06 September 2020: Clinical Research
Plasma β-Endorphin Concentration and Antipsychotic Treatment Outcome in Schizophrenia: 1-Year Follow-Up
Małgorzata Urban-Kowalczyk1ABDEFG*, Magdalena Kotlicka-Antczak1BDEF, Dominik Strzelecki1BDEG, Ewa Rudecka2BDEF, Janusz Śmigielski3BCDEDOI: 10.12659/MSM.924307
Med Sci Monit 2020; 26:e924307
Abstract
BACKGROUND: Increased levels of endogenous opioids have been observed in patients with schizophrenia; however, the influence of these endogenous opioids on the biology of schizophrenia remains unclear. The aim of this study was to evaluate the impact of beta-endorphin (BE) on the course of schizophrenia and risk of relapse.
MATERIAL AND METHODS: The study included 25 patients hospitalized with schizophrenia and 47 controls. Their symptoms were evaluated using Positive and Negative Syndrome Scale (PANSS) and composite index at five points: at the onset of hospitalization; after 4, 6 and 10 weeks of treatment; and after 12 months. β-endorphin plasma concentrations were assessed in patients at study enrollment and after 6 weeks of treatment. Data regarding rehospitalization during follow-up were also collected.
RESULTS: Patients had higher BE concentration than controls at study enrollment (P=0.002) and after 6 weeks (P=0.000). BE levels increased during treatment (mean 0.538ng/mL vs. mean 0.624 ng/mL; P=0.007). No correlation was found between BE concentration and PANSS subscale score at any stage of the study. A higher BE level at study enrollment was related to a predominance of negative symptoms after 1 year, measured with composite index (R=–0.404; P=0.045). Patients who were later hospitalized again were significantly more likely to demonstrate an increase in BE levels over 6 weeks (P=0.001).
CONCLUSIONS: Individuals with schizophrenia demonstrated higher BE concentrations than healthy controls; this tendency was particularly apparent in those affected by negative symptoms. The imbalance in the endogenous opioid system might adversely alter the course of disease and predispose patients to persistence of negative symptoms, despite antipsychotic treatment.
Keywords: Neuropeptides, Opioid Peptides, Psychotic Disorders, Schizophrenia, Antipsychotic Agents, Follow-Up Studies, Recurrence, beta-Endorphin
Background
Excess levels of endogenous opioids have been widely reported in patients with schizophrenia, and it has been hypothesized that such imbalances in opioid-dopamine interaction can influence the pathogenesis of schizophrenia [1]. Although findings in this field were initially promising, interest in the topic has generally died off. In addition, studies that have been published were weakened by various limitations, mainly small, non-homogenous patient samples, short periods of observation, and less accurate methods of biochemical assay [2,3]. Although further studies have provided some additional information, the role of endogenous opioids in pathogenesis of schizophrenia remains unclear. Scientific interest in this area is once again increasing. There are more data about biochemistry of endogenous opioids and their levels may be easy to detect using more precise methods. Moreover, research in this area is expanding to and opioid receptor has been postulated to play a role in the pathogenesis of schizophrenia [4].
Beta-endorphin (BE) is believed to be the most potent endogenous opioid detectable in both the peripheral and central nervous systems. Elevated BE concentrations are thought to influence the activity of dopamine, a precursor of endogenous opioids. Dopamine release is also indirectly enhanced by presynaptic opioid μ receptors, which also decrease dopamine pathway inhibition [5]. Endogenous opioid immunoreactivity has also been detected in various brain structures, including the hippocampus, olfactory bulb, band of Broca, basal ganglia, and cerebellum [6], some of which play crucial roles in the pathogenesis of schizophrenia. In addition, opioid μ receptors modulate mesolimbic-mesocortical dopamine activity [7]. It has been suggested that some symptoms might be precipitated by alterations in BE level associated with a dysfunction in the modulatory effect of endogenous opioids on the dopaminergic system [8].
Numerous studies also indicate that opiate antagonists may have potential antipsychotic properties [9,10]. Significantly elevated BE concentrations have been found in patients with schizophrenia compared to healthy controls [11–13]. Despite symptomatic improvement, the course of schizophrenia often fluctuates and acute positive symptoms often overlap with negative and cognitive symptoms. Significantly higher BE concentrations also have been found in patients with schizophrenia who have severe negative symptoms than in those with predominantly acute positive symptoms, and that various individually-tailored antipsychotic treatments result in an observed “normalization” of BE levels [11]. It should be noted that both negative symptoms at discharge from the hospital and severe positive symptoms at admission are predictors of a chronic course of schizophrenia [14]. Individuals with a chronic course of schizophrenia recently have been shown to have significantly higher BE levels than those in whom the disease is episodic [13], hence it is likely that a specific pattern of BE secretion may be related to the predominant type of psychopathology.
The aim of this study, therefore, was to determine whether BE concentration associated with exacerbation or relapse of schizophrenia symptoms has an impact on the effects of long-term treatment and risk of rehospitalization. To our knowledge, it is the first study that evaluated the impact of BE levels on te clinical course of schizophrenia and risk of severe symptomatic relapse. Previous research considered the role of endogenous opioids mainly in acute psychosis during a short observation period.
Material and methods
PARTICIPANTS:
Forty-nine adult patients with schizophrenia diagnosed according to ICD-10 criteria and 47 healthy controls were enrolled in the study. All patients were hospitalized in the Department of Affective and Psychotic Disorders Medical University of Lodz for exacerbation or relapse of schizophrenia symptoms. The Positive and Negative Symptoms Scale (PANSS) and composite index (CI) were used to assess psychopathology severity and predominance of negative symptoms [15]. The CI is obtained by subtracting the PANSS negative symptoms subscale score from the positive subscale score. PANSS examination with CI estimation was performed at the onset of hospitalization, and after 2, 6, and 10 weeks of treatment. In controls, blood concentration of BE was assessed on enrollment in the study (BE); in the patient sample, it was measured at the onset of hospitalization (BE1) and again after 6 weeks of treatment (BE2).
Short-term results in the primary patient sample have previously been described [16]. Following the end of the first phase of the study, 25 of the 49 patients went on to continue treatment in the outpatient psychiatric unit of the Department of Affective and Psychotic Disorders. After 1-year follow-up, these 25 patients were examined again using PANSS and composite CI. Their clinical status was also compared to those from the previous stages of assessment and correlated with BE concentration. Data were also collected on the duration of illness (DOI) and number of hospitalizations (Hn) and rehospitalizations after the first phase of study.
Exclusion criteria were as follows: diagnosis other than schizophrenia, psychoactive substance abuse, chronic somatic disease, age below 18 years. All participants included in the study were somatically healthy. A full physical examination and blood testing including morphology and C-reactive protein level were performed in all participants before BE concentration assay.
Patients were receiving pharmacological treatment comprising second-generation antipsychotic therapy, either alone or combined with first-generation antipsychotics. All participants gave their informed consent prior to their inclusion in the study. The study was approved by the Ethics Committee of the Medical University of Lodz (Nr RNN/53/18/KE). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
BIOCHEMICAL ASSAY:
To measure plasma BE concentration, 2.6 mL of peripheral blood was collected from participants in EDTA tubes. All blood draws were done between 7 and 8 am. Immediately after collection, the trypsin inhibitor aprotinin was added to each tube; following this, each tube was immediately placed in storage (0.6 trypsin inhibitor unit [TIU]/100 uL of blood). The tubes were centrifuged and the obtained plasma samples were stored at −70°C for final BE concentration assay using a Phoenix Pharmaceuticals β-endorphin ELISA Kit EK-022-14CE.
STATISTICAL ANALYSIS:
The data were verified for normality of distribution using the Shapiro-Wilk test and equality of variance using Levene’s test. For parameters with normal distribution, the Student’s
Results
The control sample consisted of 25 females and 22 males, aged 25 to 59 (mean 38.95 years; SD±8.839). The final group of patients included 13 females and 12 males, aged 20 to 62 (mean 43.52; SD±11.582). The compared study groups did not differ in terms of gender (Ch2=0.01; P=0.923) or age (t=1.868; P=0.065). Mean duration of illness (DOI) in individuals with schizophrenia was 13.560 years (min=1.0 year, max=32 years; SD±8.756). Mean number of psychiatric hospitalizations before inclusion in the study was 6.56 (min=1.0, max=19; SD±4.673).
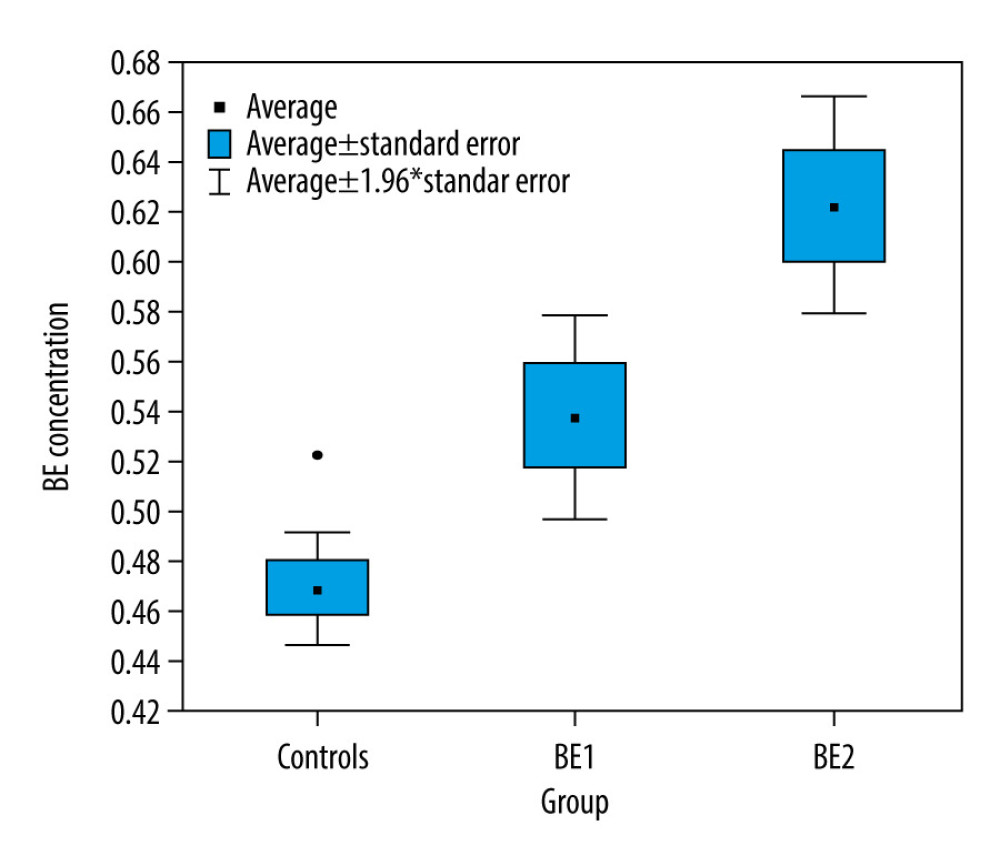
Patients had higher BE1 concentration at study enrollment than did controls (mean 0.538ng/mL SD±0.104
No correlation was observed between BE1 and PANSS total or PANSS subscale scores at any stage of the study, nor between BE2 and the PANSS total or subscale scores. However, a negative correlation was observed between BE1 and composite index after 1-year follow-up (CI 5) (R=−0.404; P=0.045): higher BE1 levels were associated with a greater predominance of negative symptoms.
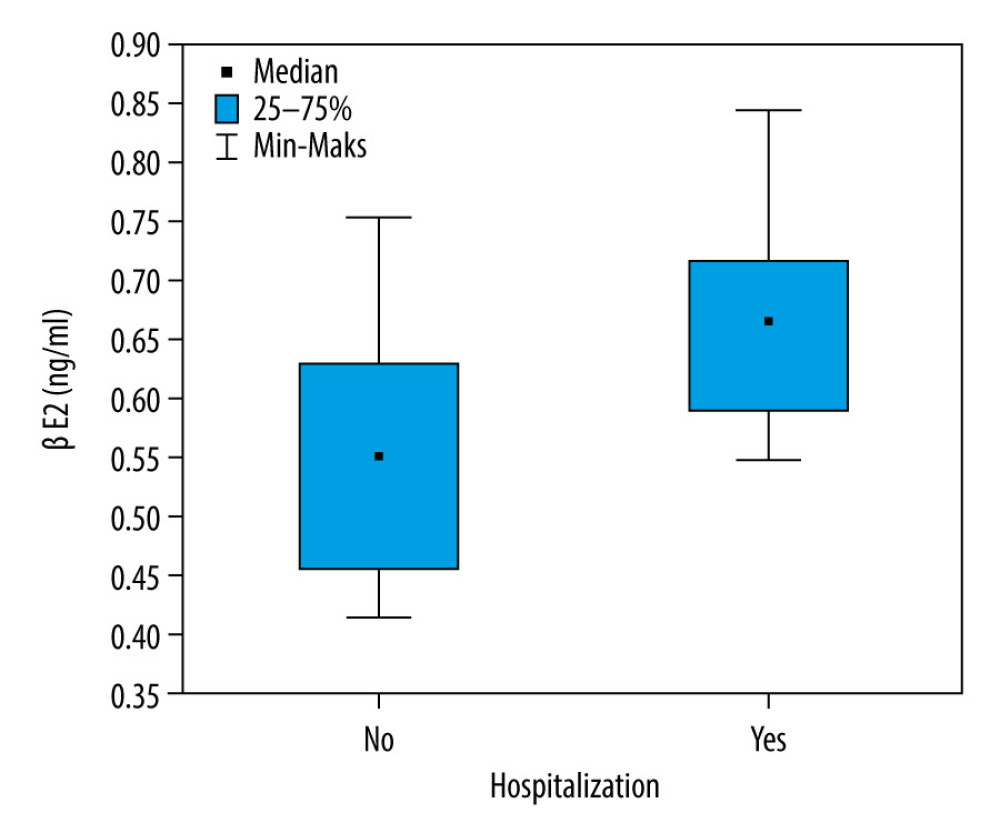
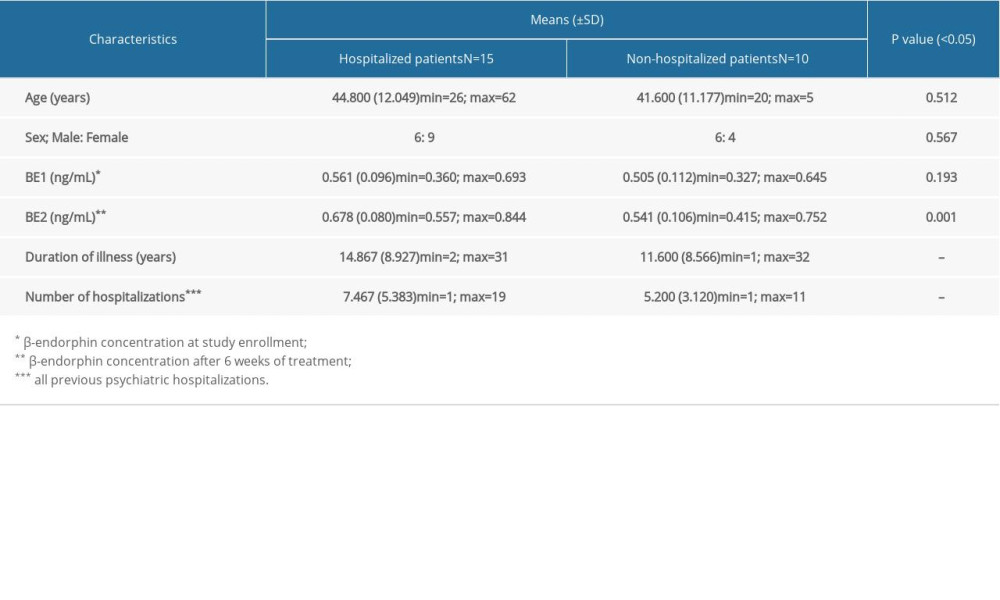
During 1-year follow-up, 60% (15/25) of patients were hospitalized again. They were significantly more likely to demonstrate higher BE2 scores than those who were not hospitalized again (P=0.038) (Figure 2). In contrast, while the patients who were not hospitalized tended not to display significant elevations of BE after 6 weeks of treatment (BE1=0.505
Discussion
Our findings provide indirect evidence for the involvement of endogenous opioids in the pathogenesis and course of schizophrenia. Initially higher BE concentration was related with a prevalence of negative symptoms after 1-year follow-up. Moreover, the presence of an elevated, and increasing, BE concentration during pharmacological treatment of an acute episode of psychosis was associated with a greater chance of rehospitalization in the following months. These findings suggest that a “hypermorphinegic” pathology could, at least partially, determine an adverse course of disease, characterized by the survival of negative symptoms and the risk of recurrence of the disease.
Although previous studies suggest that opioid antagonists may possess potentially antipsychotic properties, the majority of research on the topic have only been based on small, non-homogeneous patient samples consisting of participants with various diagnoses (schizophrenia, schizoaffective disorder, depressive disorder). Early studies have found that administration of BE produces auditory hallucinations, agitation, and conceptual disorganization, and that naloxone blocks the development of these signs; however, the final results have been inconsistent [17,18].
Opioid antagonists have been found to be effective as an adjuvant treatment rather than producing a cure. Marchesi et al. [10] reported significant therapeutic efficacy in patients with chronic schizophrenia and severe negative symptoms, and that the addition of adjuvant naltrexone to antipsychotics was especially effective in patients with negative symptoms. The weakness of this study was its small sample size (n=12) and relatively short evaluation period for combined treatment (14 days).
In another study, adding naltrexone to neuroleptic therapy yielded significant amelioration of symptoms involving deterioration and social withdrawal in the treatment compared to placebo group, which demonstrated no improvement; this therapeutic effect was particularly pronounced among patients with severe negative symptoms [19]. However, no clinical antipsychotic benefit was observed when naltrexone was added to a neuroleptic agent during a 2-week course of treatment in a group of 21 patients with schizophrenia, with a placebo group as control [20]. The total course of treatment was 3 weeks. Interestingly, it was also reported that naltrexone could decrease mannerisms and polydipsia in patients with chronic schizophrenia and improve social withdrawal [21]. Rapaport et al. [22] investigated the antipsychotic properties of nalmefene, a potent, long-acting opioid antagonist, administered as an adjuvant, in a group of 11 first-generation neuroleptic-stabilized patients. Nalmefene administration resulted in a significant decrease in psychotic symptoms in nine of the patients.
The opioid receptor system (μ) is involved in impaired sensorimotor gating, attentional set-shifting, and other critical cognitive processes in schizophrenia [23]. Dopamine metabolism, reuptake, and release are known to be altered by opioid agonists. Becerra et al. [24] reported that morphine mediates activation of the orbitofrontal cortex and hippocampus among in healthy volunteers, which suggests that opioids might potentially enhance cognitive functions. Stefano et al. [25] proposed that low doses of morphine may act as adjuvant therapy for cognitive function improvement in schizophrenia.
BE concentration also has been found to be elevated in cases of acute psychotic symptoms and decreased in those of chronic schizophrenia [26]; in addition, intracerebral administration of BE caused rigid immobility in rats, which is regarded as an analog of catatonia in humans [27]. A study of neuropeptide concentration in drug-naïve patients with schizophrenia found them to have increased levels of BE in comparison with controls [28]. The authors reported a tendency for patients to demonstrate lower BE levels, with greater improvement in positive symptoms and poorer in negative ones; however, the pharmacologically-treated sample was very small (nine individuals) and was evaluated after only a 4-week course of treatment. Panza et al. [29] noted that 15 drug-naïve patients with schizophrenia demonstrated similar BE concentrations to controls before a 2-week course of haloperidol administration, but these levels were significantly elevated after 2 and 15 days of treatment.
It has also been found that elevated BE levels decreased among inpatients with predominantly negative symptoms and increased in those with severe positive symptoms. After effective pharmacological treatment, both groups of patients demonstrated “normalized” BE levels, similar to those in healthy controls. The increase in BE concentration after treatment might be related to reduction of dopamine inhibitory tone on endogenous opioids and resolution of positive symptoms [11]. It is thought that second-generation antipsychotics might ameliorate negative symptoms via the relatively potent antagonism of 5HT2A serotonin receptors and the weaker blockade of D2 dopamine receptors in the frontal cortex [30]. In the current study, our participants displayed relatively better control of negative symptoms than in previous studies, possibly because they were receiving treatment based on second-generation antipsychotics or combined therapy with a first-generation neuroleptic; however, individuals with a higher BE concentration were more likely to demonstrate negative symptomatology, measured using the composite index.
BE concentration was only assessed in patients twice, in the early stages of the observation period: no data are available about the further dynamics of BE concentration changes. In addition, no analysis was performed of the relationship between BE level and specific pharmacological treatment. Furthermore, BE concentration in peripheral blood does not directly reflect central endogenous opioid levels; however, blood withdrawal is less invasive and more acceptable for patients than cerebrospinal fluid analysis. This was a particularly important point, assessment of biological material from our patients, who had acute schizophrenia symptoms, was required before treatment administration. However, the main limitation of our study is its small patient sample size, hence our results may only be interpreted as a “fishing expedition: rather than as a proposal for further research. Finally, the reasons for rehospitalization of patients in our study, such as uncontrolled treatment withdrawal or relapse during the natural course of disease despite regular treatment, were not analyzed.
Conclusions
An imbalance in the endogenous opioid system appears to be involved in modulation and persistence of negative symptoms. The presence of elevated levels of BE, and their further elevation during antipsychotic treatment, may further predispose patients to chronicity of disease and increase risk of significant exacerbation of symptoms.
References
1. Bodnar RJ, Endogenous opiates and behavior: 2012: Peptides, 2013; 50; 55-95
2. Volavka J, Davis LG, Ehrlich YH, Endorphins, dopamine, and schizophrenia: Schizophr Bull, 1979; 5(2); 227-39
3. De Wied D, Sigling HO, Neuropeptides involved in the pathophysiology of schizophrenia and major depression: Neurotox Res, 2002; 4; 453-68
4. Ashok AH, Myers J, Marques TR, Reduced mu opioid receptor availability in schizophrenia revealed with [11C]-carfentanil positron emission tomographic Imaging: Nat Commun, 2019; 10; 4493
5. Koneru A, Satyanarayana S, Rizwan S, Endogenous opioids: They physiological role and receptors: Global J Pharmacol, 2009; 3(3); 149-53
6. Laux-Biehlmann A, Mouheiche J, Veriepe J, Goumon Y, Endogenous morphine and its metabolites in mammals: History, synthesis, localization and perspectives: Neuroscience, 2013; 233; 95-117
7. Fricchione GL, Mendoza A, Stefano GB, Morphine and its psychiatric implications: Adv Neuroimmunol, 1994; 4(2); 117-31
8. Welch EB, Thompson BF, Opiate antagonists for the treatment of schizophrenia: J Clin Pharam Therapeutics, 1994; 19; 279-83
9. Gitlin MJ, Gerner RH, Rosenblatt M, Assessment of naltrexone in the treatment of schizophrenia: Psychopharmacology (Berl), 1981; 74; 51-53
10. Marchesi GF, Santone G, Cotani P, Troiani G, Naltrexone integrated antipsychotic treatment in schizophrenia: Biol Psychiatry, 1991; 29(Suppl); 536
11. Urban-Kowalczyk M, Śmigielski J, Strzelecki D, Comparison of beta-endorphin and CGRP levels before and after treatment for severe schizophrenia: Neuropsychiatr Dis Treat, 2016; 12; 863-68
12. Urban-Kowalczyk M, Śmigielski J, Kotlicka-Antczak M, Overrated hedonic judgment of odors in patients with schizophrenia: CNS Neurosci Ther, 2018; 24(12); 1156-62
13. Urban-Kowalczyk M, Kotlicka-Antczak M, Strzelecki D, The relationship between course of illness and β-endorphin plasma levels in patients with schizophrenia: Neuropsychatr Dis Treat, 2019; 15; 3609-14
14. Möller H-J, Jäger M, Riedel M, The Munich 15-year follow-up study (MUFUSSAD) on first-hospitalized patients with schizophrenic or affective disorders: Comparison of psychopathological and psychosocial course and outcome and prediction of chronicity: Eur Arch Psychiatry Clin Neurosci, 2010; 260; 367-84
15. Kay SR, Positive and negative syndromes in schizophrenia: Clinical and Experimental Psychiatry, 1991, New York, Brunnel/Mazer Monograph No 5
16. Urban-Kowalczyk M, Kotlicka-Anczak M, Strzelecki D, The relationship between antipsychotic treatment and plasma β-endorphin concentration in patients with schizophrenia: Brain Behavior, 2020 [submitted]
17. Bissette G, Nemeroff CB, Mackay AV, Neuropeptides and schizophrenia: Prog Brain Res, 1986; 66; 161-74
18. Pickar D, Bunney WE, Douillet P, Repeated naloxone administration in schizophrenia: A phase II World Health Organization Study: BioI Psychiatry, 1989; 25; 440-48
19. Marchesi GF, Santone G, Cotani P, The therapeutic role of naltrexone in negative symptom schizophrenia: Prog Neuropsychopharmacol Biol Psychiatry, 1995; 19(8); 1239-49
20. Sernyak MJ, Glazer WM, Heninger GR, Naltrexone augmentation of neuroleptics in schizophrenia: J Clin Psychopharm, 1998; 18(3); 248-51
21. Becker JA, Goldman MB, Alam MY, Luchins DJ, Effects of naltrexone on mannerisms and water imbalance in polydipsic schizophrenics: A pilot study: Schizophr Res, 1995; 17(3); 279-82
22. Rapaport MH, Wolkowitz O, Kelsoe JR, Beneficial effects of nalmefene augmentation in neuroleptic-stabilized schizophrenic patients: Neuropsychopharmacology, 1993; 9(2); 111-15
23. Quednow BB, Csomor PA, Chmiel J, Sensorimotor gating and attentional set-shifting are improved by the mu-opioid receptor agonist morphine in healthy human volunteers: Int J Neuropsychopharmacol, 2008; 11; 655-69
24. Becerra L, Harter K, Gonzalez RG, Borsook D, Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers: Anesth Analg, 2006; 103; 208-16
25. Stefano GB, Králíčková M, Ptacek R, Low dose morphine adjuvant therapy for enhanced efficacy of antipsychotic drug action: potential involvement of endogenous morphine in the pathophysiology of schizophrenia: Med Sci Monit, 2012; 18(7); HY23-26
26. Domschke W, Dickschas A, Mitznegg P, C.S.F. beta-endorphin in schizophrenia: Lancet, 1979; 12; 1024
27. Bloom F, Segal D, Ling N, Guillemin R, Profound behavioral effects in rats suggest new etiological factors in mental illness: Science, 1976; 194; 630-32
28. Mauri MC, Rudelli R, Vanni S, Cholecystokinin, beta-endorphin and vasoactive intestinal peptide in peripheral blood mononuclear cells of drug-naive schizophrenic patients treated with haloperidol compared to healthy controls: Psychiatry Res, 1998; 20(1–2); 45-50
29. Panza G, Monzani E, Sacerdote P, Beta-endorphin, vasoactive intestinal peptide and cholecystokinin in peripheral blood mononuclear cells from healthy subjects and from drug-free and haloperidol treated schizophrenic patients: Acta Psychiatr Scand, 1992; 85; 207-10
30. Meltzer HY, Li Z, Kaneda Y, Ichikawa J, Serotonin receptors: Their key role in drugs to treat schizophrenia: Prog Neuropsychopharmacol Biol Psychiatry, 2003; 27; 1159-72
Figures
In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952