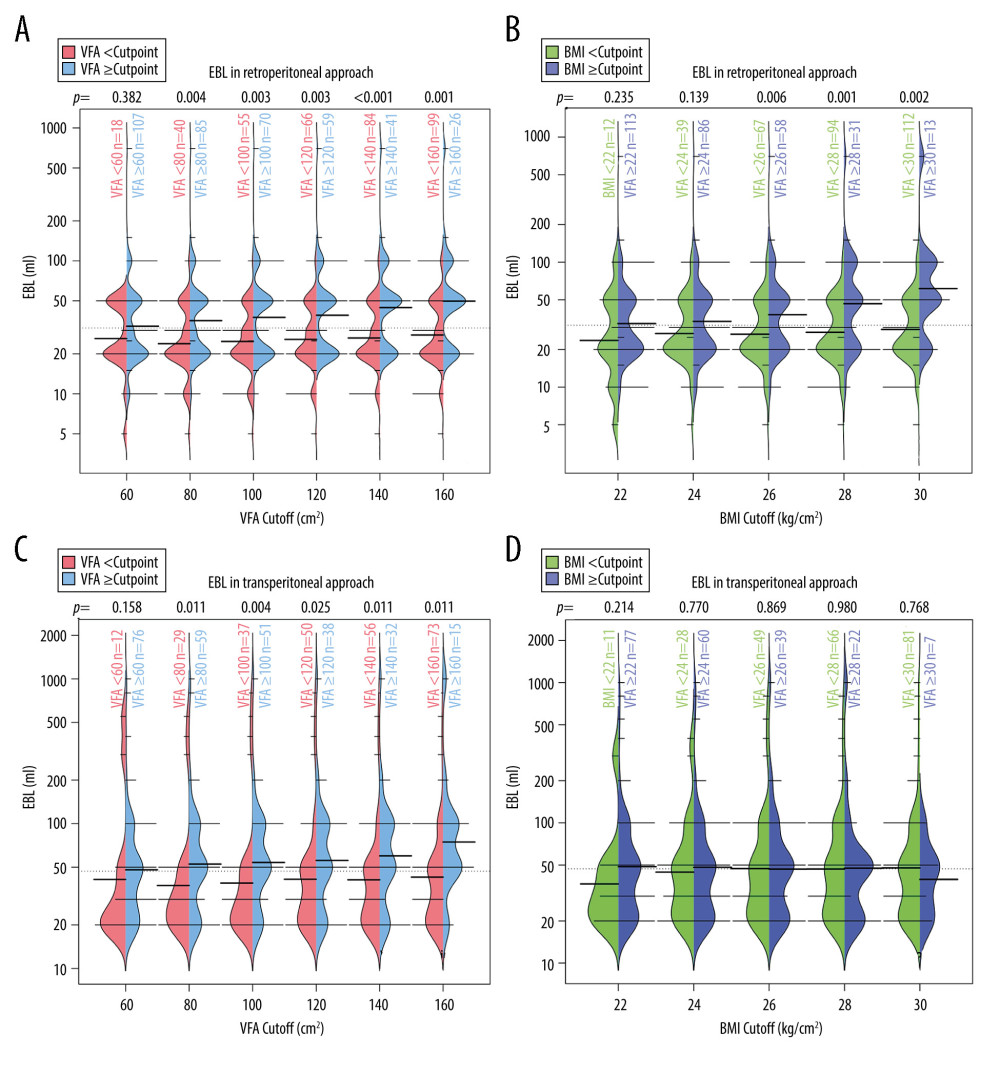 Figure 3. Bean plots of EBL under different BMI/VFA cut-offs. (A) EBL in retroperitoneal approach at different VFA cut-offs. (B) EBL in retroperitoneal approach at different BMI cut-offs. (C) EBL in transperitoneal approach at different VFA cut-offs. (D) EBL in transperitoneal approach at different BMI cut-offs. The p values at the top of each graph represent the Mann-Whitney U test results. Thick bars indicate medians of operation time and thin bars show the frequency of them. VFA – visceral fat area; BMI – body mass index; EBL – estimated blood loss. (R language, version R 4.2.3).
Figure 3. Bean plots of EBL under different BMI/VFA cut-offs. (A) EBL in retroperitoneal approach at different VFA cut-offs. (B) EBL in retroperitoneal approach at different BMI cut-offs. (C) EBL in transperitoneal approach at different VFA cut-offs. (D) EBL in transperitoneal approach at different BMI cut-offs. The p values at the top of each graph represent the Mann-Whitney U test results. Thick bars indicate medians of operation time and thin bars show the frequency of them. VFA – visceral fat area; BMI – body mass index; EBL – estimated blood loss. (R language, version R 4.2.3). Tables
Table 1. Demographic and clinicopathological parameters between visceral obese and non-obese by VFA.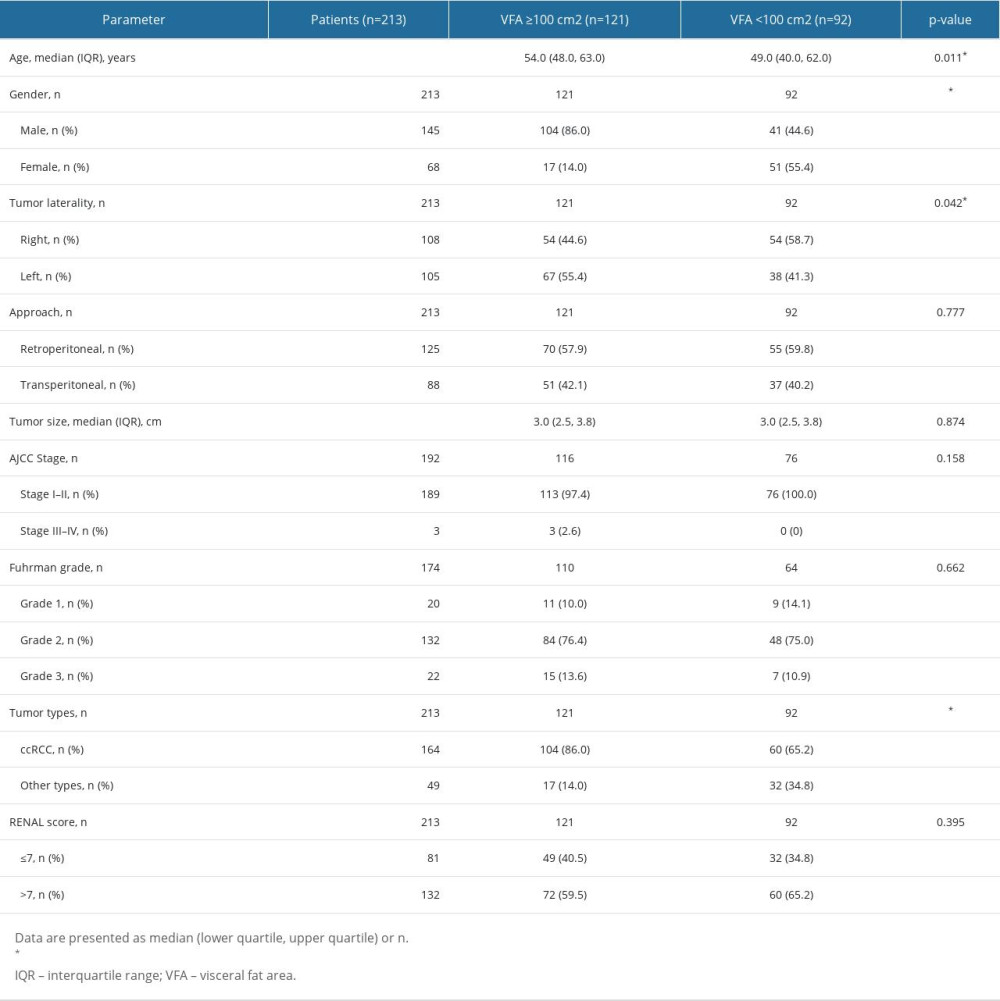 Table 2. Demographic and clinicopathological parameters between general obese and non-obese by BMI.
Table 2. Demographic and clinicopathological parameters between general obese and non-obese by BMI.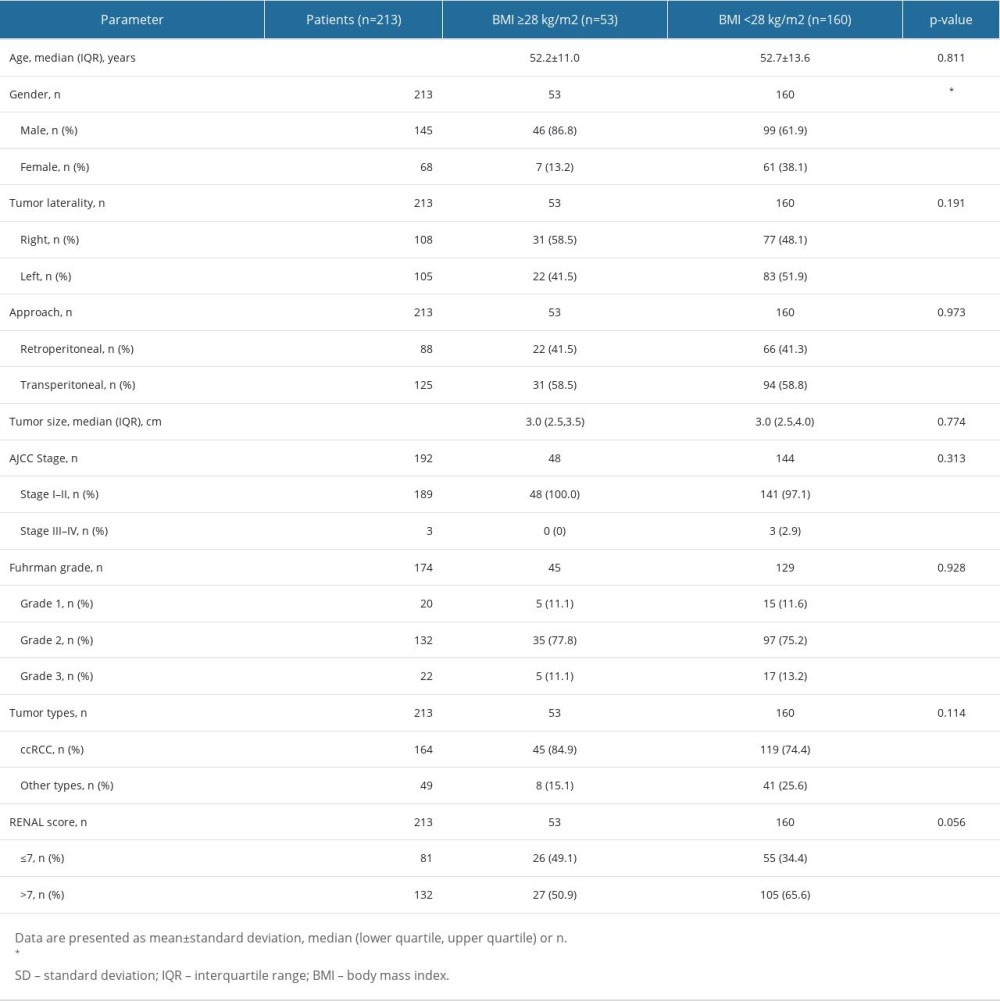 Table 3. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (retroperitoneal approach).
Table 3. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (retroperitoneal approach).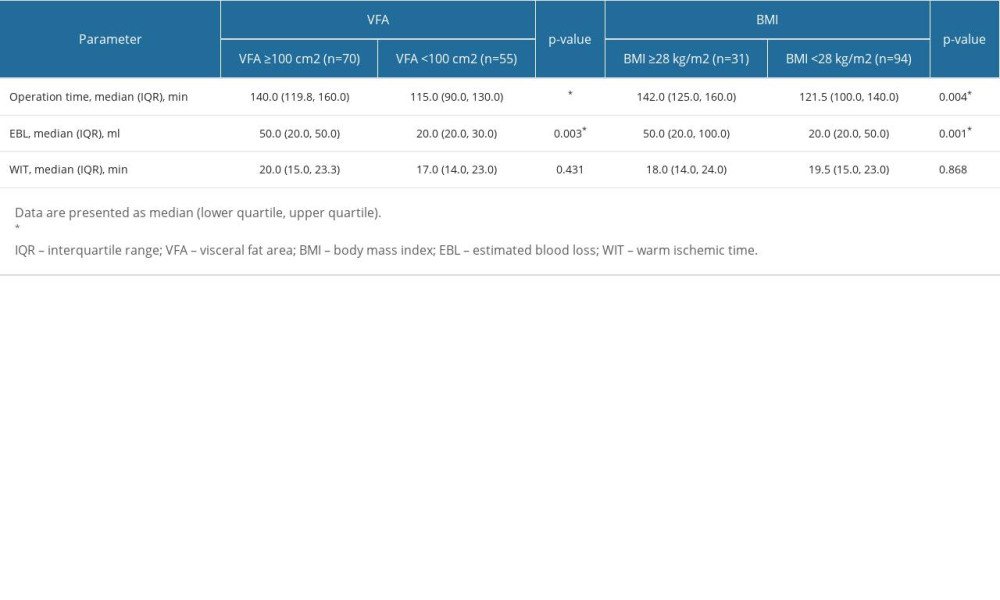 Table 4. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (transperitoneal approach).
Table 4. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (transperitoneal approach).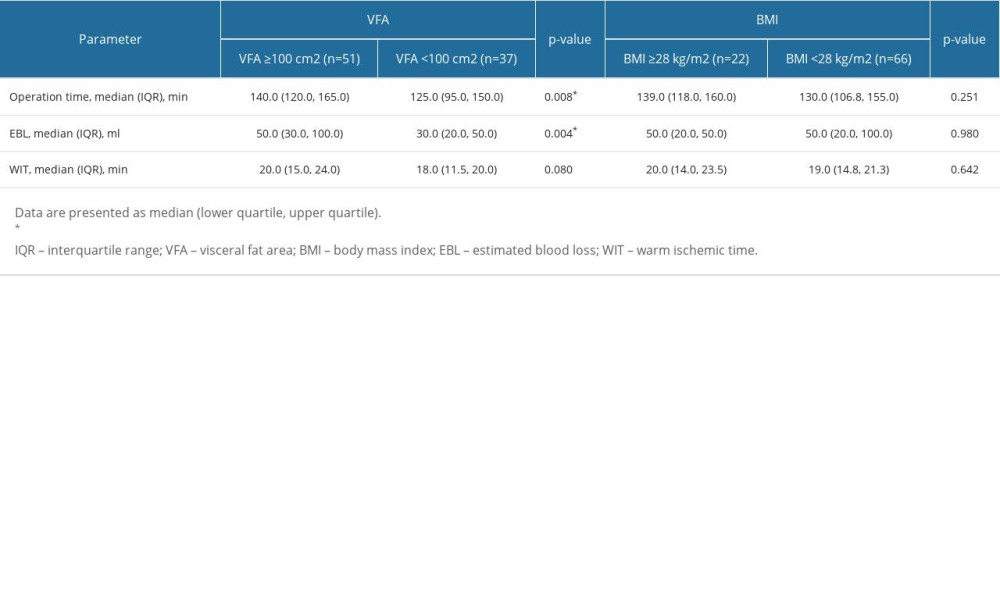 Table 5. Comparison of intraoperative complication between obese and non-obese patients by VFA/BMI.
Table 5. Comparison of intraoperative complication between obese and non-obese patients by VFA/BMI.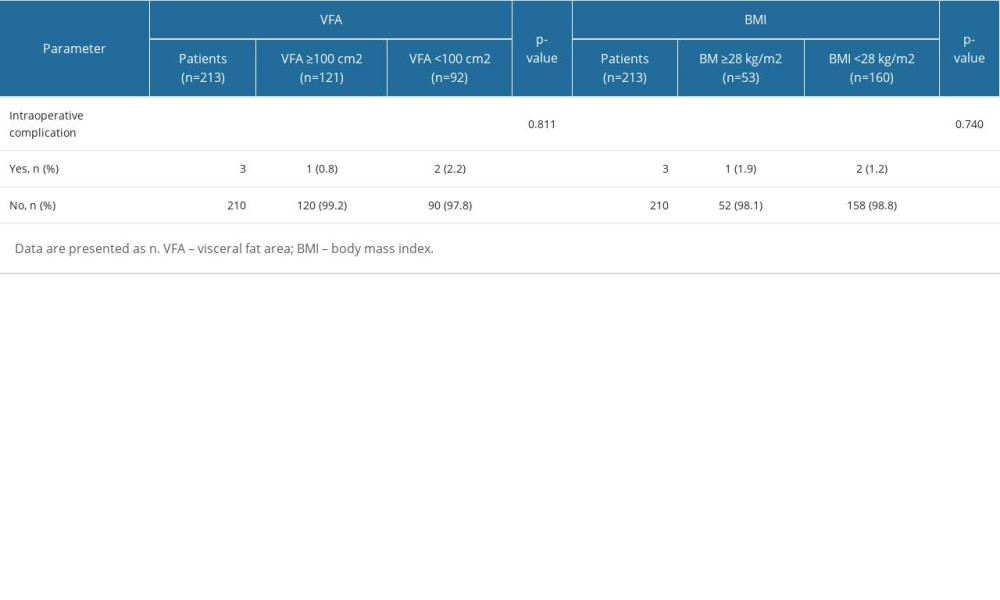
References
1. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018: Cancer J Clin, 2018; 68(1); 7-30
2. Cui Q, Wang C, Liu S, YBX1 knockdown induces renal cell carcinoma cell apoptosis via Kindlin-2: Cell Cycle, 2021; 20(22); 2413-27
3. Motzer RJ, Jonasch E, Agarwal N, Kidney cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology: J Natl Compr Canc Netw, 2022; 20(1); 71-90
4. Rodriguez C, Patel AV, Mondul AM, Diabetes and risk of prostate cancer in a prospective cohort of US men: Am J Epidemiol, 2005; 161(2); 147-52
5. Shanks JH, Pathology of renal cell carcinoma: Recent developments: Clin Oncol (R Coll Radiol), 1999; 11(4); 263-68
6. Keehn A, Srivastava A, Maiman R, The relationship between visceral obesity and the clinicopathologic features of patients with small renal masses: J Endourol, 2015; 29(3); 372-76
7. Zhai T, Zhang B, Qu Z, Elevated visceral obesity quantified by CT is associated with adverse postoperative outcome of laparoscopic radical nephrectomy for renal clear cell carcinoma patients: Int Urol Nephrol, 2018; 50(5); 845-50
8. Kuk JL, Lee S, Heymsfield SB, Waist circumference and abdominal adipose tissue distribution: Influence of age and sex: Am J Clin Nutr, 2005; 81(6); 1330-34
9. Kadowaki T, Sekikawa A, Murata K, Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study: Int J Obes (Lond), 2006; 30(7); 1163-65
10. Wang D, Li Y, Lee SG, Ethnic differences in body composition and obesity related risk factors: study in Chinese and white males living in China: PLoS One, 2011; 6(5); e19835
11. WHO Expert Consultation, Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies: Lancet, 2004; 363(9403); 157-63
12. Xu Z, Liu Y, Yan C, Measurement of visceral fat and abdominal obesity by single-frequency bioelectrical impedance and CT: A cross-sectional study: BMJ Open, 2021; 11(10); e048221
13. Kvist H, Chowdhury B, Sjöström L, Adipose tissue volume determination in males by computed tomography and 40K: Int J Obes, 1988; 12(3); 249-66
14. Wang B, Li H, Ma X, Robot-assisted laparoscopic inferior vena cava thrombectomy: Different sides require different techniques: Eur Urol, 2016; 69(6); 1112-19
15. Ozoya OO, Siegel EM, Srikumar T, Quantitative assessment of visceral obesity and postoperative colon cancer outcomes: J Gastrointest Surg, 2017; 21(3); 534-42
16. Examination Committee of Criteria for ‘Obesity Disease’ in Japan: Japan Society for the Study of Obesity, New criteria for ‘obesity disease’ in Japan: Circ J, 2002; 66(11); 987-92
17. Xu K, Zheng Q, Shao J, Sex differences in the association between visceral adipose tissue and atherosclerosis in type-2 diabetes patients with normal bodyweight: A study in a Chinese population: J Diabetes Investig, 2023; 14(1); 92-101
18. Criteria of weight for adults: Standard Number: WS/T 428-2013, 2013 Available from: [in Chinese]http://www.nhc.gov.cn/wjw/yingyang/201308/a233d450fdbc47c5ad4f08b7e394d1e8.shtml
19. Hagiwara M, Miyajima A, Hasegawa M, Visceral obesity is a strong predictor of perioperative outcome in patients undergoing laparoscopic radical nephrectomy: BJU Int, 2012; 110(11 Pt C); E980-84
20. Irving BA, Weltman JY, Brock DW, NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue: Obesity (Silver Spring), 2007; 15(2); 370-76
21. Leong KSW, Jayasinghe TN, Wilson BC, Effects of fecal microbiome transfer in adolescents with obesity: The gut bugs randomized controlled trial: JAMA Netw Open, 2020; 3(12); e2030415
22. Porter J, Blau E, Robotic-assisted partial nephrectomy: Evolving techniques and expanding considerations: Curr Opin Urol, 2020; 30(1); 79-82
23. Ioffe E, Hakimi AA, Oh SK, Effect of visceral obesity on minimally invasive partial nephrectomy: Urology, 2013; 82(3); 612-18
24. Rosen DC, Kannappan M, Kim Y, The impact of obesity in patients undergoing robotic partial nephrectomy: J Endourol, 2019; 33(6); 431-37
25. Tewari N, Awad S, Macdonald IA, Obesity-related insulin resistance: Implications for the surgical patient: Int J Obes (Lond), 2015; 39(11); 1575-88
26. Khandekar MJ, Cohen P, Spiegelman BM, Molecular mechanisms of cancer development in obesity: Nat Rev Cancer, 2011; 11(12); 886-95
27. WHO: Physical status: The use of and interpretation of anthropometry, report of a WHO expert committee, 1995 Available from: http://apps.who.int/iris/bitstream/handle/10665/37003
28. Kott O, Golijanin B, Pereira JF, The BMI paradox and robotic assisted partial nephrectomy: Front Surg, 2019; 6; 74
29. Nesbitt K, Sharma P, Visceral fat is associated with high-grade complications in patients undergoing minimally invasive partial nephrectomy for small renal masses: Curr Urol, 2021; 15(1); 52-58
30. Maurits JSF, Sedelaar JPM, Aben KKH, The association of body composition with postoperative complications and length of hospital stay after radical or partial nephrectomy in patients with renal cell cancer: A multicenter population-based cohort study: Transl Androl Urol, 2022; 11(12); 1667-79
31. Zhu Y, Wang HK, Zhang HL, Visceral obesity and risk of high grade disease in clinical t1a renal cell carcinoma: J Urol, 2013; 189(2); 447-53
32. Linehan WM, Srinivasan R, Schmidt LS, The genetic basis of kidney cancer: A metabolic disease: Nat Rev Urol, 2010; 7(5); 277-85
33. Yao Y, Xu Y, Gu L, The Mayo adhesive probability score predicts longer dissection time during laparoscopic partial nephrectomy: J Endourol, 2020; 34(5); 594-99
Figures
 Figure 1. Preoperative Axial CT images of 2 different patients at the level of umbilicus used for evaluation of visceral obesity (represented). The green area indicated by the red arrow is the VFA. (A) Visceral obesity by VFA (B) Non-visceral obesity by VFA. CT – computed tomography; VFA – visceral fat area. Medcare software (AnyPACS2.0).
Figure 1. Preoperative Axial CT images of 2 different patients at the level of umbilicus used for evaluation of visceral obesity (represented). The green area indicated by the red arrow is the VFA. (A) Visceral obesity by VFA (B) Non-visceral obesity by VFA. CT – computed tomography; VFA – visceral fat area. Medcare software (AnyPACS2.0). Figure 2. Bean plots of operation time under different BMI/VFA cut-offs. (A) Operation time in retroperitoneal approach at different VFA cut-offs. (B) Operation time in retroperitoneal approach at different BMI cut-offs. (C) Operation time in transperitoneal approach at different VFA cut-offs. (D) Operation time in transperitoneal approach at different BMI cut-offs. The P values at the top of each graph represent the Mann-Whitney U test results. Thick bars indicate medians of operation time and thin bars show the frequency of them. VFA – visceral fat area; BMI – body mass index. (R language, version R 4.2.3).
Figure 2. Bean plots of operation time under different BMI/VFA cut-offs. (A) Operation time in retroperitoneal approach at different VFA cut-offs. (B) Operation time in retroperitoneal approach at different BMI cut-offs. (C) Operation time in transperitoneal approach at different VFA cut-offs. (D) Operation time in transperitoneal approach at different BMI cut-offs. The P values at the top of each graph represent the Mann-Whitney U test results. Thick bars indicate medians of operation time and thin bars show the frequency of them. VFA – visceral fat area; BMI – body mass index. (R language, version R 4.2.3). Figure 3. Bean plots of EBL under different BMI/VFA cut-offs. (A) EBL in retroperitoneal approach at different VFA cut-offs. (B) EBL in retroperitoneal approach at different BMI cut-offs. (C) EBL in transperitoneal approach at different VFA cut-offs. (D) EBL in transperitoneal approach at different BMI cut-offs. The p values at the top of each graph represent the Mann-Whitney U test results. Thick bars indicate medians of operation time and thin bars show the frequency of them. VFA – visceral fat area; BMI – body mass index; EBL – estimated blood loss. (R language, version R 4.2.3).
Figure 3. Bean plots of EBL under different BMI/VFA cut-offs. (A) EBL in retroperitoneal approach at different VFA cut-offs. (B) EBL in retroperitoneal approach at different BMI cut-offs. (C) EBL in transperitoneal approach at different VFA cut-offs. (D) EBL in transperitoneal approach at different BMI cut-offs. The p values at the top of each graph represent the Mann-Whitney U test results. Thick bars indicate medians of operation time and thin bars show the frequency of them. VFA – visceral fat area; BMI – body mass index; EBL – estimated blood loss. (R language, version R 4.2.3). Tables
 Table 1. Demographic and clinicopathological parameters between visceral obese and non-obese by VFA.
Table 1. Demographic and clinicopathological parameters between visceral obese and non-obese by VFA. Table 2. Demographic and clinicopathological parameters between general obese and non-obese by BMI.
Table 2. Demographic and clinicopathological parameters between general obese and non-obese by BMI. Table 3. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (retroperitoneal approach).
Table 3. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (retroperitoneal approach). Table 4. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (transperitoneal approach).
Table 4. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (transperitoneal approach). Table 5. Comparison of intraoperative complication between obese and non-obese patients by VFA/BMI.
Table 5. Comparison of intraoperative complication between obese and non-obese patients by VFA/BMI. Table 1. Demographic and clinicopathological parameters between visceral obese and non-obese by VFA.
Table 1. Demographic and clinicopathological parameters between visceral obese and non-obese by VFA. Table 2. Demographic and clinicopathological parameters between general obese and non-obese by BMI.
Table 2. Demographic and clinicopathological parameters between general obese and non-obese by BMI. Table 3. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (retroperitoneal approach).
Table 3. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (retroperitoneal approach). Table 4. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (transperitoneal approach).
Table 4. Comparison of intraoperative measures between obese and non-obese by VFA/BMI (transperitoneal approach). Table 5. Comparison of intraoperative complication between obese and non-obese patients by VFA/BMI.
Table 5. Comparison of intraoperative complication between obese and non-obese patients by VFA/BMI. In Press
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








