24 February 2024: Clinical Research
Performance of Subcutaneous Implantable Cardioverter Defibrillator (S-ICD) in Chinese Population with Primary Prevention Indications: A Prospective Observational Cohort Study
Yaodong Li1AEF, Yangxin Chen2BCD, Jingfeng Wang2BCD, Jian Xu3BCD, Ruogu Li4BCD, Zhaohui Qiu5BCD, Lingyun Jiang6BCD, Farong Shen7BCD, Shubin Jiang8BCD, Bin Li9BCD, Yingjie Chu10BCD, Lang He11BCD, Lijin Pu12BCD, Xuebin Han13BCD, Xianping Long14BCD, Xiaolin Xue15BCD, Jianhong Tao16BCD, Yongquan Wu17BCD, Tao Guo18BCD, Yiqiang Yuan19BCD, Xianqing Wang20BCD, Jiang Wang21BCD, Jing Xu22BCD, Yujie Zhao23BCD, Zhihui Zhang24BCD, Wei Hua25ABCD*, Yangang Su26ABCD, Baopeng Tang27ABCDEFGDOI: 10.12659/MSM.942747
Med Sci Monit 2024; 30:e942747
Abstract
BACKGROUND: International studies have shown that use of a subcutaneous implantable cardioverter defibrillator (S-ICD) could reduce lead-related complications while maintaining adequate defibrillation performance; however, data from the Chinese population or other Asian groups are limited.
MATERIAL AND METHODS: SCOPE is a prospective, multicenter, observational cohort study. Two hundred patients with primary prevention indication for sudden cardiac death (SCD), who are candidates for S-ICD, will be enrolled. From the same population, another 200 patients who are candidates for transvenous implantable cardioverter defibrillator (TV-ICD) will be enrolled after being matched for age, sex, SCD high-risk etiology (ischemic cardiomyopathy, and non-ischemic cardiomyopathy, ion channel disease, and other) and atrial fibrillation in a 1: 1 ratio with enrolled S-ICD patients. All the patients will be followed for 18 months under standard of care.
RESULTS: The primary endpoint is proportion of patients free from inappropriate shock (IAS) at 18 months in the S-ICD group. The lower 95% confidence bound of the proportion will be compared with a performance goal of 90.3%, which was derived from the previous meta-analysis. The comparisons between S-ICD and TV-ICD on IAS, appropriate shock, and complications will be used as secondary endpoints without formal assumptions.
CONCLUSIONS: This is the first prospective multicenter study focusing on the long-term performance of S-ICD in a Chinese population. By comparing with the data derived from international historical studies and a matched TV-ICD group, data from SCOPE will allow for the assessment of S-ICD in the Chinese population in a contemporary real-world implantation level and programming techniques, which will help us to further modify the device implantation and programming protocol in this specific population in the future.
Keywords: Asians, Death, Defibrillators, Implantable, Primary Prevention
Background
Use of an implantable cardioverter defibrillator (ICD) is an integral part of treating patients deemed to be at high risk of SCD [1]. Although device function and implant technique are being optimized, it is still challenging for physicians to deal with complications related to TV-ICD leads. Cumulative data show that about 25% of transvenous ICD leads had mechanical complications when followed up to 10 years [2,3]. S-ICD, an entirely subcutaneous system that does not require intraprocedural vascular access, was designed to reduce the risk of transvenous leads-related complications [4]. Unlike TV-ICD, which requires permanent transvenous implantation of defibrillation electrodes into the cardiac chamber, the electrode of S-ICD is placed subcutaneously beside the sternum, with no exposure to most of the risks associated with intravascular or cardiac locations (Figure 1).
Numerous studies have shown that S-ICD can dramatically reduce lead-related complications while maintaining adequate ICD performance [5–7]. However, almost all these data were derived from patients in Western sites, and data from Chinese or other Asian groups with generally lower body mass index (BMI) are limited.
S-ICD was introduced in China in August 2015, while only several retrospective cases series with small sample size were published to show its procedural characteristics and short-term performance. Clinical features of S-ICD in this specific population have not been well studied, especially with modern implantation techniques and programming strategy. Thus, we performed this 18-month observational study to show the long-term performance of S-ICD. With published international data and data from TV-ICD implanted contemporarily in a similar population as a benchmark, this study will provide a full picture of S-ICD in the Chinese population.
Material and Methods
PATIENT SELECTION:
Patients older than 18 years with a Class I or IIa indication for ICD therapy for SCD primary prevention will be screened. The major exclusion criterion is an indication for pacing therapy, including bradycardia pacing, cardiac resynchronization therapy, and anti-tachycardiac pacing (ATP) for refractory monomorphic ventricular tachycardia, which could not be managed with medication or ablation therapy. Patients who fail screening for S-ICD surface ECG will not be enrolled. Detailed inclusion and exclusion criteria are listed in Table 1.
ALLOCATION AND TREATMENT:
The study flowchart is shown in Figure 2. This is an observational study. All the patients with SCD primary prevention indication will be informed about the study in detail by the investigator after they made their therapeutic decision to accept S-ICD or TV-ICD implantation after the discussion with their physicians. The informed consent with signature will be obtained after the patients agree to participate in the study. If a patient is ultimately unable to be implanted with the selected device, alternative treatment options will be used to manage the disease, including but not limited to receiving the other device. All patients will continue to receive medication therapy for their comorbidities.
A total of 400 subjects will be enrolled, and 200 eligible patients implanted with S-ICD will be enrolled prospectively. Since the amount of TV-ICD implantation is much larger than that of S-ICD implantation in the real-world practice, eligible patients who are candidates for TV-ICD will be enrolled after matching for age (<30/30-49/50-69/≥70), sex (male/female), SCD high-risk etiology, and atrial fibrillation (without/paroxysmal atrial fibrillation/persistent atrial fibrillation) in a 1: 1 ratio with enrolled S-ICD patients. SCD high-risk etiology will be categorized as ischemic cardiomyopathy, non-ischemic cardiomyopathy, ion channel disease, and other diseases (eg, congenital heart disease).
DEVICE IMPLANTATION AND PROGRAMMING:
TV-ICD implantation will be performed under routine workflow in each site. For S-ICD, the generator is highly suggested to be placed in an intermuscular position and the tube exercise test is suggested to be performed before discharge, if appropriate.
Programming of S-ICD will be set with zone cut-offs at 200 bpm (Conditional Shock Zone) and 230 bpm (Shock Zone), as suggested. The programming for TV-ICD in this trial is recommend as follows: turning off VT-1 zone or setting ATP only for therapy, >200 beats/min for fast VT zone detection, and >230 beats/min for VF zone detection. The therapy settings for VT zone and VF zone will be determined at the physician’s discretion, but is strongly suggested to be consistent with the 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on optimal ICD programming and testing. The minimum pacing rate is recommended to be set to 40 beats/min with the SAV interval of 250 ms or turning on minimum ventricular pacing function instead. The physicians can deviate from these recommendations, according to their discretion to accommodate specific patient concerns, with the rationale recorded.
FOLLOW-UP SCHEDULE:
The follow-up schedule is consistent with the recommendations in the Chinese expert consensus on ICD 2021 [8]. All patients will be followed at the clinic 1 month and 3 months after the implantation, and thereafter at an interval of 6 months to 18 months after the procedure. The follow-up duration of SCOPE is the same as the UNTOUCHED trial, a famous S-ICD study performed in the USA, but shorter than the PAS, a post-approval study in the USA with 3-year follow-up data, and EFFPRTLESS, a post-market registry outside the USA with 5-year follow-up data. Longer follow-up data may be produced in the future if appropriate. All the therapeutic events, device programming parameters, and adverse events will be recorded for the participates. All therapeutic episodes will be reviewed and judged by an independent clinical event committee (CEC) who will be blinded to group assignment. The physician will counsel the patient on the importance of maintaining the visit schedule at every visits. If a patient fails to return to the original clinic for a required standard-of-care visit, a local clinic visit will be scheduled, and the follow-up information will be collected remotely. If the patient still cannot attend a clinic visit, a phone call will be made to get as much information as possible, and the physician will reschedule the missed visit and counsel the patient on the importance of maintaining the assigned visit schedule and ascertain if the patient wishes to continue in the study. The patient will be considered lost to follow-up if continuing to be unreachable. If the patient decides to withdraw from the study, he/she will still be followed up under standard of care, but no data will be collected.
STUDY ENDPOINTS:
The primary endpoint is the proportion of patients free from IAS in the S-ICD group at 18 months. The secondary endpoints include the comparisons between the S-ICD group and TV-ICD group on the occurrences of IAS, AS, or device- and procedure-related complications, respectively. Detailed information on endpoints is shown in Table 2.
STUDY HYPOTHESES:
The primary hypothesis is that the IAS-free rate in the S-ICD group will be higher than the historical performance goal determined from a meta-analysis of trials with patients implanted with TV-ICD.
SAMPLE SIZE CALCULATION:
The sample size is calculated for the primary endpoint. The lower 95% confidence bound of the observed rate will be compared with a performance goal of 90.3%. The performance goal is derived from a meta-analysis of IAS incidence in patients implanted with TV-ICD with annual incidence of IAS at 5.29% (242/4572) [9], equal to 7.94% (5.29%*1.5≈7.94%) for 18 months, and resulting in an IAS-free incidence rate of 92.06% (100-7.94%=92.06%) with lower 95% confidence bound at 91.3% (Wald confidence limits). The performance goal is finally achieved after accounting for a clinical equivalence of 1% and set to 90.3%. The sample size is set to get a 90% power with a one-sided alpha of 2.5%. The settings of the power and alpha here are in accord with related expert consensus, which recommends that one-sided alpha is set at 2.5% with power equal to or higher than 0.8, which is also used in most trials with single-arm objective performance criteria design. The expected IAS-free rate is set to 96.8% based on the results from subgroup analysis with SMART Pass ON in the UNTOUCHED trial [10], a trial observing IAS incidence in 18 months in S-ICD patients with SCD primary prevention indication, in which the IAS rate was 2.2% with the higher 95% confidence bound at 3.2%, resulting in an IAS-free rate of 96.8%. Total attrition is expected to be 15%. Under those assumptions, a minimum of 181 subjects should be enrolled, so 200 was set as the final sample size for the S-ICD group.
For the secondary endpoints, there is no formal statistical assumption. Comparisons between the S-ICD group and TV-ICD group will be performed to explore any potential difference. Thus, 200 eligible subjects with TV-ICD will be enrolled concurrently, which results in the total sample size of 400.
STATISTICAL ANALYSIS PLAN:
For continuous variables, normally distributed data will be represented by means±standard deviation, and the comparison between groups will be performed by t-test. Skewed distribution data will be described as medium and four-quartile range, and the rank sum test will be performed for comparison. For categorical variables, the absolute and relative frequencies based on non-missing data will be calculated, and the chi-square test will be performed for comparison.
All analyses will be conducted in an intention-to-treat population that included all enrolled patients. The primary endpoint will be analyzed by Kaplan-Meier curve analysis. Subjects who withdraw from the study or die prior to 18-month follow-up without experiencing an event will be censored on the date of their last study visit. The Cox proportional hazards model will be used to explore the predictors for IAS if appropriate. Kaplan–Meier curve with log-rank test will be used to evaluate group differences in secondary endpoints. A p value of <5% and <2.5% will be considered statistically significant for two-sided and one-sided statistical tests, respectively. Subgroup analyses will be done based on matching variables if appropriate.
DATA MANAGEMENT:
Data management and central and onsite monitoring will be provided by a contract research organization (Beijing Yjheal Medical Research Center, Beijing, China). Only authorized staffs with fixed roles are permitted to add and review the data. The data will be checked against source data by clinical monitors during monitoring visits. All ICD manufacturers that provided support during implantation will have no role in the oversight, design of the study, analyses, or interpretation of the data.
ETHICS:
SCOPE has been approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University, and the local ethics committee of all participating sites will be required to approve the study protocol before enrollment in the site. Important protocol amendments, including eligibility criteria, outcomes, follow-up period, and sample size, will be reported to all the investigators and local ethics committee and need to be approved. During the research process, the investigators will be required to strictly follow guidelines of the Declaration of Helsinki and Human Biomedical Research Ethical Issues. All the subjects enrolled will be treated and followed under standard of care. This will be an observational cohort study, and all the adverse events that occur during the procedure will be reported in accordance with relevant laws and regulations.
SCOPE has registered at ISRCTN registry with trial ID ISRCTN13448502.
Discussion
Although algorithms and techniques have been improved over past decades, TV-ICD still comes at the risk of device-related complications. S-ICD has been introduced to address the problems related to transvenous leads. Many clinical studies have demonstrated the safety and effectiveness of S-ICD for preventing SCD [5,10–12], but limited data have been published in Chinese populations. SCOPE will be the first multicenter prospective study performed in a Chinese population with primary prevention indication for SCD to evaluate the long-term performance of S-ICD. By comparing with the data derived from international historical studies and a matched TV-ICD group, data from SCOPE will allow assessment of the performance of S-ICD in a Chinese population with a contemporary real-world implantation level and programming techniques, which will promote comprehensive understanding the S-ICD performance and may help to modify or standardize S-ICD implantation and/or programming protocol in the future in this population.
The S-ICD IDE trial first verified its safety and efficiency in 2013 [13], whereafter numerous studies have been published to expand the understanding of this device, including EFFORTLESS [11], PRAETORIAN [5], PAS [14], and UNTOUCHED [10]. However, none of these studies involved Asian populations, who generally have lower BMI and a different lifestyle. The mean BMI of an Asian population was reported as 23.0 kg/m2 [15], considerably lower than 30.2 kg/m2 in UNTOUCHED [10] and 27 kg/m2 in EFFORTLESS [16]. S-ICD started clinical use in China in 2015, but only a few retrospective analyses with limited cases were published to demonstrate the procedural characteristics and short-term performance [17–20]. Up to now, the follow-up data for ICD implantation in Chinese population, including S-ICD and TV-ICD, are both very scarce. For S-ICD, published data with the largest sample size was from a retrospective case series of 111 cases with median follow-up time of 158 days [20]. Almost all the other studies were single-arm studies. SCOPE will be the first prospective multicenter study focusing on the long-term performance of S-ICD in this specific population.
Patients implanted with S-ICD have largely been skewed towards patients with more co-morbidities. SCD primary prevention patients have become the major candidates for S-ICD in Europe and the USA, with the proportion being around 70% [11,14]. The variation trend is similar in China, but the proportion may be lower at this stage. The proportion of SCD primary prevention patients was reported as 35% in a retrospective multicenter study [20]. Limited data could be used for reference when considering the long-term outcomes in this specific group, especially for Chinese populations. Thus, SCOPE will focus on patients with SCD primary prevention indication to provide the performance of this device in patients with more co-morbidities, who are becoming the main candidates for S-ICD. The results will provide the outcome data in Chinese patients with SCD primary prevention, which could be used as reference information for physicians when they discuss therapeutic strategies with patients in the future, so that physicians could make medical suggestions after comprehensively considering the patient’s conditions.
Besides filling the evidence gap of this specific population, the device algorithm and implantation techniques keep being modified while clinical data are published [21–23]. The intermuscular position has been proved to be superior to subcutaneous pockets [24]. Then, a new high-pass filter (SMART Pass) was introduced that significantly reduced IAS. Subsequently, the physicians found tube exercise testing could be used as post-implant screening for myopotential interference and may be used to guide optimal programming. PRAETORIAN trial, the first randomized controlled study, has shown the benefit of S-ICD, while this trial was performed at early stage, SMART Pass was not activated or was unavailable in 78% of the first IASs [5]. The UNTOUCHED trial is the first study focusing on patients with SCD primary prevention indication, with 60.4% of the subjects equipped with SMART Pass [10]. However, patients with S-ICD were programmed with zone cut-offs at 200/250 bpm in the UNTOUCHED trial, which is different from the current real-world setting of 200/230 bpm. Thus, subjects enrolled in the SCOPE study will be implanted and programmed under optimal strategies, and all the generators will be positioned in an intermuscular position with SMART Pass ON, and the tube excise test will be performed after implantation. Data from SCOPE will demonstrate the performance of S-ICD in accordance with the state of the art and current practice, which will help to modify or standardize S-ICD implantation and/or programming protocol in the future in this population.
Moreover, SCOPE will enroll eligible patients implanted with TV-ICD contemporaneously, which will provide the opportunity to evaluate the performance of S-ICD with TV-ICD as a control. The programming strategy for TV-ICD here is set to be consistent with the high-rate arm of MADIT RIT [25], which has been proved to be markedly reduced in inappropriate therapies and is now recommended in consensus documents for primary prevention patients [26]. The similarity of the programming of these 2 groups will also preserve the comparability of the results. Meanwhile, patients with TV-ICD could only be enrolled after matching with enrolled patients with S-ICD, which will somewhat compensate for the defect of non-randomized design and make the data even more comparable. The results will show the characteristics of procedural and long-term outcomes after S-ICD and TV-ICD implantations, which will provide physicians and patients with a reference for consideration when making medical decisions in the future, and may also provide the evidence for future device programming and potential technological improvements. A randomized controlled study with formal assumptions may be performed in the future based on the results from SCOPE.
Our study design has certain limitations. SCOPE is not a randomized controlled study, and no formal assumption is designed for the comparisons between S-ICD group and TV-ICD group, which is the less rigorous scientific approach to assess the role of the S-ICD. However, the matching process of the study may partially compensate for the defect of a non-randomized design. Among the 4 matching variables, age and sex will compensate for overall demographic characteristics, which may affect the outcomes of device implantation, and SCD high-risk etiology and atrial fibrillation will compensate for different electrical activity mechanisms, which may affect the occurrence of appropriate and/or inappropriate shocks. Nevertheless, a well-designed prospective randomized controlled study with formal assumptions will be the best way to confirm the results from our study in the future. Another limitation is that although this project started in 2021, the enrollment was slow until recently due to the impact of the Covid-19 pandemic. However, the strategy and practice of S-ICD implantation and programming have not been changed during this period, thus the extension of enrollment time may have a limited impact on the results. To date, the study has completed 50% of enrollment. The study is estimated to be completed at the middle of 2025, and the results may be available around September 2025.
Conclusions
In summary, SCOPE is the first prospective observational cohort study focusing on the performance of S-ICD in a Chinese population with primary prevention indication for SCD with state-of-the-art techniques. By comparing with historical international data and data from TV-ICD implanted concurrently, the results of this trial will help understand the performance of S-ICD in a Chinese population and may be used to further modify the device implantation and programming protocol in this specific population in the future.
Figures
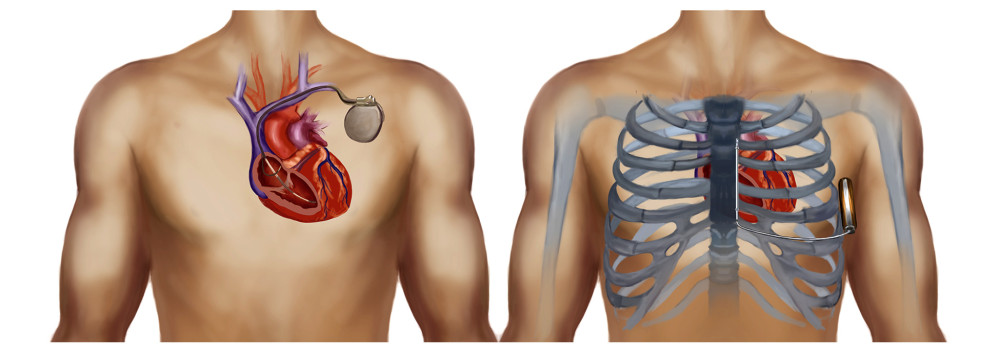 Figure 1. Schematic diagram of S-ICD (right) and TV-ICD (left).
Figure 1. Schematic diagram of S-ICD (right) and TV-ICD (left). 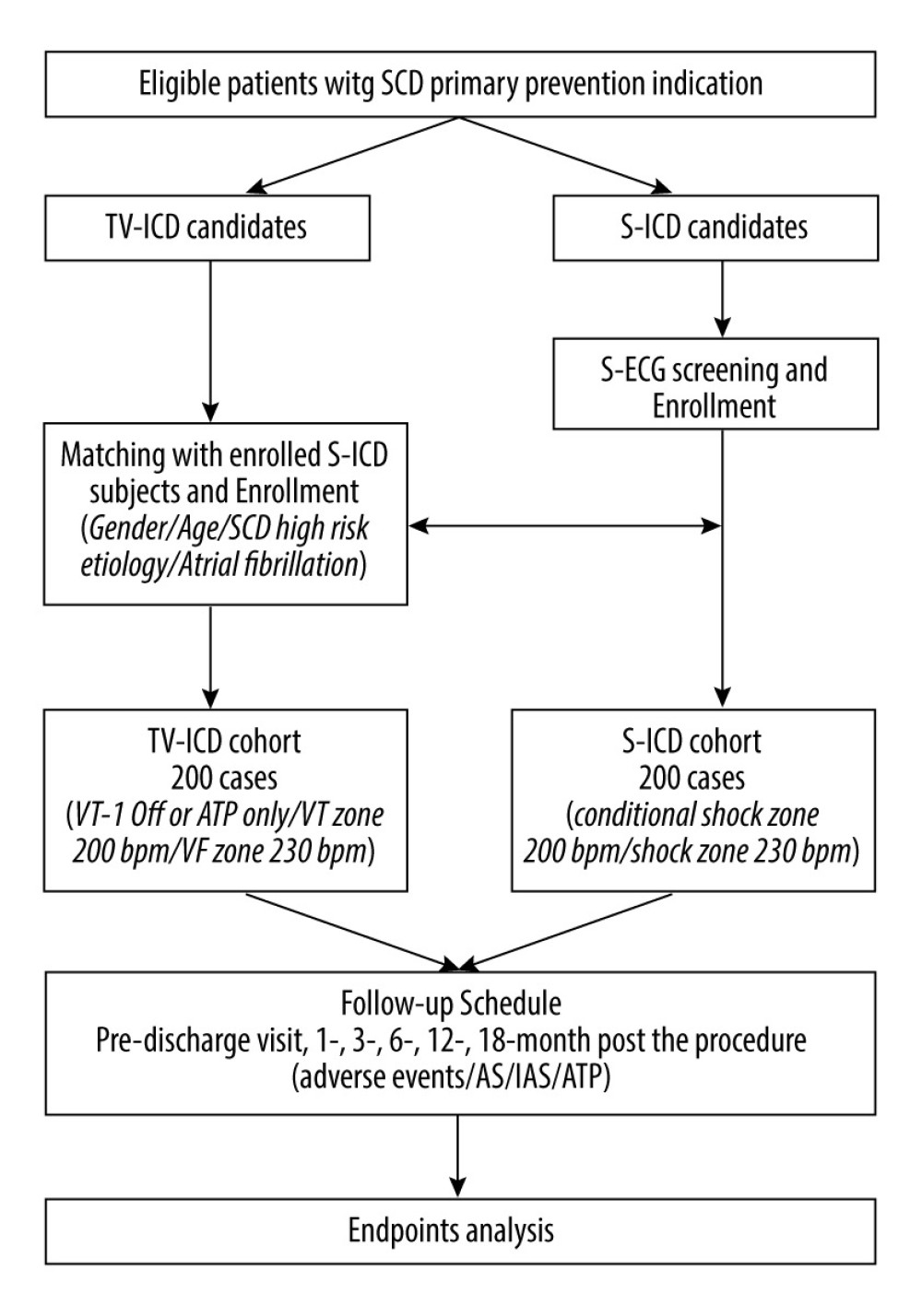 Figure 2. Flowchart of SCOPE trial. S-ICD – subcutaneous implantable cardioverter defibrillation; TV-ICD, transvenous implantable cardioverter defibrillation; S-ECG – surface electrocardiograph; AS – appropriate shock; IAS – inappropriate shock; ATP – anti-tachycardiac pacing.
Figure 2. Flowchart of SCOPE trial. S-ICD – subcutaneous implantable cardioverter defibrillation; TV-ICD, transvenous implantable cardioverter defibrillation; S-ECG – surface electrocardiograph; AS – appropriate shock; IAS – inappropriate shock; ATP – anti-tachycardiac pacing. References
1. Zeppenfeld K, Tfelt-Hansen J, de Riva M, 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Eur Heart J, 2022; 43(40); 3997-4126
2. Koneru JN, Jones PW, Hammill EF, Risk factors and temporal trends of complications associated with transvenous implantable cardiac defibrillator leads: J Am Heart Assoc, 2018; 7(10); e007691
3. Kleemann T, Becker T, Doenges K, Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years: Circulation, 2007; 115(19); 2474-80
4. Healey JS, Krahn AD, Bashir J, Perioperative safety and early patient and device outcomes among subcutaneous versus transvenous implantable cardioverter defibrillator implantations: A randomized, multicenter trial: Ann Intern Med, 2022; 175(12); 1658-65
5. Knops RE, Olde Nordkamp L, Delnoy P, Subcutaneous or transvenous defibrillator therapy: N Engl J Med, 2020; 383(6); 526-36
6. Knops RE, Pepplinkhuizen S, Delnoy P, Device-related complications in subcutaneous versus transvenous ICD: A secondary analysis of the PRAETORIAN trial: Eur Heart J, 2022; 43(47); 4872-83
7. Russo V, Rago A, Ruggiero V, Device-related complications and inappropriate therapies among subcutaneous vs. transvenous implantable defibrillator recipients: Insight monaldi rhythm registry: Front Cardiovasc Med, 2022; 9; 879918
8. Chinese Society of Pacing and Electrophysiology Chinese Society of Arrythmias., Chinese expert consensus on implantable cardioverter defibrillator therapy (2021): Chin J Cardiac Arrhyth, 2021; 25(4); 280-99
9. Scott PA, Silberbauer J, McDonagh TA, Murgatroyd FD, Impact of prolonged implantable cardioverter-defibrillator arrhythmia detection times on outcomes: A meta-analysis: Heart Rhythm, 2014; 11(5); 828-35
10. Gold MR, Lambiase PD, El-Chami MF, Primary results from the understanding outcomes with the s-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial: Circulation, 2021; 143(1); 7-17
11. Lambiase PD, Theuns DA, Murgatroyd F, Subcutaneous implantable cardioverter-defibrillators: Long-term results of the EFFORTLESS study: Eur Heart J, 2022; 43(21); 2037-50
12. Gold MR, Aasbo JD, Weiss R, Infection in patients with subcutaneous implantable cardioverter-defibrillator: Results of the S-ICD post approval study: Heart Rhythm, 2022; 19(12); 1993-2001
13. Weiss R, Knight BP, Gold MR, Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator: Circulation, 2013; 128(9); 944-53
14. Burke MC, Aasbo JD, El-Chami MF, 1-year prospective evaluation of clinical outcomes and shocks: The subcutaneous ICD post approval study: JACC Clin Electrophysiol, 2020; 6(12); 1537-50
15. Hai JJ, Lim ET, Chan CP, First clinical experience of the safety and feasibility of total subcutaneous implantable defibrillator in an Asian population: Europace, 2015; 17(Suppl 2); ii63-68
16. Boersma L, Barr C, Knops R, Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: The EFFORTLESS study: J Am Coll Cardiol, 2017; 70(7); 830-41
17. Li MH, Zhang L, Liang YX, A single-center analysis of the safety and efficiency of subcutaneous implantable cardioverter defibrillator implantation technique: Chin J Cardiac Arrhyth, 2020; 24(06); 566-70
18. Wu CJ, Li YD, Zhang L, Clinical observation of subcutaneous implantable cardioverter defibrillator implanted in a single center: Chin J Cardiac Arrhyth, 2020; 24(06); 561-65
19. He L, Chen ZP, Jin J, Three years of single-center experience of subcutaneous implantable cardioverter defibrillator: Chin J Cardiac Arrhyth, 2020; 24(6); 571-75
20. Hua W, Su YG, Tang BP, Application of subcutaneous implantable cardioverter defibrillator in China: Chin J Cardiac Arrhyth, 2020; 24(6); 556-60
21. Ishida Y, Sasaki S, Toyama Y, A novel screening test for inappropriate shocks due to myopotentials from the subcutaneous implantable cardioverter-defibrillator: Heart Rhythm O2, 2020; 1(1); 27-34
22. Theuns D, Brouwer TF, Jones PW, Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator: Heart Rhythm, 2018; 15(10); 1515-22
23. Gold MR, Theuns DA, Knight BP, Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: The START study: J Cardiovasc Electrophysiol, 2012; 23(4); 359-66
24. Botto GL, Ziacchi M, Nigro G, Intermuscular technique for implantation of the subcutaneous implantable defibrillator: A propensity-matched case-control study: Europace, 2023; 25(4); 1423-31
25. Ruwald AC, Schuger C, Moss AJ, Mortality reduction in relation to implantable cardioverter defibrillator programming in the Multicenter Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy (MADIT-RIT): Circ Arrhythm Electrophysiol, 2014; 7(5); 785-92
26. Wilkoff BL, Fauchier L, Stiles MK, 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing: Heart Rhythm, 2016; 13(2); e50-86
Figures
 Figure 1. Schematic diagram of S-ICD (right) and TV-ICD (left).
Figure 1. Schematic diagram of S-ICD (right) and TV-ICD (left). Figure 2. Flowchart of SCOPE trial. S-ICD – subcutaneous implantable cardioverter defibrillation; TV-ICD, transvenous implantable cardioverter defibrillation; S-ECG – surface electrocardiograph; AS – appropriate shock; IAS – inappropriate shock; ATP – anti-tachycardiac pacing.
Figure 2. Flowchart of SCOPE trial. S-ICD – subcutaneous implantable cardioverter defibrillation; TV-ICD, transvenous implantable cardioverter defibrillation; S-ECG – surface electrocardiograph; AS – appropriate shock; IAS – inappropriate shock; ATP – anti-tachycardiac pacing. In Press
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952