19 February 2024: Review Articles
Resibufogenin: An Emerging Therapeutic Compound with Multifaceted Pharmacological Effects – A Comprehensive Review
Hao Zhang1AEG, Baiyu Jian2AG*DOI: 10.12659/MSM.942783
Med Sci Monit 2024; 30:e942783
Abstract
ABSTRACT: Resibufogenin (RBG), a significant bufadienolide compound found in the traditional Chinese medicine Chansu, has garnered increasing attention in recent years for its wide range of pharmacological effects. This compound has shown promising potential in various therapeutic areas, including oncology, cardiology, and respiratory medicine. Among its notable properties, the anticancer effects of RBG are particularly striking, positioning it as a potential candidate for innovative cancer treatments. The mechanism of action of RBG is diverse, impacting various cellular processes. Its anticancer efficacy has been observed in different types of cancer cells, where it induces apoptosis and inhibits cell proliferation. Beyond its oncological applications, RBG also demonstrates substantial anti-inflammatory and antiviral activities. These properties suggest its utility in managing chronic inflammatory disorders and viral infections, respectively. The compound’s cardiotonic effects are also noteworthy, providing potential benefits in cardiovascular health, particularly in heart failure management. Additionally, RBG has shown effectiveness in blood pressure regulation and respiratory function improvement, making it a versatile agent in the treatment of hypertension and respiratory disorders. However, despite these promising aspects, systematic reviews focusing specifically on RBG are limited. This article aims to address this gap by comprehensively reviewing RBG’s origin, physiological, and pharmacological effects. The review will serve as a crucial reference for clinicians and researchers interested in the therapeutic applications of RBG, highlighting its potential in various medical domains. By synthesizing current research findings, this review will facilitate a deeper understanding of RBG’s role in medicine and encourage further investigation into its clinical uses.
Keywords: Amphibian Venoms, Bufogenin
Background
PHYSICOCHEMICAL PROPERTIES:
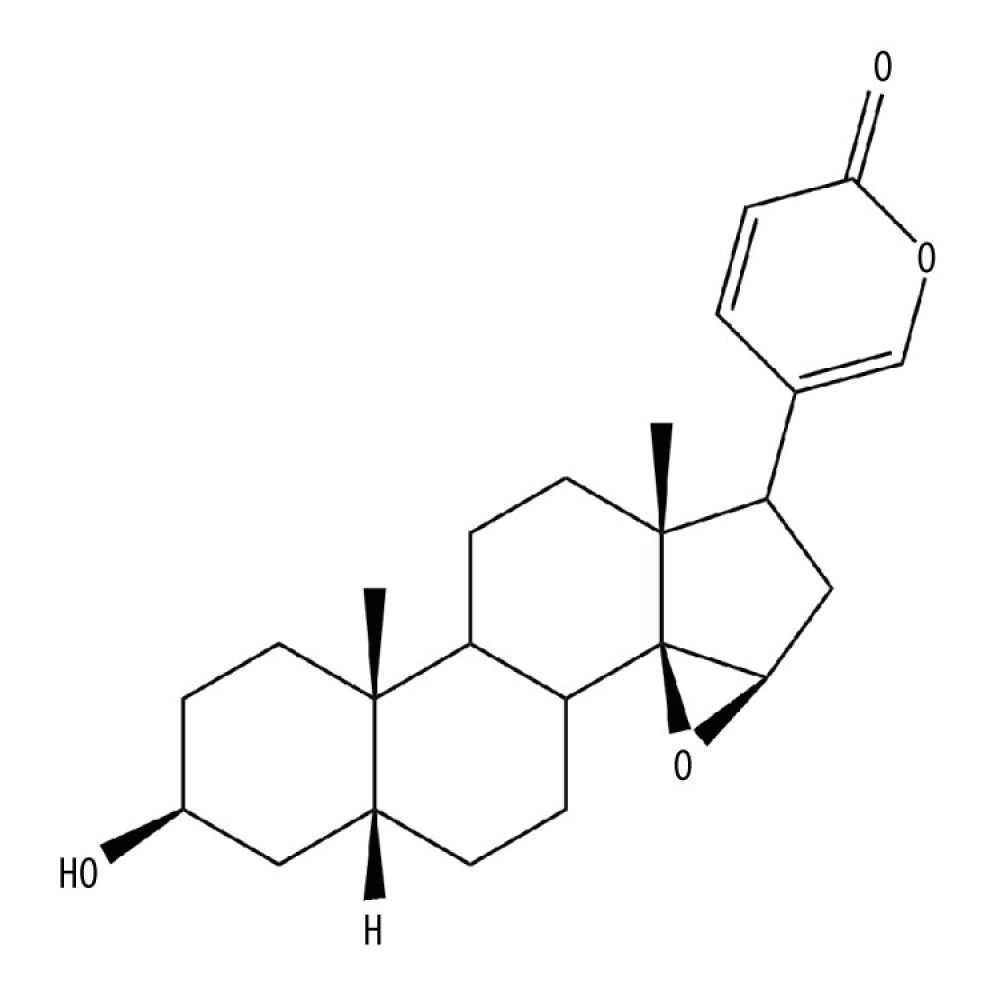
The chemical structure of RBG is similar to that of digitoxigenin, as shown in Figure 1. Its IUPAC name is 5-[(1R,2S,4R,6R,7R,10S,11S, 14S,16R)-14-hydroxy-7,11-dimethyl-3-oxapentacyclo [8.8.0.02,4.02,7.011,16] octadecan-6-yl] pyran-2-one, molecular formula is C24H32O4 and molecular weight is 384.5 g/mol [8]. It is insoluble in water. It has a melting point of 155°C and a boiling point of 431.17°C (data were retrieved from Chemical Book (http://www.chemicalbook.com/). RBG is unstable under strong acid or alkaline conditions. It was reported that RBG is not stable in gastric fluid but is relatively stable in intestinal fluid [9].
PHARMACOLOGICAL ACTIVITIES OF RBG:
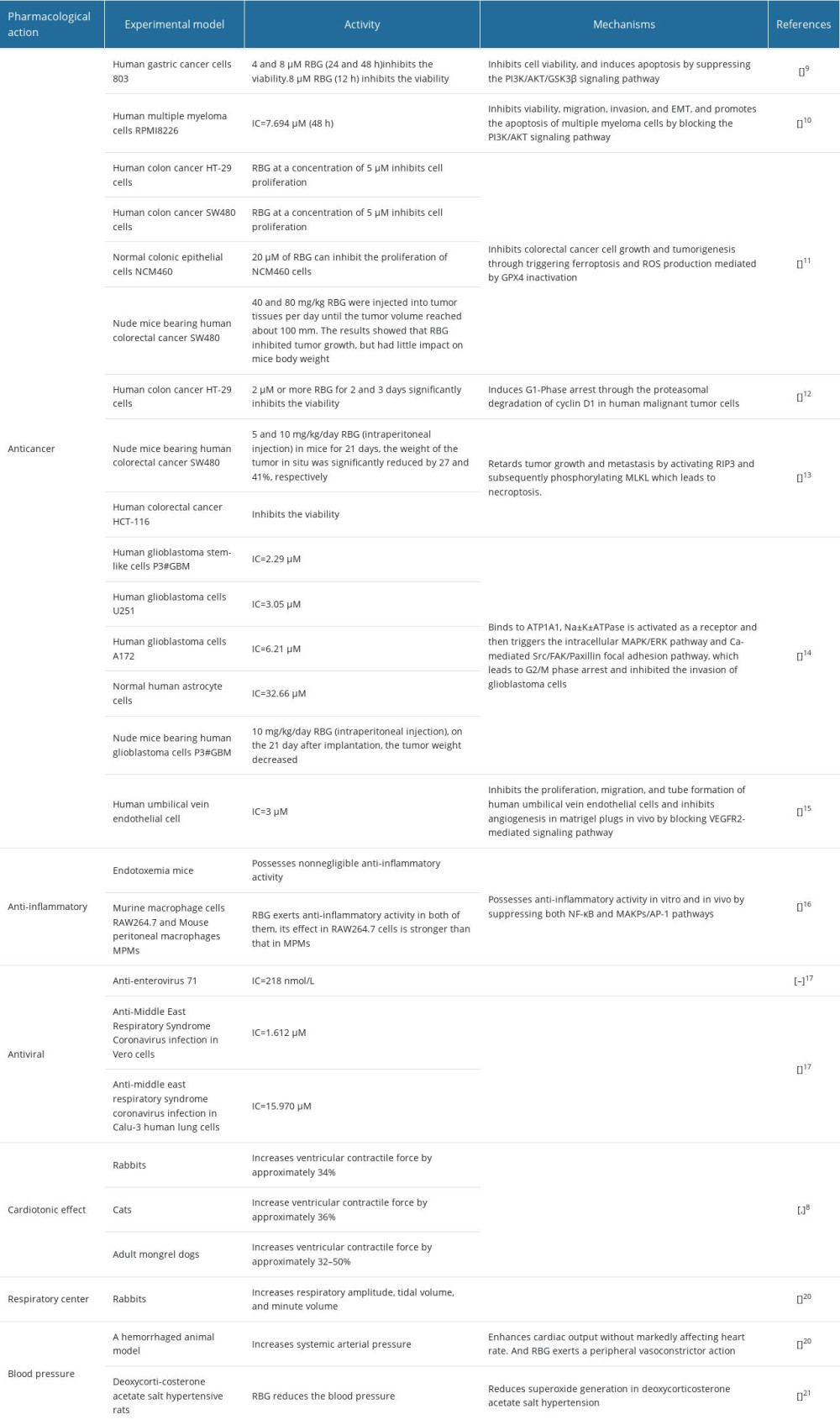
RBG exhibits a wide range of pharmacological activities, including anticancer, anti-inflammatory, antiviral, and cardiotonic effects, as well as regulation of blood pressure and improvement of respiration. Table 1 provides an overview of the effects and related mechanisms of RBG.
ANTICANCER ACTIVITY:
RBG has been found to exert cytotoxic or inhibitory effect on various cancers, including gastric cancer, multiple myeloma, colon cancer, and glioblastoma. The anticancer effects of RBG have been attributed to a multitude of mechanisms. In gastric carcinoma cells, RBG inhibited proliferation and induced apoptosis through the PI3K/AKT/GSK3β signaling pathway [10]. Similarly, RBG exerted its effects on multiple myeloma cells by blocking the PI3K/AKT pathway, resulting in reduced viability, migration, invasion, and increased apoptosis [11]. In colorectal cancer cells, RBG has been shown to inhibit tumor growth and tumorigenesis by triggering ferroptosis and reactive oxygen species production mediated by GPX4 inactivation [12]. Another study revealed that RBG induced G1-phase arrest in human colon cancer HT-29 cells by promoting the proteasomal degradation of cyclin D1 [13]. Furthermore, RBG has been found to suppress colorectal cancer growth and metastasis through RIP3-mediated necroptosis [14]. In glioblastoma cells, RBG was discovered to bind ATP1A1, Na+-K+-ATPase was activated as a receptor and then triggered the intracellular MAPK/ERK pathway and Ca2+-mediated Src/FAK/Paxillin focal adhesion pathway, which led to G2/M phase arrest and inhibition of cell invasion [15]. Additionally, RBG exhibited inhibitory effects on angiogenesis, including the proliferation, migration, and tube formation of human umbilical vein endothelial cells, as well as suppressing vascular endothelial growth factor (VEGF)-mediated vascular network formation in in vivo experiments [16]. RBG achieved this by inhibiting the phosphorylation of VEGFR2 and downstream protein kinases FAK and Src in endothelial cells [16]. Importantly, RBG demonstrated promising antitumor effects through antiangiogenesis in vivo, without significant toxicity [16]. The findings underscore the potential of RBG as a promising therapeutic agent in cancer treatment. However, it is crucial to conduct further research to fully elucidate its mechanism of action, evaluate its efficacy in various disease models, and determine its safety profile in humans.
ANTI-INFLAMMATORY ACTIVITY:
Recent studies have demonstrated that RBG has significant anti-inflammatory effects in vitro and in vivo. In endotoxemia mice, a single intraperitoneal administration of RBG resulted in a significant reduction in serum levels of key pro-inflammatory cytokines, namely tumor necrosis factor (TNF)-α, interleukin (IL)-6, and monocyte chemoattractant protein (MCP)-1 [17]. In addition, RBG demonstrated notable inhibitory effects on the production of pro-inflammatory mediators such as inducible nitric oxide synthase, IL-6, TNF-α, and MCP-1 in lipopolysaccharide-stimulated macrophages through the suppression of their transcriptional activity [17]. Mechanistic studies have shed light on the underlying molecular pathways responsible for RBG’s anti-inflammatory effects. RBG was found to inhibit the phosphorylation of IκBα and prevent the nuclear translocation of p65, effectively inactivating the NF-κB signaling pathway [17]. Furthermore, RBG mitigated activator protein 1 signaling by reducing the phosphorylation levels of JNK and ERK [17]. Collectively, these studies suggest that RBG is a promising anti-inflammatory candidate.
ANTIVIRAL ACTIVITY:
RBG also displays antiviral activity. Enterovirus 71 (EV71) is one of the main causative pathogens of hand, foot, and mouth disease. In vitro studies have demonstrated the anti-EV71 activity of RBG. The half-maximal inhibitory concentration (IC50) value of RBG was (218±31) nmol/L. Middle East respiratory syndrome coronavirus (MERS-CoV), has been confirmed to be the etiological agent of MERS. It has been reported that RBG inhibited MERS-CoV infection in Vero cells (IC50=1.612 μM) and in Calu-3 human lung cells (IC50=15.970 μM) [18–20].
CARDIOTONIC EFFECT:
RBG has been demonstrated to exert noteworthy cardiotonic effects in various animal experiments [8,21]. Specifically, it has been observed to increase ventricular contractile force by approximately 34% in rabbits and approximately 36% in cats. Furthermore, in adult mongrel dogs, RBG was shown to enhance ventricular contractile force by approximately 32% to 50%. It increases the contractility of cardiac muscle in a dose-dependent manner, a positive inotropic effect. These findings highlight the potential positive impact of RBG on cardiac function, suggesting its relevance in cardiovascular research and therapeutic applications.
EFFECT ON THE RESPIRATORY SYSTEM:
RBG has been reported to be an efficacious respiratory stimulator [21]. In animal experiments, RBG was found to significantly increase respiratory amplitude, tidal volume, and minute volume. Importantly, the excitatory effect of RBG on respiration persisted even after the administration of procaine, suggesting that it acts through the central nervous system. Moreover, additional results demonstrated that RBG acts as a central respiratory stimulant, and its action remained unaffected even after intravenous injection of procaine or the removal of the carotid sinus nerve and the ganglion nodosum. This contrasts with the actions of nicotine and lobeline, which were blocked following the same procedures. These findings indicate that RBG has a unique mechanism of action as a central respiratory stimulant.
EFFECT ON BLOOD PRESSURE:
Several reports have indicated a significant increase in mean systemic arterial pressure following the administration of RBG in a hemorrhaged animal model [8,21]. The underlying mechanism for this effect is believed to involve 2 factors [8,21]. First, RBG enhances cardiac output without markedly affecting heart rate. Second, RBG exerts a peripheral vasoconstrictor action. In addition, in a study on hypertensive rats, it was reported that RBG was capable of reducing blood pressure in deoxycorticosterone acetate (DOCA)-salt hypertensive rats [22]. However, its effects were not observed in angiotensin-infused rats [22]. Additionally, the production of superoxide anion, which was elevated in the aortas of both groups of hypertensive rats, compared with the control group, was effectively normalized by RBG only in the volume-expanded (DOCA-salt) animals [22]. RBG did not show any impact on the superoxide anion levels in the angiotensin-infused rats [22]. These findings suggest that RBG may have specific mechanisms of action in different types of hypertension. Further research is warranted to explore these differential effects and elucidate the precise mechanisms involved.
PHARMACOKINETICS OF RBG:
Drug pharmacokinetics is one of the most important issues in pharmacological therapy. The pharmacokinetic profile of RBG in rats after oral administration in a dose of 20 mg/kg was reported [23]. An effective method was established to determine RBG and its metabolites in plasma by liquid chromatography-tandem mass spectrometry. Results revealed that RBG was rapidly absorbed and reached its maximum concentration at approximately 0.25 h with a Cmax of 37.63±10.52 ng/mL, and it could be rapidly metabolized and eliminated. RBG had rapid metabolic profiles according to the half-life (t1/2) of 1.72 ±0.49. The Tmax, AUC0-t and MRT0-t of RBG were 0.25±0 h, 38.52±7.61 ug/1*h, and 1.51±0.19 h, respectively. In addition, the main metabolic reactions of RBG were hydroxylation, dihydroxylation, dehydrogenation, and isomerization. 3-epi-RBG, hydroxylated-RBG, and dihydroxylated-RBG were the main metabolites that possessed high blood concentrations. According to the reports, the metabolite 5β-hydroxylated-resibufogenin has demonstrated significant inhibitory effects on cell growth and the induction of apoptosis in A549 and H1299 cells by facilitating apoptosome assembly and caspase activation [24]. It also has been found to induce biochemical, morphological, and cell cycle alterations in human neoplasms and vegetal cells [25]. Moreover, it acts as an inhibitor of Na+-K+-ATPase and is associated with potential cardiovascular risks [26]. However, our knowledge regarding the pharmacological effects and physiological toxicity of other metabolites is still limited. Hence, further studies are required to explore the correlation between metabolism, toxicity, and activity.
ELECTROPHYSIOLOGIC EFFECTS OF RBG:
The electrophysiological effects of RBG have been studied in various animal models, including dog, sheep, rabbit, guinea pig, and human heart tissues [27]. The experimental results reveal 3 significant effects on the electrophysiological parameters: (1) RBG induces a reduction in the absolute values of resting potential and the maximum rate of rise of action potential phase 0 (Vmax); (2) RBG leads to a shortening of action potential duration in both membrane potential and monophasic potential; and (3) RBG causes a decrease in action potential amplitude both in vitro and in vivo [21,28,29]. The results suggest the electropharmacologic characteristics of RBG are similar to those of acetylstrophanthidin. These findings imply that RBG can be classified within the digitalis-like drug family [21].
Discussion
RBG demonstrates diverse pharmacological activities, including anticancer, anti-inflammatory, antiviral, and cardiotonic, as well as blood pressure regulation, and respiratory improvement effects. While considerable focus has been placed on the anticancer activity of RBG in recent years, research on its other pharmacological effects remains limited. RBG has exhibited cytotoxic and inhibitory effects against various types of cancer, such as gastric cancer, multiple myeloma, colon cancer, and glioblastoma, making it a promising candidate for cancer therapy. Previous studies have indicated that the primary molecular mechanism underlying the anticancer activity of RBG involves cell cycle arrest, induction of cell apoptosis, and inhibition of angiogenesis. However, further investigations are warranted in this area. Reprogramming of energy metabolism is a critical hallmark in the multistep development and progression of cancer. Unfortunately, there is a lack of available data on the potential effects of RBG on energy metabolism. Therefore, it is crucial to identify the specific molecular target of RBG to comprehensively understand its pharmacological and toxicological mechanisms. Such knowledge will provide valuable guidance and evidence for drug development and clinical applications. However, the molecular target underlying the anticancer activity of RBG remains unclear. Recent advancements in technologies from diverse fields, including chemistry, biology, and others, have provided novel methods for the direct target identification of active ingredients in traditional Chinese medicine. These novel techniques, such as responsive target stability assays and protein microarrays, hold promise in facilitating the discovery of direct molecular targets for RBG [30–32].
Drug-induced toxicity is a vital problem in drug development and clinical drug application [33,34]. The main drawback of RBG is its potential cardiotoxicity [21]. Several animal experimental studies have demonstrated that RBG can induce delayed afterdepolarizations and trigger arrhythmia both in vitro and in vivo [27,28]. Currently, there is a relative lack of long-term toxicological studies and pharmacodynamics studies on various dosages and administration routes. Therefore, more research is required in this regard.
Drugs with poor water solubility pose a challenge in terms of low bioavailability [35,36]. Similarly, RBG is insoluble in water. One strategy involves modifying the chemical structure of RBG to increase its water solubility. It is important to note that modifying the chemical structure of a drug can impact its pharmacological activity. Another approach to improve the bioavailability and solubility of RBG is through the development of novel pharmaceutical formulations. Several techniques have been used to enhance solubility, including solid dispersion [37,38], microemulsion [39] and submicron emulsion [40], cyclodextrin complexation [41,42], and nanoscale drug delivery systems [43–45]. However, these techniques do present certain challenges in their application. For instance, issues of drug stability in solid dispersions and the preparation and stability of microemulsion and submicroemulsion systems need to be addressed. Furthermore, the safety concerns associated with cyclodextrins and nanoparticles require further evaluation and research. Overall, structural modifications and innovative formulation techniques hold promise for improving the solubility and bioavailability of RBG. However, further research and development are needed to overcome the challenges associated with these techniques and realize the broader application of RBG.
Conclusions and Future Directions
In summary, based on current research findings, RBG has demonstrated significant promise as a compound with diverse pharmacological activities. To fully unveil the mechanism of action of RBG, establish precise dosage guidelines, evaluate its safety profile, and assess its efficacy, it is imperative to conduct continuous and rigorous scientific research, coupled with extensive clinical trials. Furthermore, efforts to enhance the drug’s bioavailability are essential. These endeavors will not only contribute to a deeper understanding of RBG’s characteristics, but will also lay a solid scientific foundation for its future clinical applications.
References
1. Park JS, Shin DY, Lee YW, Apoptotic and anti-metastatic effects of the whole skin of Venenum bufonis in A549 human lung cancer cells: Int J Oncol, 2012; 40(4); 1210-19
2. Wang ZJ, Sun L, Heinbockel T, Resibufogenin and cinobufagin activate central neurons through an ouabain-like action: PLoS One, 2014; 9(11); e113272
3. Commission CP: Pharmacopoeia of the People’s Republic of China, 2020; 402, Beijing, China Medical Science Press
4. Shao H, Li B, Li H, Novel strategies for solubility and bioavailability enhancement of bufadienolides: Molecules, 2021; 27(1); 51
5. Tian X, Wang C, Dong P, Arenobufagin is a novel isoform-specific probe for sensing human sulfotransferase 2A1: Acta Pharm Sin B, 2018; 8(5); 784-94
6. Zou D, Wang Q, Chen T, Bufadienolides originated from toad source and their anti-inflammatory activity: Front Pharmacol, 2022; 13; 1044027
7. Chu Q, Xu H, Gao M, Liver-targeting Resibufogenin-loaded poly(lactic-co-glycolic acid)-D-alpha-tocopheryl polyethylene glycol 1000 succinate nanoparticles for liver cancer therapy: Int J Nanomedicine, 2016; 11; 449-63
8. Iwatsuki K, Yusa T, Kataoka Y, Sato K, Experimental and clinical studies of resibufogenin: Tohoku J Exp Med, 1965; 86(2); 93-101
9. Xin S: Study on the Druggability of Resibufogenin that the active ingredient in Chansu, 2012, China, Shandong University of Traditional Chinese Medicine
10. Lu Z, Xu A, Yuan X, Anticancer effect of resibufogenin on gastric carcinoma cells through the phosphoinositide 3-kinase/protein kinase B/glycogen synthase kinase 3beta signaling pathway: Oncol Lett, 2018; 16(3); 3297-302
11. Zhou Y, Hong Z, Jin K, Resibufogenin inhibits the malignant characteristics of multiple myeloma cells by blocking the PI3K/Akt signaling pathway: Exp Ther Med, 2022; 24(1); 441
12. Shen LD, Qi WH, Bai JJ, Resibufogenin inhibited colorectal cancer cell growth and tumorigenesis through triggering ferroptosis and ROS production mediated by GPX4 inactivation: Anat Rec (Hoboken), 2021; 304(2); 313-22
13. Ichikawa M, Sowa Y, Iizumi Y, Resibufogenin induces G1-phase arrest through the proteasomal degradation of cyclin D1 in human malignant tumor cells: PLoS One, 2015; 10(6); e0129851
14. Han Q, Ma Y, Wang H, Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis: J Transl Med, 2018; 16(1); 201
15. Zhang X, Yao Z, Xue Z, Resibufogenin targets the ATP1A1 signaling cascade to induce G2/M phase arrest and inhibit invasion in glioma: Front Pharmacol, 2022; 13; 855626
16. Yang T, Jiang YX, Wu Y, Resibufogenin suppresses triple-negative breast cancer angiogenesis by blocking VEGFR2-mediated signaling pathway: Front Pharmacol, 2021; 12; 682735
17. Gao Y, Xu Z, Li X, Resibufogenin, one of bufadienolides in toad venom, suppresses LPS-induced inflammation via inhibiting NF-kappaB and AP-1 pathways: Int Immunopharmacol, 2022; 113(Pt A); 109312
18. El-Seedi HR, Yosri N, El-Aarag B, Chemistry and the potential antiviral, anticancer, and anti-inflammatory activities of cardiotonic steroids derived from toads: Molecules, 2022; 27(19); 6586
19. Jin YH, Jeon S, Lee J, Broad spectrum antiviral properties of cardiotonic steroids used as potential therapeutics for emerging coronavirus infections: Pharmaceutics, 2021; 13(11); 1839
20. Chen JW, Xu L, Sun SY, Identification of cinobufagin and resibufogenin as inhibitors of enterovirus 71 infection: Chemical Research in Chinese Universities, 2014; 30(6); 953-58
21. Xie JT, Dey L, Wu JA, Lowell TK, Yuan CS, Cardiac toxicity of resibufogenin: Electrophysiological evidence: Acta Pharmacol Sin, 2001; 22(4); 289-97
22. Danchuk S, Sukhanov S, Horvat D, Effects of resibufogenin in experimental hypertension: Am J Nephrol, 2008; 28(1); 8-13
23. Wei WL, An YL, Li ZW, Simultaneous determination of resibufogenin and its eight metabolites in rat plasma by LC-MS/MS for metabolic profiles and pharmacokinetic study: Phytomedicine, 2019; 60; 152971
24. Ning J, Yu ZL, Hu LH, Characterization of phase I metabolism of resibufogenin and evaluation of the metabolic effects on its antitumor activity and toxicity: Drug Metab Dispos, 2015; 43(3); 299-308
25. Machado KDC, Sousa LQ, Lima DJB, Marinobufagin, a molecule from poisonous frogs, causes biochemical, morphological and cell cycle changes in human neoplasms and vegetal cells: Toxicol Lett, 2018; 285; 121-31
26. Strauss M, Smith W, Fedorova OV, Schutte AE, The Na(+)K(+)-ATPase inhibitor marinobufagenin and early cardiovascular risk in humans: A review of recent evidence: Curr Hypertens Rep, 2019; 21(5); 38
27. Xie JT, January CT, The monophasic action potential technique and its application in cardiac electropharmacology: Methods Find Exp Clin Pharmacol, 1993; 15(8); 557-67
28. Xie JT, Cunningham P, Shorofsky S, Induction of delayed afterdepolarizations and triggered arrhythmias in isolated Purkinje fibers: Comparison of resibufogenin and acetylstrophanthidin: Zhongguo Yao Li Xue Bao, 1994; 15(2); 97-102
29. Xie JT, Wang H, Attele AS, Yuan CS, Effects of resibufogenin from toad venom on isolated Purkinje fibers: Am J Chin Med, 2000; 28(2); 187-96
30. Li CH, Zhou Y, Tu PF, Zeng KW, Jiang Y, Natural carbazole alkaloid murrayafoline A displays potent anti-neuroinflammatory effect by directly targeting transcription factor Sp1 in LPS-induced microglial cells: Bioorg Chem, 2022; 129; 106178
31. Ye S, Luo W, Khan ZA, Celastrol attenuates angiotensin ii-induced cardiac remodeling by targeting STAT3: Circ Res, 2020; 126(8); 1007-23
32. Dai X, Yin C, Zhang Y, Osthole inhibits triple negative breast cancer cells by suppressing STAT3: J Exp Clin Cancer Res, 2018; 37(1); 322
33. Jiang J, Wang R, Wei GW, GGL-Tox: Geometric graph learning for toxicity prediction: J Chem Inf Model, 2021; 61(4); 1691-700
34. MohammadiPeyhani H, Chiappino-Pepe A, Haddadi K, NICEdrug.ch, a workflow for rational drug design and systems-level analysis of drug metabolism: Elife, 2021; 10; e65543
35. Ainurofiq A, Choiri S, Azhari MA, Improvement of meloxicam solubility using a beta-cyclodextrin complex prepared via the kneading method and incorporated into an orally disintegrating tablet: Adv Pharm Bull, 2016; 6(3); 399-406
36. Dadej A, Wozniak-Braszak A, Bilski P, Modification of the release of poorly soluble sulindac with the APTES-modified SBA-15 mesoporous silica: Pharmaceutics, 2021; 13(10); 1693
37. Iqbal B, Ali A, Ali J, Recent advances and patents in solid dispersion technology: Recent Pat Drug Deliv Formul, 2011; 5(3); 244-64
38. Huang BB, Liu DX, Liu K, Wu G, Application of solid dispersion technique to improve solubility and sustain release of emamectin benzoate: Molecules, 2019; 24(23); 4315
39. Jadhav KR, Shaikh IM, Ambade KW, Kadam VJ, Applications of microemulsion based drug delivery system: Curr Drug Deliv, 2006; 3(3); 267-73
40. Nicolaos G, Crauste-Manciet S, Farinotti R, Brossard D, Improvement of cefpodoxime proxetil oral absorption in rats by an oil-in-water submicron emulsion: Int J Pharm, 2003; 263(1–2); 165-71
41. Saokham P, Muankaew C, Jansook P, Loftsson T, Solubility of cyclodextrins and drug/cyclodextrin complexes: Molecules, 2018; 23(5); 1161
42. Manne ASN, Hegde AR, Raut SY, Hot liquid extrusion assisted drug-cyclodextrin complexation: A novel continuous manufacturing method for solubility and bioavailability enhancement of drugs: Drug Deliv Transl Res, 2021; 11(3); 1273-87
43. Wong KH, Yang D, Chen S, Development of nanoscale drug delivery systems of dihydroartemisinin for cancer therapy: A review: Asian J Pharm Sci, 2022; 17(4); 475-90
44. Da Silva FLO, Marques MBF, Kato KC, Carneiro G, Nanonization techniques to overcome poor water-solubility with drugs: Expert Opin Drug Discov, 2020; 15(7); 853-64
45. Wang K, Qi J, Weng T, Enhancement of oral bioavailability of cyclosporine A: comparison of various nanoscale drug-delivery systems: Int J Nanomedicine, 2014; 9; 4991-99
In Press
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952