01 May 2024: Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Patients with Interstitial Lung Diseases Undergoing Transbronchial Cryobiopsy: A Randomized Trial
Liya Lu1AB*, Xiaobo Chen2EF, Shiyue Li2F, Yanyi Cen1CDDOI: 10.12659/MSM.942773
Med Sci Monit 2024; 30:e942773
Abstract
BACKGROUND: While many studies have been conducted on sugammadex sodium and neostigmine in patients undergoing general anesthesia, few have explored their effects in patients with interstitial lung diseases (ILDs).
MATERIAL AND METHODS: Sixty-three patients who underwent transbronchial cryobiopsy under general anesthesia were enrolled in a prospective randomized study. The patients were randomly divided into 2 groups: neostigmine combined with atropine group (group C, n=32) and sugammadex group (group S, n=31). Induction and maintenance of anesthesia were the same in both groups. Patients received rocuronium during anesthesia. At the end of the procedure, when the T2 of the train-of-four stimulation technique (TOF) monitoring appeared, neostigmine 0.04 mg/kg combined with atropine 0.02 mg/kg was injected intravenously in group C, and sodium sugammadex 2 mg/kg was injected intravenously in group S. Time from administration of muscle relaxant antagonist to recovery of TOF ratio (TOFr) to 0.9 and extubation time were recorded. The residual rate of neuromuscular blockade at 1, 3, 5, 7, and 10 min after extubation was calculated.
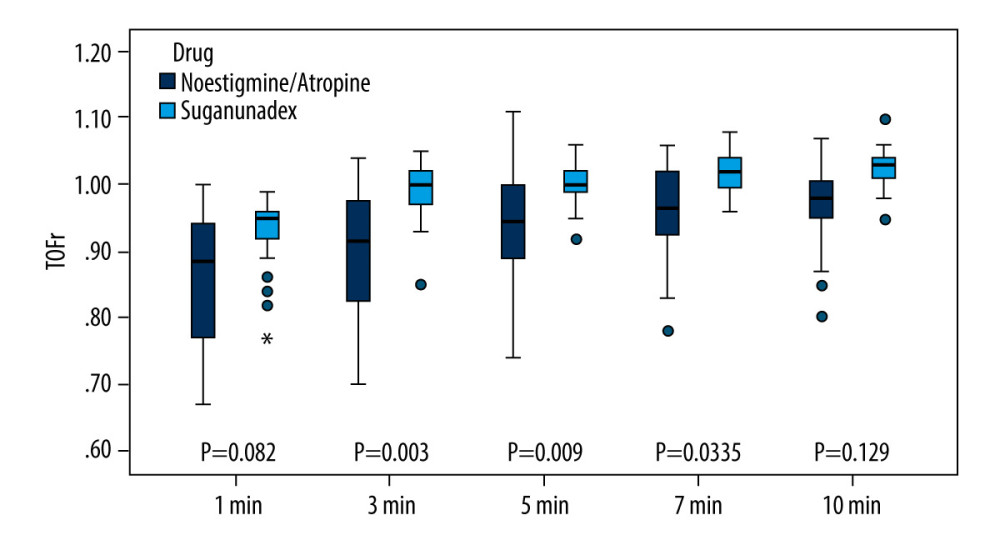
RESULTS: Compared to group C, group S had a significantly shorter recovery time of TOFr to 0.9 (4.0[2.0] min vs 14.0[11.0] min, P<0.001) and extubation time (4.0[3.0] min vs 11.0[7.0] min, P<0.001). The residual rate of neuromuscular blockade was remarkably lower in group S than in group C at 3, 5, and 7 min after extubation (3.2% vs 31%, 0% vs 25%, 0% vs 6%, P<0.05).
CONCLUSIONS: Sugammadex is more effective than neostigmine in reversing the muscle-relaxant effect of rocuronium bromide in patients with ILDs.
Keywords: Delayed Emergence from Anesthesia, sugammadex
Introduction
Interstitial lung diseases (ILDs) are a prevalent group of clinical lung diseases with a high mortality rate. Early diagnosis is crucial for optimal prognosis. In recent years, transbronchial cryobiopsy (TBCB) has become the main diagnostic technique for ILDs. Due to hypoxemia, most patients with ILDs experience progressive lung function decline during exacerbation of the disease. It is widely believed that patients with poorer preoperative lung function are more susceptible to postoperative pulmonary complications (PPCs) [1].
ILDs are characteristic of inflammation and interstitial fibrosis in the alveolar units. With the progression of the disease, patients experience decreased lung function and high mortality rates. Early diagnosis and timely treatment are crucial for improving the prognosis and symptoms of patients with ILDs. In recent years, TBCB has emerged as the primary diagnostic technique for interstitial lung diseases. Some studies have demonstrated the efficacy and safety of TBCB in diagnosing interstitial lung diseases [2]. However, due to the larger size of tissue specimens obtained from TBCB, there is an increased risk of potential bleeding and pneumothorax. Therefore, the current international expert consensus recommends performing TBCB under general anesthesia, with tracheal intubation [3].
Most patients with ILDs have hypoxemia, progressive dyspnea, and reduced diffusion function leading to restrictive ventilatory impairment. In a meta-analysis comprising 15 studies with 994 patients, the incidence of respiratory failure after TBCB was reported as 0.4% [2]. The prevention of postoperative complications in patients with ILDs, such as respiratory failure, pulmonary infection, and pulmonary atelectasis, is a crucial clinical concern. Several risk factors, including age, American Society of Anesthesiologists (ASA) classification, body mass index, history of respiratory tract infection, and chronic obstructive pulmonary disease, can affect the development of PPCs. Patients with poorer preoperative pulmonary function, coupled with procedure, anesthesia, and inadequate postoperative respiratory recovery, have an increased likelihood of developing PPCs after procedures [1,4].
Muscle relaxation residuals have been found to be closely associated with PPCs. These residuals weaken the pharyngeal and respiratory muscles, reduce the efficacy of coughing, and increase the risk of hypoxemia, pulmonary atelectasis, and pulmonary infections. Residual perioperative muscle relaxation, often defined by a train-of-four ratio (TOFr) <0.9, is a well-known anesthesia-related risk factor for PPCs. Tracheal extubation with a TOFr <0.9 is associated with increased occurrences of hypoxia, upper airway obstruction, oxygen desaturation, microaspiration, and the need for re-intubation [5]. Clinical studies have revealed that residual perioperative muscle relaxation incidence is significant, and even a slight residual of neuromuscular blockade (TOFr≤0.9) has been shown to impair peripheral chemoreflex of the carotid body and respiratory muscle strength, thus imposing a significant impact on lung function [6,7]. In fact, a TOFr of 0.9 does not indicate complete recovery. At this point, approximately 75% to 80% of nicotinic receptors can still be blocked, and patients can exhibit signs of neuromuscular (or chemical) imbalance, especially in high-risk patients. A prospective study in Europe on PPCs has shown that a TOFr >0.95 before extubation, as compared to TOFr >0.9, reduces the occurrence of PPCs [8], indicating that a TOFr of 0.9 does not necessarily equate to complete neuromuscular or chemical recovery. Therefore, residual muscle relaxation is the primary cause of PPCs, and rapid and effective reversal of muscle relaxation is key to reducing PPCs.
Currently, neuromuscular blockade antagonists used in general anesthesia are classified into 2 groups: non-selective neuromuscular blockade antagonists (cholinesterase inhibitors), such as neostigmine, and the new selective neuromuscular blockade antagonist, sugammadex sodium, a gamma-cyclodextrin drug that reverses non-depolarizing neuromuscular blockade induced by aminosteroids, particularly rocuronium [9].
Compared with neostigmine, sugammadex sodium better reduces residual neuromuscular blockade and thus dramatically decreases the incidence of consequential PPCs, particularly in critically ill patients [5,9]. While many studies have been conducted on sugammadex sodium and neostigmine in patients undergoing general anesthesia, only a few have explored their effects in patients with ILDs. Therefore, this study aimed to investigate the impact of sugammadex sodium on postoperative neuromuscular blockade recovery in patients with ILDs undergoing TBCB.
Material and Methods
GENERAL INFORMATION:
This was a randomized, assessor-blinded trial. The study was approved by the Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (Medical Research and Development Ethics Audit No.164, 2020) and was registered (
BLINDING:
Before the procedure, patients were randomly assigned to 1 of 2 groups: group C (neostigmine combined with atropine), and group S (sugammadex sodium). Patient grouping was accomplished using an opaque envelope method. The envelopes, prepared by a blinded outcomes assessor, adhered to a 1: 1 randomization ratio and were opened by the investigator only after patient enrollment. The anesthesiologist was aware of the study drug allocation, but the patients and independent outcomes assessors were not.
GROUPING AND TREATMENT:
The patients included in the study were randomly assigned to the 2 groups: group C, which received neostigmine combined with atropine, and group S, which received sugammadex sodium. At the end of the procedure, when the postoperative train-of-four stimulation (TOF) count reached 2, group C received intravenous neostigmine at a dose of 0.04 mg/kg combined with atropine at a dose of 0.02 mg/kg, while group S received intravenous sugammadex sodium at a dose of 2 mg/kg.
ANESTHESIA METHODS:
Patients were pre-oxygenated for 5 min via a nasal cannula, with an oxygen flow rate of 4 to 5 min/L. Both groups underwent tracheal intubation under general anesthesia. Intravenous access was established prior to admission, and subsequent monitoring included tracking the 5-lead ECG, heart rate, mean arterial pressure, peripheral oxygen saturation (SpO2), bispectral index, and body surface temperature of thenar eminence. Dexmedetomidine (0.2 μg/kg for ≥5 min) was intravenously infused 15 min before anesthesia induction, followed by induction with intravenous sufentanil (0.1 μg/kg) and propofol (1–3 μg/mL plasma target-controlled infusion), allowing for a bispectral index decrease to 40 to 60. After connecting the muscle relaxation monitoring device, the patient’s arm was secured in abduction on the arm rest, with a displacement sensor installed on the thumb and index finger, and the remaining 3 fingers were properly immobilized using adhesive tape. The adductor pollicis muscle’s TOF was monitored at a frequency of 2 Hz, pulse width of 0.2 ms, current intensity of 60 mA, and stimulation interval of 0.5 s.
A series of stimuli was administered at 20-s intervals. After completing neuromuscular blockade monitoring, intravenous injection of rocuronium bromide was performed at a dose of 0.45 mg/kg, with no additional muscle relaxant administered during the procedure. Subsequently, volume-controlled mechanical ventilation was initiated, using a tidal volume of 6 to 8 mL/kg, respiratory rate of 12 to 16 breaths per min, inspiratory to expiratory ratio of 1: 2, fraction of inspired oxygen (FiO2) of 60%, and end-tidal CO2 (PETCO2) of 35 to 45 mmHg. Total intravenous anesthesia was maintained throughout the procedure with a continuous infusion of propofol (1–3 μg/mL) and remifentanil (0.05–0.2 μg/kg/min), with infusion rates adjusted to maintain a bispectral index level of 45 to 60. Vasoactive drugs were administered based on changes in blood pressure and heart rate.
Propofol and remifentanil infusions were maintained to achieve bispectral index levels between 60 and 80 at the end of the procedure. Muscle relaxation reversal agents were administered in both groups upon the appearance of T2 on the muscle relaxation monitoring. Administration of all anesthetic drugs was ceased when the patient’s TOFr recovered to 0.75, while mechanical ventilation was continued for respiratory support. The tracheal tube was removed once the patient regained consciousness, exhibited an active swallowing reflex, had strong limb movement, and ventilation volume returned to preoperative levels. Following extubation, patients were observed for 10 min to ensure stabilization of heart rate, mean arterial pressure, and SpO2 prior to transfer to the post-anesthesia care unit (PACU). During the PACU stay, nasal oxygen was administered at a rate of 2 L/min.
OBSERVATION PARAMETERS:
The study documented the mean arterial pressure and heart rate at various time points: T0 (immediately before tracheal intubation), T1 (end of the procedure), T2 (immediately after extubation), T3 (3 min after extubation), T4 (5 min after extubation), and T5 (10 min after extubation). The time intervals from the end of the procedure to T2, from administration of neuromuscular blockade antagonist to TOFr 0.9, and from administration of neuromuscular blockade antagonist to extubation were also recorded. Residual muscle relaxation was recorded at 1, 3, 5, 7, and 10 min after extubation, with TOFr <0.9 indicating residual muscle relaxation. The study also recorded the incidence of hypoxemia (SpO2 of 90% to 93%) during the PACU stay. The occurrence of adverse reactions, such as postoperative nausea and vomiting (PONV), skin pruritus, and allergies, was recorded. Finally, the incidence of pulmonary complications, including aspiration, pulmonary atelectasis, pulmonary infection, and respiratory failure, at 7 consecutive days after the procedure was monitored and documented.
STATISTICAL ANALYSIS:
Data analysis was performed using SPSS 23.0 software. Normally distributed data were expressed as mean±standard deviation and analyzed using an independent samples
The sample size was calculated according to the authors’ preliminary study; 30 patients in each group would have an 80% power to detect a difference time of TOFr≤0.9 between the 2 groups using 2-sided analysis with an error of 0.05.The investigators aimed to enroll 33 patients for both groups in case of missing data.
Results
Sixty-eight patients were enrolled in the study, with 3 cases in group C and 2 cases in group S being excluded from the analysis due to intraoperative pneumothorax. Therefore, a total of 63 patients were included in the study, with 32 patients in group C and 31 patients in group S. There were no statistically significant differences between the 2 groups in terms of sex, age, body mass index, ASA classification, preoperative lung function, and blood gas analysis (Table 1).
Results showed no statistically significant differences in the length of procedure and time of T2 appearance between the 2 groups. However, group S demonstrated significantly shorter time of recovery of TOFr to 0.9 (4.0[2.0] min vs 14.0[11.0] min,
There were no significant differences in heart rate and mean arterial pressure at T0, T1, T2, T3, T4, and T5 between the 2 groups. Likewise, there were no statistically significant differences in the incidence of PONV, skin pruritus, or allergy between the 2 groups (
The incidence of mild hypoxia during the PACU stay was slightly higher in group C than in group S (
Discussion
Currently, the commonly used neuromuscular blockade antagonist, neostigmine, requires a longer time for TOFr to recover to 0.9. In the present study, residual neuromuscular blockade was still observed within 10 min after extubation in the neostigmine group, which is consistent with the findings of Nemes et al [10]. This indicates that the use of neostigmine for neuromuscular blockade reversal cannot eradicate recurrent residual blockade in the short term. Sugammadex, an improved cyclodextrin molecule, forms complexes with rocuronium or vecuronium in the plasma, rendering them inactive and dissociating them from acetylcholine receptors, thereby facilitating rapid restoration of normal receptor function [11]. A study demonstrated that sugammadex reverses rocuronium-induced neuromuscular blockade significantly faster than does neostigmine and is associated with lower PaCO2 levels [12]. When used in tracheal intubation and interventional procedures, sugammadex can rapidly eliminate residual muscle relaxation effects. Several meta-analyses have also reported that sugammadex sodium is associated with a lower incidence of residual muscle relaxation with respiratory events than is neostigmine [13–15]. The results of our study confirmed these advantages, showing that the time to recovery of TOFr to 0.9 and the time to extubation were shorter in the sugammadex group than in the neostigmine group. Moreover, the residual rate of neuromuscular blockade was significantly lower 3 to 7 min after extubation in the sugammadex group, indicating a reduced risk of postoperative residual muscle relaxation and faster recovery after procedure.
Sugammadex sodium logically prevents the development of pulmonary atelectasis by improving the electromyographic activity of the diaphragm and intercostal muscles, which leads to higher tidal volumes and improved clearance of secretions, compared with neostigmine [16]. According to a multicenter study [17], sugammadex sodium reduces the risk of pulmonary complications by 30%, risk of pneumonia by 47%, and risk of respiratory failure by 55%, compared with neostigmine. However, the evidence from various studies on the effect of different neuromuscular blockade antagonists on PPCs is controversial, with some studies suggesting that there is no reduction with the use of different neuromuscular blockade antagonists [10,18,19].
The occurrence of PPCs can be attributed to multiple factors, encompassing patient-related aspects, such as their overall health status, age, preoperative lung function, nature and duration of the procedure, perioperative opioid usage, and variations in anesthesia techniques. For example, a retrospective study published in 2021 [20] on the association of sugammadex sodium and neostigmine with PPCs revealed no statistically significant difference in the incidence of PPCs between the 2 drugs. Moreover, it was observed that the overall occurrence of PPCs declined progressively over time, with an adjusted annual reduction rate of 0.91, which could be attributed to the extensive duration of data collection spanning 10 years. Additionally, the implementation of various quality improvement measures, such as enhanced postoperative recovery protocols, use of TOF monitoring, and the establishment of objective criteria for diagnosing ventilator-associated pneumonia, played a role in diminishing the occurrence of PPCs. A retrospective observational study revealed that the incidence of PPCs did not significantly differ between patients administered sugammadex and neostigmine during femur fracture repair under general anesthesia [21]. There are several factors contributing to this outcome. First, femoral fracture repair surgeries pose a high risk of PPCs, due to the substantial number of elderly patients and the potential for embolism. Second, the retrospective nature of the study may have influenced the results, as there may have been a tendency to prefer sugammadex for high-risk patients or those expected to experience PPCs upon emerging from anesthesia. Moreover, the results could have been impacted by various uncontrolled influencing factors, when compared with prospective studies.
The incidence of PPCs due to multiple perioperative causes is approximately 5% [17]. Our study focused on patients with relatively poor pulmonary function and hypothesized that sugammadex sodium would reduce the incidence of residual postoperative neuromuscular blockade and therefore reduce PPCs in such patients. However, in this study, the incidence of hypoxia during PACU stay was slightly higher in the neostigmine group than in the sugammadex sodium group. One case of pulmonary atelectasis occurred in the neostigmine group, whereas none occurred in the sugammadex sodium group, although the difference was not statistically significant. Recent research has indicated that sugammadex is linked to a decrease in PPCs among patients with respiratory dysfunction undergoing laparoscopic gastric or intestinal surgery [4]. This could be attributed to the use of deep neuromuscular blockade, which enhanced surgical conditions and prolonged procedure duration. The limited sample size of certain patients might account for these negative outcomes, emphasizing the need for a comprehensive prospective study to delineate the variations in clinical outcomes between the 2 groups.
Our study had several limitations. First, it was conducted at a single center with a small sample size, which can limit its generalizability to the wider population. A larger sample size may have yielded more conclusive clinical results. Second, our study focused only on the management of realistic conditions, and did not consider other factors that can contribute to PPCs, such as opioid dosage and fluid therapy [22]. Therefore, further well-designed, large-scale prospective studies are needed to gain a comprehensive understanding of PPCs following procedures.
Nausea and vomiting are additional concerns in this context. Initially, acetylcholinesterase inhibitors were not recommended, due to their association with increased PONV. While studies have explored the effects of sugammadex and neostigmine on PONV, existing evidence indicates that sugammadex can reduce the occurrence of PONV; however, these findings did not reach statistical significance in a meta-analysis [15]. Our study revealed no significant difference in PONV incidence between the 2 groups; however, it is essential to note that PONV risk is influenced by various factors, such as age, history of diabetes, and type of surgery [23,24]. Therefore, further research is warranted to determine any potential association between sugammadex and neostigmine and PONV.
Conclusions
Sugammadex demonstrated superior efficacy compared with neostigmine in reversing the muscle-relaxant effects of rocuronium bromide in patients with ILDs who underwent TBCB. Furthermore, it effectively decreased the occurrence of residual muscle relaxation.
References
1. Sabaté S, Mazo V, Canet J, Predicting postoperative pulmonary complications: Implications for outcomes and costs: Curr Opin Anaesthesiol, 2014; 27; 201-9
2. Ravaglia C, Bonifazi M, Wells AU, Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: A comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature: Respiration, 2016; 91(3); 215-27
3. Hetzel J, Maldonado F, Ravaglia C, Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: Expert statement from the Cryobiopsy Working Group on safety and utility and a call for standardization of the procedure: Respiration, 2018; 95; 188-200
4. Ji Y, Yuan H, Chen Y, Sugammadex is associated with reduced pulmonary complications in patients with respiratory dysfunction: J Surg Res, 2023; 290; 133-4
5. Abad-Grumeta A, Kipolles-Melchor J, Casans-France R, A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade: Anaesthesia, 2015; 70; 1441-52
6. Kumar GV, Nair AP, Murthy HS, Residual neuromuscular blockade affects postoperative pulmonary function: Anesthesiology, 2012; 117; 1234
7. Broens SJL, Boon M, Martini CH, Reversal of partial neuromuscular block and the ventilatory response to hypoxia: A randomized controlled trial in healthy volunteers: Anesthesiology, 2019; 131; 467-76
8. Blobner M, Hunter JM, Meistelman C, Hoeft A, Use of a train-of-four ratio of 0.95 versus 0.9 for tracheal extubation: An exploratory analysis of Br J Anaesth, 2020; 124; 63-72
9. Ledowski T, Falke L, Johnston F, Retrospective investigation of postoperative outcome after reversal of residual neuromuscular blockade: Sugammadex: Anaesthesiology, 2014; 31; 423-29
10. Nemes R, Fülesdi B, Pongracz A, Impact of reversal strategies on the incidence of postoperative residual paralysis after rocuronium relaxation without neuromuscular monitoring: A partially randomised placebo controlled trial: Eur J Anaesthesiol, 2017; 34; 609-16
11. Bailey CR, Sugammadex: When should we be giving it?: Anaesthesia, 2017; 72; 1170-75
12. Kogler J, Chalfe N, Karaman Ilić M, Hodoba N, Sugammadex reversal of rocuronium-induced neuromuscular block in interventional bronchoscopy: Eur J Anaesthesiol, 2012; 29; 146
13. Hristovska AM, Duch P, Allingstrup M, Afshari A, The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta-analysis and trial sequential analysis: Anaesthesia, 2018; 73; 631-41
14. Carron M, Zarantonello F, Tellaroli P, Ori C, Efficacy and safety of sugammadex compared to neostigmine for reversal of neuromuscular blockade: A meta-analysis of randomized controlled trials: J Clin Anesth, 2016; 35; 1-12
15. Abad-Gurumeta A, Ripollés-Melchor J, Casans-Francés R, Evidence Anaesthesia Review Group. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade: Anaesthesia, 2015; 70; 1441-52
16. Cammu G, Schepens T, De Neve N, Diaphragmatic and intercostal electromyographic activity during neostigmine, sugammadex and neostigmine-sugammadex-enhanced recovery after neuromuscular blockade: A randomised controlled volunteer study: Eur J Anaesth, 2017; 34; 8-15
17. Kheterpal S, Vaughn MT, Dubovoy TZ, Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): A multicenter matched cohortanalysis: Anesthesiology, 2020; 132; 1371-81
18. Unterbuchner C, Neuromuscular block and blocking agents in 2018: Turk J Anaesthesiol Reanim, 2018; 46; 75-80
19. Togioka BM, Yanez D, Aziz MF, Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged procedure: Br J Anaesth, 2020; 124; 553-61
20. Li G, Freundlich RE, Gupta RK, Postoperative pulmonary complications’ association with sugammadex versus neostigmine: A retrospective registry analysis: Anesthesiology, 2021; 134(6); 862-73
21. Cho SA, Kim JH, Cho CK, Sung TY, The effect of neuromuscular blockade reversal agents on postoperative pulmonary complications in patients undergoing femur fracture repair surgery: A retrospective observational study: Ann Geriatr Med Res, 2023; 27(3); 212-19
22. Colomina MJ, Ripollés-Melchor J, Guilabert P, Observational study on fluid therapy management in surgical adult patients: BMC Anesthesiol, 2021; 21(1); 316
23. Ding X, Zhu X, Zhao C, Use of sugammadex is associated with reduced incidence and severity of postoperative nausea and vomiting in adult patients with obesity undergoing laparoscopic bariatric surgery: A post-hoc analysis: BMC Anesthesiol, 2023; 23(1); 163
24. Ding X, Che J, Xu S, A nomogram to predict postoperative nausea and vomiting in the ward following laparoscopic bariatric surgery: Surg Endosc, 2023; 37(12); 9217-27
Tables
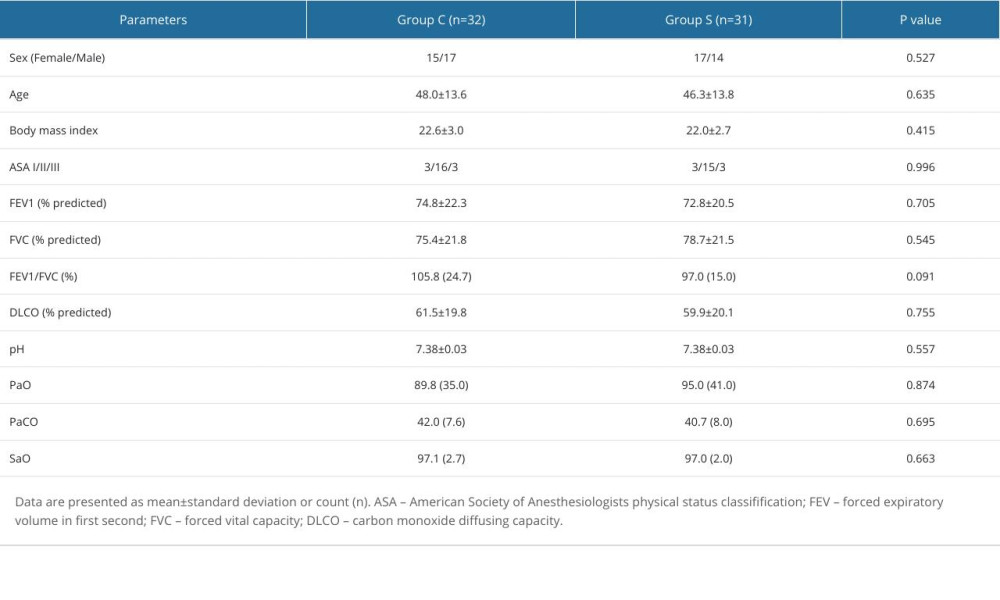 Table 1. Demographics and patients’ general characteristics.
Table 1. Demographics and patients’ general characteristics.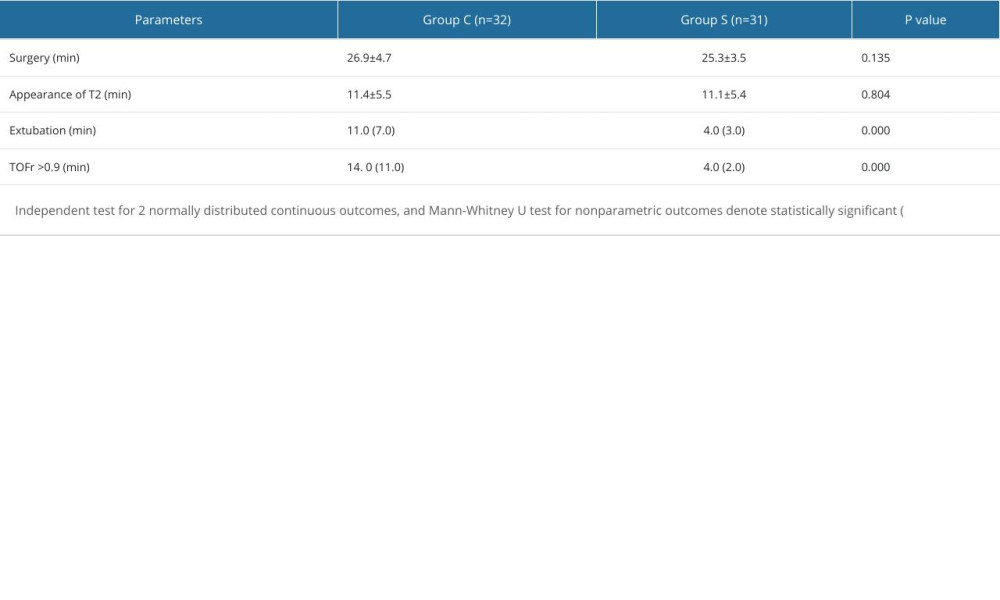 Table 2. Intraoperative manifestations.
Table 2. Intraoperative manifestations.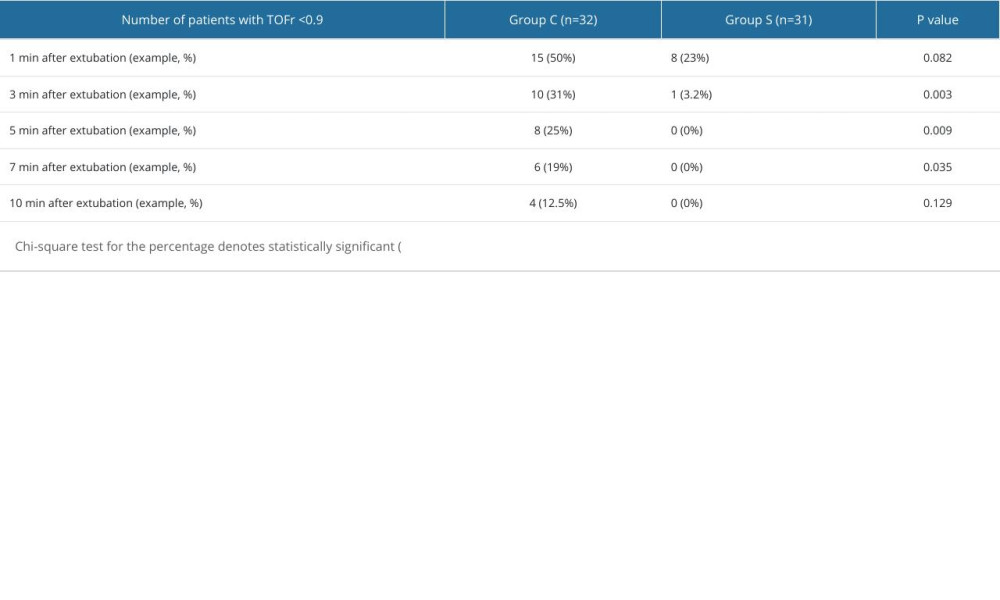 Table 3. Comparison of the residual rate of neuromuscular blockade.
Table 3. Comparison of the residual rate of neuromuscular blockade. Table 1. Demographics and patients’ general characteristics.
Table 1. Demographics and patients’ general characteristics. Table 2. Intraoperative manifestations.
Table 2. Intraoperative manifestations. Table 3. Comparison of the residual rate of neuromuscular blockade.
Table 3. Comparison of the residual rate of neuromuscular blockade. In Press
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952