31 May 2020: Clinical Research
Comparative Study of Drug-eluting Beads versus Conventional Transarterial Chemoembolization for Treating Peculiar Anatomical Sites of Gastric Cancer Liver Metastasis
Hao Xu1ABCDEFG*, Xuli Min1BCDE, Yongjun Ren1BCDG, Lin Yang1DEF, Fang Liu2CDDOI: 10.12659/MSM.922988
Med Sci Monit 2020; 26:e922988
Abstract
BACKGROUND: This study aimed to assess the relative safety and short-term efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE) and conventional transarterial chemoembolization (c-TACE) for treating peculiar anatomical sites of gastric cancer liver metastasis.
MATERIAL AND METHODS: Of the 68 patients with gastric cancer liver metastases confirmed by imaging and pathology, 35 were treated with DEB-TACE and 33 with c-TACE. The DEB-TACE group comprised 26 males and 9 females aged 28–75 years (56.8±6.3), and the c-TACE group included 19 males and 14 females aged 33–77 (60.2±9.4) years. Liver functions of the 2 groups were compared between pre-TACE and 1-week and 1-month after TACE. Computed tomography and magnetic resonance imaging were reexamined at 1, 3, and 6 months after TACE, and short-term efficacy was assessed based on modified response evaluation criteria in solid tumors.
RESULTS: One month following DEB-TACE and c-TACE, the number of cases with objective response (OR) was 29 cases (29 out of 35 cases, 82.9%) and 20 cases (20 out of 33 cases, 60.6%) and disease control (DC) in the 2 groups was 33 cases (33 out of 35 cases, 94.3%) and 26 cases (26 out of 33 cases, 78.8%) respectively (P=0.041, P=0.031). Alanine transaminase (ALT) and Aspartate transaminase (AST) significantly increased in the DEB-TACE and c-TACE groups 1 week later (P<0.001). There were no serious complications in the 2 groups; incidences of nausea and vomiting were significantly lower, but instances of fever were markedly elevated in the DEB-TACE group (P=0.023, P=0.016, respectively).
CONCLUSIONS: The safety, feasibility, and short-term efficacy of DEB-TACE and c-TACE in the treatment of gastric cancer liver metastasis are clear. DEB-TACE leads to less incidences of nausea and vomiting but more incidences of fever than c-TACE.
Keywords: Chemoembolization, Therapeutic, Microspheres, Radiology, Interventional, Carcinoma, Hepatocellular, Liver Neoplasms
Background
The hematogenous metastasis of tumors frequently affects the liver. Since liver metastatic tumors are often multiple lesions and the surgical resection rate is low, the main treatment methods are currently non-surgical. These treatment methods include systemic chemotherapy, molecular-targeted therapy, immunotherapy, transarterial chemoembolization (TACE), radiofrequency ablation (RFA), cryoablation and radiotherapy [1], but the therapeutic effects have been relatively unsuccessful [2]. Gastric cancer and liver metastasis, especially when the metastatic lesion is located near great vessels, the subcapsular of the liver, the hilar area (hepatogastric space), or other special anatomical sites, is an unresectable surgical condition, and ablation and other local treatments also have certain limits [3]. The special anatomical site is thought to be a key prognostic factor associated with patient quality of life and survival, and patients with metastases in these sites have significantly different 5-year survival rates to those without liver metastasis [4,5].
Owing to the fact that it is minimally invasive, reproducible, and effective, TACE has become an important local treatment for metastatic liver cancer, especially in patients with colorectal cancer liver metastasis [6–10]. The embolic material used is one of the main factors affecting the therapeutic efficacy of TACE. Conventional (c)TACE uses iodized oil and a chemotherapy-drug mixture emulsion as the embolization material, but the iodized oil deposition is poor for liver metastasis, resulting in an unsatisfactory chemoembolization effect. In recent years, a new type of embolic agent, drug-eluting beads (DEBs), have been increasingly used in the clinical setting, especially for patients with primary liver cancer. However, there is still no consensus on the embolic effectiveness of DEB for patients with gastric cancer and liver metastasis [11]. In this paper, the safety, embolic response, and short-term efficacy of DEB-TACE and c-TACE for treating gastric cancer liver metastasis in special anatomical sites was compared via a case-control approach.
Material and Methods
CLINICAL DATA:
Inclusion criteria were as follows: patients who had gastric cancer liver metastasis in a special anatomical position, as confirmed by imaging and pathology, that could not be removed, or patients who refused surgery; patients with a recurring liver metastatic tumor following an operation; patients without heart, brain, kidney, or other important organ dysfunctions. Exclusion criteria were as follows: patients with severe hepatic abnormalities (Child-Pugh C grade), such as refractory ascites, hepatic encephalopathy, jaundice, or hepatorenal syndrome; patients with severe coagulation abnormalities; patients with complete portal vein trunk occlusion and few collateral vessels; patients with cachexia or multiple organ failure; patients with renal dysfunction (urinary creatinine >20 mg/L or creatinine clearance rate <30 mL/minute); patients in pregnancy. From January 2016 to February 2019, 68 patients with liver metastasis of gastric cancer met the inclusion criteria, including 35 patients with DEB-TACE and 33 patients with c-TACE. The mean age of 35 patients undergoing DEB-TACE treatment was 56.8±6.3 years. There were 9 female patients and 26 male patients. Patients undergoing c-TACE treatment had a mean age of 60.2±9.4 years. There were 14 female patients and 19 male patients (Table 1).
INTERVENTIONAL PROCEDURES:
Before the operation, imaging examinations, electrocardiogram (ECG), and laboratory examinations were completed, and the pathological diagnosis was confirmed by biopsy. All patients signed an informed consent.
Intervention surgical instruments were as follows: 5F artery sheath, 5F RH, or Yashiro catheter; 0.035-inch guide wire (Terumo Company, Japan); 2.5F microcatheter and 0.018-inch micro guide wire (COOK Company, USA); CalliSpheres® drug-eluting beads (100–300 μm) (Jiangsu Hengrui Medicine Co., Ltd., China); Lipiodol® Ultra Fluide (Guerbet Aulnay-sous-Bois France).
CHEMOTHERAPY: FOLEIRI:
Preparation of DEBs was as follows. Drug loading began 30 minutes before embolization. All microspheres were taken up with a 20 mL syringe, the syringe was placed upright for 5 minutes to allow the microspheres to settle and form a layer, and the supernatant was removed. A 10 mL syringe was used to dissolve 100–200 mg of irinotecan in water for injection, and we confirmed the chemotherapy drugs were completely dissolved. The microspheres were mixed with chemotherapy drugs and gently shaken once every 5 minutes, 6 times. After loading the drug, the contrast agent was added in the proportion of 1: 1 and mixed well, and the stopcock and another syringe were connected (Figure 1).
Interventional operation was as follows. The area of the groin to be operated on was disinfected and the towel placed around the area. A femoral artery puncture was conducted followed by the 5F artery sheath being inserted. With the aid of a super-slide guide wire, the catheter was inserted, and the celiac trunk, superior mesenteric artery, hepatic artery, and left gastric artery were imaged. If necessary, the inferior phrenic artery was imaged to determine the number of tumor blood supply arteries and other information, and the super-selective angiography was performed by microcatheter. According to the size, number, and perfusion of metastases, the size of DEB and the amount of iodized oil were selected. The prepared DEBs were slowly pushed and injected to embolize the tumor vascular bed until the contrast showed that the tumor had no staining. The end point of c-TACE was the same as that of DEB-TACE. After the operation, the patients with post-embolism syndrome (nausea, vomiting, abdominal pain, and fever) were treated regularly with antiemetic and pain relief and drugs for liver protection and infection prevention.
EFFICACY AND SAFETY ASSESSMENT:
The results of computed tomography (CT) or magnetic resonance imaging (MRI) were recorded before treatment and 1, 3, and 6 months postoperatively. The therapeutic effect was evaluated according to the modified response evaluation criteria in solid tumors (mRECIST) [12,13]. Complete response (CR) is considered a loss of any visible arterial enhancement within the tumor site. Partial response (PR) is considered a decrease in tumor sum diameters by >30% relative to baseline sum diameters in tumors exhibiting arterial enhancement. Stable disease (SD) is considered a decrease of less than PR and an increase of less than progressive disease (PD) in the tumor sum diameters for those tumors exhibiting arterial enhancement. PD is considered an increase in arterial enhancement-exhibiting tumor sum diameters by >20% relative to the smallest viable lesion diameter. CR+PR is the objective response of the disease, CR+PR+SD is the disease control. ([CR+PR]/total cases)×100% is the objective response rate (ORR), and ([CR+PR+SD]/total cases)×100% is the disease control rate (DCR).
The liver function, coagulation function, and alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBIL), and prothrombin time (PT), were analyzed before the operation and 1-week and 1-month post-operation to compare the embolic reaction after interventional therapy.
STATISTICAL ANALYSIS:
Data are means±standard deviations or counts (rates,%). Independent-sample
Results
One month after DEB-TACE and c-TACE, the number of cases with OR was 29 cases (29 out of 35 cases, 82.9%) and 20 cases (20 out of 33 cases, 60.6%), and the number of cases with DC were 33 cases (33 out of 35 cases, 94.3%) and 26 cases (26 out of 33 cases, 78.8%), respectively (
Discussion
The liver is the most common organ to lead to malignant tumor metastasis of the digestive system. Colorectal cancer and gastric cancer are the most common primary lesions [4,14,15]. The main therapeutic methods are surgical resection, systemic chemotherapy, molecular targeted therapy, immunotherapy, and interventional therapy [1,14,16]. Simultaneous and heterogeneous liver metastases of gastric cancer can be treated by surgical resection or ablation, and both can respond to treatment [17,18]. There are some difficulties involved in the surgical treatment and ablation of multiple lesions and metastasis of special anatomical sites. Most of these cases received neoadjuvant chemotherapy or immunotherapy and underwent surgery when they became resectable; however, TACE was used as a trial treatment for those cases that did not become resectable or patients who refused to undergo surgical resection. As a representative technique of interventional therapy, c-TACE has been applied to the treatment of liver cancer and has achieved remarkable results. However, there are some clinical problems with the treatment of metastatic liver cancer with c-TACE, such as repeated liver function damage due to the chemotherapy drugs leaking from the iodized oil. The drugs easily enter the circulation, causing systemic toxicity [11].
DEB have advantages that ultra-liquid iodized oil does not have. The microspheres are symmetrical and uniform in shape and size and can better embolize the artery supplying the tumor. DEB can release anti-tumor drugs slowly and continuously into the tumor tissue, increasing the drug concentration significantly, and effectively controlling the recurrence of metastatic cancer [11]. The interaction between microspheres and anti-tumor drugs is strong, which can significantly reduce the amount of drugs entering the systemic circulation at one time and reduce side effects. DEB have a significant effect on primary liver cancer, and although their economic cost is higher than c-TACE, this is acceptable for these types of patients [19,20].
In recent years, there have been an increasing number of studies into using DEB-TACE to treat liver metastasis of colorectal cancer. It is reported to be safe and feasible to embolize patients with liver metastasis of colorectal cancer with DEB loaded with irinotecan (DEBIRI) [7,8]. A comparative study of the efficacies of embolization (DEBIRI) and intravenous chemotherapy (FOLFIRI regimen) showed that there were longer progression free survival (PFS) and overall survival (OS) in the DEBIRI group than in the FOLFIRI group, and there were less systemic adverse reactions in the DEBIRI group [9]. DEBIRI combined with FOLFOX in the treatment of liver metastasis cancer can improve patient quality of life, shorten the length of stay, increase the tumor necrosis rate, and prolong the survival period [6,19]. However, there are few studies on DEB-TACE in patients with liver metastasis of gastric cancer, especially for special anatomical sites of the liver, such as subcapsular, perivascular, and hilar tumors, and some of the existing literature only summarizes empirical treatment [21,22].
In this study, DEB-TACE and c-TACE were used to treat 35 and 33 respective cases of special anatomical site liver metastasis of gastric cancer. The results showed that there was a significant difference in DCR and ORR between the 2 groups 1 month after operation. There were no significant differences in the DCR and ORR between the 2 groups at 3-months and 6-months post-operation. This may be because the tumor blood vessels are blocked within 1 month after an operation, and the slow release of chemotherapeutic drugs leads to increased tumor necrosis. The tumor produces angiogenic factors in the anoxic environment after 3 months, which may explain the enhancement of the CT and MRI scans. Incomplete embolization is also an important factor in the enhancement and progression of the disease [23,24]. One week after the operation, the ALT and AST of the 2 groups increased, which indicated that both DEB-TACE and c-TACE caused transient injury to the hepatocytes, possibly due to chemotherapeutic drug injury, ectopic embolization, etc. C-TACE has been used for metastatic liver cancer for many years, but the side effect of embolization affects the treatment compliance and efficacy, which is closely related to the skill of the clinician [8]. DEB-TACE and c-TACE may increase the risk of ectopic embolism and hepatocyte injury in patients with poor hepatic artery supply.
The incidence of post-embolization syndrome was not high in either group, but the incidences of nausea and vomiting were higher, and fever was lower in the DEB-TACE group than in the c-TACE group, and the differences were statistically significant. A possible reason is that DEB-TACE causes a high tumor necrosis rate and a series of pathophysiological inflammatory changes [25,26]. The symptoms were relieved after symptomatic treatment, such as liver protective treatment, anti-inflammatories, analgesics, and antiemetics. No serious complications, such as pulmonary embolism, liver abscess, bile duct injury, or gastric mucosal injury, were reported in this study.
Conclusions
DEB-TACE was effective for treating gastric cancer liver metastasis, and the embolization reaction was mild. DEB-TACE leads to more thorough tumor necrosis than c-TACE without increasing adverse reactions, except for fever which can be controlled by physical cooling. However, the price of DEB is high, which limits the clinical application. It is important to reiterate that local treatment should be combined with systemic therapy, such as molecular targeted therapy and immunotherapy, and a combination of local and whole provides the most effective cure. However, long-term DEB-TACE effects on liver metastasis of gastric cancer needs further study.
Figures
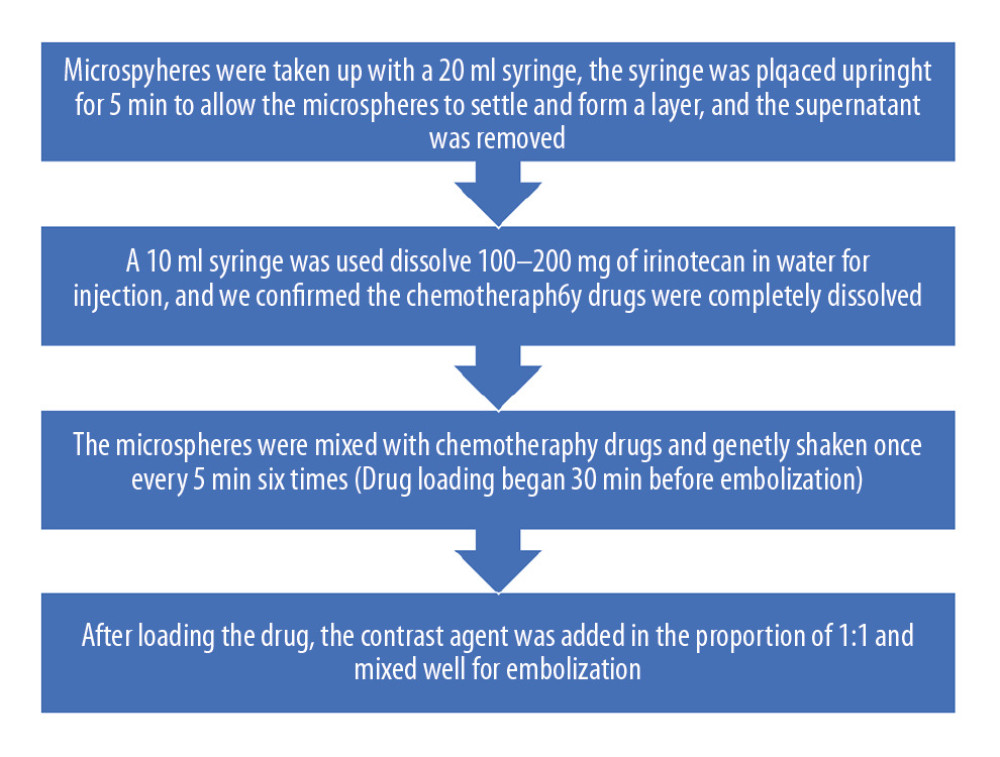 Figure 1. Preparation of drug-eluting beads (irinotecan-eluting beads).
Figure 1. Preparation of drug-eluting beads (irinotecan-eluting beads). 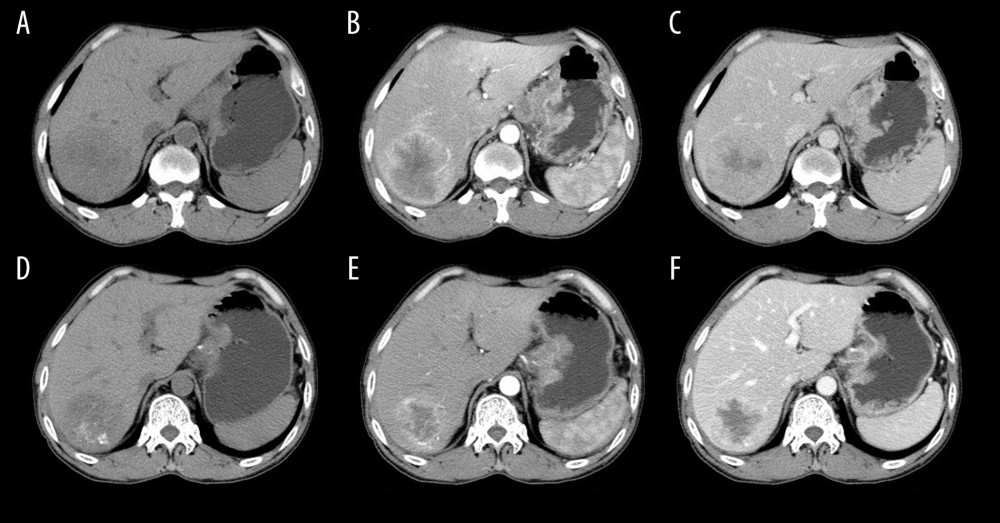 Figure 2. A 54-year-old male with liver metastasis of gastric cancer. Computed tomography (CT) was performed before (A–C) and 1 month after (D–F) c-TACE (conventional transarterial chemoembolization). Partial response (PR) is evaluated according to the mRECIST (E versus B; B is baseline; E is follow-up 1-month post c-TACE). The tumor necrosis was increased (F vs. C).
Figure 2. A 54-year-old male with liver metastasis of gastric cancer. Computed tomography (CT) was performed before (A–C) and 1 month after (D–F) c-TACE (conventional transarterial chemoembolization). Partial response (PR) is evaluated according to the mRECIST (E versus B; B is baseline; E is follow-up 1-month post c-TACE). The tumor necrosis was increased (F vs. C). 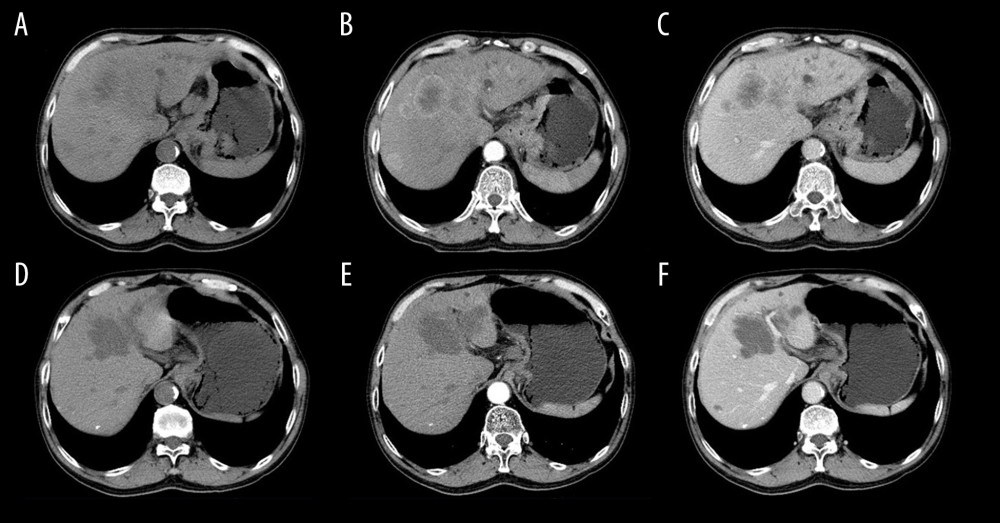 Figure 3. A 60-year-old male with liver metastasis of gastric cancer. Computed tomography (CT) was performed before (A–C) and 1 month after (D–F) irinotecan-eluting beads-transarterial chemoembolization (TACE). Partial response (PR) is evaluated according to the mRECIST (E versus B; B is baseline; E is follow-up 1-month post irinotecan-eluting beads-TACE). The tumor necrosis was increased (F vs. C).
Figure 3. A 60-year-old male with liver metastasis of gastric cancer. Computed tomography (CT) was performed before (A–C) and 1 month after (D–F) irinotecan-eluting beads-transarterial chemoembolization (TACE). Partial response (PR) is evaluated according to the mRECIST (E versus B; B is baseline; E is follow-up 1-month post irinotecan-eluting beads-TACE). The tumor necrosis was increased (F vs. C). References
1. Lv WH, Zhao Y, Li XD, Zhang MQ, Clinical effects of bevacizumab targeted treatment on advanced colorectal cancer with liver metastasis: Eur Rev Med Pharmacol Sci, 2016; 20(11); 2249-55
2. Makino H, Kunisaki C, Izumisawa Y, Indication for hepatic resection in the treatment of liver metastasis from gastric cancer: Anticancer Res, 2010; 30(6); 2367-76
3. Fisichella R, Sparta D, Berretta S, Combined microwave thermal ablation and liver resection for single step treatment of otherwise unresectable colorectal liver metastases; A mono-institutional experiences: Eur Rev Med Pharmacol Sci, 2015; 19(2); 180-81
4. De Greef K, Rolfo C, Russo A, Multidisciplinary management of patients with liver metastasis from colorectal cancer: World J Gastroenterol, 2016; 22(32); 7215-25
5. Negoi I, Runcanu A, Paun S, Resection of large metachronous liver metastasis with gastric origin: Case report and review of the literature: Cureus, 2016; 8(10); e814
6. Akinwande O, Dendy M, Ludwig JM, Kim HS, Hepatic intra-arterial injection of irinotecan drug eluting beads (DEBIRI) for patients with unresectable colorectal liver metastases: A systematic review: Surg Oncol, 2017; 26(3); 268-75
7. Akinwande O, Scoggins C, Martin RC, Early experience with 70–150 mum irinotecan drug-eluting beads (M1-DEBIRI) for the treatment of unresectable hepatic colorectal metastases: Anticancer Res, 2016; 36(7); 3413-18
8. Iezzi R, Marsico VA, Guerra A, Trans-arterial chemoembolization with irinotecan-loaded drug-eluting beads (DEBIRI) and capecitabine in refractory liver prevalent colorectal metastases: A phase II single-center study: Cardiovasc Intervent Radiol, 2015; 38(6); 1523-31
9. Fiorentini G, Aliberti C, Tilli M, Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: Final results of a phase III study: Anticancer Res, 2012; 32(4); 1387-95
10. Aliberti C, Fiorentini G, Muzzio PC, Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead(R), drug-eluting bead loaded with irinotecan: Results of a phase II clinical study: Anticancer Res, 2011; 31(12); 4581-87
11. Malagari K, Iezzi R, Goldberg SN, The ten commandments of chemoembolization: Expert discussion and report from Mediterranean Interventional Oncology (MIOLive) congress 2017: Eur Rev Med Pharmacol Sci, 2018; 22(2); 372-81
12. Prajapati HJ, Spivey JR, Hanish SI, mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE): Ann Oncol, 2013; 24(4); 965-73
13. Lencioni R, Llovet JM, Modified RECIST (mRECIST) assessment for hepatocellular carcinoma: Semin Liver Dis, 2010; 30(1); 52-60
14. Oki E, Tokunaga S, Emi Y, Surgical treatment of liver metastasis of gastric cancer: A retrospective multicenter cohort study (KSCC1302): Gastric Cancer, 2016; 19(3); 968-76
15. Ajani JA, D’Amico TA, Almhanna K, Gastric cancer, Version 3.2016, NCCN clinical practice guidelines in oncology: J Natl Compr Canc Netw, 2016; 14(10); 1286-312
16. Milano MT, Katz AW, Zhang H, Okunieff P, Oligometastases treated with stereotactic body radiotherapy: Long-term follow-up of prospective study: Int J Radiat Oncol Biol Phys, 2012; 83(3); 878-86
17. Zhou F, Yu XL, Liang P, Microwave ablation is effective against liver metastases from gastric adenocarcinoma: Int J Hyperthermia, 2017; 33(7); 830-35
18. Hwang JE, Kim SH, Jin J, Combination of percutaneous radiofrequency ablation and systemic chemotherapy are effective treatment modalities for metachronous liver metastases from gastric cancer: Clin Exp Metastasis, 2014; 31(1); 25-32
19. Cucchetti A, Trevisani F, Cappelli A, Cost-effectiveness of doxorubicin-eluting beads versus conventional trans-arterial chemoembolization for hepatocellular carcinoma: Dig Liver Dis, 2016; 48(7); 798-805
20. Megias VJ, Garcia MR, Lopez BE, Trans-arterial chemoembolization with doxorubicin-eluting particles versus conventional trans-arterial chemoembolization in unresectable hepatocellular carcinoma: A study of effectiveness, safety and costs: Radiologia, 2015; 57(6); 496-504
21. Hirasawa T, Asahara S, Fujisaki STranscatheter arterial chemoembolization (TACE) using degradable starch microspheres (DSM) for metastatic liver tumors in patients with gastric cancer: Nihon Shokakibyo Gakkai Zasshi, 2008; 105(3); 367-72 [in Japanese]
22. Tarazov PG, Transcatheter therapy of gastric cancer metastatic to the liver: Preliminary results: J Gastroenterol, 2000; 35(12); 907-11
23. Xu H, Ren YJ, Liu K, Correlations of serum VEGF and MMP-2 levels with CLM in CRC patients and effects of TACE on their expressions: Eur Rev Med Pharmacol Sci, 2018; 22(11); 3394-401
24. Jia ZZ, Jiang GM, Feng YL, Serum HIF-1alpha and VEGF levels pre- and post-TACE in patients with primary liver cancer: Chin Med Sci J, 2011; 26(3); 158-62
25. Marcacuzco QA, Nutu OA, San RMR, Complications of transarterial chemoembolization (TACE) in the treatment of liver tumors: Cir Esp, 2018; 96(9); 560-67
26. Tu J, Jia Z, Ying X, The incidence and outcome of major complication following conventional TAE/TACE for hepatocellular carcinoma: Medicine (Baltimore), 2016; 95(49); e5606
Figures
 Figure 1. Preparation of drug-eluting beads (irinotecan-eluting beads).
Figure 1. Preparation of drug-eluting beads (irinotecan-eluting beads). Figure 2. A 54-year-old male with liver metastasis of gastric cancer. Computed tomography (CT) was performed before (A–C) and 1 month after (D–F) c-TACE (conventional transarterial chemoembolization). Partial response (PR) is evaluated according to the mRECIST (E versus B; B is baseline; E is follow-up 1-month post c-TACE). The tumor necrosis was increased (F vs. C).
Figure 2. A 54-year-old male with liver metastasis of gastric cancer. Computed tomography (CT) was performed before (A–C) and 1 month after (D–F) c-TACE (conventional transarterial chemoembolization). Partial response (PR) is evaluated according to the mRECIST (E versus B; B is baseline; E is follow-up 1-month post c-TACE). The tumor necrosis was increased (F vs. C). Figure 3. A 60-year-old male with liver metastasis of gastric cancer. Computed tomography (CT) was performed before (A–C) and 1 month after (D–F) irinotecan-eluting beads-transarterial chemoembolization (TACE). Partial response (PR) is evaluated according to the mRECIST (E versus B; B is baseline; E is follow-up 1-month post irinotecan-eluting beads-TACE). The tumor necrosis was increased (F vs. C).
Figure 3. A 60-year-old male with liver metastasis of gastric cancer. Computed tomography (CT) was performed before (A–C) and 1 month after (D–F) irinotecan-eluting beads-transarterial chemoembolization (TACE). Partial response (PR) is evaluated according to the mRECIST (E versus B; B is baseline; E is follow-up 1-month post irinotecan-eluting beads-TACE). The tumor necrosis was increased (F vs. C). Tables
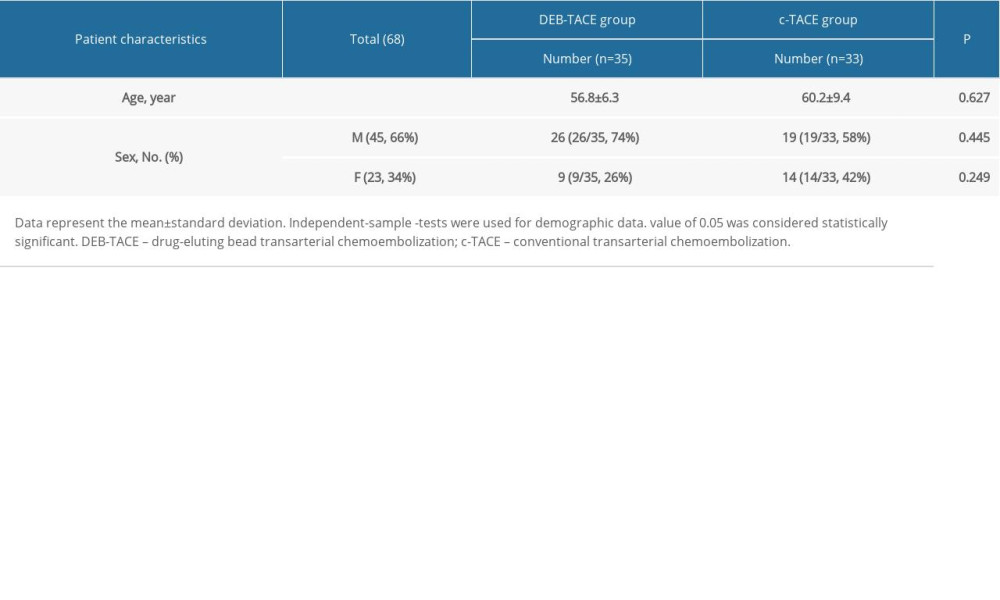 Table 1. Demographic data.
Table 1. Demographic data.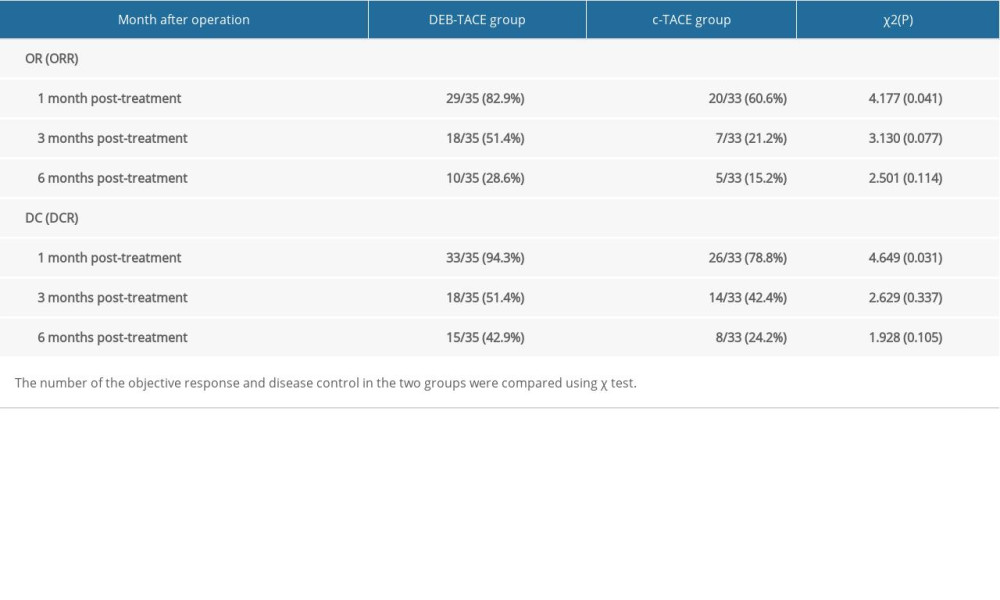 Table 2. Comparison of objective response (OR) and disease control (DC) at different follow-up times (number of cases).
Table 2. Comparison of objective response (OR) and disease control (DC) at different follow-up times (number of cases).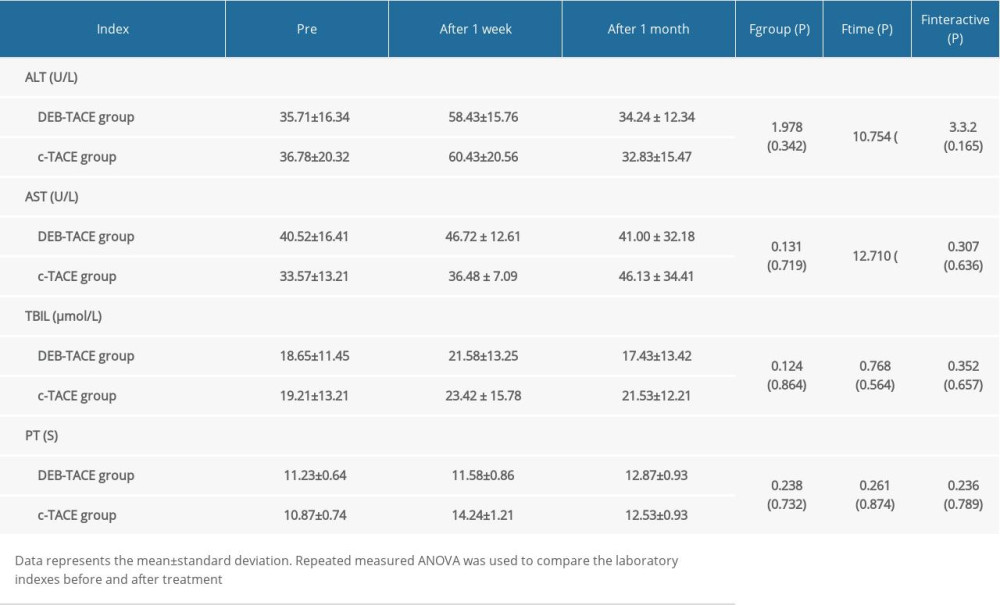 Table 3. Comparison of liver function indexes.
Table 3. Comparison of liver function indexes.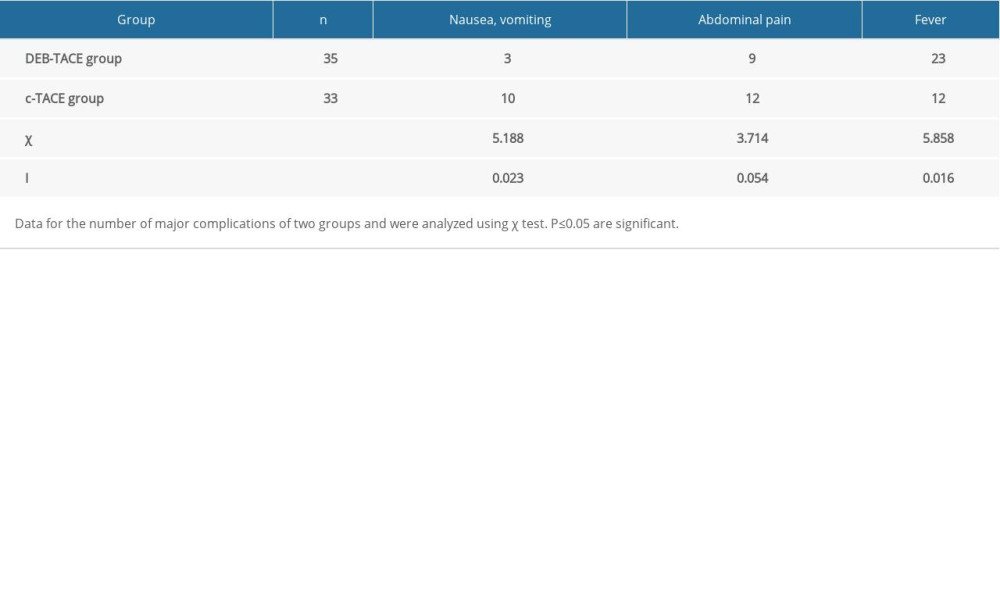 Table 4. Comparison of the occurrence of post-embolization syndrome (number of cases).
Table 4. Comparison of the occurrence of post-embolization syndrome (number of cases). Table 1. Demographic data.
Table 1. Demographic data. Table 2. Comparison of objective response (OR) and disease control (DC) at different follow-up times (number of cases).
Table 2. Comparison of objective response (OR) and disease control (DC) at different follow-up times (number of cases). Table 3. Comparison of liver function indexes.
Table 3. Comparison of liver function indexes. Table 4. Comparison of the occurrence of post-embolization syndrome (number of cases).
Table 4. Comparison of the occurrence of post-embolization syndrome (number of cases). In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








