15 July 2020: Clinical Research
Radiofrequency Ablation (RFA) Combined with Transcatheter Arterial Chemoembolization (TACE) for Patients with Medium-to-Large Hepatocellular Carcinoma: A Retrospective Analysis of Long-Term Outcome
Weiwen Liu1ABC*, Huihong Xu2DE, Xihui Ying1EFG, Dengke Zhang1CD, Linqiang Lai1BC, Linyou Wang3EF, Jianfei Tu1CFG, Jiansong Ji1BDGDOI: 10.12659/MSM.923263
Med Sci Monit 2020; 26:e923263
Abstract
BACKGROUND: The aim of this study was to investigate the prognostic value of radiofrequency ablation (RFA) plus transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC) patients with tumor size ranging from 3.0 to 10.0 cm.
MATERIAL AND METHODS: We retrospectively analyzed data on 201 patients with medium-to-large HCC. According to treatment procedure, the patients were divided into the TACE group (n=124) and the TACE+RFA group (n=77). We recorded data on patient safety, subcapsular hepatic hematoma, large amount of ascites, liver abscess, gallbladder injury, and local skin infection. The overall survival (OS) and progression-free survival (PFS) in the 2 groups were analyzed and compared between groups.
RESULTS: The median PFS was 4.00 months (3.00–5.00 months) in the TACE group and 9.13 months (6.64–11.62 months) in the TACE+RFA group (P<0.001). Median OS was 12.00 months (8.88–15.13 months) in the TACE group and 27.57 months (20.06–35.08 months) in the TACE+RFA group (P<0.001). In the TACE+RFA group, multivariate Cox regression analysis showed that tumor size ≤5 cm) (HR: 1.952, 95% CI: 1.213-3.143, P=0.006), hepatitis B (HR: 2.323, 95% CI: 1.096–4.923, P=0.028), TACE times (1 or >1) (HR: 1.867, 95% CI: 1.156–3.013, P=0.011), alpha-fetoprotein (AFP) level >200 ng/ml (HR: 2.426, 95% CI: 1.533–3.839, P<0.001), and AST level >40 U/L (HR: 1.946, 95% CI: 1.196-3.166, P=0.007) were independent prognostic factors for overall survival.
CONCLUSIONS: Combination therapy of TACE with RFA is a safe and effective treatment for patients with medium-to-large HCC, with the long-term beneficial effect of retarding tumor progression and improving PFS and OS.
Keywords: Carcinoma, Hepatocellular, Catheter Ablation, Chemoembolization, Therapeutic, Aged, 80 and over, Combined Modality Therapy, Liver, Liver Neoplasms, Progression-Free Survival, radiofrequency ablation, alpha-Fetoproteins
Background
Hepatocellular carcinoma (HCC) is one of the leading cause of cancer-related death globally [1] and especially in China [2–4]. TACE is widely accepted as the an effective treatment method in patients with intermediate HCC [5,6]. The median 2-year survival for subjects treated by TACE is 60%
Clinical management guidelines regard RFA as an effective treatment modality for early-stage HCC [9,10]. RFA and microwave ablation (MWA) have been shown as a potential curative treatment modality for HCC measuring larger than 3 cm [11–14]. Yin et al. reported that RFA or MWA achieved a complete ablation rate more than 90% with 1-, 3-, and 5-year survival rates of 75.8%, 30.9%, and 15.4%, respectively [11]. Pusceddu et al. assessed 15 patients (33.3%) diagnosed with medium HCC measuring 3–5 cm and 13 patients (28.9%) with large HCCs measuring >5 cm who were treated with RFA, finding that complete ablation was achieved in 80% of patients with medium-sized tumors and in 53.8% of patients with large tumors (p=0.03) [14], suggesting that RFA can be extended to treat medium and large tumors. However, the complete response rate dropped to 45–70% for medium-sized HCCs (3.1–5.0 cm) and was only 23–45% for large HCCs (>5.0 cm) [15]. The monotherapy by RFA may pose the risk of tumor relapse for the patients with relatively large HCC [16].
Recent studies have shown that RFA combined with TACE can have a synergistic effect on HCCs [17,18]. Meta-analyses showed that TACE in combination with RFA significantly improved local tumor control and overall survival rates compared with TACE or RFA alone for patients with HCC larger than 3 cm [17–19]. However, most studies reported the short-term outcomes of the involved patients with HCC ≤7 cm [20,21] and few studies investigated the prognostic factors of RFA in combination with TACE for patients with HCC.
The aim of the present study was to evaluate the prognostic factors of RFA in combination with TACE for patients with medium-to-large HCCs, as well as the risk factors affecting the safety, PFS, and OS.
Material and Methods
PATIENTS:
All the included HCCs were diagnosed according to AASLD guidelines [22]. This study included patients treated in our department from Feb 2009 to May 2016. Only patients who met the following inclusion criteria were enrolled: age 18–80 years; tumors at BCLC A or B stage, with at least 1 tumor measuring 3–10 cm and the total number of tumors less than 4; patients with well-compensated cirrhosis but unsuitable for or declined surgical resection; KPS >70; liver function of Child-Pugh A or B; adequate hematologic function; and complete clinical follow-up.
The exclusion criteria were: BCL C or D stage; cardiac, pulmonary, cerebral, and renal dysfunction; history of another malignancy.
This work was approved by the Ethics Committee of Lishui Central Hospital, and written informed consent was obtained from all the included subjects.
TACE AND RFA PROCEDURES:
All patients underwent an initial evaluation of tumor arteries after a hepatic arterial angiography via a 5-Fr Rosch hepatic catheter (Cook, Bloomington, IN, USA). A 2.7-Fr micro-catheter (Progreat; Terumo, Japan) was carefully inserted into the tumor-feeding arteries for embolization, infusing an emulsion of 10 ml iodized oil mixed with 10 mg epirubicin/50 mg oxaliplatin. The injected dose of emulsion was determined by the tumor size and vascularity of the tumors (from 5 to 30 ml). Once complete stasis of tumor arterial blood flow was achieved, we stopped the infusion of iodized oil emulsion, followed by additional embolization using 350–560 or 560–710 μm gelatin sponge particles (Alicon Pharm Science and Technology Co., HangZhou, China) or 300–500 or 500–700 μm polyvinyl alcohol (PVA). Successful embolization was determined when no contrast staining in the tumor was identified on the post-embolization angiography.
In the TACE alone group, contrast-enhanced CT scans or MRI were performed 1 month after chemoembolization. If viable lesions were identified, repeat TACEs (2–4 times) were performed, followed by contrast-enhanced CT or MRI examinations.
In the TACE+RFA group, RFA was applied to patients with less than 3 viable lesions or a primary viable tumor ≤5 cm in size after contrast-enhanced CT or MRI. RFA was exclusively carried out after cTACE, and the time interval (usually 7–15 days) between the 2 treatments was determined based on liver function, Barcelona Clinic Liver Cancer (BCLC) score, and patient intention to treatment.
The RFA technique was as described in a previous study [23] Briefly, an RF electrode with an active tip that can induce a 5-cm ablation zone (RITA STARBURST XL; RITA Medical Systems, Mountain View, USA) was used for patients who had a lesion size of less than 5 cm; otherwise, an electrode with an active tip that can induce a 7-cm ablation zone (RITA STARBURST XL; RITA Medical Systems, Mountain View, USA) was used for patients with lesion sizes >5 cm. Overlap ablations were allowed to cover the whole tumor mass to achieve an adequate safety margin of 0.5–1.0 cm (Figure 1).
CLINICAL FOLLOW-UP:
Outpatient clinic visits were scheduled at 1, 2, and 3 months after treatments and every 3 months thereafter. Symptoms associated with post-treatment complications was assessed, serum AFP level and liver function and whole blood cells were tested, and contrast-enhanced CT scans or MRI were performed. Repeated TACE or RFA was performed when an enhanced viable tumor foci or tumor recurrence was identified on follow-up MRI or CT imaging (Figure 2). Patients were followed up by either telephone survey or our electronic medical chart system.
TREATMENT EFFECT EVALUATION:
Residual “viable tumor” tissue was defined as the arterially enhancing tissue within the treated hepatocellular carcinoma using the evaluation criteria as proposed by AASLD, which is a modified RECIST criteria (mRECIST) [24]. The safety profile of RFA and TACE for the patients was defined using the criteria proposed by the Society of Interventional Radiology (SIR) [25]. The post-treatment morbidities that required a higher level of care or resulted in a substantially longer hospital stay (>48 h) were regarded as the major complications according to the guidelines proposed by SIR24.
STATISTICAL ANALYSIS:
Data were analyzed by SPSS 23.0 software (SPSS, Inc., Chicago, IL, USA). Survival data comparison was made by log-rank test and demonstrated by survival curves. Risk factors relevant to survival were analyzed by Cox regression test. Two-tailed P<0.05 was deemed as a statistically significant difference.
Results
SAFETY OF THE COMBINATION THERAPY:
There were no significant differences in liver function and whole blood cell counts between the pre-RFA and 1-week post-RFA. Major complications after RFA occurred in 4.8% (6/124) of patients, including subcapsular hepatic hematoma (n=3), a large amount of ascites (n=2), liver abscess (n=1), gallbladder injury (n=1), and local skin infection (n=1). The complications were resolved with appropriate medications and there were no procedure-related deaths.
OVERALL SURVIVAL AND LOCAL TUMOR PROGRESSION:
We followed up 201 patients for a median time period of 21.4±16.1 months (range 1–77.2 months), and 44 patients were still alive at the time of data collection.
The 1-, 3-, and 5-year PFS were 11.9% vs. 43.2%, 0 vs. 18.0%, and 0 vs. 9.5% in TACE group and the TACE+RFA group. The median PFS was 4.00 months (3.00–5.00 months) in TACE group and 9.13months (6.64–11.62 months) in the TACE+RFA group (P<0.001) (Figure 3).
The 1-, 3-, 5-year OS rates were 48.1% vs. 76.2%, 6.5% vs. 37.1%, and 0 vs. 16.4% between the TACE group and the TACE+RFA group. Median OS was 12.00 months (8.88–15.13 months) in the TACE group and 27.57 months (20.06–35.08 months) in the TACE+RFA group (P<0.001) (Figure 4).
PROGNOSTIC FACTORS OF OS:
Univariate Cox regression analysis was performed to identify the prognostic factors for overall survival of patients: age (≤60 or >60), sex, hepatitis B status (active or non-active), cirrhosis, history of surgery, tumor size (≤5 cm or 5–10 cm), number of nodules (1 or more than 1), Child-Pugh stage, BCLC stage, times of TACE (1 or multiple), RF ablation (completely or incompletely ablated), AFP (ng/ml) (≤200 or >200), ALT (U/L) (≤40 or >40), and AST (U/L) (≤40 or >40). The results demonstrated that tumor size (P=0.007), times of TACE (1 or multiple) (P=0.015), AFP level (ng/ml) (≤200, >200) (P=0.001), AST level (U/L) (≤40, >40) (P=0.008), and cirrhosis (P=0.051) were the significant prognostic factors. The number of nodules (1 or more than 1) (P=0.152), RF ablation (complete or uncomplete) (P=0.165), and HBV infection status (active or non-active) (P=0.123) were unfavorable prognostic factors (Table 2).
The independent factors identified above were assessed by multivariate Cox proportional regression analysis. The results confirmed that tumor size (≤5 cm or 5–10 cm)(HR: 1.952, 95% CI: 1.213–3.143, P=0.006), hepatitis B status (HR: 2.323, 95% CI: 1.096–4.923, P=0.028), TACE times (1 or multiple) (HR: 1.867, 95% CI: 1.156–3.013, P=0.011), AFP level (ng/ml) (≤200 or >200) (HR: 2.426, 95% CI: 1.533–3.839, P<0.001), and AST level (U/L) (≤40 or >40) (HR: 1.946, 95% CI: 1.196–3.166, P=0.007) were significant prognostic risk factors.
Patients with smaller tumor sizes (≤5 cm) had much better survival than those with large tumor sizes (5–10 cm), (Figure 5). Patients who received TACE+RFA had longer survival compared to those with recurrent tumors (Figure 6). The 1-, 2-, 3-, 5- and 6-year post-ablation survival rates were significantly higher in subjects with a pre-ablation AFP level ≤200 ng/mL than in those with an AFP level >200 ng/mL (Figure 7). Patients with ≤40 U/L AST had longer 1-, 2-, 3-, 5-, and 6-year survival rates compared with those with AST >40 U/L (Figure 8).
PROGNOSTIC FACTORS OF PFS:
Univariate Cox regression analysis was performed to evaluate the impact of the following factors on progression-free survival: age (≤60 or >60), sex, hepatitis B status (active or non-active), cirrhosis, history of surgery, tumor size (≤5 cm or 5–10 cm), number of nodules (1 or more than 1), Child-Pugh stage, BCLC stage, times of TACE (1 or multiple), RF ablation (completely or incompletely ablated), AFP (ng/ml) (≤200 or >200), ALT (U/L) (≤40 or >40), and AST (U/L) (≤40 or >40). The results demonstrated that HBV infection (HR: 0. 0.560, 95% CI: 0.334–0.936, P=0.027), TACE times (1 or more than 1) (HR: 0.624, 95% CI: 0.417–0.933, P=0.021), pre-ablation AFP level (HR: 0.550, 95% CI: 0.349–0.866, (P=0.010), and AST (HR: 0.610, 95% CI: 0.407–0.915, P=0.017) were prognostic factors for PFS (Table 3).
Multivariate Cox regression analysis confirmed that hepatitis B status (HR: 0.209, 95% CI: 1.078–4.048, P=0.029), TACE times (1 or more than 1) (HR: 1.646, 95% CI: 1.081–2.507, P=0.020), AFP level (ng/ml) (≤200 or >200) (HR: 1.732, 95% CI: 1.136–2.639, P=0.011), and AST level (U/L) (≤40 or >40) (HR: 1.741, 95% CI: 1.144–2.650, P=0.010) were independent prognostic factors for prognosis.
Discussion
The management for HCC with tumor diameter more than 3.0 cm or multiple HCCs remains a major clinical challenge. Previous studies have shown a low tumor necrosis rate in HCC patients treated with TACE or RFA alone [11,26]. Our study demonstrated that a complete ablation rate of 74.2% (92 of 124 patients) was achieved in patients with medium-to-large HCCs treated with TACE plus RFA. In the patients with tumor size ≤5 cm, the complete ablation rate was 88.6%, as compared to the patients with the tumor size of 5–10 cm, who had a complete ablation rate of 55.6%. Our study also shows that the median PFS was 4.0 months (3.00–5.00) in the TACE group and 9.13 months (6.64–11.62) in the TACE+RFA group (P<0.001). Median OS was 12.00 months (8.88–15.13) in the TACE group and 27.57 months (20.06–35.08) in the TACE+RFA group (P<0.001). Patients with small-sized tumors (≤5 cm) treated with combination therapy had much better OS than those with larger tumors (5–10 cm). Cox proportional univariate and multivariate analyses identified tumor size, hepatitis B, TACE times, AFP level, and AST as the significant prognostic factors affecting overall survival.
The results of our study are consistent with those of previous studies, showing that tumor size was an important factor that influences OS after RFA [3,11,15,27,28]. Applying many sessions of TACE for treating patients with large tumors would not benefit the patients with complete necrosis of the tumor, but would rather lead to deterioration of liver function and increased VEGF. In the current clinical guidelines, RFA is not recommended for treating the tumors larger than 5 cm, but some authors have reported that RFA can be used to treat large tumors of up to 7 cm [11,15]. However, combination therapy may offer some hope for these patients by enlarging the complete necrosis of tumor. El-Kady et al. included 8/8 (100%) patients with medium HCC and successfully treated with TACE+RFA and 9/12 (75%) patients with larger lesions that were successfully ablated. After 6 months, 83.3% of patients in the TACE+RFA group maintained the ablated status [12]. In our study, 55.6% of patients with large tumors (5–10 cm) achieved complete ablation. The patients in our study were initially treated with TACE exclusively using microcatheters, which are useful for embolizing all the tumor-feeding vessels, with the outcome of reducing the tumor size and increasing the success rate of RFA. In addition, TACE (1–3 times) prior to RFA can induce partial necrosis of large lesions and decrease the volume of a viable tumor, thus facilitating complete ablation [28]. TACE prior to RFA is helpful for identifying the micro-metastatic foci due to the contrast staining of tumor vessels or lipiodol deposit, which are usually neglected by contrast-enhanced CT or MRI. Our study suggests that combination therapy is suitable for large lesions. However, the 1- and 3-year post-ablation survival rates were 66.5% and 28.0%, respectively, and the overall survival rate was still not ideal, suggesting that further study is needed. Lin et al. found no significant differences in overall survival and local tumor progression rates between medium-to-large tumors and in patients with tumors of BCLC stages A, B1, and B2 [15], which may be due to the 58 medium-sized and 17 large-sized tumors included in their study.
Numerous studies have reported a relationship between the serum AFP level and the prognosis of HCC [27–30]. The results of our study are consistent with that of the study by Yin et al. [11], which showed that patients with AFP ≤200 ng/mL had significantly better survival than those with a pre-ablation AFP >200 ng/mL, with 5- and 6-year survival rates of 24.7%
Sohn et al. found that the clinical outcomes of RFA for recurred HCCs after TACE were inferior to those of the first-treated tumors [30], suggesting that overall survival of HCC patients who received RFA after TACE may be associated with the pathophysiologic features of tumors. We hypothesized that the repeated TACE or surgeries change the biological behavior of tumors and the sensitivity of tumors to subsequent treatments, thus potentially resulting in poor prognosis.
Our study found that complete ablation had no definite association with overall survival, which is consist with one reported study [31], but is in the conflict with the study reported by Lin et al., which showed that a complete response to RFA was the only significant factor affecting the OS [15]. However, Nakazawa et al. reported a result different from that of ours, in which a complete response to ME-RFA was significantly associated with local tumor progression [32], which may be due to the different demographic characteristics of patients.
Yin, et al. [11] reported that the tumor recurrence after hepatectomy, incomplete tumor ablation, and pre-ablation AFP level predicted poor prognosis. The study by Zhao et al. [33] indicated that the survival of HCC was associated with pathology type, tumor numbers, clinical stages, and Child-Pugh score. Ke et al. [34] reported that tumor size, ablative margin, serum AFP level, and number of ablation sessions to achieve a imaging-confirmed complete ablation were the significant factors predictive of OS. Peng et al. [35] found that tumor size and tumor number were the significant prognostic factors of OS. Sohn et al. [30] reported that the factors relevant to prognosis were Child-Pugh class and serum AFP concentration. However, our study demonstrated that tumor size, hepatitis B status, TACE times (1 or more than 1), and AFP and AST levels were independent prognostic factors relevant to prognosis. The differences between the results of our study and previous studies may be due to the patient selection and tumors numbers. Additionally, we found that patients with or without a history of liver surgery had the same OS, suggesting that patients with a postoperative recurrence may have the same therapeutic results. Xie et al. [36] reported that lesion number was the prognostic factor most strongly affecting the post-RFA prognosis, but our study found no significant association between the prognosis and tumor numbers, which may be due to the inclusion of different numbers of patients with a maximum of 4 tumor nodules.
This work had several limitations. Firstly, this study was retrospectively designed, which inevitably entails patient selection bias. Secondly, we included different numbers of patients with newly diagnosed HCC or recurrent HCCs after other treatments, such as TACE or surgical resection, which may have influenced the comparison between the 2 groups of patients with TACE alone and TACE+RFA. Thirdly, more patients in the TACE alone group had HCC >5 cm than in the TACE+RFA group (p<0.001). This significant difference in tumor size between the 2 groups may have contributed to bias in the result of survival analysis.
Conclusions
Combination therapy of TACE plus RFA for patients with medium-to-large hepatocellular carcinoma is safe and effective, and this combination therapy can delay tumor progression and improve PFS and OS. Tumor size, TACE times, AFP, and AST level, and HBV infection status are independents risk prognostic factors for overall survival.
Figures
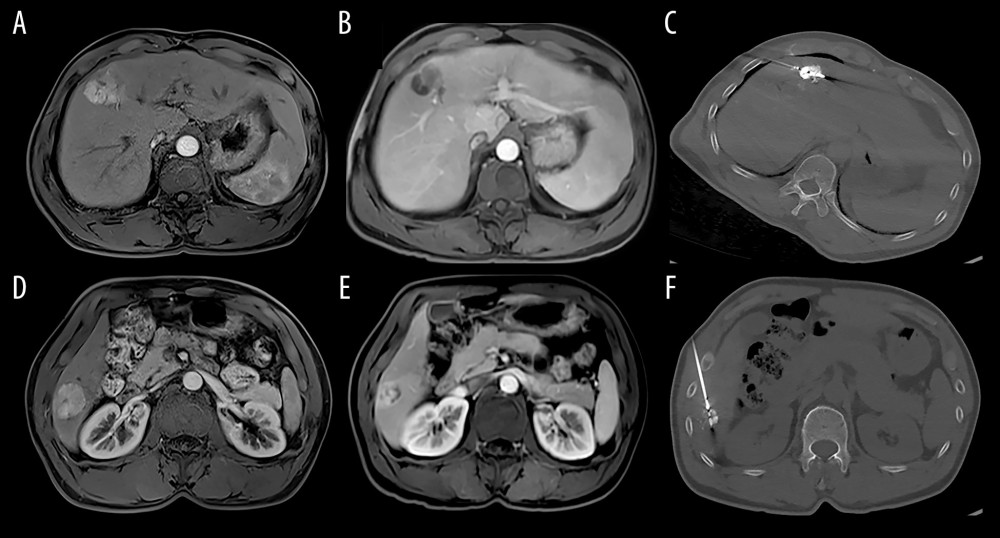 Figure 1. Image of the hepatocellular carcinoma before and after TACE and RFA (A, D: MRI of hepatocellular carcinoma before TACE; B, E: MRI of hepatocellular carcinoma after TACE; C, F: CT image of MRI of hepatocellular carcinoma received RFA).
Figure 1. Image of the hepatocellular carcinoma before and after TACE and RFA (A, D: MRI of hepatocellular carcinoma before TACE; B, E: MRI of hepatocellular carcinoma after TACE; C, F: CT image of MRI of hepatocellular carcinoma received RFA). 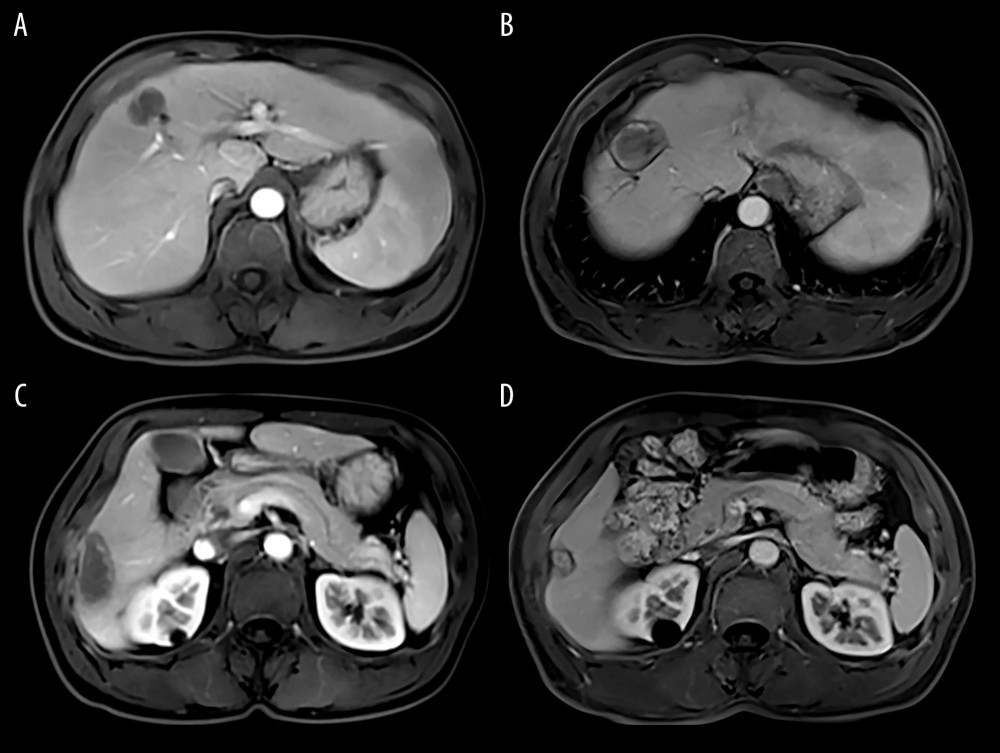 Figure 2. MRI of the hepatocellular carcinoma 1 months after TACE+RFA (A, C: MRI of hepatocellular carcinoma 1 moth after TACE+RFA; B, D: MRI of hepatocellular carcinoma 6 moth after TACE+RFA).
Figure 2. MRI of the hepatocellular carcinoma 1 months after TACE+RFA (A, C: MRI of hepatocellular carcinoma 1 moth after TACE+RFA; B, D: MRI of hepatocellular carcinoma 6 moth after TACE+RFA). 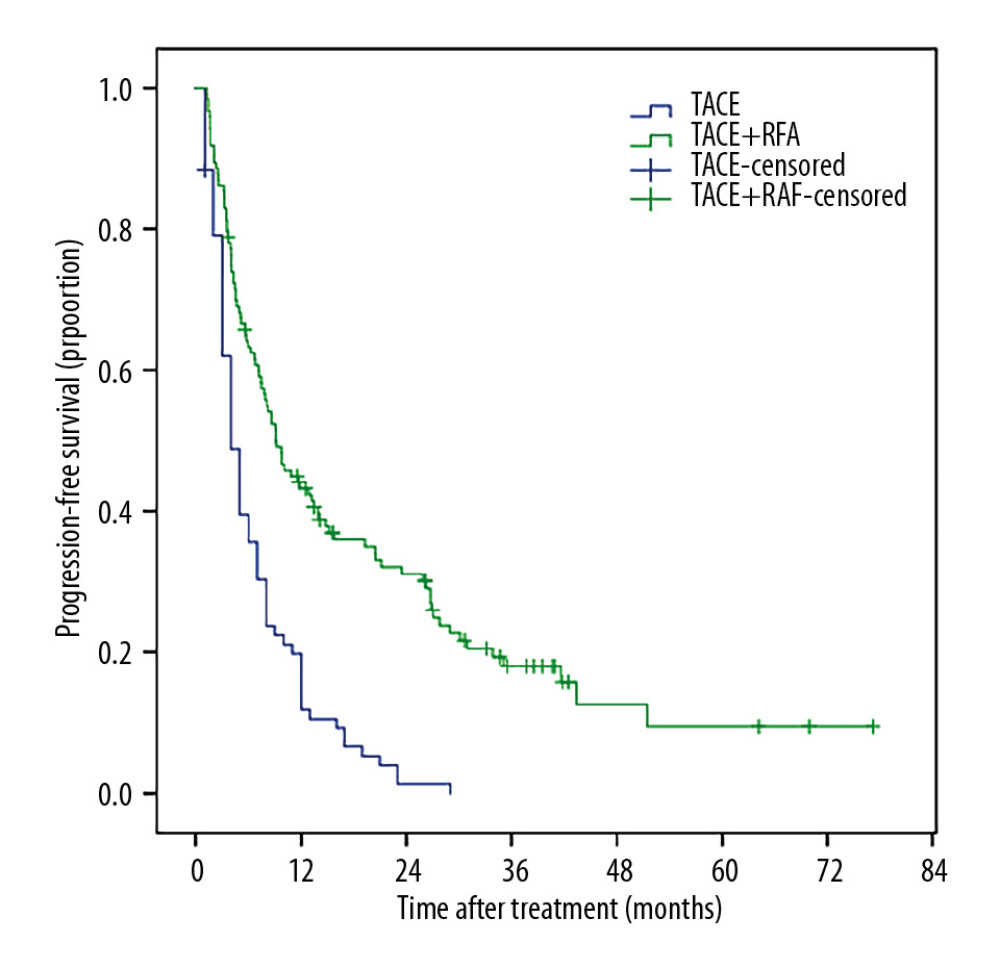 Figure 3. The Kaplan-Meir curve for PFS of the study population.
Figure 3. The Kaplan-Meir curve for PFS of the study population. 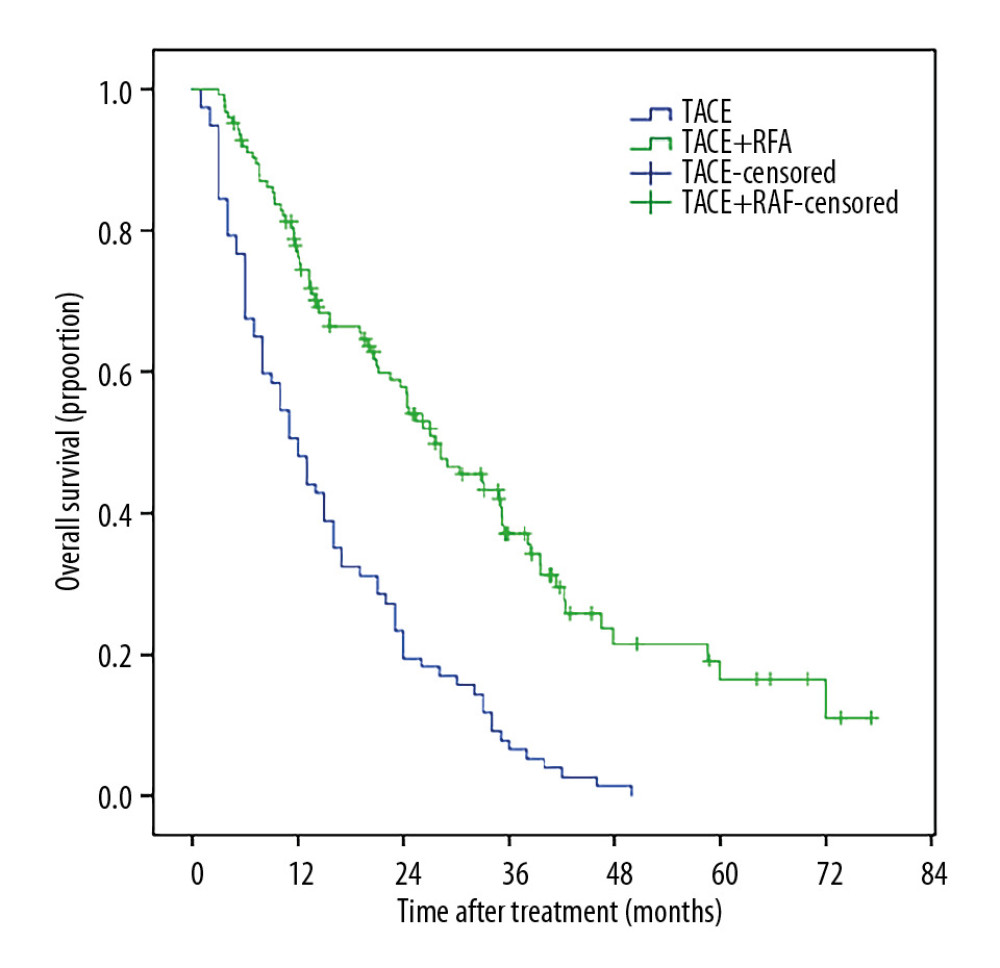 Figure 4. The Kaplan-Meir curve for OS of the study.
Figure 4. The Kaplan-Meir curve for OS of the study. 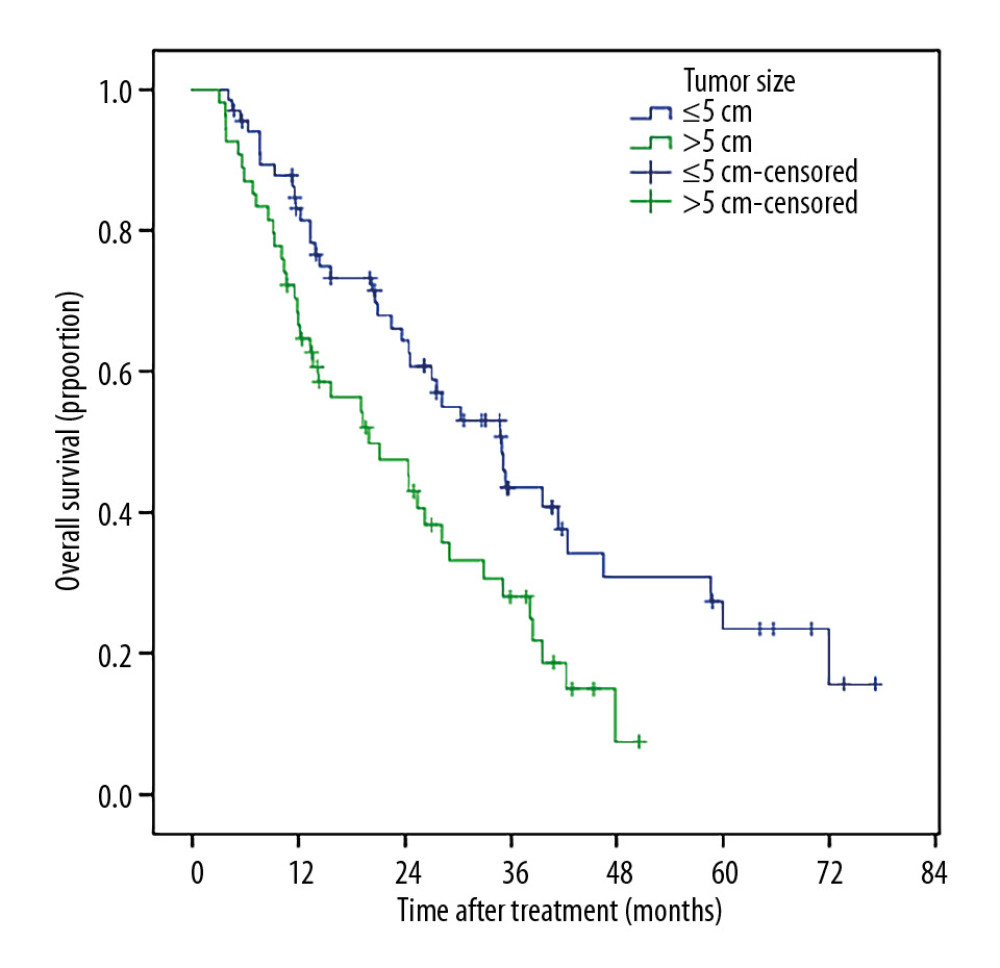 Figure 5. Patients with smaller tumor sizes (≤5 cm) had much better OS than those with large tumor sizes (5–10 cm).
Figure 5. Patients with smaller tumor sizes (≤5 cm) had much better OS than those with large tumor sizes (5–10 cm). 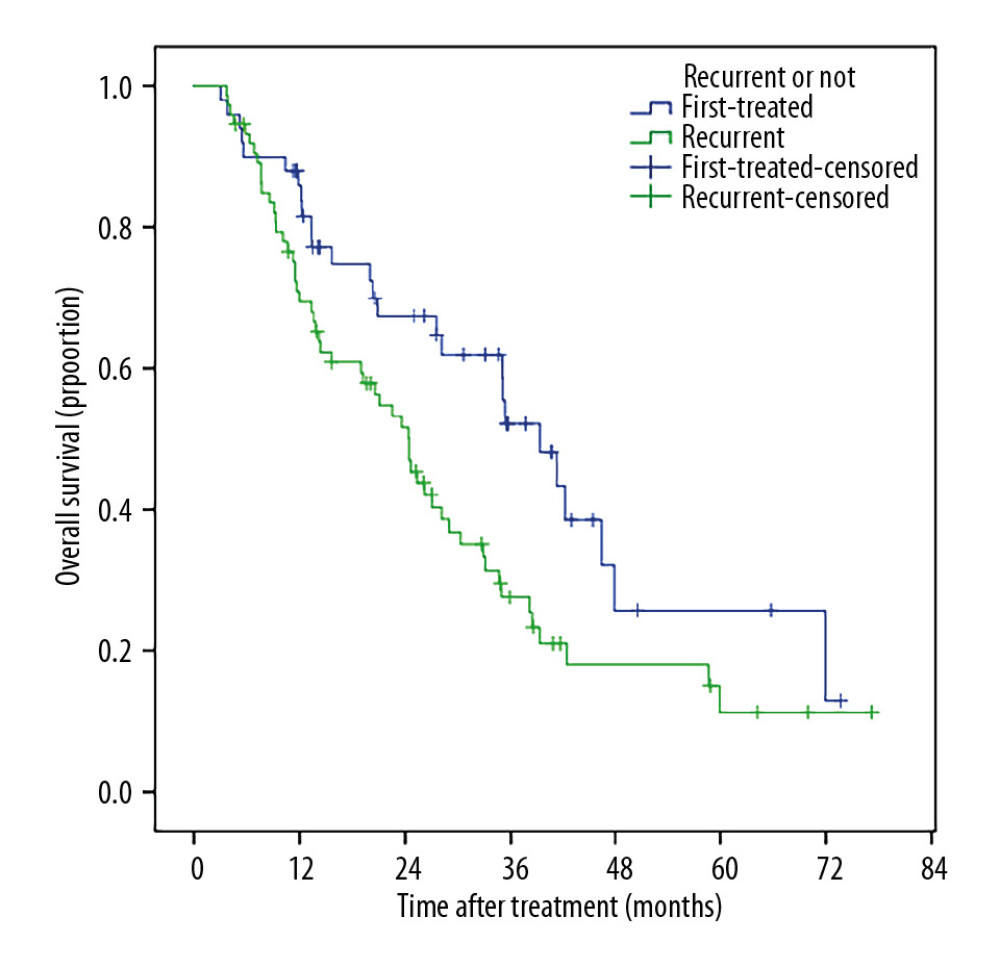 Figure 6. Patients who received TACE+RFA as the primary treatment had markedly longer OS compared with patients treated for recurrent tumors.
Figure 6. Patients who received TACE+RFA as the primary treatment had markedly longer OS compared with patients treated for recurrent tumors. 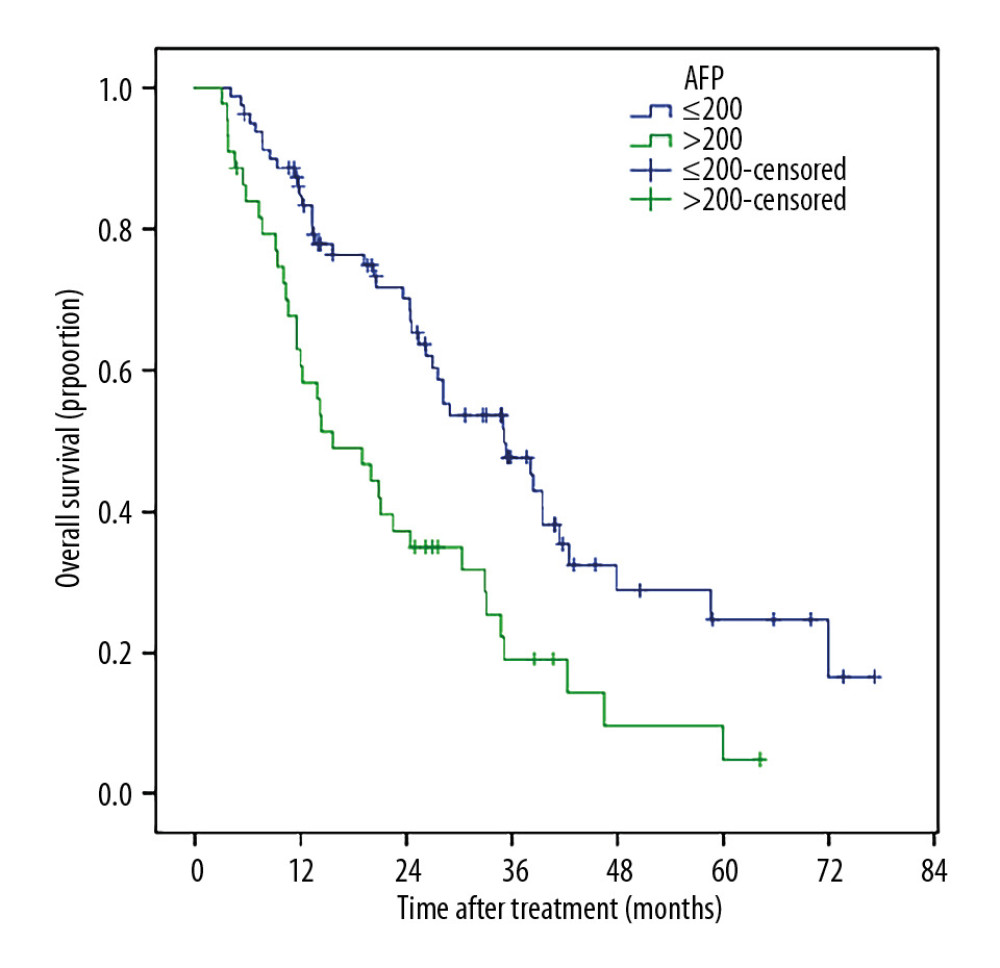 Figure 7. The 1-, 2-, 3-, 5-, and 6-year post-ablation survival rates were significantly higher in patients with a pre-ablation AFP level v200 ng/mL than in those with a pre-ablation AFP level >200 ng/mL.
Figure 7. The 1-, 2-, 3-, 5-, and 6-year post-ablation survival rates were significantly higher in patients with a pre-ablation AFP level v200 ng/mL than in those with a pre-ablation AFP level >200 ng/mL. 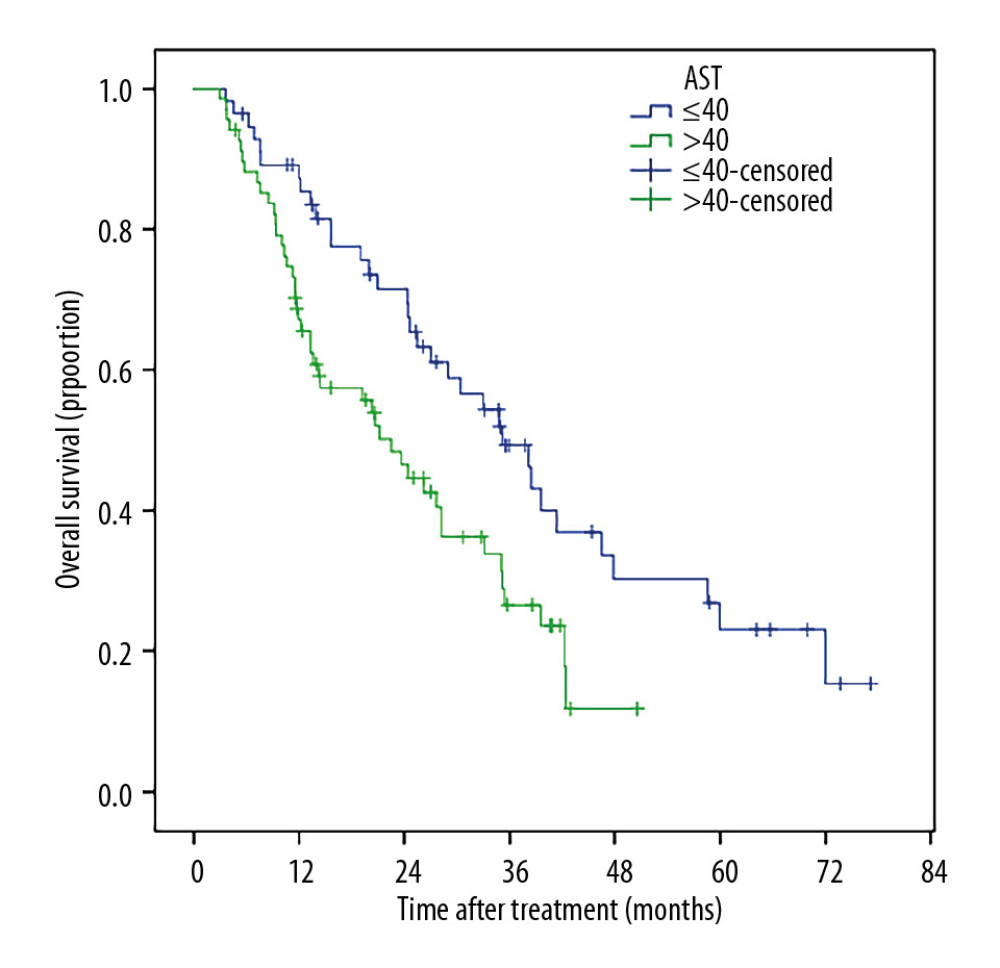 Figure 8. Patients with ≤40 U/L AST had longer 1-, 2-, 3-, 5-, and 6-year survival rates compared with those with AST >40 U/L (87.3% vs. 67.1%, 71.5% vs. 46.5%, 49.4% vs. 26.5%, 23.0% vs. 11.8%, and 15.4% vs. 0%.
Figure 8. Patients with ≤40 U/L AST had longer 1-, 2-, 3-, 5-, and 6-year survival rates compared with those with AST >40 U/L (87.3% vs. 67.1%, 71.5% vs. 46.5%, 49.4% vs. 26.5%, 23.0% vs. 11.8%, and 15.4% vs. 0%. References
1. Torre LA, Bray F, Siegel RL, Global cancer statistics, 2012: Cancer J Clin, 2015; 65(2); 87-108
2. Chen W, Zheng R, Baade PD, Cancer statistics in China, 2015: Cancer J Clin, 2016; 66(2); 115-32
3. Zhao M, Wang JP, Pan CC, CT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvement: Eur J Radiol, 2012; 81(10); 2717-25
4. Kudo M, Izumi N, Ichida T: Hepatol Res, 2016; 46(5); 372-90
5. Sieghart W, Hucke F, Peck-Radosavljevic M, Transarterial chemoembolization: Modalities, indication, and patient selection: J Hepatol, 2015; 62(5); 1187-95
6. Lewandowski RJ, Mulcahy MF, Kulik LM, Chemoembolization for hepatocellular carcinoma: Comprehensive imaging and survival analysis in a 172-patient cohort: Radiology, 2010; 255(3); 955-65
7. Takayasu K, Arii S, Ikai I, Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients: Gastroenterology, 2006; 131(2); 461-69
8. Wang B, Xu H, Gao ZQ, Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization: Acta Radiol, 2008; 5; 523-29
9. Bruix J, Sherman MAmerican Association for the Study of Liver Diseases, Management of hepatocellular carcinoma: An update: Hepatology, 2011; 53(3); 1020-22
10. Bruix J, Sherman M, Llovet JM, Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona – 2000 EASL conference. European Association for the Study of the Liver: J Hepatol, 2001; 35(3); 421-30
11. Yin XY, Xie XY, Lu MD, Percutaneous thermal ablation of medium and large hepatocellular carcinoma: Long-term outcome and prognostic factors: Cancer, 2009; 115(9); 1914-23
12. El-Kady NM, Esmat G, Mahmoud EH, Hypertonic saline-enhanced radiofrequency versus chemoembolization sequential radiofrequency in the treatment of large hepatocellular carcinoma: Eur J Gastroenterol Hepatol, 2013; 25(5); 628-33
13. Livraghi T, Goldberg SN, Lazzaroni S, Hepatocellular carcinoma: Radio-frequency ablation of medium and large lesions: Radiology, 2000; 214(3); 761-68
14. Pusceddu C, Melis L, Ballicu N, Percutaneous microwave ablation under ct guidance for hepatocellular carcinoma: A single institutional experience: J Gastrointest Cancer, 2018; 49(3); 295-301
15. Lin CC, Cheng YT, Chen MW, Lin SM, The effectiveness of multiple electrode radiofrequency ablation in patients with hepatocellular carcinoma with lesions more than 3 cm in size and Barcelona Clinic Liver Cancer Stage A to B2: Liver Cancer, 2016; 5(1); 8-20
16. Ueno M, Hayami S, Shigekawa Y, Prognostic impact of surgery and radiofrequency ablation on single nodular HCC 5 cm: Cohort study based on serum HCC markers: J Hepatol, 2015; 63(6); 1352-59
17. Zhao M, Wang JP, Pan CC, CT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvement: Eur J Radiol, 2012; 81(10); 2717-25
18. Chu HH, Kim JH, Yoon HK, Chemoembolization combined with radiofrequency ablation for medium-sized hepatocellular carcinoma: A propensity-score analysis: J Vasc Interv Radiol, 2019; 30; 1533-43
19. Lu Z, Wen F, Guo Q, Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A meta-analysis of randomized-controlled trials: Eur J Gastroenterol Hepatol, 2013; 25(2); 187-94
20. Peng ZW, Zhang YJ, Liang HH, Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: A prospective randomized trial: Radiology, 2012; 262(2); 689-700
21. Mori Y, Tamai H, Shingaki N, Diffuse intrahepatic recurrence after percutaneous radiofrequency ablation for solitary and small hepatocellular carcinoma: Hepatol Int, 2009; 3(3); 509-15
22. Bruix J, Sherman M, Management of hepatocellular carcinoma: An update: Hepatology, 2011; 53(3); 1020-22
23. Tu J, Ji J, Wu F, Effectiveness of combined 131I-chTNT and radiofrequency ablation therapy in treating advanced hepatocellular carcinoma: Cell Biochem Biophys, 2015; 71(2); 777-84
24. Lencioni R, Llovet JM, Modified RECIST (mRECIST) assessment for hepatocellular carcinoma: Semin Liver Dis, 2010; 30(1); 52-60
25. Sacks D, Mcclenny TE, Cardella JF, Lewis CA, Society of Interventional Radiology clinical practice guidelines: J Vasc Interv Radiol, 2003; 14(9 Pt 2); S199
26. Seror O, N’Kontchou G, Ibraheem M, Large (> or =5.0-cm) HCCs: Multipolar RF ablation with three internally cooled bipolar electrodes – initial experience in 26 patients: Radiology, 2008; 248(1); 288-96
27. Toro A, Ardiri A, Mannino M, Effect of pre- and post-treatment alpha-fetoprotein levels and tumor size on survival of patients with hepatocellular carcinoma treated by resection, transarterial chemoembolization or radiofrequency ablation: A retrospective study: BMC Surgery, 2014; 14; 40
28. Zhang L, Yin X, Gan YH, Radiofrequency ablation following first-line transarterial chemoembolization for patients with unresectable hepatocellular carcinoma beyond the Milan criteria: BMC Gastroenterology, 2014; 14; 11
29. Tena I, Gupta G, Tajahuerce M, Successful second-line metronomic temozolomide in metastatic paraganglioma: Case reports and review of the literature: Clin Med Insights Oncol, 2018; 12; 1179554918763367
30. Sohn W, Choi MS, Cho JY, Role of radiofrequency ablation in patients with hepatocellular carcinoma who undergo prior transarterial chemoembolization: Long-term outcomes and predictive factors: Gut Liver, 2014; 8(5); 543-51
31. Kong W, Xu H, Cheng J, The prognostic role of a combined fibrinogen and neutrophil-to-lymphocyte ratio score in patients with resectable hepatocellular carcinoma: A retrospective study: Med Sci Monit, 2020; 26; e918824
32. Nakazawa T, Kokubu S, Shibuya A, Radiofrequency ablation of hepatocellular carcinoma: Correlation between local tumor progression after ablation and ablative margin: Am J Roentgenol, 2007; 188(2); 480-88
33. Zhao M, Wang J-p, Pan C-c, CT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvement: Eur J Radiol, 2012; 81(10); 2717-25
34. Ke S, Ding X-M, Qian X-J, Radiofrequency ablation of hepatocellular carcinoma sized >3 and ≤5 cm: Is ablative margin of more than 1 cm justified?: World J Gastroenterol, 2013; 19(42); 7389-98
35. Peng Z-W, Zhang Y-J, Chen M-S, Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: A prospective randomized trial: J Clin Oncol, 2013; 31(4); 426-32
36. Xie H, Wang H, An W, The efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization for primary hepatocellular carcinoma in a cohort of 487 patients: PloS One, 2014; 9(2); e89081
Figures
 Figure 1. Image of the hepatocellular carcinoma before and after TACE and RFA (A, D: MRI of hepatocellular carcinoma before TACE; B, E: MRI of hepatocellular carcinoma after TACE; C, F: CT image of MRI of hepatocellular carcinoma received RFA).
Figure 1. Image of the hepatocellular carcinoma before and after TACE and RFA (A, D: MRI of hepatocellular carcinoma before TACE; B, E: MRI of hepatocellular carcinoma after TACE; C, F: CT image of MRI of hepatocellular carcinoma received RFA). Figure 2. MRI of the hepatocellular carcinoma 1 months after TACE+RFA (A, C: MRI of hepatocellular carcinoma 1 moth after TACE+RFA; B, D: MRI of hepatocellular carcinoma 6 moth after TACE+RFA).
Figure 2. MRI of the hepatocellular carcinoma 1 months after TACE+RFA (A, C: MRI of hepatocellular carcinoma 1 moth after TACE+RFA; B, D: MRI of hepatocellular carcinoma 6 moth after TACE+RFA). Figure 3. The Kaplan-Meir curve for PFS of the study population.
Figure 3. The Kaplan-Meir curve for PFS of the study population. Figure 4. The Kaplan-Meir curve for OS of the study.
Figure 4. The Kaplan-Meir curve for OS of the study. Figure 5. Patients with smaller tumor sizes (≤5 cm) had much better OS than those with large tumor sizes (5–10 cm).
Figure 5. Patients with smaller tumor sizes (≤5 cm) had much better OS than those with large tumor sizes (5–10 cm). Figure 6. Patients who received TACE+RFA as the primary treatment had markedly longer OS compared with patients treated for recurrent tumors.
Figure 6. Patients who received TACE+RFA as the primary treatment had markedly longer OS compared with patients treated for recurrent tumors. Figure 7. The 1-, 2-, 3-, 5-, and 6-year post-ablation survival rates were significantly higher in patients with a pre-ablation AFP level v200 ng/mL than in those with a pre-ablation AFP level >200 ng/mL.
Figure 7. The 1-, 2-, 3-, 5-, and 6-year post-ablation survival rates were significantly higher in patients with a pre-ablation AFP level v200 ng/mL than in those with a pre-ablation AFP level >200 ng/mL. Figure 8. Patients with ≤40 U/L AST had longer 1-, 2-, 3-, 5-, and 6-year survival rates compared with those with AST >40 U/L (87.3% vs. 67.1%, 71.5% vs. 46.5%, 49.4% vs. 26.5%, 23.0% vs. 11.8%, and 15.4% vs. 0%.
Figure 8. Patients with ≤40 U/L AST had longer 1-, 2-, 3-, 5-, and 6-year survival rates compared with those with AST >40 U/L (87.3% vs. 67.1%, 71.5% vs. 46.5%, 49.4% vs. 26.5%, 23.0% vs. 11.8%, and 15.4% vs. 0%. Tables
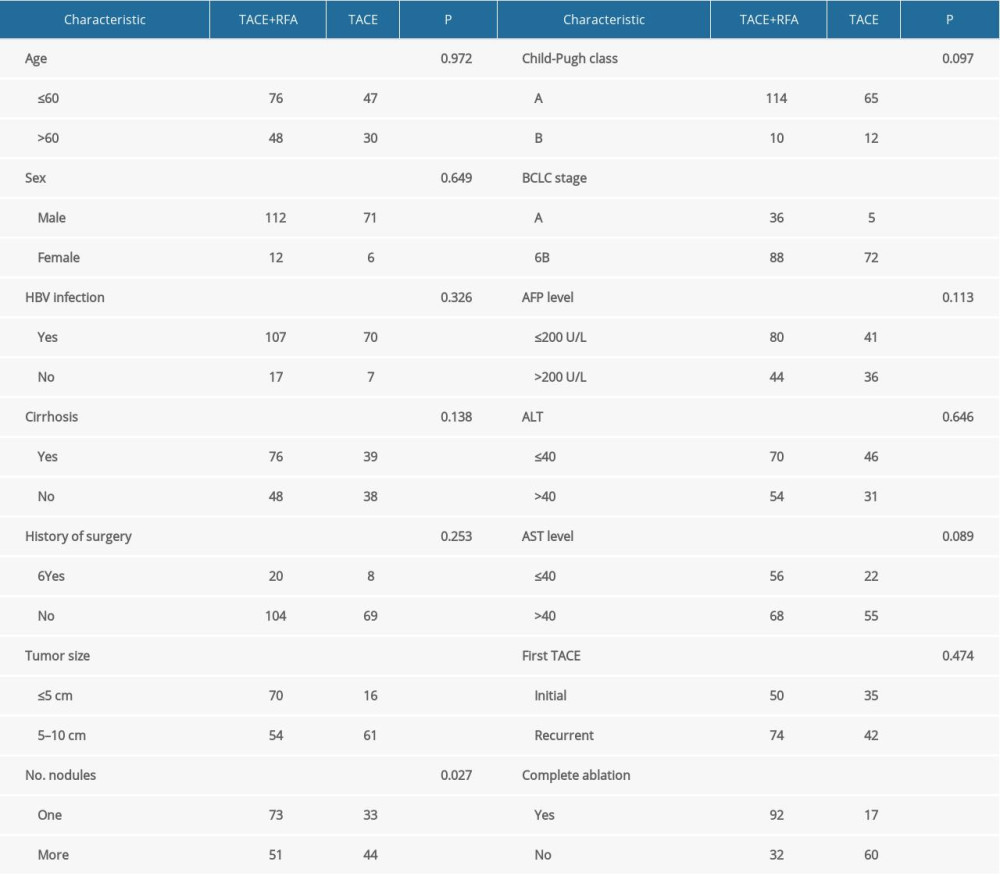 Table 1. The demographic information and clinical characteristics of the patients.
Table 1. The demographic information and clinical characteristics of the patients.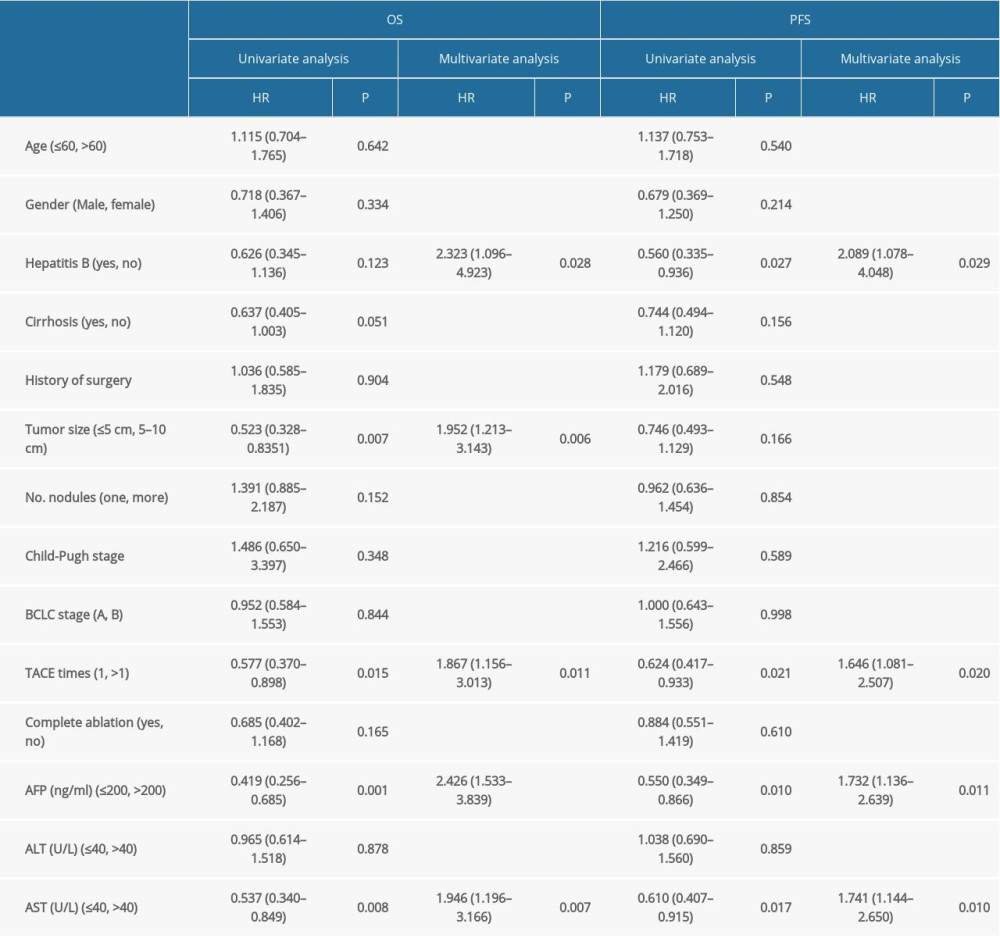 Table 2. Univariate and multivariate cox model for OS and PFS (this analysis was done in the TACE plus RFA group).
Table 2. Univariate and multivariate cox model for OS and PFS (this analysis was done in the TACE plus RFA group).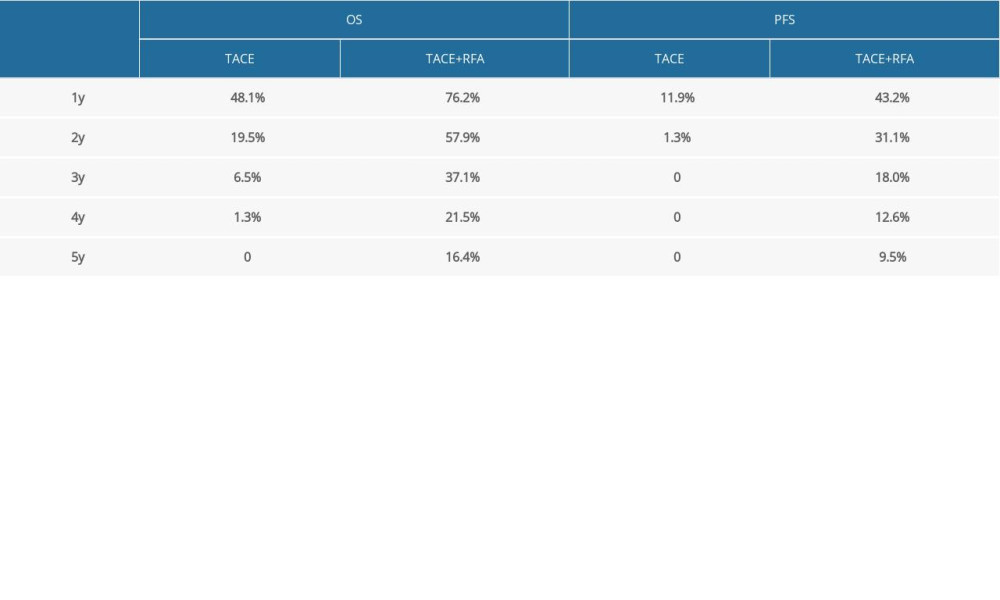 Table 3. The overall survival and progression free survival of the two group of patients.
Table 3. The overall survival and progression free survival of the two group of patients. Table 1. The demographic information and clinical characteristics of the patients.
Table 1. The demographic information and clinical characteristics of the patients. Table 2. Univariate and multivariate cox model for OS and PFS (this analysis was done in the TACE plus RFA group).
Table 2. Univariate and multivariate cox model for OS and PFS (this analysis was done in the TACE plus RFA group). Table 3. The overall survival and progression free survival of the two group of patients.
Table 3. The overall survival and progression free survival of the two group of patients. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








