10 December 2023: Clinical Research
Impact of Internal Carotid Stenosis Treatment on Cerebral Blood Flow Volume: A Comparative Study between Preoperative and Postoperative Values
Piotr KaszczewskiDOI: 10.12659/MSM.941958
Med Sci Monit 2023; 29:e941958
Abstract
BACKGROUND: Among patients with ICA stenosis, there are some cases with elevated, undisrupted, and diminished cerebral blood flow (CBF). The aim of this study was to assess the influence of ICA stenosis treatment on postoperative CBF changes in relation to preoperative CBF values.
MATERIAL AND METHODS: We qualified 58 patients ≥65 years old (28 males, 30 females, mean age 71.02±6.34 years) for surgical intervention due to symptomatic ≥70% ICA stenosis. In all patients, a flow volume in all extracranial arteries (internal carotid [ICA], external carotid [ECA], and vertebral arteries [VA]) was measured preoperatively and 2-3 days following the surgery. The CBF values were compared with the ones established for a healthy population of the same age.
RESULTS: Preoperatively, there were 3 subgroups of patients, comparing to healthy population: with elevated CBF – “significant compensation,” with undisrupted CBF – “mild compensation,” and with diminished CBF – “no compensation.” Postoperatively, a significant CBF increase was observed in patients with preoperative “no” and “mild compensation” – 277.18±154.26 ml/min (P=0.0000001) and 221.56±98.8 ml/min (P=0.0000001). In a “significant compensation” group, there was no flow increase observed (CBF change of 2.57±58.5 ml/min, P=0.954) – a redistribution of flow was observed.
CONCLUSIONS: In patients with lower preoperative CBF values, surgical treatment caused a significant increase in global cerebral inflow, which was more prominent in patients with the lowest preoperative CBF. In patients with high preoperative CBF, surgical treatment resulted in a flow redistribution, but did not cause a CBF increase. Volumetric flow assessment in DUS can predict hemodynamic benefit from surgery in terms of CBF increase.
Keywords: Carotid Arteries, Carotid Artery Diseases, carotid stenosis, Endarterectomy, Carotid, ischemic stroke, Ultrasonography, Doppler, Ultrasonography, Doppler, Duplex
Background
Atherosclerotic lesions of carotid arteries are one of the leading causes of ischemic stroke and transient ischemic attack (TIA), accounting for up to 20% of cases [1,2].
Surgical treatment reduces the risk of ipsilateral stroke and is recommended for patients with 70–99% symptomatic ICA stenosis and is suggested for symptomatic patients with 50–69% ICA stenosis, as well as for patients with 60–99% asymptomatic carotid stenosis who are at increased risk of stroke on best medical treatment (BMT) alone [1].
Diagnostic modalities including magnetic resonance imaging (MRI), computed tomography (CT), or Doppler ultrasonography (DUS) allow us to identify features associated with increased risk of neurological symptoms in asymptomatic patients [2].
Cerebral blood flow (CBF) correlates with cerebrovascular reserve (CVR) and with the risk of forthcoming ischemic events regardless of other risk factors or testing method [3]. The risk of occurrence of ischemic symptoms (including stroke), their severity, and clinical outcomes depend on the collateral circulation [4–7].
Cerebral hemodynamic is related to neurocognitive functioning in patients with ICA lesions and with its improvement following carotid revascularization [8,9].
Recently, a series of studies evaluating flow volume in extracranial arteries in healthy population and patients with carotid lesions have been published [10–15]. The reference CBF values for healthy population >65 years old were proposed. Among patients with significant ICA stenosis and occlusion, 3 subgroups with significant flow differences were identified, depending on the development of collateral circulation and flow increase in other extracranial arteries: patients with significant flow volume increase in other extracranial arteries resulting in CBF higher than in healthy equally aged population – “significant compensation,” patients with mild increase of the flow in other extracranial arteries resulting in CBF similar to healthy population – “mild compensation,” and patients without flow increase in other extracranial arteries, with diminished CBF – “no compensation” [10–15].
CBF values are correlated with the risk of occurrence of ischemic symptoms. CBF may be altered by the presence of the ICA stenosis and increases after revascularization [10–15].
In this study we present a novel approach to carotid stenosis diagnostics with DUS – a global cerebral inflow assessment. It combines the measurements of ICA stenosis degree and flow volume in extracranial arteries, which allows for evaluation of the compensatory mechanisms and collateral circulation.
The aim of this study was to examine the association between postoperative CBF increase in relation to preoperative CBF values in patients who underwent carotid endarterectomy due to >70% symptomatic ICA stenosis.
Material and Methods
BIOETHICS COMMITTEE APPROVAL:
All procedures were approved by the Medical University of Warsaw Bioethics Committee (approval no. AKBE/1239/2018).
REFERENCE CBF AND FLOW VOLUME VALUES IN EXTRACRANIAL ARTERIES:
Reference values established by our team on the group of healthy volunteers over 65 years old and published in 2020 are presented in Table 1 (adapted from a previous publication [11]).
STUDY DESIGN:
The prospective study was conducted in 2022. Study participants were symptomatic patients referred to the Department of General, Vascular, Endocrine, and Transplant Surgery with the diagnosis of significant ICA stenosis, with the narrowing exceeding 70%. After confirming the initial diagnosis with a second ultrasound examination conducted by an experienced sonographer from our department, the patients were qualified for surgical treatment.
Since we describe and propose a new method of carotid ultrasound examination in the assessment of the ICA stenosis based on volumetry, a maximally homogenous study group was enrolled. Strict inclusion and exclusion criteria were created to eliminate the potential influence of concomitant disorders on the cerebral perfusion and obtained results.
CBF MEASUREMENT:
In patients qualified for surgical treatment, meeting the inclusion and exclusion criteria, ultrasound examination with volumetric flow assessment in extracranial arteries was performed before the surgery and 2–3 days following surgical treatment. They were conducted according to the protocol, which is described in detail (eg, patient position and preparation) in the previous study published by our team [11].
CBF was assessed as a grand total of the flow volumes bilaterally in ICAs, external carotid arteries (ECA), and vertebral arteries (VA). Flow volume was measured in the ECA distally to the origin of the superior thyroid artery (STA). Flow volume in the common carotid artery (CCA) was measured as a control of measurement accuracy – when CCA flow volume slightly exceeded sum of flow volumes in the ICA and ECA, the measurement was considered accurate.
The diameter of each vessel (inner-to-inner) was measured 3 times with 3 different imaging modalities: B-mode, Superb Microvascular Imaging (SMI), and combined B-mode/SMI. The average of 3 measurements was considered the vessel diameter. The flow volume in the vessel was calculated from vessel diameter and spectral Doppler using the ultrasound scanner’s semiautomatic program. Volumetric calculation was repeated 2–3 times for each artery and their average was considered the flow volume in the artery.
To maintain high accuracy and eliminate interobserver variability, volumetric measurements were conducted by the same sonographer (authors initials: PK), using a Canon Aplio i800 ultrasound scanner with Linear i11LX3 transducer (Canon Medical Systems Corporation, Otawara, Tochigi, Japan).
STUDY GROUP:
There were 58 patients (28 male, 30 female) aged ≥65 years old (mean age 71.02±6.34 years) qualified to surgical intervention: carotid endarterectomy (CEA) or carotid artery stenting (CAS) due to symptomatic ≥70% ICA stenosis. All patients were carefully assessed to exclude pathologies that could potentially influence cerebral perfusion.
In this study, patients with preoperative CBF values exceeding the proposed reference values (Table 1) are referred to as having “significant compensation.” Patients with CBF within reference range are referred to as having “mild compensation,” and patients with CBF lower than the proposed reference range are referred as having “no compensation.”
STATISTICAL ANALYSIS:
Statistical analysis was performed with Statistica 13 (StatSoft Polska Sp. z o.o., Cracow, Poland). For the comparison of the 2 groups, the
For multiple groups, the sets of data in which all variables were normally distributed were analyzed with one-way analysis of variance. When the data did not follow a Gaussian distribution, the non-parametric Kruskal-Wallis one-way analysis of variance test was performed. The statistical significance was established with post hoc tests. The significance level in all tests was <0.05.
Results
PREOPERATIVE CBF VALUES:
In the study group there were:
POSTOPERATIVE CBF CHANGES IN COMPARISON TO PREOPERATIVE CBF AND COMPENSATORY STATUS:
In 2 out of 3 subgroups (“no compensation” and “mild compensation”) the statistically significant flow increase was observed:
In patients with preoperative significant compensation the surgery did not cause significant CBF changes (preoperatively 1115.50±112.89 ml/min, postoperatively 1118.10±121.01 ml/min, CBF change of 2.57±58.5 ml/min),
The differences in the CBF increase were statistically significant between:
The difference in the CBF increase between “mild compensation” and “no compensation” group (277.18±154.26 ml/min vs 221.56±98.80 ml/min) did not reach statistical significance, p=0.303. The postoperative CBF changes are presented in the Figure 1A–1C.
COMPARISON OF THE FLOW PATHWAYS BEFORE AND AFTER THE SURGERY:
The flow pathways, and postoperative flow changes in extracranial arteries are shown in the Figure 2 (2A – “no compensation group”, 2B – “mild compensation group”, 2C – “significant compensation” group) and in Table 2.
SIDE TO SIDE FLOW COMPARISON BEFORE AND AFTER THE SURGERY:
In the ipsilateral side, to postoperative flow increase was observed in:
The flow increase in the ipsilateral side was mainly caused by the significant flow increase in the revascularized ICA.
Carotid revascularization slightly improved the flow on the contralateral side in:
Postoperative flow increase was not statistically significant, however in both groups it was close to statistical significance.
In “significant compensation” group no significant differences were noticed on both sides pre- and postoperatively. On the ipsilateral side a non-significant flow increase was observed (from 506.14 ml/min to 563.28 ml/min, P=0.16 – Figure 3E) while a simultaneous decline was noticed on the contralateral side (from 609.36 ml/min to 576.14 ml/min, P=0.42 – Figure 3F).
NUMBER OF ARTERIES WITH COMPENSATORY INCREASED FLOW VOLUME BEFORE AND AFTER THE SURGERY:
Similar tendencies were observed in “no” and “mild compensation” groups.
In both groups the number of arteries with elevated flow increased significantly after the surgery, while a simultaneous decrease in the number of arteries with decreased flow was observed. The number of arteries with flow within reference range remained relatively unchanged.
In “significant compensation group” a number of arteries with decreased flow changed from 0.71 to 0.14 (P=0.016). There was slight, but non-significant increase in the number of arteries with the flow similar to reference values (from 1.86 to 2.36, P=0.257), and with increased flow (from 3.43 to 3.5, P=0.875). The data are presented in the Table 3 and Figure 4. This resulted in the flow redistribution – there was no change in CBF, while the flow in ipsilateral ICA, and ipsilateral side increased and was accompanied with slight decrease on the contralateral side (see results section: Side to side flow comparison before and after the surgery and Figure 3).
Discussion
Collateral circulation, which allows maintaining CBF and CVR, is a key factor facilitating undisrupted brain function. Improvement of cognitive performance was observed after carotid endarterectomy (CEA) in patients with TIA and ipsilateral high-grade ICA stenosis, who initially had decreased values of CVR. The improvement was correlated inversely with age and preoperative CVR values [8,9].
Yamane et al proved that CEA can increase CBF and improve CVR in patients with low CBF or low CVR by restoring blood flow through the ICA [16].
The efficiency of collateral circulation correlates with the risk of forthcoming ischemic symptoms (including stroke), their severity, and clinical outcomes of treatment and rehabilitation [4–7]. In patients with well-developed collateral circulation receiving BMT (best medical therapy), the risk of ischemic stroke was lower than in the group without collateralization (6.3% versus 13.3% for disabling or fatal stroke; 11.3% versus 27.8% for hemispheric stroke) [17].
The method presented in this article allows for indirect assessment of collateral circulation. Patients with high preoperative CBF, caused by significant flow increase in non-stenosed extracranial arteries have well-developed collateral circulation, while patients with low CBF values have poor collateralization.
In our research, in the groups with preoperative “no compensation” and “mild compensation,” the CEA resulted in both ipsilateral and contralateral flow volume increases. Several authors have also reported changes in cerebral circulation following carotid stenosis treatment.
A postoperative increase in CBF on the contralateral side after CAS or CEA has been reported in patients with >50% contralateral ICA stenosis and is presumed to be related with anterior communicating artery increased flow [18–20]. Sadato et al reported that following CAS there was an increase in hemispheric CBF on both the operated and contralateral sides independent of age, postoperative hyperperfusion, cerebral steal syndrome after preoperative acetazolamide-challenge SPECT, and morphology of the anterior communicating artery [21]. It may be related to leptomeningeal anastomoses – the increase of the flow on the previously stenotic side reduces the need for collateralization increasing contralateral flow. Another explanation may be transhemispheric diaschisis, which can cause CBF reduction on the non-stenotic side. Recovery from the diaschisis after revascularization may result in CBF increase [21,22].
Shimada et al reported reduction in hypoxic neural tissue in 1-(2–18F-fluoro-1-[hydroxymethyl]ethoxy) methyl-2-nitroimidazole (18F-FRP170) positron emission tomography (PET), which is associated with cognitive improvement in patients after carotid revascularization [23].
Yoshida et al examined changes in glucose metabolism in brain tissues in PET with (18)F-fluorodeoxyglucose (FDG). Following carotid revascularization, they observed improvement in cognitive functions, correlated with increased glucose metabolism. Such improvement was not observed in patients who experienced cerebral hyperperfusion [24]. Oka et al reported a long-term increase in CBF and CVR and its normalization in patients following carotid artery stenting due to near occlusion [25].
In our study we were able to show that there was a different hemodynamic response, in terms of CBF increase, after carotid stenosis treatment in symptomatic patients, which was strongly correlated with preoperative CBF values and compensatory status. We found that in “no- compensation” patients with lowest preoperative CBF, the postoperative flow volume increase was most prominent – these patients benefit hemodynamically most from CEA. In patients with “mild compensation,” who had preoperatively CBF similar to the healthy population, the postoperative flow increase was less prominent, but was still significant, and there was hemodynamic benefit from surgery. In these 2 groups the CEA results not only in ipsilateral flow increase, but also in contralateral flow improvement. The flow increase was statistically significant in “no compensation” both on the ipsilateral and contralateral sides. In the “mild compensation group” the flow increase was close to statistical significance on both sides, but did not reach it; however, the total CBF increase was statistically significant.
In patients with “significant compensation,” who had the highest CBF values before the surgery, meaning that the central nervous system was well supplied with blood, there was no flow volume increase after the surgery. This may indicate that in patients with high CBF values, the collateral circulation was well developed and provided adequate blood supply to the brain, despite the stenosis. After CEA there was no need for collateralization and the blood supply could be provided with the physiological pathways, which is why redistribution of flow was observed.
In the significant compensation group the flow redistribution was most prominent in the ipsilateral ICA and ipsilateral ECA. Before surgery there was an elevated flow in the ipsilateral ECA. The external carotid artery is an important collateral circulation pathway in case of ICA stenosis or occlusion – the flow in the ECA can significantly change in this situation, and the resistance may decrease and the flow velocity may increase. In the DUS examination such flow changes in the ECA are described as internalization of the ECA. Following the surgery, when the physiological blood supply pathway is regained, there is no need for further collateralization, which is why the increase of flow in the revascularized ICA was accompanied by the simultaneous decrease of flow in the ipsilateral ECA. This flow pattern has been previously reported by Russel et al [26].
Similar flow changes may be observed in the contralateral ECA, which is also an important collateral pathway, and after revascularization of the ICA the flow in this artery may decrease. However, these flow changes seem to depend on the development of collateral circulation, preoperative flow volume, and compensatory status. In the “significant compensation” and “mild compensation” groups a decrease in ipsilateral ECA flow postoperatively was observed. In the “no compensation” group the flow volume increased in ipsilateral ECA, contralateral ECA, and both ICA following revascularization.
Examples of the flow changes are shown in Figures 5 and 6.
Our study has several limitations. It was a single-center study; therefore, the sample size was relatively smaller than in multicenter randomized studies. This number of patients does not allow for comparison of different age groups, sexes, or revascularization methods (CAS versus CEA), especially between the groups of patients at 5-year intervals (as it is in the reference values). The clarity of our results indicates for the need for multicenter cooperation on this issue.
Our method has, however, several advantages. Almost all studies in the medical literature examining CBF changes are based on expensive and invasive methods, with limited accessibility, reserved for large reference medical centers using PET and SPECT. We used ultrasonography, which is cheap, easily accessible, and non-invasive, and allows assessing preoperative CBF values and measuring the postoperative flow increase. It also facilitates predicting who will benefit from surgery in terms of flow increase and who will not. This may help in deciding which asymptomatic should be selected and which will benefit from carotid revascularization.
Our exclusion and inclusion criteria were strict and it may seem difficult for this study to be generalizable to a larger population. We would like to stress that our criteria aimed to exclude patients in whom results might be biased by concomitant disorders and that NASCET and ACST trials, on which current guidelines are based, used much more severe patient and hospital selection criteria [27].
The lower CBF values are 2 times more frequent among symptomatic patients and significantly correlate with the risk of forthcoming ischemic symptoms [10–15].
The patients with lower CBF may have higher risk of developing ischemic symptoms, and lower CBF may help provide earlier surgical intervention in such patients.
We hope that our results will provide crucial information in diagnostics and decision making in patients with ICA lesions and doubtful indications for surgical treatment. This may also play an important role in asymptomatic patients – lower values of CBF may become one of the factors (like hypoechoic plaques, plaque ulceration, and microembolization in transcranial Doppler) promoting surgical intervention, while increased CBF may indicate the need of BMT.
The biggest novelty of our method is the ability to predict with high confidence who will benefit from carotid endarterectomy and how large the CBF increase will be. It is the first study proposing such a method, which has never been described previously.
Conclusions
ICA stenosis treatment improves cerebral hemodynamics, resulting in CBF increase, especially in patients with lower preoperative values, while in patients with high preoperative CBF it causes only flow redistribution, without flow volume increase. Volumetric flow assessment in DUS can predict haemodynamic benefit from the surgery in terms of flow increase.
Figures
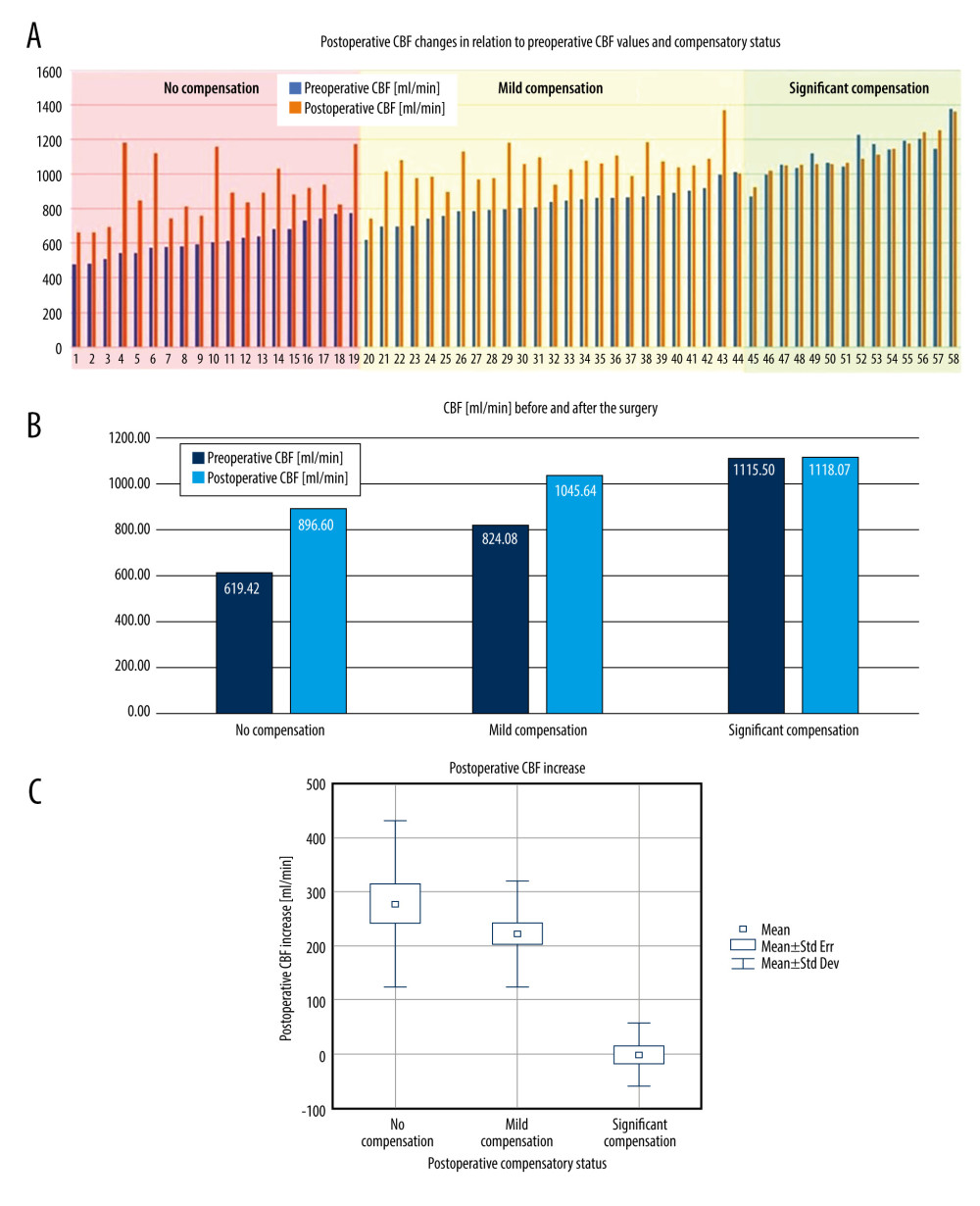 Figure 1. A – pre- and postoperative CBF comparison in each patient in the study group. B – average pre- and postoperative CBF comparison in “no compensation,” “mild compensation” and “significant compensation” groups. C – comparison of the postoperative CBF increase in “no compensation,” “mild compensation” and “significant compensation” groups.
Figure 1. A – pre- and postoperative CBF comparison in each patient in the study group. B – average pre- and postoperative CBF comparison in “no compensation,” “mild compensation” and “significant compensation” groups. C – comparison of the postoperative CBF increase in “no compensation,” “mild compensation” and “significant compensation” groups. 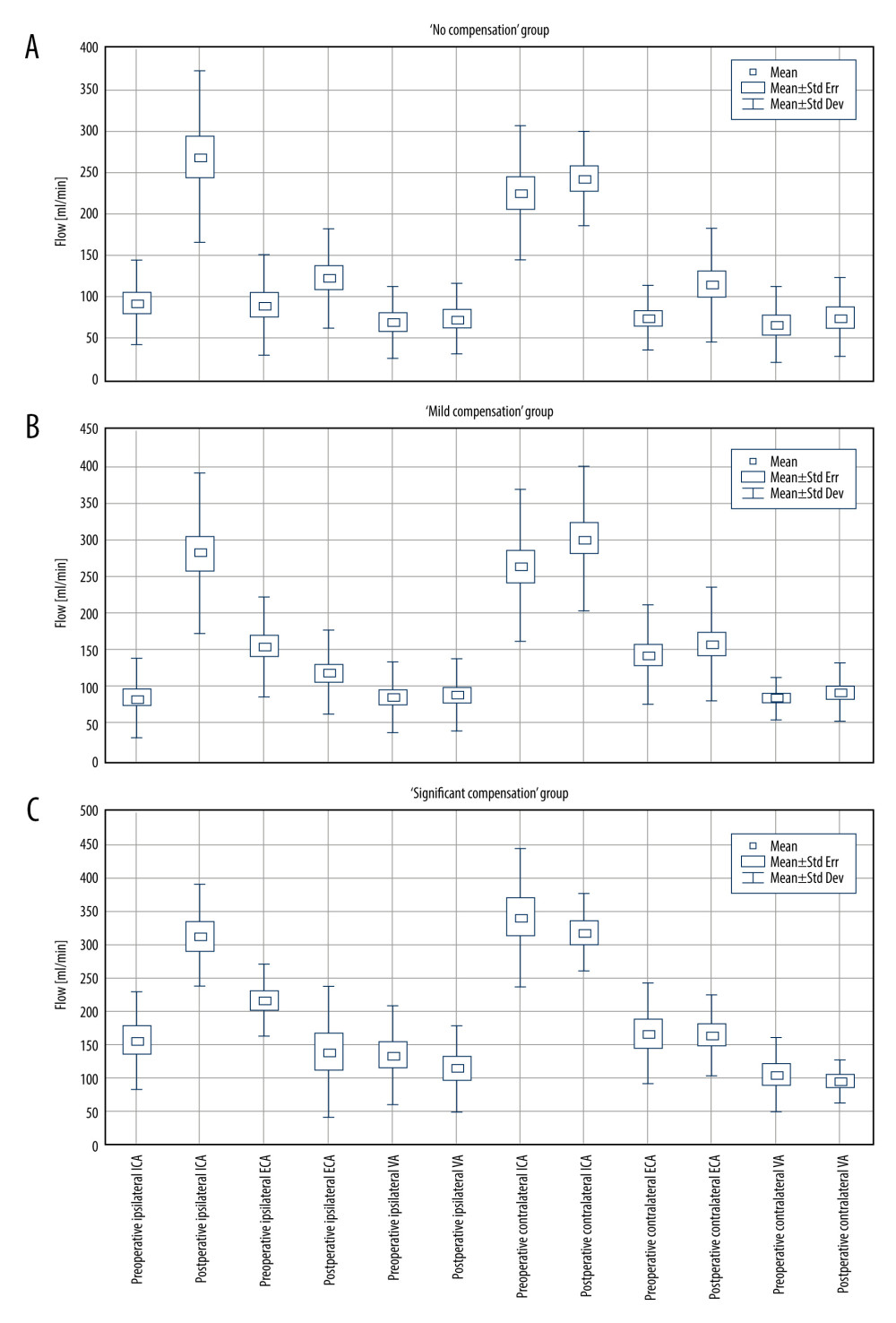 Figure 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries. A – “no compensation group,” B – “mild compensation group,” C – “significant compensation” group.
Figure 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries. A – “no compensation group,” B – “mild compensation group,” C – “significant compensation” group. 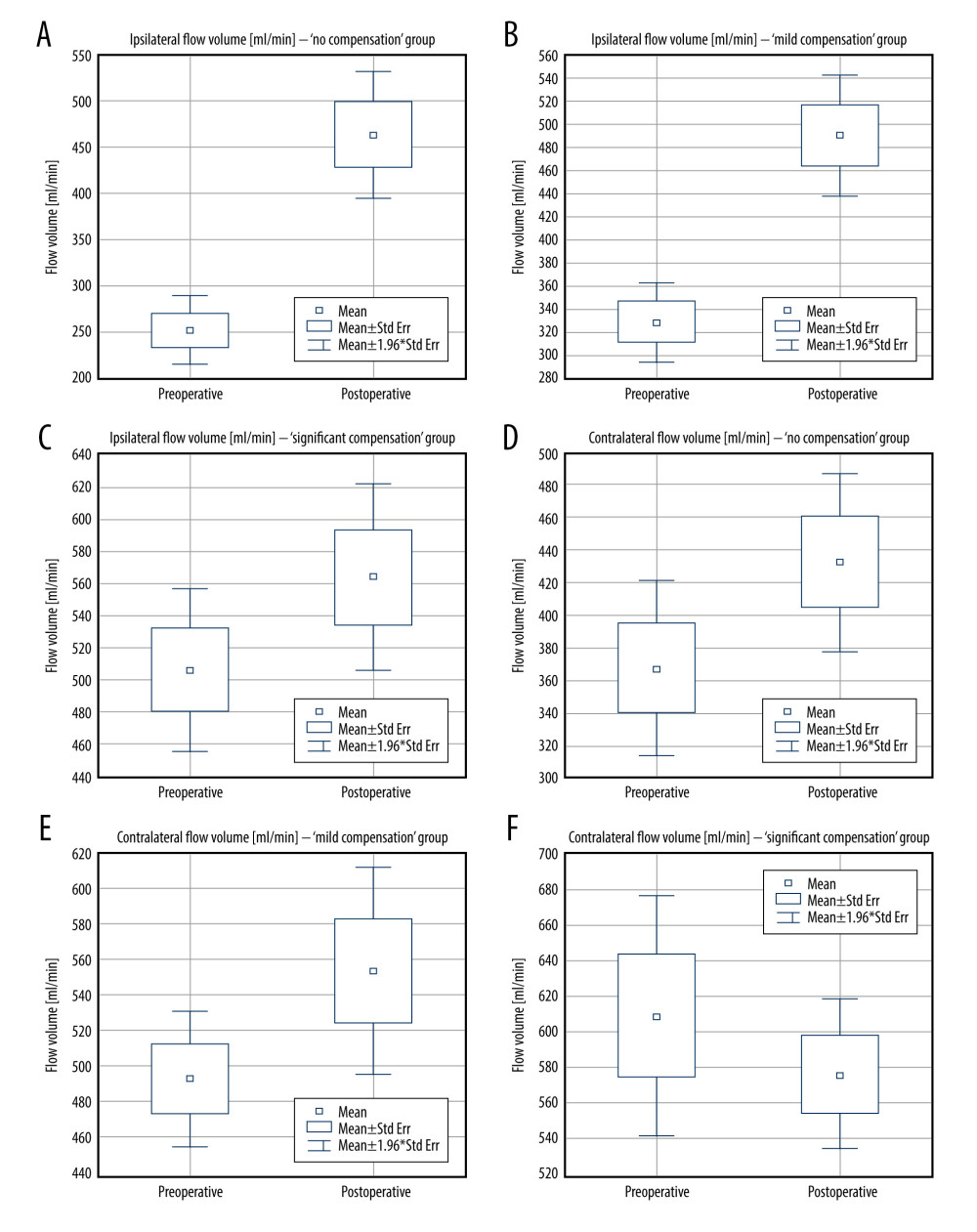 Figure 3. Pre- and postoperative flow volume changes in the ipsi- and contralateral side. “No compensation” group: ipsilateral side – A, contralateral side – B. “Mild compensation group: ipsilateral side – C, contralateral side – D. “Significant compensation” group: ipsilateral side – E, contralateral side – F.
Figure 3. Pre- and postoperative flow volume changes in the ipsi- and contralateral side. “No compensation” group: ipsilateral side – A, contralateral side – B. “Mild compensation group: ipsilateral side – C, contralateral side – D. “Significant compensation” group: ipsilateral side – E, contralateral side – F. 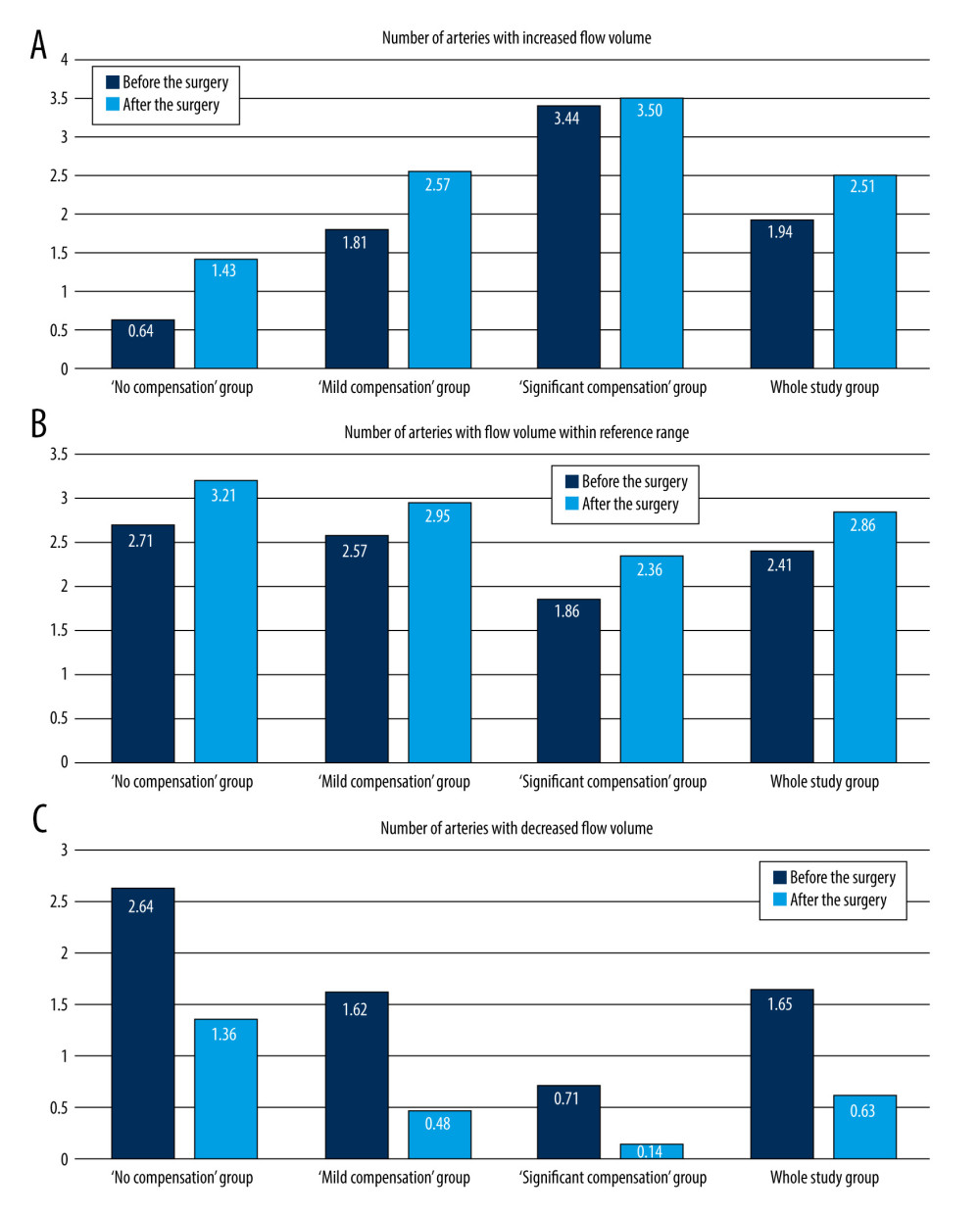 Figure 4. Changes in the average number of arteries with increased flow volume, flow volume within reference range, and decreased flow volume in patients with “no compensation,” “mild compensation, and “significant compensation.”
Figure 4. Changes in the average number of arteries with increased flow volume, flow volume within reference range, and decreased flow volume in patients with “no compensation,” “mild compensation, and “significant compensation.” 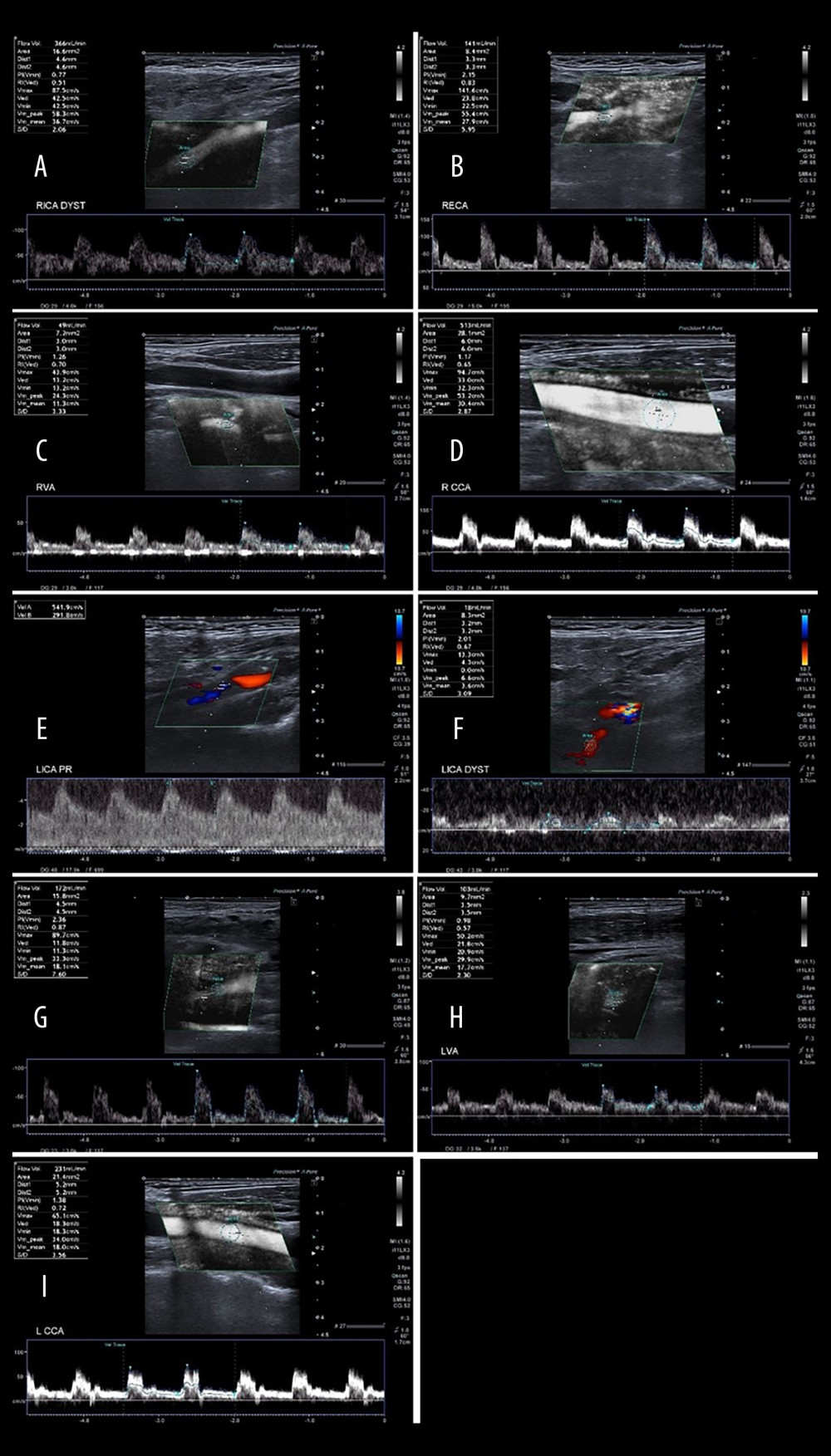 Figure 5. A 65-year-old patient with severe left ICA stenosis. Preoperative measurements: A – RICA flow 366 ml/min, B – RECA – flow 141 ml/min, C – RVA – flow 49 ml/min, D – RCCA flow 513 ml/min, E – LICA stenosis with flow velocity 5.42/2.92 m/s, F – distal part of the LICA with flow reduction 18 ml/min, G – LECA flow 172 ml/min, H – LVA flow 103 ml/min, I – LCCA flow 231 ml/min. Total preoperative CBF of 849 ml/min.
Figure 5. A 65-year-old patient with severe left ICA stenosis. Preoperative measurements: A – RICA flow 366 ml/min, B – RECA – flow 141 ml/min, C – RVA – flow 49 ml/min, D – RCCA flow 513 ml/min, E – LICA stenosis with flow velocity 5.42/2.92 m/s, F – distal part of the LICA with flow reduction 18 ml/min, G – LECA flow 172 ml/min, H – LVA flow 103 ml/min, I – LCCA flow 231 ml/min. Total preoperative CBF of 849 ml/min. 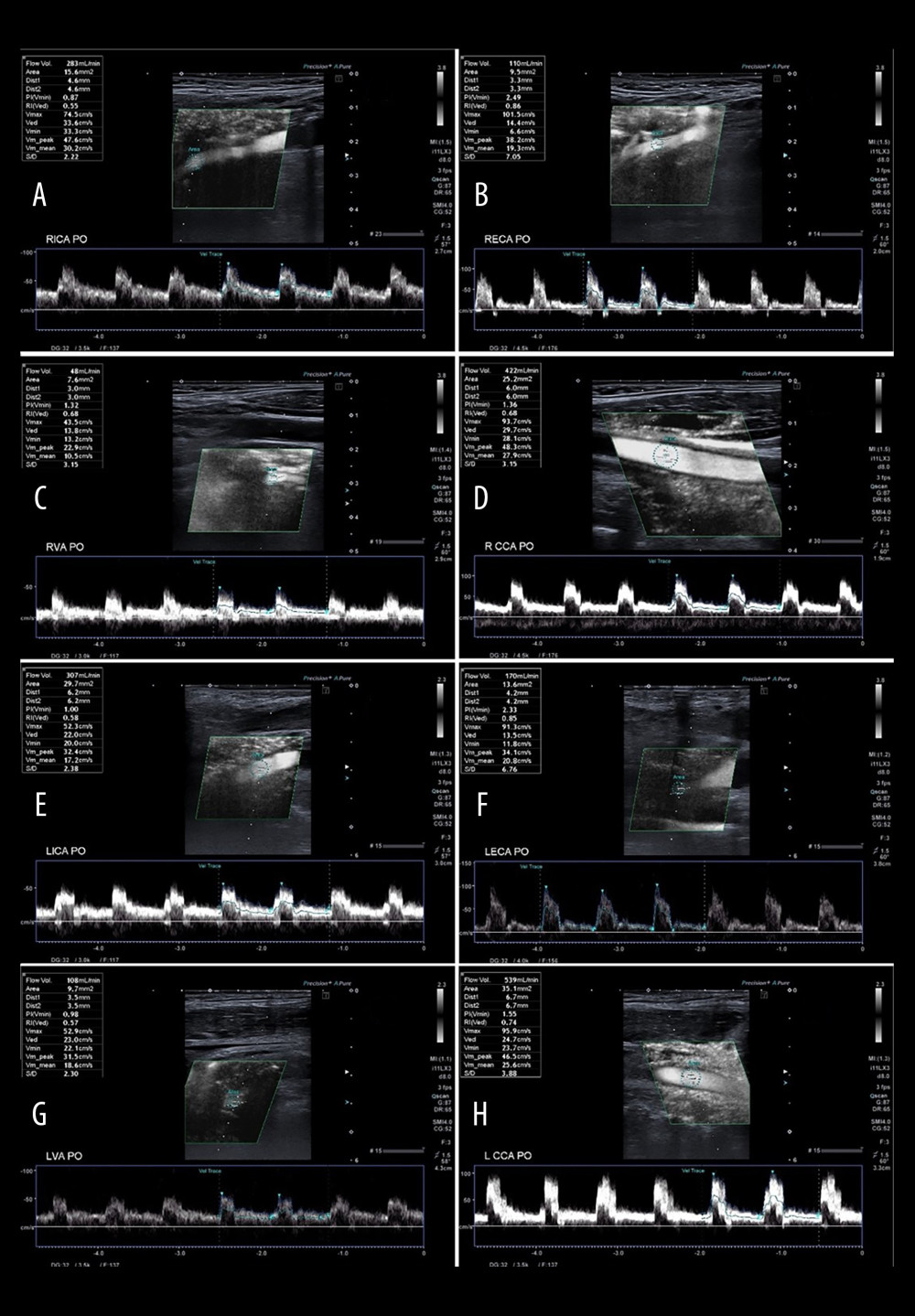 Figure 6. A 65-year-old patient 3 days after successful endarterectomy of the left ICA. A – RICA flow 283 ml/min, B – RECA – flow 110 ml/min, C – RVA – flow 48 ml/min, D – RCCA flow 422 ml/min, E – LICA after EA flow 307 ml/min, F – LECA flow 170 ml/min, G – LVA flow 108 ml/min, H – LCCA flow 539 ml/min. Total postoperative CBF of 1026 ml/min.
Figure 6. A 65-year-old patient 3 days after successful endarterectomy of the left ICA. A – RICA flow 283 ml/min, B – RECA – flow 110 ml/min, C – RVA – flow 48 ml/min, D – RCCA flow 422 ml/min, E – LICA after EA flow 307 ml/min, F – LECA flow 170 ml/min, G – LVA flow 108 ml/min, H – LCCA flow 539 ml/min. Total postoperative CBF of 1026 ml/min. Tables
Table 1. Reference values of the CBF and the flow volume in extracranial arteries.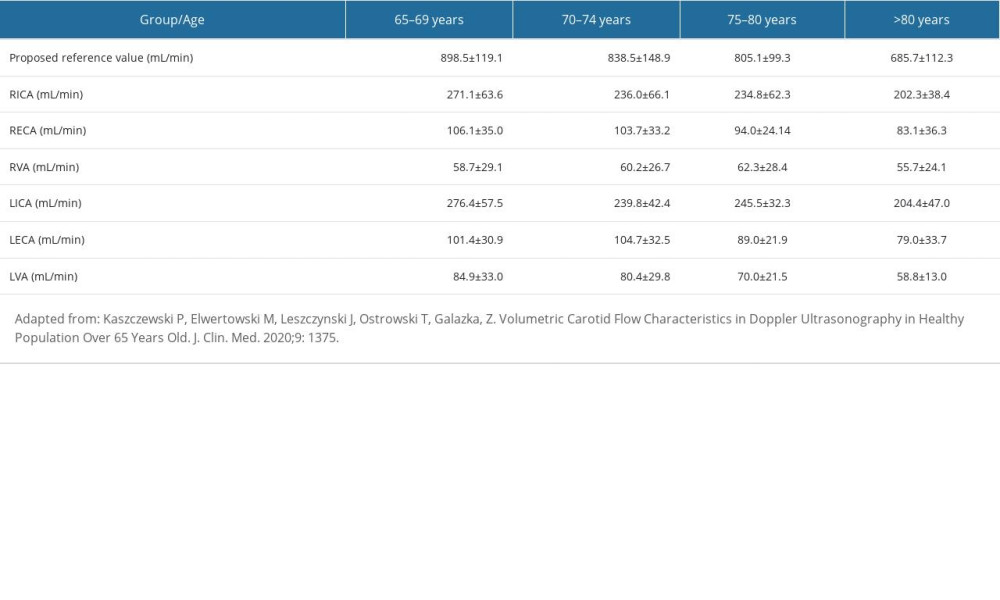 Table 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries in the groups with different preoperative compensatory status.
Table 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries in the groups with different preoperative compensatory status.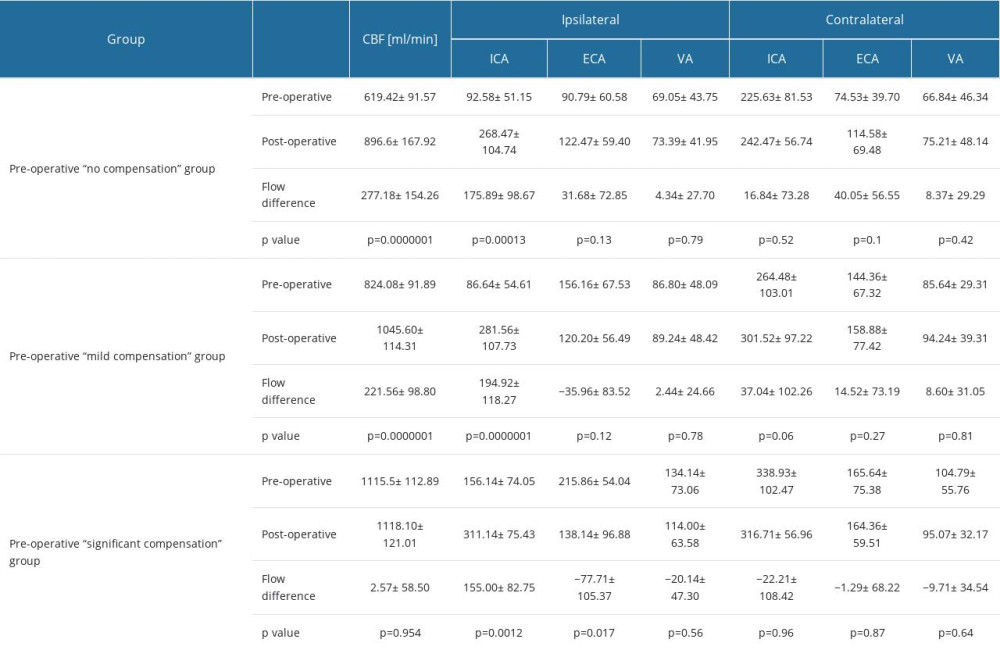 Table 3. Changes in the average number of arteries with increased flow volume, flow volume within reference range and decreased flow volume in patients with “no compensation,” “mild compensation: and “significant compensation.”
Table 3. Changes in the average number of arteries with increased flow volume, flow volume within reference range and decreased flow volume in patients with “no compensation,” “mild compensation: and “significant compensation.”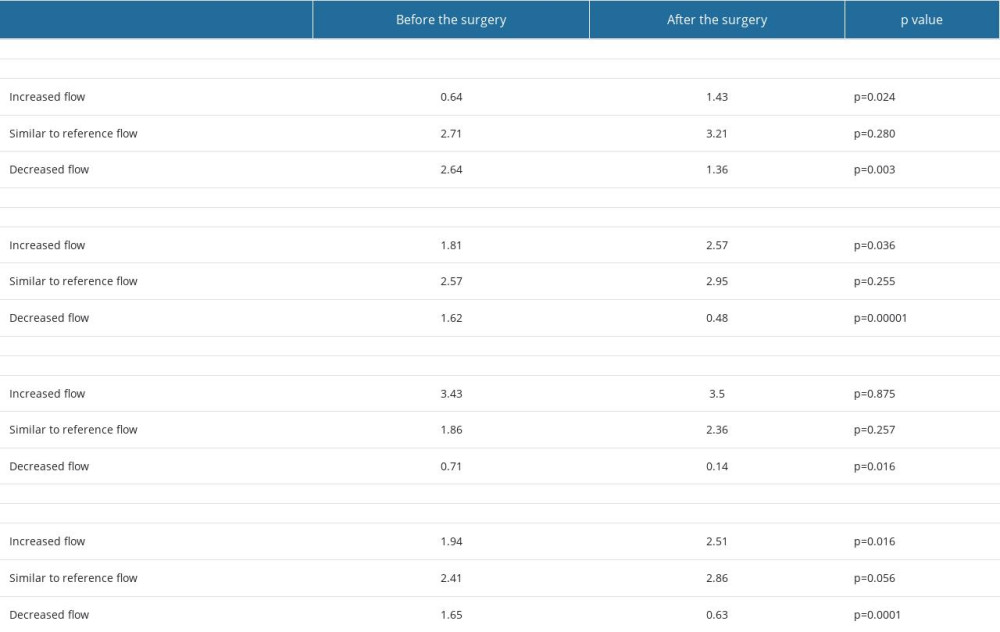
References
1. Bonati LH, Kakkos S, Berkefeld J, European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis: Eur Stroke J, 2021; 6(2); I-XLVII
2. Aboyans V, Ricco JB, Bartelink MEL, Editor’s choice – 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Eur J Vasc Endovasc Surg, 2018; 55(3); 305-68
3. Gupta A, Chazen J, Hartman M, Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis: Stroke, 2012; 43(11); 2884-91
4. Sobczyk O, Sam K, Mandell DM, Cerebrovascular reactivity assays collateral function in carotid stenosis: Front Physiol, 2020; 11; 1031
5. Bang OY, Saver JL, Buck BH, Impact of collateral flow on tissue fate in acute ischaemic stroke: J Neurol Neurosurg Psychiatry, 2008; 79; 625-29
6. Sheth SA, Sanossian N, Hao Q, Starkman S, Collateral flow as causative of good outcomes in endovascular stroke therapy: J NeuroInterv Surg, 2014; 8; 2-7
7. Tan B, Wan-Yee K, Paliwal P, Good Intracranial Collaterals Trump Poor ASPECTS (Alberta Stroke Program Early CT Score) for intravenous thrombolysis in anterior circulation acute ischemic stroke: Stroke, 2016; 47; 2292-98
8. Lattanzi S, Carbonari L, Pagliariccio G, Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy: Neurology, 2018; 90; e307-e15
9. Lattanzi S, Carbonari L, Pagliariccio G, Predictors of cognitive functioning after carotid revascularization: J Neurol Sci, 2019; 405; 116435
10. Elwertowski M, Leszczyński J, Kaszczewski P, The importance of blood flow volume in the brain-supplying arteries for the clinical management – the impact of collateral circulation: J Ultrason, 2018; 18; 112-19
11. Kaszczewski P, Elwertowski M, Leszczynski J, Volumetric carotid flow characteristics in Doppler iltrasonography in healthy population over 65 years old: J Clin Med, 2020; 9; 1375
12. Leszczyński J, Kaszczewski P, Elwertowski M, Volumetric flow changes in extracranial arteries in a symptomatic patient with significant bilateral carotid artery stenosis: A case study and literature review: Am J Case Rep, 2020; 21; e927202
13. Kaszczewski P, Elwertowski M, Leszczyński J, Intracranial flow volume estimation in patients with internal carotid artery occlusion: Diagnostics, 2022; 12; 766
14. Kaszczewski P, Elwertowski M, Leszczyński J, Volumetric flow assessment in doppler ultrasonography in risk stratification of patients with in-ternal carotid stenosis and occlusion: J Clin Med, 2022; 11; 531
15. Kaszczewski P, Elwertowski M, Leszczyński J, Volumetric flow assessment in extracranial arteries in patients with 70–99% internal carotid artery stenosis: Diagnostics, 2022; 12; 2216
16. Yamane K, Shima T, Nishida M, Changes in cerebral blood flow after carotid endarterectomy: Keio J Med, 2000; 49(Suppl 1); A80-A82
17. Henderson RD, Eliasziw M, Fox AJ, Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group: Stroke, 2000; 31; 128-32
18. Oka F, Ishihara H, Kato S, Cerebral hemodynamic benefits after contralateral carotid artery stenting in patients with internal carotid artery occlusion: Am J Neuroradiol, 2013; 34; 616-21
19. Baracchini C, Meneghetti G, Manara R, Cerebral hemodynamics after contralateral carotid endarterectomy in patients with symptomatic and asymptomatic carotid occlusion: A 10-year follow-up: J Cereb Blood Flow Metab, 2006; 26; 899-905
20. Kataoka T, Hyogo T, Hayase K, Nakamura H, Cerebral blood flow change before and after carotid angioplasty and stenting (CAS) in cases with contralateral carotid artery occlusion: Interv Neuroradiol, 2006; 12; 201-4
21. Sadato A, Maeda S, Hayakawa M, Carotid stenting for unilateral stenosis can increase contralateral hemispheric cerebral blood flow: J Neurointerv Surg, 2018; 10(4); 351-54
22. Lagrèze HL, Levine RL, Pedula KL, Contralateral flow reduction in unilateral stroke: Evidence for transhemispheric diaschisis: Stroke, 1987; 18; 882-26
23. Shimada Y, Kobayashi M, Yoshida K, Reduced hypoxic tissue and cognitive improvement after revascularization surgery for chronic cerebral ischemia: Cerebrovasc Dis, 2019; 47(1–2); 57-64
24. Yoshida K, Ogasawara K, Saura H, Post-carotid endarterectomy changes in cerebral glucose metabolism on (18)F-fluorodeoxyglucose positron emission tomography associated with postoperative improvement or impairment in cognitive function: J Neurosurg, 2015; 123(6); 1546-54
25. Oka F, Ishihara H, Kato S, Cerebral hemodynamic benefits after carotid artery stenting in patients with near occlusion: J Vasc Surg, 2013; 58(6); 1512-17
26. Russel D, Dybevold S, Kjartansson O, Cerebral vasoreactivity and blood flow before and 3 months after carotid endarterectomy: Stroke, 1990; 21; 1029-32
27. Wennberg DE, Lucas FL, Birkmeyer JD, Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics: JAMA, 1998; 279(16); 1278-81
Figures
 Figure 1. A – pre- and postoperative CBF comparison in each patient in the study group. B – average pre- and postoperative CBF comparison in “no compensation,” “mild compensation” and “significant compensation” groups. C – comparison of the postoperative CBF increase in “no compensation,” “mild compensation” and “significant compensation” groups.
Figure 1. A – pre- and postoperative CBF comparison in each patient in the study group. B – average pre- and postoperative CBF comparison in “no compensation,” “mild compensation” and “significant compensation” groups. C – comparison of the postoperative CBF increase in “no compensation,” “mild compensation” and “significant compensation” groups. Figure 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries. A – “no compensation group,” B – “mild compensation group,” C – “significant compensation” group.
Figure 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries. A – “no compensation group,” B – “mild compensation group,” C – “significant compensation” group. Figure 3. Pre- and postoperative flow volume changes in the ipsi- and contralateral side. “No compensation” group: ipsilateral side – A, contralateral side – B. “Mild compensation group: ipsilateral side – C, contralateral side – D. “Significant compensation” group: ipsilateral side – E, contralateral side – F.
Figure 3. Pre- and postoperative flow volume changes in the ipsi- and contralateral side. “No compensation” group: ipsilateral side – A, contralateral side – B. “Mild compensation group: ipsilateral side – C, contralateral side – D. “Significant compensation” group: ipsilateral side – E, contralateral side – F. Figure 4. Changes in the average number of arteries with increased flow volume, flow volume within reference range, and decreased flow volume in patients with “no compensation,” “mild compensation, and “significant compensation.”
Figure 4. Changes in the average number of arteries with increased flow volume, flow volume within reference range, and decreased flow volume in patients with “no compensation,” “mild compensation, and “significant compensation.” Figure 5. A 65-year-old patient with severe left ICA stenosis. Preoperative measurements: A – RICA flow 366 ml/min, B – RECA – flow 141 ml/min, C – RVA – flow 49 ml/min, D – RCCA flow 513 ml/min, E – LICA stenosis with flow velocity 5.42/2.92 m/s, F – distal part of the LICA with flow reduction 18 ml/min, G – LECA flow 172 ml/min, H – LVA flow 103 ml/min, I – LCCA flow 231 ml/min. Total preoperative CBF of 849 ml/min.
Figure 5. A 65-year-old patient with severe left ICA stenosis. Preoperative measurements: A – RICA flow 366 ml/min, B – RECA – flow 141 ml/min, C – RVA – flow 49 ml/min, D – RCCA flow 513 ml/min, E – LICA stenosis with flow velocity 5.42/2.92 m/s, F – distal part of the LICA with flow reduction 18 ml/min, G – LECA flow 172 ml/min, H – LVA flow 103 ml/min, I – LCCA flow 231 ml/min. Total preoperative CBF of 849 ml/min. Figure 6. A 65-year-old patient 3 days after successful endarterectomy of the left ICA. A – RICA flow 283 ml/min, B – RECA – flow 110 ml/min, C – RVA – flow 48 ml/min, D – RCCA flow 422 ml/min, E – LICA after EA flow 307 ml/min, F – LECA flow 170 ml/min, G – LVA flow 108 ml/min, H – LCCA flow 539 ml/min. Total postoperative CBF of 1026 ml/min.
Figure 6. A 65-year-old patient 3 days after successful endarterectomy of the left ICA. A – RICA flow 283 ml/min, B – RECA – flow 110 ml/min, C – RVA – flow 48 ml/min, D – RCCA flow 422 ml/min, E – LICA after EA flow 307 ml/min, F – LECA flow 170 ml/min, G – LVA flow 108 ml/min, H – LCCA flow 539 ml/min. Total postoperative CBF of 1026 ml/min. Tables
 Table 1. Reference values of the CBF and the flow volume in extracranial arteries.
Table 1. Reference values of the CBF and the flow volume in extracranial arteries. Table 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries in the groups with different preoperative compensatory status.
Table 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries in the groups with different preoperative compensatory status. Table 3. Changes in the average number of arteries with increased flow volume, flow volume within reference range and decreased flow volume in patients with “no compensation,” “mild compensation: and “significant compensation.”
Table 3. Changes in the average number of arteries with increased flow volume, flow volume within reference range and decreased flow volume in patients with “no compensation,” “mild compensation: and “significant compensation.” Table 1. Reference values of the CBF and the flow volume in extracranial arteries.
Table 1. Reference values of the CBF and the flow volume in extracranial arteries. Table 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries in the groups with different preoperative compensatory status.
Table 2. Pre- and postoperative flow volume comparison in the ipsi- and contralateral extracranial arteries in the groups with different preoperative compensatory status. Table 3. Changes in the average number of arteries with increased flow volume, flow volume within reference range and decreased flow volume in patients with “no compensation,” “mild compensation: and “significant compensation.”
Table 3. Changes in the average number of arteries with increased flow volume, flow volume within reference range and decreased flow volume in patients with “no compensation,” “mild compensation: and “significant compensation.” In Press
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








