26 June 2020: Clinical Research
Hepatitis B Surface Antigen (HBsAg) Kinetics in Chronic Hepatitis B Patients during Peginterferon Treatment
Kaifa Wang1AEG, Guangyu Huang2BCF*, Yagang Chen2DF, Yuming Wang34BFDOI: 10.12659/MSM.921487
Med Sci Monit 2020; 26:e921487
Abstract
BACKGROUND: Hepatitis B surface antigen (HBsAg) loss/seroconversion is considered to be an ideal endpoint for antiviral therapy and a final therapeutic target for chronic hepatitis (CHB). This study aimed to evaluate the HBsAg kinetics in CHB patients during peginterferon-α (Peg-IFN-α) treatment.
MATERIAL AND METHODS: A retrospective cohort study was performed using a case database, which included 151 patients who received Peg-IFN-α treatment and with HBsAg load of no less than 4 time points from May 1, 2018 to January 31, 2019. The HBsAg kinetic pattern was analyzed by Q-type clustering, and a clinical prognostic empirical model was constructed based on the HBsAg kinetic pattern of uncured patients.
RESULTS: Changes of HBsAg in 13 functionally cured patients were attributed to 3 kinetic patterns by cluster analysis, and there was a significant positive correlation between functionally cure time and baseline HBsAg. For uncured 116 patients with treatment duration longer than or equal to 56 days, 5 HBsAg kinetic patterns were obtained by cluster analysis, and the clinical prognosis empirical model was established. Finally, 13 new functionally cured patients preliminarily confirmed the rationality of the proposed empirical model.
CONCLUSIONS: According to empirical model, we recommend that the therapeutic regime should be timely adjusted to improve sustained response rate and reduce patients’ medical burden for patients with second (Z type) and fifth (Z+W type) kinds of patterns. While for the rest of patterns’ patients, it is recommended to continue treatment for a longer period of time to achieve the desired therapeutic goal.
Keywords: Hepatitis B Surface Antigens, Hepatitis, Chronic, Kinetics, Antiviral Agents, Cluster Analysis, Duration of Therapy, Hepatitis B, Chronic, Interferon alpha-2, Interferon-alpha, Polyethylene Glycols, Recombinant Proteins, Time Factors
Background
Hepatitis B virus (HBV) infection remains a major global public health problem, especially in developing countries, including China. Chronic hepatitis B (CHB) is an important risk factor for developing cirrhosis and hepatocellular carcinoma. Therefore, the goal of CHB treatment is to delay the progression of the disease, reduce the incidence of cirrhosis and hepatocellular carcinoma, and improve the survival rate of patients [1–4].
In clinical practice, the existing antiviral drugs of CHB mainly include 2 types, one is immunomodulators, such as peginterferon-a a2a/b (Peg-IFN-a), and another is the drug with direct antiviral effects, such as nucleos(t)ide analogues (NAs). However, due to the persistence of covalently closed circular DNA (cccDNA) in the nucleus of infected hepatocytes and the lack of therapeutic drugs directed against cccDNA, complete elimination of the virus from the host, i.e., complete sterilizing cure, is almost impossible to achieve clinically [5–7]. Therefore, the concept of functional cure, also referred to as clinical or immunologic cure, was recently proposed and widely recognized in the clinic [5–7]. The clinical efficacy index of hepatitis B functional cure mainly refers to hepatitis B surface antigen (HBsAg). The HBsAg load at the initial treatment is the baseline antigen level, and the standard of functional cure is HBsAg load lower than or equal to 0.05 IU/mL [5–7].
Obviously, HBsAg kinetics during treatment is closely related to the outcome of treatment, and it is important to predict the effect of long-term treatment within a short period of time. A retrospective study [8] found that CHB patients with a baseline HBsAg ≤25 000 IU/mL had a higher rate of negative conversion of hepatitis B e antigen (HBeAg, 35% versus 16.3%,
Since May 1, 2018, we started to use Peg-IFN-a to treat CHB in The Fourth Affiliated Hospital Zhejiang University School of Medicine (ZJU4H). More than 200 patients with CHB have been admitted. The present study systematically analyzed HBsAg kinetics during the interferon therapy based on retrospective sample data. Preliminary questions and evidence such as whether long-term sustained interferon therapy is helpful to control infection progression, whether the functional cure time is related to the baseline HBsAg, the HBsAg kinetics during interferon therapy and how to predict the clinical prognosis of different HBsAg kinetic patterns were investigated and provided in the current study under the setting of interferon treatment of CHB.
Material and Methods
STUDY DESIGN AND SETTING:
A retrospective cohort study was conducted using the case database from ZJU4H. Informed consent was received from patients and the experiment in the study was approved by the Ethical Committee of the hospital.
STUDY POPULATION:
In the case bank of ZJU4H, from May 1, 2018 to January 31, 2019, there were 151 patients, with a minimum of 4 time points at their HBsAg levels, among them 13 cases reached the functional cure standard, and 116 uncured patients with more than or equal to 8 time points (i.e., treatment time longer than or equal to 56 days, approximately 2 months). The HBsAg kinetic pattern was analyzed for the functionally cured patients and uncured patients, respectively. Based on the kinetic patterns, a clinical prognostic empirical model was constructed, which was preliminarily validated using 13 new functionally cured patients by May 4, 2019.
DATA COLLECTION AND DEFINITION:
Effective data included case ID, gender, age, clinical diagnosis, time of detection, HBsAg load, and the baseline of HBeAg, alanine aminotransferase (ALT) and neutrophils. HBsAg and HBeAg were detected by Architecti 2000 Chemiluminescence (Abbott, USA) using Abbott AxSYM Chemiluminescence (Abbott, USA). ALT was quantified by conventional biochemical analyzer, while neutrophils was quantified by conventional blood analyzer. HBV DNA was detected by Fosun hepatitis B virus nucleic acid detection reagent (magnetic bead method hypersensitivity reagent). HBV genotypes, not detected, were commonly B and C in China.
Including criteria: 1) CHB patients have been treated by NAs for more than 12 months, at the same time with HBsAg ≤1500 IU/mL. 2) HBeAg negative; as long as HBsAg negative conversion occurs, functional cure standards on HBeAg-negative patients can be achieved. When HBeAg is positive, even HBsAg converts to negative, but HBeAg is not negative, functional cure cannot be achieved. Hence, only HBeAg negative patients were enrolled to make the research more rigorous. 3) HBV DNA <100 IU/mL.
Excluding criteria was as follows: 1) patients allergic to interferon; 2) patients with decompensated cirrhosis, or who had experienced decompensation cirrhosis; 3) leukocytes in the peripheral blood is <3.5×109/L and/or platelets is <80×109/L; 4) cardiovascular diseases, lung, kidney, brain lesions and fundus lesions in critical organs; 5) patients with autoimmune disorders, mental illness, diabetes, and thyroid dysfunction (hyperactivity or low); 6) patients diagnosed with or suspected of liver cancer and other malignant tumors; 7) patients with organ transplantation or are prepared for organ transplantation; 8) patients using immunosuppressants; 9) pregnancy or planning pregnancy within 2 years; 10) patients addicted to alcohol or drug; 11) patients with HIV, HCV, HAV, HEV infection or other liver diseases; and 12) the attending physician believed that there were other situations in which interferon therapy should not be used.
OUTCOME MEASUREMENT:
According to the latest expert consensus on the concept of functional cure [5–7], a functional cure is diagnosed when the HBsAg load is lower than or equal to 0.05 IU/mL. HBsAg was detected by Architecti 2000 Chemiluminescence (Abbott, USA) using Abbott AxSYM Chemiluminescence (Abbott, USA).
STATISTICAL ANALYSIS:
Note that the duration of treatment varies from patient to patient, and the average duration of treatment for 13 functionally cured patients out of a total of 151 patients was 96.38±36.29 days. We used 100 days as the reference treatment duration, and an affine transformation between actual duration and reference duration was performed on the detection time points of each patient. As a result, the treatment duration of each patient after the transformation was unified into the reference duration, and then the linear interpolation method was used to obtain the patients’ daily HBsAg from the start of treatment to the end of reference duration. Note that the HBsAg kinetics is mainly related to the profile of HBsAg in each patient, regardless of their distance. The unified HBsAg per patient per day was used to obtain the corresponding symbol sequence (the increase was recorded as 1, stable was recorded as 0, and the decrease was recorded as −1). Then, the correlation was used as the metric between the symbol sequences of HBsAg, and the centroid clustering method of Q-type cluster analysis was used to explore its kinetic pattern.
The Shapiro-Wilk test was used to check the normality of quantitative data. Quantitative data were expressed as mean±standard deviation (SD) or median with its corresponding first and third quartiles (Q1–Q3) according to whether they followed normal distribution, and categorical data were expressed as frequencies or percentages. Categorical data were subjected to chi-square test. If the total sample size was smaller than 40 or the theoretical frequency was smaller than 5, Fisher’s exact test was further performed. Independent sample
The data were processed by Excel 2017 and Matlab R2018a. The statistical test was performed by IBM SPSS Statistics Version 22, and
Results
DEMOGRAPHIC INFORMATION:
From May 1, 2018 to January 31, 2019, a total of 151 patients with HBsAg levels of no less than 4 time points, and the average age was 36.79±6.99 years, including 120 males (average age 37.54±6.57 years) and 31 females (average age 33.90±7.77 years).
According to the functional cure criteria, a total of 13 patients met the criteria in our statistical cycle (Table 1), of which 6 patients had baseline HBsAg of no higher than 10 IU/mL (all male, average age 38.83±6.67 years), minimal baseline HBsAg was 0.1 IU/mL, while the maximum baseline HBsAg was 3.4 IU/mL. The shortest cure time was 42 days, while the longest was 108 days. The remaining 7 patients had baseline HBsAg level higher than 10 IU/mL (2 females, 5 males, average age 34.43±4.14 years), the minimal baseline HBsAg was 34.65 IU/mL, whereas the maximum baseline HBsAg was 550 IU/mL, the shortest cure time was 77 days, while the longest cure time was 167 days.
During the statistical cycle, a total of 138 patients did not meet the functional cure criteria, and their age, gender, and baseline HBsAg are shown in Table 2. Note that the shortest treatment duration in the 138 uncured patients was only 21 days, and the analysis of such patient has limited clinical significance except for more information interference. We further screened the uncured patients with more than or equal to 8 time points (i.e., treatment time longer than or equal to 56 days, approximately 2 months). As a result, 116 uncured patients were included in the follow-up kinetic pattern analysis, and the distributions of age, sex, baseline HBsAg are also listed in Table 2.
RELATIONSHIP BETWEEN CURE DURATION AND BASELINE HBSAG:
According to the Shapiro-Wilk test, the baseline HBsAg of the 13 functionally cured patients did not subject to the normal distribution (
Based on the Shapiro-Wilk test again, the cure durations of both patients with baseline HBsAg less than 10 IU/mL (n=6) and greater than 10 IU/mL (n=7) were subject to normally distributed (P>0.05). Thus, t-test was performed, and the results showed that their difference was statistically significant (Table 3), suggesting that the higher baseline HBsAg means the longer functional cure duration.
HBSAG KINETICS OF THE FUNCTIONALLY CURED PATIENTS: The original time series of HBsAg in all 13 functionally cured patients are shown in Supplementary Figure 1. Taking the reference treatment duration as 100 days, performing the affine transformation and linear interpolation, we can find the affine transformation in time do not change the pattern of HBsAg itself (Supplementary Figure 2).
Q-type clustering analysis of their symbol sequences revealed that all HBsAg in 13 functionally cured patients can be attributed to 3 kinetic patterns. Both patterns 1 and 2 contain 6 patients, while pattern 3 contains only 1 patient (ID 103232). In order to comprehensively characterize the kinetic patterns of each type, we first use the median time series of daily HBsAg of all patients to illustrate their kinetic patterns (Figure 1A, 1C, 1E). Then, according to clinical experience, we focus on the daily change proportion of HBsAg relative to its baseline HBsAg, which is defined as 100×(day ith antigen amount–baseline antigen amount)/baseline antigen amount. A positive value indicates an increase in antigen, while a negative value indicates a decrease in antigen. Similarly, the median time series of daily change proportions of all patients were used to illustrate their kinetic patterns (Figure 1B, 1D, 1F). From Figure 1, 3 kinetic patterns can be summarized as follows: 1) a sharp rise at first, then continue to fall (inverted V type, Figure 1A, 1B); 2) a slow rise at first, and then a wave/platform period, finally, a continuous decline (M or inverted U type, Figure 1C, 1D); 3) continued decline (L type, Figure 1E, 1F).
Furthermore, to understand the differences between HBsAg kinetic patterns of the functional cured patients, stratified analysis was made on the baseline of HBeAg, ALT and neutrophils. Because there was only 1 patient in pattern 3 (L type), Mann-Whitney U test was performed between pattern 1 (inverted V type) and pattern 2 (M or inverted U type). The results showed that there was no significant difference between pattern 1 and 2 (Table 4, P>0.05).
TIME SERIES OF HBSAG LEVELS: For the screened 116 cases of uncured patients, their time series of HBsAg were shown in Supplementary Figure 3. Comparing with Supplementary Figure 1, we can find that the uncured patients’ HBsAg kinetics is far more complex than that in the cured patients.
HBSAG KINETICS OF THE UNCURED PATIENTS: Similar to functionally cured patients, we first unified the treatment duration to the reference duration (100 days) and then obtained all 116 uncured patients’ symbol sequences. After that, Q-type clustering analysis was performed. Considering that there may be more kinetic patterns in uncured patients, we first set the cluster number range to 3–10, and then combined with clinical experience, 5 clusters were finally selected to be studied. The distribution of patients in each cluster was shown in Table 5. One-way ANOVA showed that the age was no significant difference among the 5 clusters of patients (F(4,114)=0.648, P=0.630). Determined by Kruskal-Wallis one-way ANOVA, the difference of baseline HBsAg and length of treatment up to January 31, 2019 were statistically significant among the 5 clusters patients (χ2(4)=10.411, P=0.034 and χ2(4)=11.529, P=0.021).
Similarly, based on the daily HBsAg of each patient after affine transformation, both the median time series of daily HBsAg (Figure 2A, 2C, 2E, 2G, 2I) and change proportions (Figure 2B, 2D, 2F, 2H, 2J) of all patients were used to illustrate their kinetic patterns. From Figure 2, 5 kinetic patterns can be summarized as follows: 1) a sharp rise at first, and then continues to fall (inverted V/inverted U type, Figures 2A, 2B); 2) a relatively long refractory/platform period at first, and then slowly declines (Z-type, Figure 2C, 2D); 3) a slow rises at first, then a fluctuation period during which the level continues to gradually decrease, and finally continues to decline (M+W+M type, Figure 2E, 2F); 4) a short refractory period at first, then a sudden sharp increase, follows a short fluctuation/platform period, then quickly decreased and finally remains in platform period (inverted Z+W+Z type, Figure 2G, 2H); 5) a short refractory period at first, follows a sudden and sharp decrease, and then continued to gradually reduce during a short period of fluctuation/platform (Z+W type, Figure 2I, 2J).
Note that there were 5 kinetic patterns in the uncured patients. Based on the Kruskal-Wallis one-way ANOVA, stratified analysis for the patterns was made on the baseline of HBeAg, ALT and neutrophils. The results showed that there was no significant difference among the patterns (Table 6, P>0.05).
PROGNOSIS EMPIRICAL MODEL BASED ON THE KINETIC PATTERNS OF UNCURED PATIENTS:
Note that the goal of functional cure is to make HBsAg less than or equal to 0.05 IU/mL. Thus, a long rising or refractory/platform period during the treatment may correspond to a poor clinical outcome. Conversely, a continued gradual decline or a gradually decreased fluctuation indicates a better clinical outcome, even if there is a transient sharp rise or refractory/platform period. According to the kinetic patterns in Figure 2, with different baseline HBsAg, we can roughly propose the following prognosis empirical model: 1) patients who show kinetic pattern 1 have the most satisfactory prognosis; 2) patients who show kinetic patterns 3 or 4 have acceptable prognosis; 3) patients who show kinetic patterns 2 or 5 have poor prognosis, and may require longer treatment to have a better prognosis. Especially, for the pattern 2 (Z type), patients may require other therapeutic intervention strategies to overcome the long-term platform/refractory period, so as to achieve a better clinical outcome.
It is worth noting that there were another 13 patients in the present study who had reached the functional cure criteria up to May 4, 2019, among them 8 cases belonged to pattern 1, both patterns 3 and 4 included 2 cases, and only 1 case belonged to pattern 5 (Table 7). This was generally consistent with the aforementioned empirical model.
Discussion
During the treatment process of CHB, the detection of HBV particles in peripheral blood serves as the main indicator of complete sterilizing cure in the past, in which the kinetics of HBV particles is the focus of attention [11,12]. Nowadays, the concept of functional cure has been increasingly accepted by clinicians and become expert consensus [5–7]. Therefore, the study of kinetic pattern of HBsAg during the treatment has gradually gained more and more attention.
The present study reviewed the clinical cohort data of interferon treatment on CHB patients in ZJU4H from May 1, 2018 and analyzed the kinetics of HBsAg. According to cluster analysis, we found that there were 3 and 5 kinetic patterns in the cured and uncured patients, respectively. Furthermore, a prognosis empirical model was roughly proposed based on the kinetic patterns of uncured patients. Using the empirical model and combining the information in Table 5, we believe that long-term interferon therapy will be benefit to control HBV infection, though longer fluctuation or refractory/platform period may occur during the treatment (Figure 2C, 2D, 2I, 2J). However, according to the baseline HBsAg shown in Tables 1 and 7, we further recommend that patients with lower baseline HBsAg will have a better prognosis if they are treated by interferon therapy as early as possible, which is also verified in a recent study [10].
According to the relationship between cure duration and baseline HBsAg, we can conclude that the clinical cure duration is closely related to baseline HBsAg [13,14]. In general, the higher baseline HBsAg, the longer it takes to reach functional cure, which is consistent with the conclusions in [8,10,15].
For the 5 kinetic patterns of HBsAg in uncured patients, we found that there were statistically significant in baseline HBsAg and length of treatment up to January 31, 2019. For baseline of HBeAg, ALT and neutrophils, although current stratified analyses indicated that there was no significant difference between the 5 kinetic patterns (Table 6), we could find a trend from the absolute value of the medians in each pattern, that was, smaller baseline HBeAg/ALT and larger neutrophils might lead to better clinical prognosis. Those may be related to the difference in HBeAg seroconversion rates in patients with different baseline HBsAg during treatment [16]. Note that the safety of Peg-IFN-a treatment for 72–96 weeks has been confirmed [15] and extending Peg-IFN-a treatment to 72–96 weeks allows more patients to obtain HBsAg clearance [17]. A continue longer treatment is recommended to achieve the desired treatment endpoint except for pattern 2 (Z type) and pattern 5 (Z+W type), in which the therapeutic regime should be timely adjusted to improve the sustained response rate and reduce patients’ medical burden [7]. Furthermore, a prognosis empirical model was roughly proposed and preliminarily tested by the renewed clinical cohort data.
Though the obtained HBsAg kinetic patterns and the proposed prognosis empirical model may provide practical evidence to achieve higher therapeutic goals through continuous interferon treatment, the present study still had certain limitations. For example, as the systemic interferon therapy for CHB has been implemented for a relatively short period in our hospital, the effective sample size was still small, especially the number of functionally cured patients. Thus, we will continue to collect clinical data and obtain more robust kinetic patterns of HBsAg. Furthermore, as the sample size increases, we will consider more potential factors, such as gene stratification, etc. Those will be left to us for future study.
Conclusions
Generally speaking, during the early treatment with Peg-IFN-a, there was a significant positive correlation between functional cure time and baseline HBsAg, and the treatment is conducive to the control of the progress of virus infection, especially in patients with lower baseline HBsAg. The HBsAg level will show several specific kinetic patterns in patients under long-term interferon therapy, and the kinetic patterns are related to patients’ clinical outcome. The continuous decline or decreasing fluctuation of HBsAg means better clinical outcome, even if there was a brief sharp rise or non-response/platform period during the treatment. According to the empirical model, for patients with second (Z type) and fifth (Z+W type) kinds of patterns, we recommend that the therapeutic regime should be timely adjusted to improve the sustained response rate and reduce patients’ medical burden. While for the rest of patterns’ patients, it is recommended to continue treatment for a longer period of time to achieve the desired therapeutic goal.
Figures
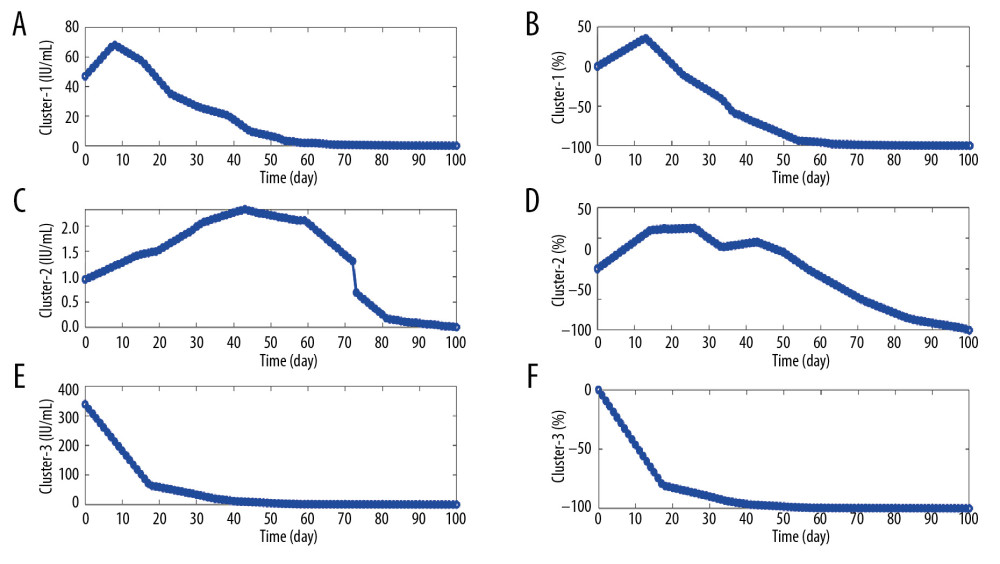 Figure 1. Illustration of the kinetic patterns of HBsAg in functionally cured patients. (A, C, E) The median time series of daily HBsAg for each pattern. (B, D, F) The median time series of daily change proportions for each pattern.
Figure 1. Illustration of the kinetic patterns of HBsAg in functionally cured patients. (A, C, E) The median time series of daily HBsAg for each pattern. (B, D, F) The median time series of daily change proportions for each pattern. 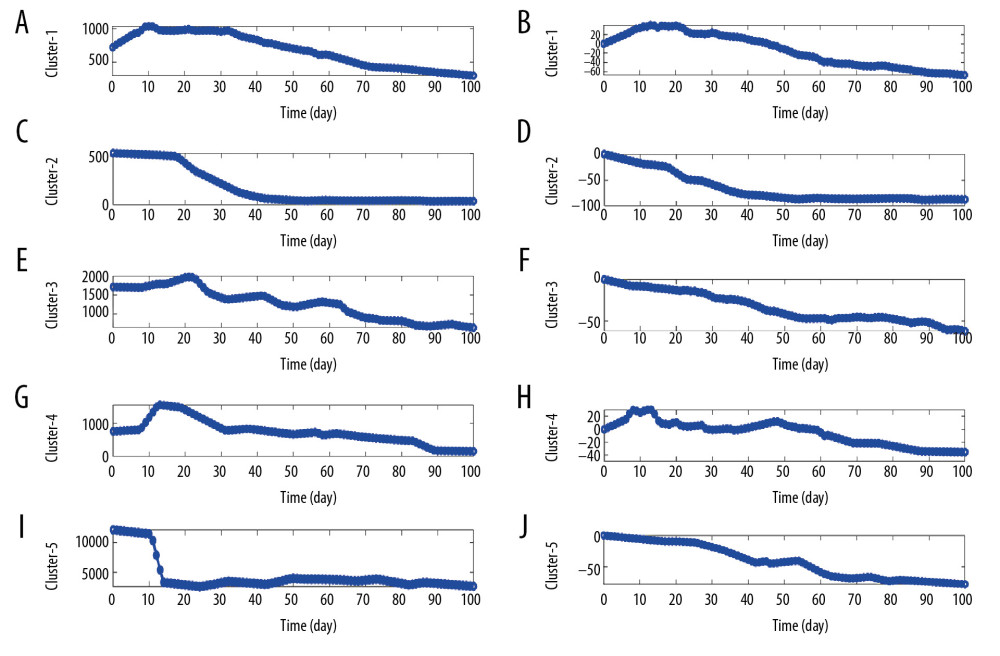 Figure 2. Illustration of the kinetic patterns of HBsAg in uncured patients. (A, C, E, G, I) The median time series of daily HBsAg for each pattern. (B, D, F, H, J) The median time series of daily change proportions for each pattern.
Figure 2. Illustration of the kinetic patterns of HBsAg in uncured patients. (A, C, E, G, I) The median time series of daily HBsAg for each pattern. (B, D, F, H, J) The median time series of daily change proportions for each pattern. Tables
Table 1. Basic information of functionally cured patients.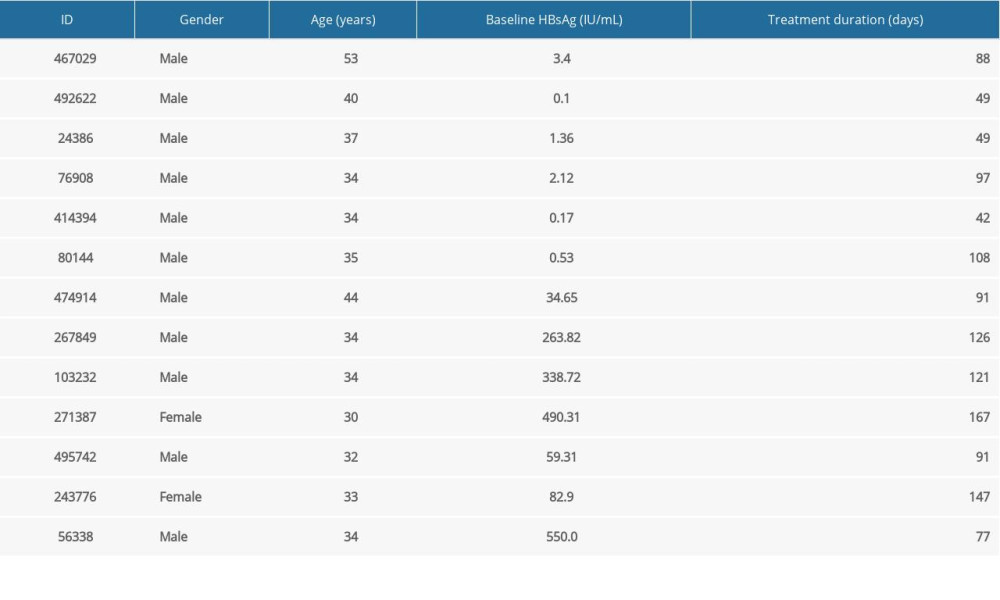 Table 2. The distributions of age, sex, baseline HBsAg for uncured patients.
Table 2. The distributions of age, sex, baseline HBsAg for uncured patients.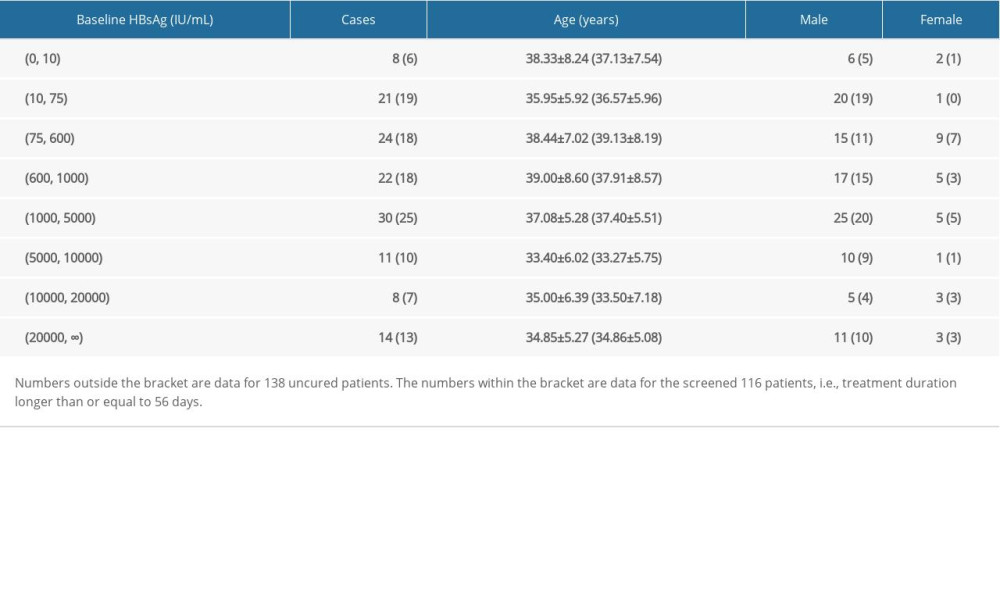 Table 3. Comparison of cure duration between different baseline HBsAg groups (mean±standard deviation).
Table 3. Comparison of cure duration between different baseline HBsAg groups (mean±standard deviation).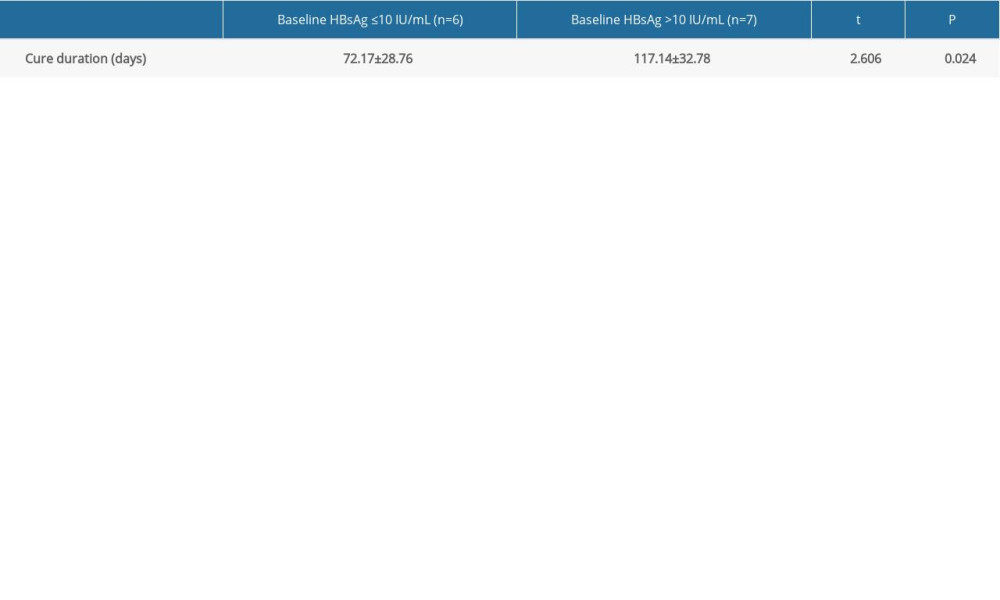 Table 4. Stratified analysis for HBsAg Kinetics of the functionally cured patients.
Table 4. Stratified analysis for HBsAg Kinetics of the functionally cured patients.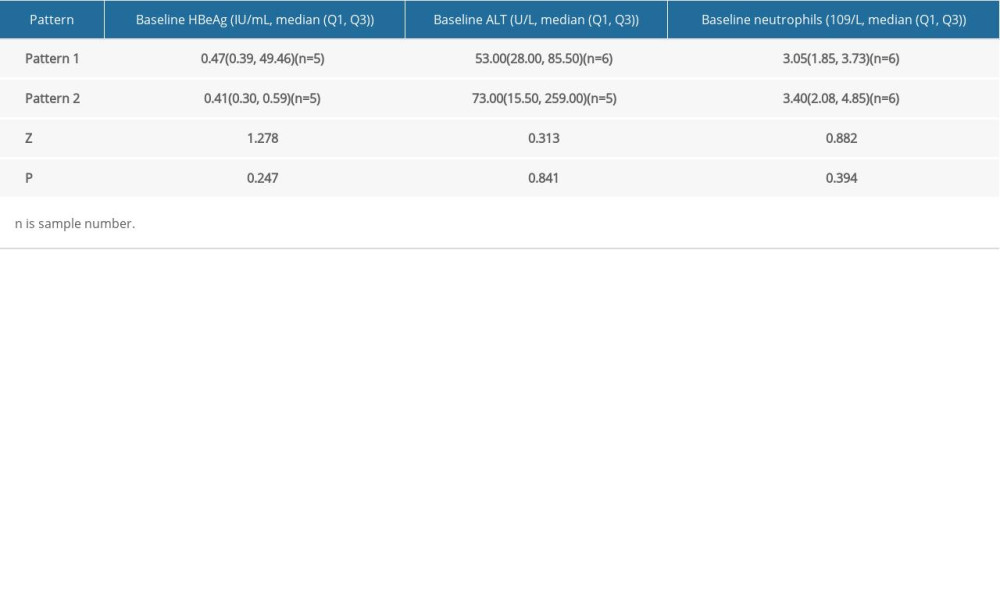 Table 5. Distribution of five clusters from 116 uncured patients.
Table 5. Distribution of five clusters from 116 uncured patients.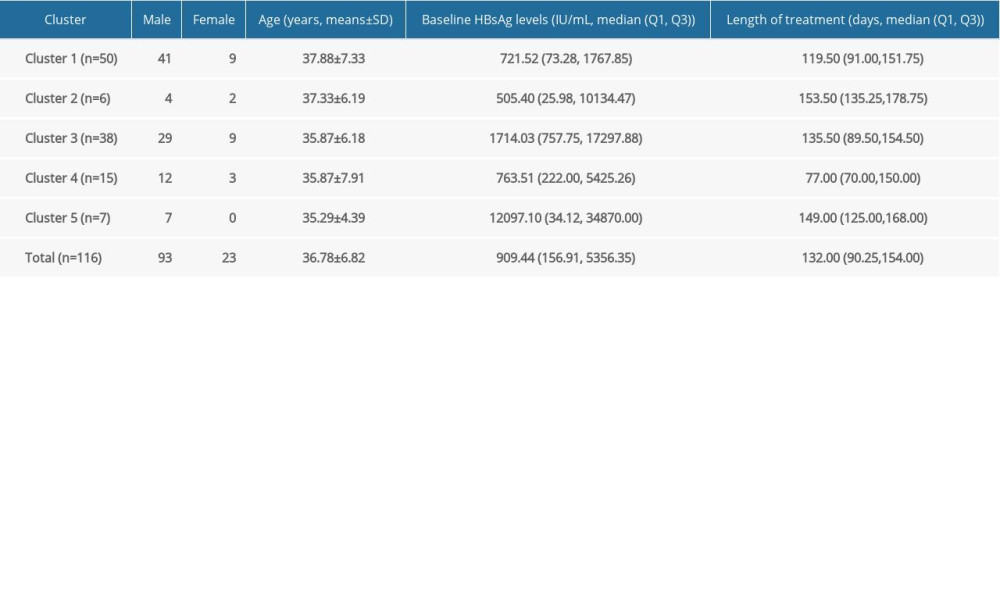 Table 6. Stratified analysis for HBsAg kinetics of the uncured patients.
Table 6. Stratified analysis for HBsAg kinetics of the uncured patients.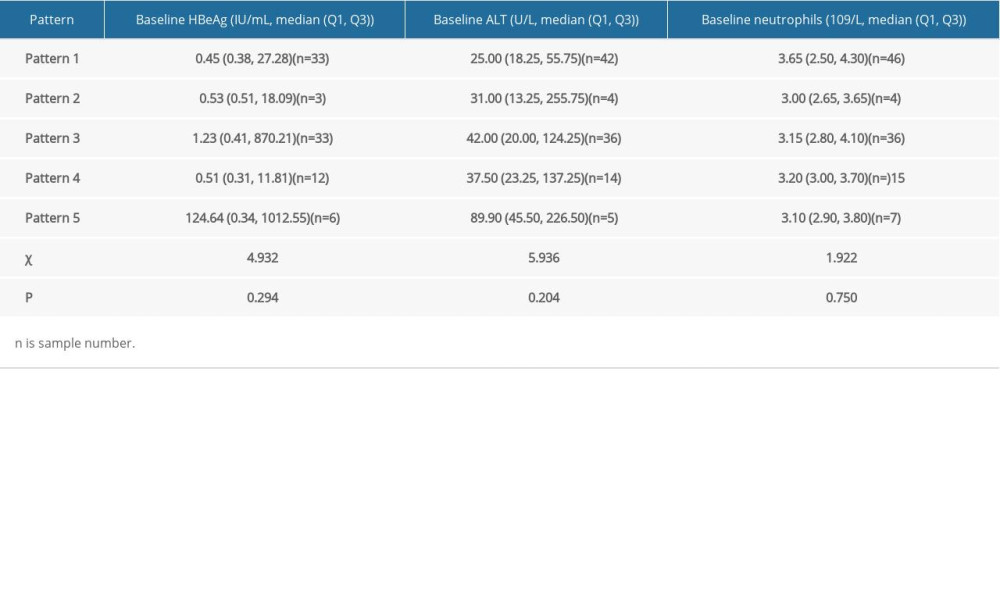 Table 7. Information of 13 new functionally cured patients.
Table 7. Information of 13 new functionally cured patients.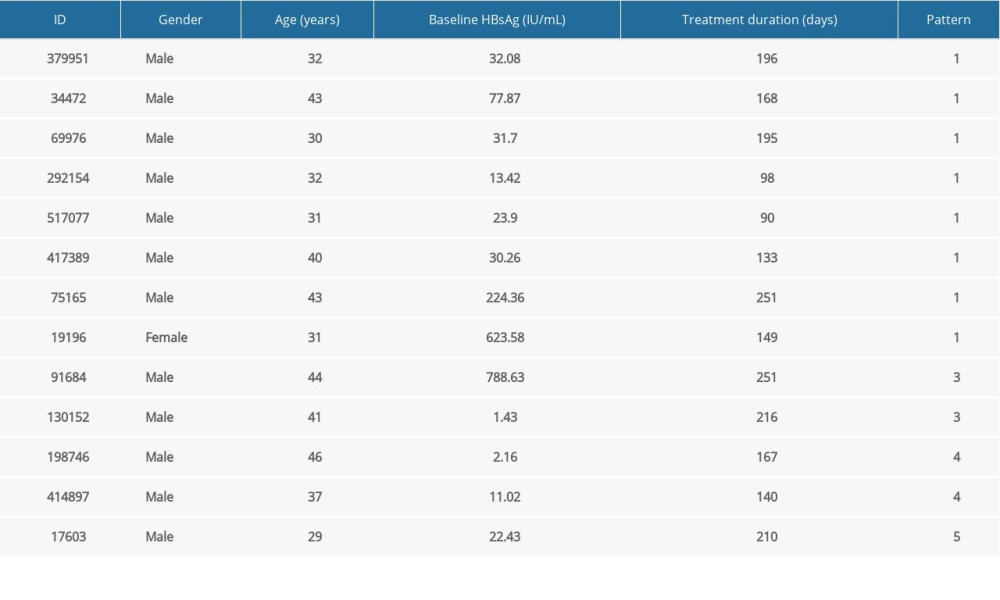
References
1. Sarin SK, Kumar M, Lau GK, Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update: Hepatol Int, 2016; 10(1); 1-98
2. Terrault NA, Lok ASF, McMahon BJ, Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance: Hepatology, 2018; 67(4); 1560-99
3. European Association for the Study of the Liver, European Association for the Study of the Liver, EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection: J Hepatol, 2017; 67(2); 370-98
4. Hou J, Wang G, Wang F, Guideline of prevention and treatment for chronic hepatitis B (2015 update): J Clin Transl Hepatol, 2017; 5(4); 297-318
5. Lok AS, Zoulim F, Dusheiko G, Ghany MG, Hepatitis B cure: From discovery to regulatory approval: J Hepatol, 2017; 67(4); 847-61
6. Zhang W, Zhang D, Dou X, Consensus on pegylated interferon alpha in treatment of chronic hepatitis B: J Clin Transl Hepatol, 2018; 6(1); 1-10
7. Ning Q, Wu D, Wang GQ, Roadmap to functional cure of chronic hepatitis B: An expert consensus: J Viral Hepat, 2019; 26(10); 1146-55
8. Chan HLY, Messinger D, Papaptheodoridis GV, A baseline tool for predicting response to peginterferon alfa in HBeAg, positive patients with chronic hepatitis B: Aliment Pharmacol Ther, 2018; 48(5); 547-55
9. Wang YCYS, Su CW, Predictors of response to pegylated interferon in chronic hepatitis B: A real-world hospital-based analysis: Sci Rep, 2016; 6; 29605
10. Chan HLY, Chan FWS, Hui AJ, Switching to peginterferon for chronic hepatitis B patients with hepatitis B e antigen seroconversion on entecavir – A prospective study: J Viral Hepat, 2019; 26(1); 126-35
11. Liu B, Li J, Cairns MJ, Identifying miRNAs, targets and functions: Brief Bioinform, 2014; 15(1); 1-19
12. Tang LSY, Covert E, Wilson E, Kottilil S, Chronic hepatitis B infection: A review: JAMA, 2018; 319(17); 1802-13
13. Honer Zu Siederdissen C, Cornberg M, The role of HBsAg levels in the current management of chronic HBV infection: Ann Gastroenterol, 2014; 27(2); 105-12
14. Martinot-Peignoux M, Lapalus M, Asselah T, Marcellin P, HBsAg quantification: Useful for monitoring natural history and treatment outcome: Liver Int, 2014; 34(Suppl 1); 97-107
15. Sun J, Ma H, Xie Q, Response-guided peginterferon therapy in patients with HBeAg-positive chronic hepatitis B: A randomized controlled study: J Hepatol, 2016; 65(4); 674-82
16. Piratvisuth T, Marcellin P, Popescu M, Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients: Hepatol Int, 2013; 7(2); 429-36
17. Hu PDX, Xie Q, High HBsAg loss rate in HBeAg loss CHB patients SWITCH from NUC to Peg-INF alfa-2a (NEW SWITCH study): Hepatol Int, 2017; 11; S1-1093
Figures
 Figure 1. Illustration of the kinetic patterns of HBsAg in functionally cured patients. (A, C, E) The median time series of daily HBsAg for each pattern. (B, D, F) The median time series of daily change proportions for each pattern.
Figure 1. Illustration of the kinetic patterns of HBsAg in functionally cured patients. (A, C, E) The median time series of daily HBsAg for each pattern. (B, D, F) The median time series of daily change proportions for each pattern. Figure 2. Illustration of the kinetic patterns of HBsAg in uncured patients. (A, C, E, G, I) The median time series of daily HBsAg for each pattern. (B, D, F, H, J) The median time series of daily change proportions for each pattern.
Figure 2. Illustration of the kinetic patterns of HBsAg in uncured patients. (A, C, E, G, I) The median time series of daily HBsAg for each pattern. (B, D, F, H, J) The median time series of daily change proportions for each pattern. Tables
 Table 1. Basic information of functionally cured patients.
Table 1. Basic information of functionally cured patients. Table 2. The distributions of age, sex, baseline HBsAg for uncured patients.
Table 2. The distributions of age, sex, baseline HBsAg for uncured patients. Table 3. Comparison of cure duration between different baseline HBsAg groups (mean±standard deviation).
Table 3. Comparison of cure duration between different baseline HBsAg groups (mean±standard deviation). Table 4. Stratified analysis for HBsAg Kinetics of the functionally cured patients.
Table 4. Stratified analysis for HBsAg Kinetics of the functionally cured patients. Table 5. Distribution of five clusters from 116 uncured patients.
Table 5. Distribution of five clusters from 116 uncured patients. Table 6. Stratified analysis for HBsAg kinetics of the uncured patients.
Table 6. Stratified analysis for HBsAg kinetics of the uncured patients. Table 7. Information of 13 new functionally cured patients.
Table 7. Information of 13 new functionally cured patients. Table 1. Basic information of functionally cured patients.
Table 1. Basic information of functionally cured patients. Table 2. The distributions of age, sex, baseline HBsAg for uncured patients.
Table 2. The distributions of age, sex, baseline HBsAg for uncured patients. Table 3. Comparison of cure duration between different baseline HBsAg groups (mean±standard deviation).
Table 3. Comparison of cure duration between different baseline HBsAg groups (mean±standard deviation). Table 4. Stratified analysis for HBsAg Kinetics of the functionally cured patients.
Table 4. Stratified analysis for HBsAg Kinetics of the functionally cured patients. Table 5. Distribution of five clusters from 116 uncured patients.
Table 5. Distribution of five clusters from 116 uncured patients. Table 6. Stratified analysis for HBsAg kinetics of the uncured patients.
Table 6. Stratified analysis for HBsAg kinetics of the uncured patients. Table 7. Information of 13 new functionally cured patients.
Table 7. Information of 13 new functionally cured patients. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








