06 July 2020: Clinical Research
Comparison on the Efficacy of Double Tract Gastric Interposition Reconstruction Versus Jejunal Interposition Reconstruction After Proximal Gastrectomy
Chao Yue1A, Rui Peng1B, Wei Wei1D, Bin Zhou1F, Xu Wen1F, Rongmin Gu1G, Xuezhi Ming1B, Gang Li1E*, Huanqiu Chen1EDOI: 10.12659/MSM.922504
Med Sci Monit 2020; 26:e922504
Abstract
BACKGROUND: This study aimed to compare the efficacy of antrum-preserving double tract gastric interposition reconstruction (ADGR) versus antrum-preserving double tract jejunal interposition reconstruction (ADJR) after proximal gastrectomy (PG).
MATERIAL AND METHODS: In a retrospective study, 62 cases of proximal gastric cancer undergoing proximal gastrectomy were divided into an ADJR group (n=32) and an ADGR group (n=30) according to reconstruction methods. Perioperative outcomes and postoperative complications were compared between the 2 groups, and the changes in hemoglobin (Hb), total protein (TP), body weight, and quality of life (QOL) were observed at 1, 3, 6, and 12 months postoperatively. Endoscopy was given at 12 months postoperatively for assessing reflux esophagitis and residual food.
RESULTS: Differences were indistinct in the 2 groups regarding the operation time, intraoperative blood loss, postoperative length of stay (LOS), first flatus time, and postoperative complications (P>0.05). At 1, 3, 6, and 12 months after operation, no evident differences were shown between the 2 groups regarding weight loss and Visick scores (P>0.05). Compared with the ADJR group, the Hb level at 6 and 12 months after operation and TP level at 12 months after operation were increased markedly in the ADGR group (P<0.05). No apparent difference was detected between the 2 groups in reflux esophagitis (P=0.467). The incidence of residual food in the ADGR group was significantly lower than that in the ADJR group (6.67% versus 31.25%, P=0.014).
CONCLUSIONS: ADGR was superior to ADJR in improving nutritional status and preventing residual food of patients with proximal gastric cancer after proximal gastrectomy.
Keywords: Gastrectomy, Nutritional Status, Anastomosis, Surgical, Postoperative Complications, Quality of Life, Reconstructive Surgical Procedures
Background
To date, gastric cancer is still the third leading cause of cancer-related deaths globally [1]. It was estimated there were 1 033 701 new cases of gastric cancer and 782 685 deaths in 2018 [2]. Despite an overall decline in its incidence, that of proximal (cardia/gastro-esophageal) gastric cancer, which refers to a tumor located within the proximal third of the stomach, has persistently increased over past 2 decades [3,4]. By comparison to distal (corpus and antrum) gastric cancer, the prognosis of proximal gastric cancer is usually worse because it is more aggressive and more likely to be at an advanced stage at the time of diagnosis [5,6].
Total gastrectomy and proximal gastrectomy, the major resection types for proximal gastric cancer, can both induce multiple postoperative complications like dyspepsia, malnutrition, and anemia, thus influencing the quality of life (QOL) [7–9]. Digestive tract reconstruction is one of the common procedures after gastrectomy, such as esophagogastrostomy and Roux-en-Y anastomosis [10–12]. Among these reconstruction methods, an esophagogastrostomy is most prevalent due to its simple operation, less operation time and blood loss, but esophageal reflux easily occurs [12,13]. Compared with jejunal interposition, both short- and mid-term outcomes were better in patients with early proximal gastric cancer who received jejunal pouch interposition after proximal gastrectomy [14]. In one study patients treated with jejunal interposition reconstruction after total gastrectomy were shown to have an association with a reduced risk of long-term complications and improved QOL by comparison to Roux-en-Y anastomosis [15]. There is as yet, no consensus about the optimal reconstruction method after gastrectomy.
In our study, we tried to develop a method of double tract reconstruction (DTR) for proximal gastrectomy, namely antrum-preserving double tract gastric interposition reconstruction (ADGR), and compared its efficacy with antrum-preserving double tract jejunal interposition reconstruction (ADJR) after proximal gastrectomy, with the purpose of identifying the optimal surgical technique for QOL improvement.
Material and Methods
PATIENTS:
The clinical data of 62 patients undergoing proximal gastrectomy at Jiangsu Cancer Hospital were retrospectively analyzed between December 2015 and December 2017. According to different reconstruction methods, the patients were divided into an ADJR group (n=32) and an ADGR group (n=30). All of the study participants provided informed consent. The Institutional Review Board of Jiangsu Cancer Hospital approved this study, and the approval number was 2019-010.
Inclusion criteria were as follows: 1) patients with gastric adenocarcinoma; 2) primary tumors located within the upper one-third of the stomach; 3) no macroscopic presence of lymphatic metastasis at stations #4d, #5, and #6 during operation; 4) patients who underwent either ADJR or ADGR along with D1+ lymphadenectomy following open surgery; 5) patients undergoing ADGR who were older and had poor physical condition, or those with shorter mesentery of small intestine and difficulties in anastomosis between jejunum and esophagus, or those incomplete obstruction of cardia preoperatively and obvious dilatation of distal esophagus. Exclusion criteria were as follows: 1) patients with distant metastasis, remnant gastric cancer, hematological diseases or previous neoplastic diseases; 2) patients undergoing emergency procedures, neoadjuvant treatments, or those requiring a transthoracic esophagectomy.
SURGICAL TECHNIQUES:
Japanese gastric cancer treatment guidelines were used to determine the range of lymph node dissection [16]. D1+ or D2 lymphadenectomy was performed, in which the lymph nodes at stations of 1, 2, 3a, 4a, 4sb, 7, 8a, 9, and 11p were dissected. For the remnant distal stomach, the lymph nodes along the infra-pyloric area and right gastric artery were preserved to keep the blood supply. It was not mandatory to preserve vagal nerves. In addition, antecolic anastomosis was performed on the patients in both the ADGR group and the ADJR group.
ADGR surgical procedures are shown in Figures 1 and 2. Firstly, the proximal gastrectomy was performed at least 3 cm distance to the inferior tumor margin, and the remnant gastric antrum was kept. Secondly, a circular stapler was used to mechanically perform end-to-side esophagogastrostomy (E-Gstomy), and the jejunum was separated approximately 15 cm distance to Treitz ligament. The distal jejunal limb was brought via the transverse mesocolon along the antecolic route. Thirdly, end-to-side G-Jstomy, which was 5 cm from the pylorus tube and about 10 to 15 cm below the E-Gstomy, was carried out mechanically with a 25-mm-diameter circular stapler. At last, end-to-side J-Jstomy, 30 cm below the G-Jstomy, was carried out with a circular stapler. Meanwhile, 1 to 2 drainage tubes were put around the E-Gstomy, and incisions were closed.
ADJR surgical procedures (Figure 1) were as follows: first, the remnant gastric antrum was preserved at least 3 cm far from the inferior tumor margin and closed using a linear stapler after proximal gastrectomy. The jejunum was mobilized and separated 15 cm distance approximately to Treitz ligament. Secondly, end-to-side esophagojejunostomy (E-Jstomy) was implemented between the stump closing of the distal jejunal limb and esophagus using a circular stapler, and a linear stapler was employed to close the jejunal stump. Thirdly, end-to-side gastrojejunostomy (G-Jstomy), 15 cm below the E-Jstomy, was conducted using linear staplers. Finally, circular staplers were selected to mechanically conduct end-to-side jejunojejunostomy (J-Jstomy), 20 cm below the G-Jstomy. After checking the abdominal cavity and placing 1 to 2 drainage tubes around the E-Jstomy, incisions were closed. Postoperatively, the patients in both the ADGR group and the ADJR group routinely took omeprazole enteric-coated capsules (20 mg, twice daily).
OUTCOME MEASURES:
In the perioperative period, the operation time, intraoperative blood loss, postoperative length of stay (LOS), and the first flatus time were recorded. The occurrence of postoperative complications was closely observed, including wound infection, pleural effusion, lymphorrhagia, and so on. At 1, 3, 6, and 12 months after operation, the changes of hemoglobin (Hb), total protein (TP), body weight, and QOL were observed in the 2 groups. The QOL was assessed using Visick scores. At 12 months after operation, endoscopy was given for assessing reflux esophagitis and residual food, in which reflux esophagitis was described using Los-Angeles classification. Additionally, the survival data were also recorded.
STATISTICAL ANALYSIS:
SPSS 18.0 software (IBM, Chicago, IL, USA) was used. Categorical variables described as n (%) were compared by chi-square test or Fisher’s exact test, while continuous variables manifested as mean±standard deviation (χ̄±s) were assessed using paired-samples
Results
BASELINE CHARACTERISTICS OF PATIENTS:
Between December 2015 and December 2017, 62 cases of proximal gastric cancer underwent proximal gastrectomy; patient mean age was 65.53±7.74 years old. There were 44 males and 18 females. As shown in Table 1, there was no statistical significance between the ADJR group and the ADGR group regarding age, gender, body mass index (BMI), and TNM stage (all P>0.05).
PERIOPERATIVE OUTCOMES:
The perioperative outcomes of patients in the ADJR group and the ADGR group are compared in Table 2. No evidence differences were presented between the 2 groups in operation time, intraoperative blood loss, postoperative LOS, and first flatus time (all P>0.05; Table 2).
CHANGES OF BODY WEIGHT, HB, TP, AND QOL AT DIFFERENT TIME POINTS:
No statistical significance was identified between the ADJR group and the ADGR group in the preoperative Hb and TP levels, body weight, and Visick scores (P>0.05, Table 3). At 1, 3, 6, and 12 months after operation, the weight loss in the 2 groups showed no significant difference (Figure 3A, Table 3). Both Hb and TP levels exhibited a gradual decrease in the 2 groups during the first 3 months, but at 6 and 12 months after operation, the Hb level was markedly higher in the ADGR group than in the ADJR group (Figure 3B, Table 3); the TP level was notably increased in the ADGR group at 12 months postoperatively compared with the ADJR group (Figure 3C, Table 3). During 1-month post operation, the Visick scores exhibited an obvious upward tendency. The Visick scores in the ADGR group were slightly higher than in the ADJR group at 6 months and 12 months after operation, but no significant difference was shown (Figure 3D, Table 3).
POSTOPERATIVE COMPLICATIONS:
Table 2 shows the primary postoperative complications in the 2 groups. As shown, no significant differences were detected between the 2 groups in postoperative complications (all P>0.05). At 12 months postoperatively, the endoscopic examination revealed that 1 case (3.33%) in the ADGR group and 1 case (3.13%) in the ADJR group respectively suffered from reflux esophagitis of grade C and grade B, but no apparent difference was detected in incidence of reflux esophagitis (P=0.467). Residual food was found in 2 cases (6.67%) in the ADGR group and 10 cases (31.25%) in the ADJR group. The incidence of residual food in the ADGR group was decreased obviously in comparison to the ADJR group (P=0.014).
During the 12-month follow-up, 1 case (3.33%) in the ADGR group and 1 case (3.13%) in the ADJR group were respectively subjected to recurrence and metastasis; the case in the ADGR group died.
Discussion
As a function-preserving procedure, proximal gastrectomy is valuable for proximal gastric cancer, superior to total gastrectomy [17–19]. Recently, proximal gastrectomy has been reported to be related to a high probability of anastomotic stricture, reflux esophagitis, and mortality despite good results in survival [20]. Although there are various reconstruction methods after gastrectomy, it remains controversial about the optimal reconstruction method. In this study, we developed a technique of ADGR, and compared its efficacy with ADJR after proximal gastrectomy. The results showed compared with the ADJR group, the Hb level at 6 and 12 months after operation and TP level at 12 months after operation were increased obviously in the ADGR group, and the incidence of residual food was notably lower at 12 months after operation. These findings suggest that ADGR can effectively improve the patients’ nutritional status and prevent the occurrence of residual food, superior to ADJR.
As a classical reconstruction approach after proximal gastrectomy, esophagogastrostomy has simple and safe operations. It is considered as the best reconstruction approach due to low invasiveness and better surgical outcomes [21]. Nevertheless, direct operations can easily induce severe gastroesophageal reflux, leading to different degrees of esophagitis [22]. To avoid this, a variety of anti-reflux procedures, such as gastric tube reconstruction [23], valvuloplasty plus fundoplasty [24], and esophagojejunostomy [25,26] have been used, in which esophagojejunostomy is considered a better alternative after proximal gastrectomy [27]. Study evidence suggested that the incidence of reflux esophagitis was lower in patients undergoing proximal gastrectomy with jejunal interposition than those with esophagogastrostomy [28], but the subjective symptoms like abdominal discomfort after meals were more common [29]. DTR, an approach which respectively makes end-to-side and side-to-side anastomoses between esophagus and proximal jejunum, as well as jejunum and remnant stomach, can prevent the emptying dysfunction by adding an outlet in the stomach. Although both jejunal interposition and DTR can maintain the gradual intestinal absorption and improve the QOL, DTR may achieve more stable results in intestinal absorption and hormonal secretion [30]. Additionally, Xiao et al. found that antrum preserving DTR contributed to improving the short-term QOL (lowering reflux esophagitis and promoting early recovery) of patients with esophagogastric junction adenocarcinoma after gastrectomy [31].
In the present study, we developed a novel method of ADGR after proximal gastrectomy. It is more natural than ADJR because it can maintain the physiological continuity of the alimentary tract, and the small intestine is not elevated greatly. This method is suitable for the older patients or those with shorter mesenteries. The patients in our study had a mean age of 65.53±7.74 years, and no statistical significance was shown between the ADGR group and the ADJR group in surgical outcomes and postoperative complications. Although ADGR added a gastrojejunal anastomosis, its efficacy and safety were not affected. At 12 months postoperatively, the endoscopic examination exhibited no apparent difference in reflux esophagitis between the 2 groups, but the frequency of residual food was decreased obviously in the ADGR group by comparison to the ADJR group. This may be ascribed to the fact that compared with ADJR, ADGR relatively corresponds with physiological status because it mostly keeps the original esophagus-stomach-duodenum-jejunum passage, and the remnant gastrojejunostomy corresponds to an additional food passage, thus leading to a lower risk for residual food. However, for ADJR, a segment of interposed jejunum between esophagus and remnant stomach may cause obstruction during food intake, consequently increasing the risk of residual food.
Body weight is usually used to measure the nutritional status [32]. Postoperatively, the weight loss in the first year showed no apparent difference between the 2 groups, suggesting that the 2 groups had the same effect in body weight maintenance. There may be various mechanisms that can influence body weight, such as decreased gastric acid levels and alteration of intestinal flora, but reduction of food intake is the most reliable explanation for weight loss [33]. Other nutritional indicators like Hb and TP were observed in our study. We found that Hb level at 6 at 12 months after operation and TP level at 12 months after operation were both increased markedly in the ADGR group in comparison to the ADJR group, suggesting a superiority of ADGR in improving nutritional status. During the actual surgical operation, the size of remnant stomach in patients undergoing ADGR was slightly larger than that those undergoing ADJR, which may be associated with a better nutritional status. In addition, there may be other reasons which need further studies to confirm.
At the time of performing a function-preserving gastrectomy, postoperative QOL is another key outcome. In comparison to Roux-en-Y reconstruction, proximal gastrectomy with jejunal pouch interposition reconstruction could result in a better short-term QOL (at 1 year postoperatively), but this positive influence has been shown to decline over time (at 5 years postoperatively) [34]. Regarding the necessity for additional meals, Takiguchi et al. found proximal gastrectomy was better than total gastrectomy for early upper-third gastric cancer [35]. In terms of QOL, our results showed no difference between the ADGR group and the ADJR group in the postoperative first year. This finding might be explained by a similar DTR involved in ADGR and ADJR probably had a notable impact on postoperative dietary habit and overall QOL.
The superiority of the present study was that it was the first study to explore the ADGR procedure after proximal gastrectomy, which provided an overview of the procedure for ADGR in the field of function-preserving surgeries. However, ADGR is a complicated procedure only for open surgery, which is a major limitation of the procedure because most proximal gastrectomy cases undergo laparoscopic surgery. Second, only some nutritional indicators were selected in this study, which might affect the completeness of results. Third, some differences might not be shown due to the short follow-up visit. In the future, more large-scale prospective studies are required to be carried out to further confirm the efficacy of ADGR.
Conclusions
ADGR can effectively improve the nutritional status and prevent the occurrence of residual food in patients with proximal gastric cancer after proximal gastrectomy, superior to ADJR. However, more large-scale prospective studies are required to be performed to further verify ADGR efficacy.
Figures
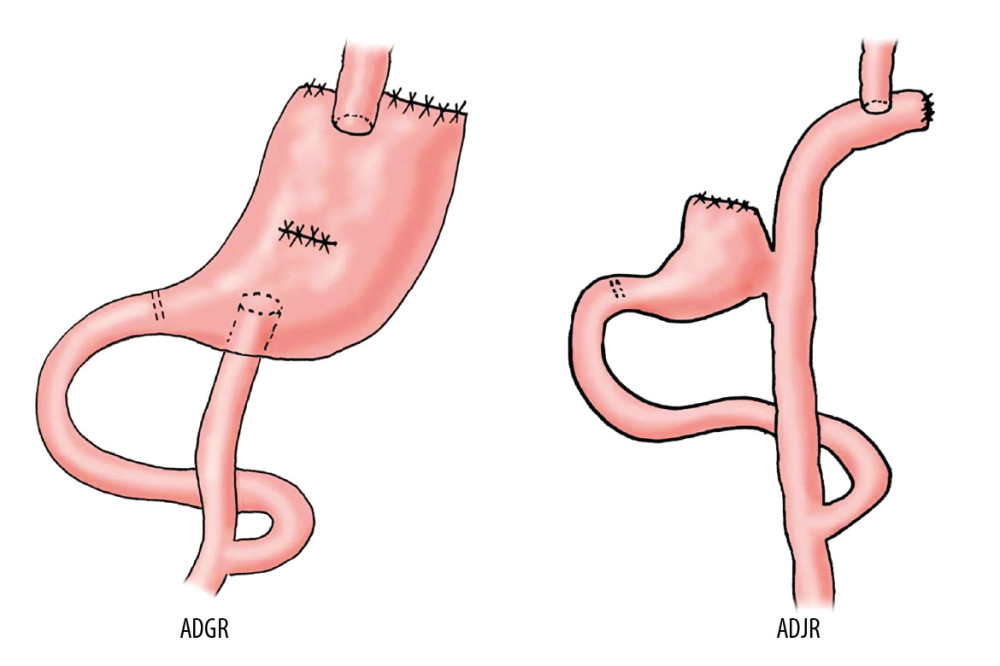 Figure 1. The surgical diagram of antrum-preserving double tract gastric interposition reconstruction (ADGR) and antrum-preserving double tract jejunal interposition reconstruction (ADJR).
Figure 1. The surgical diagram of antrum-preserving double tract gastric interposition reconstruction (ADGR) and antrum-preserving double tract jejunal interposition reconstruction (ADJR). 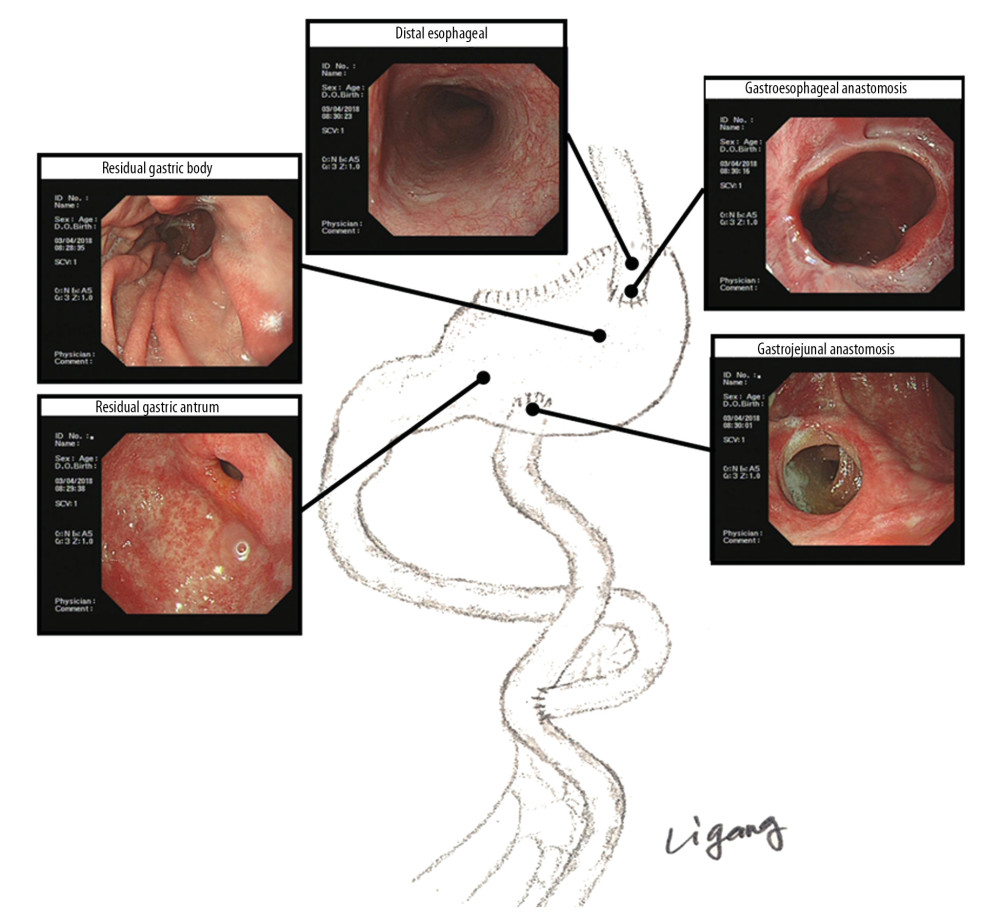 Figure 2. The gastroscopic diagram of antrum-preserving double tract gastric interposition reconstruction (ADGR).
Figure 2. The gastroscopic diagram of antrum-preserving double tract gastric interposition reconstruction (ADGR). 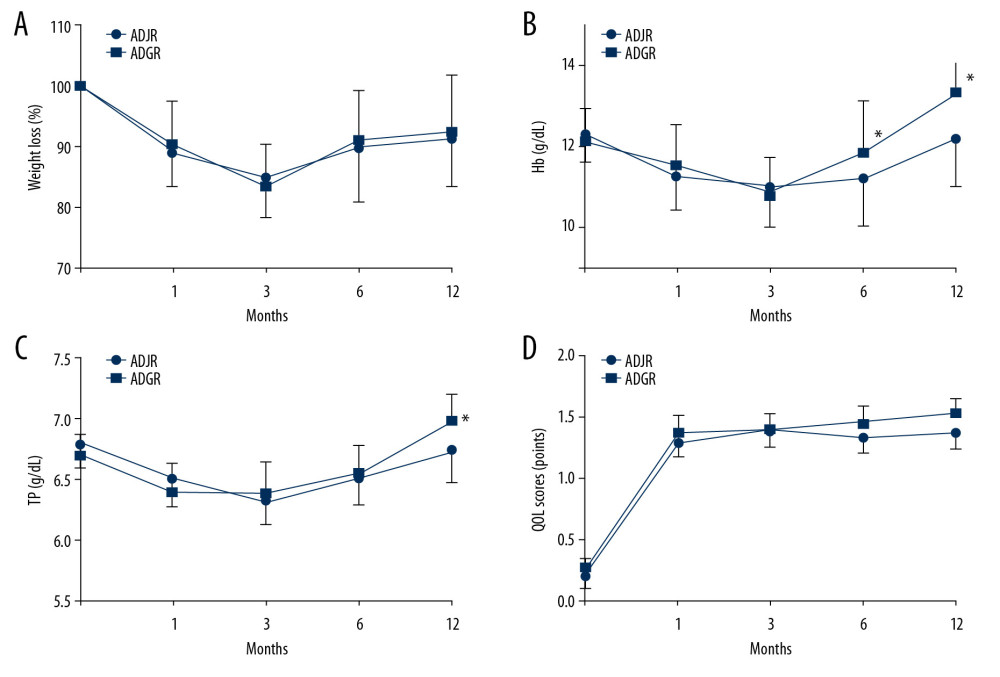 Figure 3. Comparison on the weight loss (A), Hb (B), TP (C) and QOL (D) in the ADGR group and the ADJR group at different time points after operation. ADGR – antrum-preserving double tract gastric interposition reconstruction; ADJR – antrum-preserving double tract jejunal interposition reconstruction; Hb – hemoglobin; TP – total protein.
Figure 3. Comparison on the weight loss (A), Hb (B), TP (C) and QOL (D) in the ADGR group and the ADJR group at different time points after operation. ADGR – antrum-preserving double tract gastric interposition reconstruction; ADJR – antrum-preserving double tract jejunal interposition reconstruction; Hb – hemoglobin; TP – total protein. References
1. Ferlay J, Soerjomataram I, Dikshit R, Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN2012: Int J Cancer, 2015; 136(5); E359-86
2. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68(6); 394-424
3. Borch K, Jönsson B, Tarpila E, Changing pattern of histological type, location, stage and outcome of surgical treatment of gastric carcinoma: Br J Surg, 2000; 87(5); 618-26
4. Brown LM, Devesa SS, Epidemiologic trends in esophageal and gastric cancer in the United States: Surg Oncol Clin N Am, 2002; 11(2); 235-56
5. Gulmann C, Hegarty H, Grace A, Differences in proximal (cardia) versus distal (antral) gastric carcinogenesis via the retinoblastoma pathway: World J Gastroenterol, 2004; 10(1); 17-21
6. Ding P, Gao Z, Zheng C, Risk evaluation of splenic hilar or splenic artery lymph node metastasis and survival analysis for patients with proximal gastric cancer after curative gastrectomy: A retrospective study: BMC Cancer, 2019; 19(1); 905
7. Kim J, Kim S, Min YD, Consideration of cardia preserving proximal gastrectomy in early gastric cancer of upper body for prevention of gastroesophageal reflux disease and stenosis of anastomosis site: J Gastric Cancer, 2012; 12(3); 187-93
8. Carey S, Storey D, Biankin AV, Long term nutritional status and quality of life following major upper gastrointestinal surgery – a cross-sectional study: Clin Nutr, 2011; 30(6); 774-79
9. Jung DH, Ahn SH, Park DJ, Kim HH, Proximal gastrectomy for gastric cancer: J Gastric Cancer, 2015; 15(2); 77-86
10. Japanese Gastric Cancer Association, Japanese gastric cancer treatment guidelines 2010 (ver. 3): Gastric Cancer, 2011; 14(2); 113-23
11. Zhang CD, Yamashita H, Seto Y, Gastric cancer surgery: Historical background and perspective in Western countries versus Japan: Ann Transl Med, 2019; 7(18); 493
12. Kumagai K, Shimizu K, Yokoyama N, Questionnaire survey regarding the current status and controversial issues concerning reconstruction after gastrectomy in Japan: Surg Today, 2012; 42(5); 411-18
13. Kuroda S, Nishizaki M, Kikuchi S, Double-flap technique as an antireflux procedure in esophagogastrostomy after proximal gastrectomy: J Am Coll Surg, 2016; 223(2); e7-13
14. Takagawa R, Kunisaki C, Kimura J, A pilot study comparing jejunal pouch and jejunal interposition reconstruction after proximal gastrectomy: Dig Surg, 2010; 27(6); 502-8
15. Fan KX, Xu ZF, Wang MR, Outcomes for jejunal interposition reconstruction compared with Roux-en-Y anastomosis: A meta-analysis: World J Gastroenterol, 2015; 21(10); 3093-99
16. Japanese Gastric Cancer Association, Japanese gastric cancer treatment guidelines 2014 (ver. 4): Gastric Cancer, 2017; 20(1); 1-19
17. Harrison LE, Karpeh MS, Brennan MF, Total gastrectomy is not necessary for proximal gastric cancer: Surgery, 1998; 123(2); 127-30
18. Adachi Y, Inoue T, Hagino Y, Surgical results of proximal gastrectomy for early-stage gastric cancer: Jejunal interposition and gastric tube reconstruction: Gastric Cancer, 1999; 2(1); 40-45
19. Takiguchi N, Takahashi M, Ikeda M, Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): A nationwide multi-institutional study: Gastric Cancer, 2015; 18(2); 407-16
20. Rosa F, Quero G, Fiorillo C: Gastric Cancer, 2018; 21(5); 845-52
21. Nakamura M, Nakamori M, Ojima T, Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: An analysis of our 13-year experience: Surgery, 2014; 156(1); 57-63
22. Hsu CP, Chen CY, Hsieh YH, Esophageal reflux after total or proximal gastrectomy in patients with adenocarcinoma of the gastric cardia: Am J Gastroenterol, 1997; 92(8); 1347-50
23. Shiraishi N, Hirose R, Morimoto A, Gastric tube reconstruction prevented esophageal reflux after proximal gastrectomy: Gastric Cancer, 1998; 1(1); 78-79
24. Matsushiro T, Hariu T, Nagashima H, Valvuloplasty plus fundoplasty to prevent esophageal regurgitation in esophagogastrostomy after proximal gastrectomy: Am J Surg, 1986; 152(3); 314-19
25. Katai H, Sano T, Fukagawa T, Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach: Br J Surg, 2003; 90(7); 850-53
26. Kameyama J, Ishida H, Yasaku Y, Proximal gastrectomy reconstructed by interposition of a jejunal pouch. Surgical technique: Eur J Surg, 1993; 159(9); 491-93
27. Katsoulis IE, Robotis JF, Kouraklis G, Yannopoulos PA, What is the difference between proximal and total gastrectomy regarding postoperative bile reflux into the oesophagus?: Dig Surg, 2006; 23(5–6); 325-30
28. Tokunaga M, Ohyama S, Hiki N, Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: Comparison between esophagogastric anastomosis and jejunal interposition: World J Surg, 2008; 32(7); 1473-77
29. Tokunaga M, Hiki N, Ohyama S, Effects of reconstruction methods on a patient’s quality of life after a proximal gastrectomy: Subjective symptoms evaluation using questionnaire survey: Langenbecks Arch Surg, 2009; 394(4); 637-41
30. Nomura E, Kayano H, Lee SW, Functional evaluations comparing the double-tract method and the jejunal interposition method following laparoscopic proximal gastrectomy for gastric cancer: An investigation including laparoscopic total gastrectomy: Surg Today, 2019; 49(1); 38-48
31. Xiao JW, Liu ZL, Ye PC: World J Gastroenterol, 2015; 21(34); 9999-10007
32. Braga M, Zuliani W, Foppa L, Food intake and nutritional status after total gastrectomy: results of a nutritional follow-up: Br J Surg, 1988; 75(5); 477-80
33. Bergh C, Sjöstedt S, Hellers G, Meal size, satiety and cholecystokinin in gastrectomized humans: Physiol Behav, 2003; 78(1); 143-47
34. Namikawa T, Oki T, Kitagawa H, Impact of jejunal pouch interposition reconstruction after proximal gastrectomy for early gastric cancer on quality of life: Short- and long-term consequences: Am J Surg, 2012; 204(2); 203-9
35. Takiguchi N, Takahashi M, Ikeda M, Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): A nationwide multi-institutional study: Gastric Cancer, 2015; 18(2); 407-16
Figures
 Figure 1. The surgical diagram of antrum-preserving double tract gastric interposition reconstruction (ADGR) and antrum-preserving double tract jejunal interposition reconstruction (ADJR).
Figure 1. The surgical diagram of antrum-preserving double tract gastric interposition reconstruction (ADGR) and antrum-preserving double tract jejunal interposition reconstruction (ADJR). Figure 2. The gastroscopic diagram of antrum-preserving double tract gastric interposition reconstruction (ADGR).
Figure 2. The gastroscopic diagram of antrum-preserving double tract gastric interposition reconstruction (ADGR). Figure 3. Comparison on the weight loss (A), Hb (B), TP (C) and QOL (D) in the ADGR group and the ADJR group at different time points after operation. ADGR – antrum-preserving double tract gastric interposition reconstruction; ADJR – antrum-preserving double tract jejunal interposition reconstruction; Hb – hemoglobin; TP – total protein.
Figure 3. Comparison on the weight loss (A), Hb (B), TP (C) and QOL (D) in the ADGR group and the ADJR group at different time points after operation. ADGR – antrum-preserving double tract gastric interposition reconstruction; ADJR – antrum-preserving double tract jejunal interposition reconstruction; Hb – hemoglobin; TP – total protein. Tables
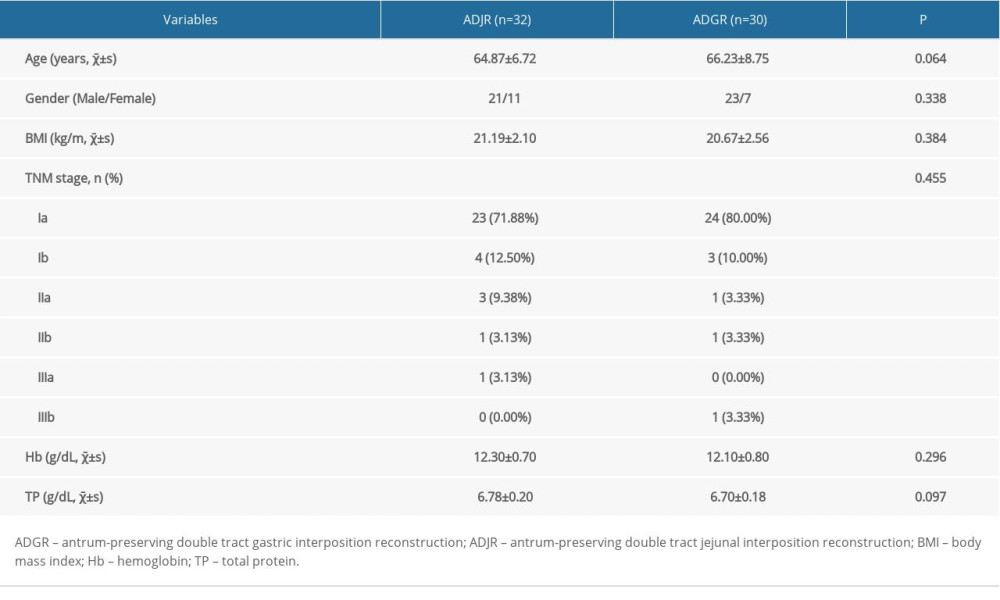 Table 1. Baseline characteristics of patients in ADJR group and ADGR group.
Table 1. Baseline characteristics of patients in ADJR group and ADGR group.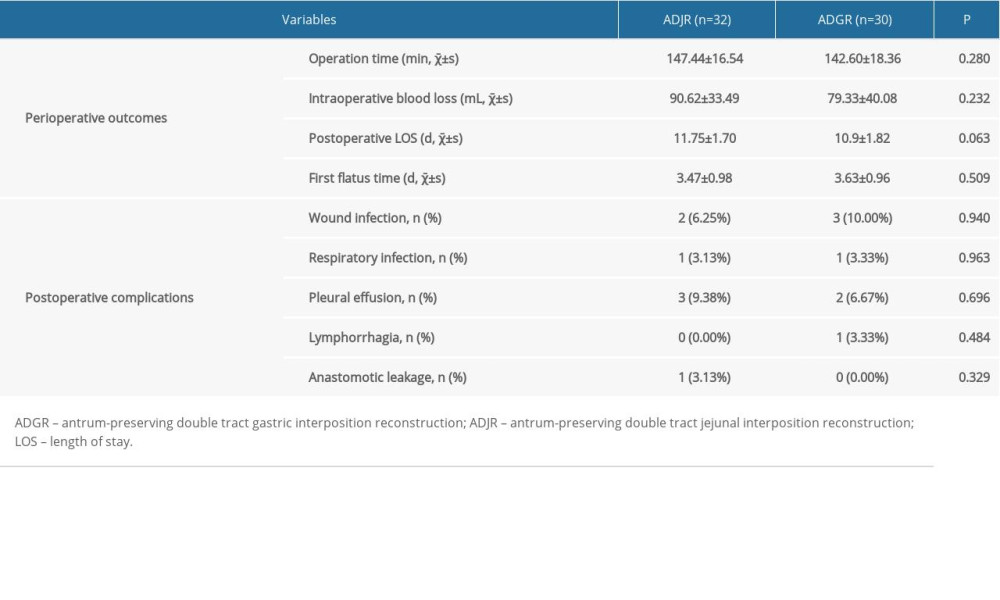 Table 2. Perioperative outcomes and postoperative complications of patients in ADJR group and ADGR group.
Table 2. Perioperative outcomes and postoperative complications of patients in ADJR group and ADGR group.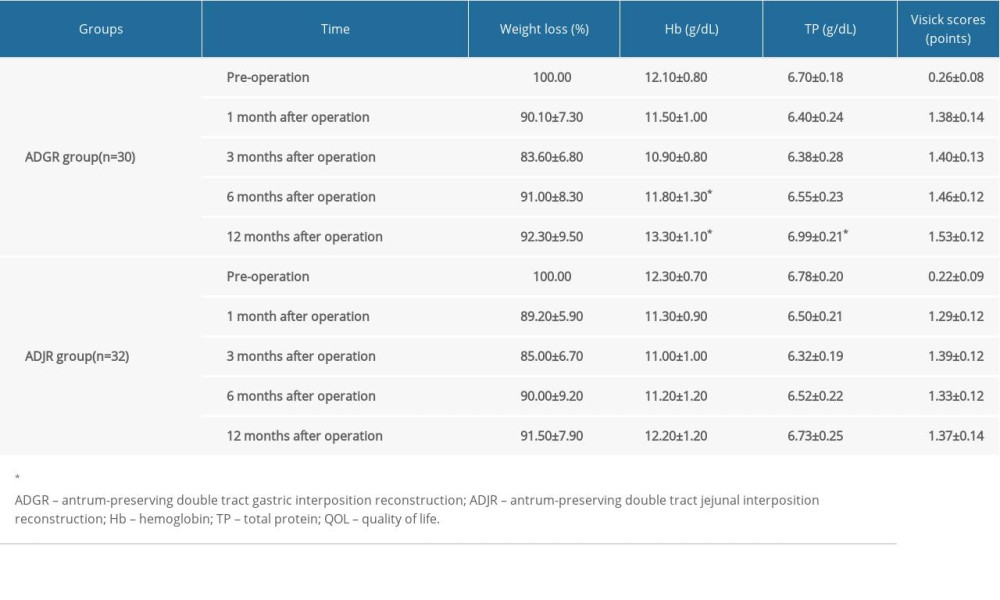 Table 3. Changes of weight loss, Hb, TP, and QOL in 2 groups at different time points (χ̄±s).
Table 3. Changes of weight loss, Hb, TP, and QOL in 2 groups at different time points (χ̄±s). Table 1. Baseline characteristics of patients in ADJR group and ADGR group.
Table 1. Baseline characteristics of patients in ADJR group and ADGR group. Table 2. Perioperative outcomes and postoperative complications of patients in ADJR group and ADGR group.
Table 2. Perioperative outcomes and postoperative complications of patients in ADJR group and ADGR group. Table 3. Changes of weight loss, Hb, TP, and QOL in 2 groups at different time points (χ̄±s).
Table 3. Changes of weight loss, Hb, TP, and QOL in 2 groups at different time points (χ̄±s). In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








