16 October 2020: Clinical Research
Single Large Nodule (>5 cm) Prognosis in Hepatocellular Carcinoma: Kinship with Barcelona Clinic Liver Cancer (BCLC) Stage A or B?
Li Wan12BCDE, Ding-Hui Dong1ABCDE, Xiao-Ning Wu1BCD, Hong-Fan Ding1BCD, Qiang Lu1BCD, Yong Tian2BC, Xu-Feng Zhang1ABCDEFG*, Wenzhi Li13ADEDOI: 10.12659/MSM.926797
Med Sci Monit 2020; 26:e926797
Abstract
BACKGROUND: The aim of the present study was to evaluate the prognosis among patients with a single large hepatocellular carcinoma (HCC) >5 cm compared with other patients in Barcelona Clinic Liver Cancer (BCLC) stage A or stage B.
MATERIAL AND METHODS: Data on patients with BCLC stage A/B HCC were collected between 2008 and 2012. BCLC stage A was subclassified as A1 (single tumor, 2-5 cm, or 2-3 nodules £3 cm), or A2 (single tumor >5 cm). Overall survival (OS) was evaluated and compared.
RESULTS: Among 1005 patients with HCC, 455 were stage A1, 188 were stage A2, and 362 were stage B. The OS of stage A2 patients was significantly worse than that of stage A1 patients (median survival, 30.6 vs. 43.2 months, p<0.001), and was similar to that of stage B patients (median survival, 30.6 vs. 33.5 months, p=0.519). After surgical resection, OS was statistically distinct between stage A1+A2 and B (median survival, 51.2 vs. 36.0 months, p=0.001), and between stage A1 and A2+B (median survival, 54.4 vs. 36.8 months, p<0.001). In contrast, when treated by transarterial chemoembolization, there was no difference in OS between patients with stage A1+A2 HCC and patients with stage B HCC (median survival, 32.4 vs. 31.3 months, p=0.310), whereas patients with stage A1 HCC showed a significantly more favorable OS than those with stage A2+B HCC (median survival, 39.6 vs. 31.8 months, p=0.023). On multivariable analysis, the groupings that showed significantly different associations with OS were BCLC stage A2+B vs. A1 (hazard ratio 1.6, p<0.001) rather than stage B vs. A1+A2.
CONCLUSIONS: Patients with solitary HCC >5 cm had a comparable survival with BCLC stage B. HCC >5 cm should therefore be classified as an intermediate stage.
Keywords: Carcinoma, Hepatocellular, general surgery, Adolescent, Aged, 80 and over, Chemoembolization, Therapeutic, Disease-Free Survival, Liver Neoplasms, Survival Rate
Background
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death worldwide, and its incidence is increasing [1–3]. Although the prognosis of HCC has improved substantially in the last decades, the recurrence rate after curative treatments is extremely high (~70%) and long-term survival remains discouraging [4–6]. Establishing effective staging systems in prognosis evaluation and treatment allocation is critical in improving the prognosis of HCC patients.
The treatments and prognosis of HCC patients depend not only on tumor status, but also on liver function. The Barcelona Clinic Liver Cancer (BCLC) system incorporates the severity of liver cirrhosis, tumor burden, and patient-performance status, and therefore is widely accepted in treatment allocation and prognosis evaluation in HCC [7,8]. BCLC is now endorsed by the American Association for the Study of Liver Disease (AASLD) and European Association for the Study of Liver (EASL) [7,9]. According to the BCLC staging system, if liver function is Child-Pugh grade A or B, but there is no macroscopic vascular invasion, lymph node involvement, or distant metastasis, a single HCC nodule >2 cm in diameter or 2–3 nodules ≤3 cm in diameter would be classified as stage A. In the case of 2–3 nodules >3 cm in diameter or >3 nodules of any size, the HCC would be classified as stage B [9]. However, a single large HCC nodule >5 cm in diameter has been ambiguously classified. Since tumor size >5 cm is associated with microvascular invasion and histological grade of HCC, the patient prognosis is poor [10,11]. Surgical resection and liver transplant are not always recommended for these patients [9]. Although some groups strongly advocate for categorizing a single large HCC (>5 cm) as BCLC stage A [12,13], some others, including the Spanish group that developed the BCLC stage system, have demonstrated that classifying a single large HCC as BCLC stage B might be more appropriate and useful in prognosis evaluation [14–18]. However, the current BCLC staging system still classifies HCC larger than 5 cm in size as stage A. As such, the objective of the current study was to evaluate, based on data from a high-volume center, the prognosis prediction when a single HCC nodule larger than 5 cm was classified as BCLC stage A
Material and Methods
STUDY COHORT:
Data for patients who were initially diagnosed with HCC within BCLC stages 0, A, and B (no macroscopic vascular invasion or extrahepatic metastasis of tumor, Child-Pugh Class A or B liver function, performance status 0) between January 2008 and December 2012 in our hospital were collected from a prospectively maintained database. All of the patients were diagnosed with HCC based on preoperative serum alpha-fetoprotein (AFP), computed tomography (CT), ultrasonography, and/or magnetic resonance imaging, and confirmed by histological examination if available. In the current study, BCLC stage A1 was defined as a single tumor ranging from 2 to 5 cm, or less than 3 nodules not exceeding 3 cm, whereas a solitary large tumor >5 cm was defined as BCLC A2. The survival rates were compared between different recombined groups (A1+A2
Demographic information and biochemical values were all collected from paper medical documents and the computerized database within 1 week before the initial treatment. Tumor status was evaluated based on pathological examination if available, or imaging studies if histologic examination was not available. Treatment modality was tailored to each patient according to tumor loads, vascular status, liver function, and patient general condition and willingness. Specifically, surgical resection was firstly considered among patients with BCLC stage A and B. Loco-regional therapies, including radiofrequency ablation (RFA) and transarterial chemoembolization (TACE), were the treatments of choice among patients with unresectable tumor or unwillingness to undergo surgical treatment. In contrast, patients who could not tolerate any invasive treatments might be treated with supportive care or traditional Chinese herb formulas (as supportive treatments). Overall survival (OS) was the primary outcome, which was defined as the time period between the initial diagnosis and the date of death or end of the followup period (December 2019). The study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF2017LSL-007), the rules of which comply with the Helsinki Declaration. Informed consent was waived, as the data were analyzed without personal identifiers.
STATISTICAL ANALYSIS:
Numerical data and nominal variables were expressed as median and range, and number and percentages, respectively. Mann-Whitney U test or
Results
BASELINE CHARACTERISTICS:
A total of 1127 patients were initially included. Among these, 80 patients with BCLC stage 0 HCC (single tumor ≤2 cm) were excluded. Patients undergoing liver transplant (n=34) or sorafenib (n=8) were also excluded from the study. After these exclusions, 1005 patients remained for further analysis. There were 455 (45.3%) patients staged as BCLC stage A1, 188 (18.7%) as stage A2, and 362 (36%) as stage B. The basic characteristics of the patients among the 3 groups were compared (Table 1). Patients did not differ in age, sex, HBV and HCV status, liver cirrhosis, AFP value, or 30-day and 90-day mortality among the 3 groups (all P>0.05). However, patients within BCLC stage A2 experienced more diabetes complications and Child-Pugh class B liver function than BCLC stage B (both P<0.05). Surgical resection, as a radical treatment, was equally performed in patients with BCLC stage A1, A2, and B HCC (P>0.05). Not surprisingly, radiofrequency ablation was more commonly performed in BCLC A1 HCC than in the other 2 groups (both P<0.05), while TACE were more common in patients within BCLC stages A2 and B HCC than in those with stage A1 HCC (both P<0.05). Moreover, more patients with stage A2 HCC received no aggressive treatment and only supportive treatment compared with those with stage A1 HCC (P=0.005).
OVERALL SURVIVAL:
After a median followup of 48.5 months, 233 (51.2%) patients in the BCLC stage A1 group, 107 (56.9%) in the stage A2 group, and 216 (59.7%) in the stage B group died. Patients in the BCLC stage A2 group had a significantly worse OS than those in the stage A1 group (median survival, 30.6 months vs. 43.2 months, P<0.001, Figure 1A), but were comparable in OS to those in the stage B group (median survival, 30.6 months vs. 33.5 months, P=0.519, Figure 1A). The 1-, 3-, and 5-year survival rates were 83.2%, 57.7%, and 33.3% for the stage A1 patients; 84.3%, 33.5%, and 18.6% for the stage A2 patients, and 82.6%, 42.3%, and 15.5% for the stage B patients.
SURVIVAL OF PATIENTS IN DIFFERENT SUBGROUPS:
We then compared the overall survival of the patients when allocating the stage A2 patients into the stage A1 or stage B groups. The OS differed significantly when patients were grouped into stage A1+A2 vs. B (median survival, 38.2 months vs. 33.5 months, P=0.003, Figure 1B), compared with when they were grouped into stage A1 vs. A2+B (median survival, 43.2 months vs. 22.7 months, P<0.001, Figure 1C).
In addition, we further stratified the patients according to different treatments. After surgical resection, the OS of the patients was statistically distinct between the stage A1+A2 and stage B groups (median survival, 51.2 months vs. 36.0 months, P=0.001, Figure 2A), and between the stage A1 and stage A2+B groups (median survival, 54.4 months vs. 36.8 months, P<0.001, Figure 2B). In contrast, there was no difference in OS between patients in the stage A1+A2 group vs. patients in the stage B group (median survival, 32.4 months vs. 31.3 months, P=0.310, Figure 3A). In contrast, patients in the stage A1 group showed a significantly more favorable OS than patients in the stage A2+B group (median survival, 39.6 months vs. 31.8 months, P=0.023, Figure 3B).
:
We also investigated the OS of patients with stage A2 HCC after different interventions. Perhaps not surprisingly, patients who underwent surgical resection showed more favorable outcomes vs. patients who received TACE only (median survival, 38.8 months vs. 35.0 months, P=0.010, Figure 4). The 1-, 3-, and 5-year OS rates were 92.3%, 52.5%, and 43.8% after surgical resection, and 83.7%, 32.1%, and 7.8% after TACE.
PROGNOSTIC FACTORS ASSOCIATED WITH SURVIVAL:
Multivariable analysis indicated that liver cirrhosis (HR 1.3, 95% CI 1.0–1.6, P=0.022), Child-Pugh B vs. A (HR 1.5, 95% CI 1.0–2.1 P=0.032) and BCLC stage A2+B vs. A1 (HR 1.6, 95% CI 1.3–2.1, P<0.001) were significantly associated with OS of the patients. In contrast, the current staging system (Stage B vs. A1+A2: HR 0.9, 95% CI 0.7–1.2, P=0.415) was not able to differentiate the survival of HCC patients (Table 2).
Discussion
BCLC is a widely accepted staging system in clinical practice that stratifies HCC patients into appropriate subgroups for treatment strategy and prognosis evaluation. However, while the current BCLC staging system takes into account liver function and tumor status, it partly overlooks tumor size, especially for single large lesions. The categorization of single HCC nodules larger than 5 cm remains controversial. The current study utilized the data of over 1000 HCC patients from a high-volume center in China, and demonstrated that it would be more appropriate to change the allocation of single tumors >5 cm in size from BCLC A2 to BCLC stage B. Specifically, patients with stage A2 HCC had significantly worse OS than patients with stage A1+A2, but similar OS to patients with stage B HCC. Significant differences in OS were also observed among patients with stage A1+A2
In fact, BCLC staging classifies solitary tumors beyond 5 cm as stage A, and surgical resection is recommended [7,8]. However, this recommendation has also been revised such that patients with resectable solitary large tumors should be considered as early-stage, whereas patients with unresectable solitary large tumors would be considered intermediate-stage [7,13,19]. In contrast, by using regret-based decision-curve analysis, Cocchetti et al. showed significant separation among physicians’ preference for surgical resection or TACE [20]. As such, using resectability as a criterion to differentiate early
The optimal treatment for a single large HCC nodule (>5 cm) is still poorly defined. Historically, RFA and liver transplantation (LT) were not recommended for patients with HCC ≥5 cm in diameter [7,9,25]. In contrast, TACE was always considered as a safe and effective treatment for a single large HCC tumor [9]. In addition, surgical resection has also been questioned, as a single large tumor is always associated with a high recurrence rate and worse survival. However, consistent with the current study, more and more studies have demonstrated improved survival of these patients after surgical resection
In the current cohort, patients with stage A2 and B HCC were more likely to receive TACE treatment than patients in stage A1. Of note, TACE was the recommended treatment for intermediate-stage HCC in the BCLC staging [7,8]. That is to say, a single large HCC nodule was always stratified as a more aggressive lesion in clinical practice. In addition, larger tumor size was closely associated with a higher incidence of microvascular invasion and worse tumor differentiation [30–32]. Of note, in the current study, allocating stage A2 patients to stage B significantly improved the OS of stage A patients compared with stage B patients after TACE. This implies that solitary HCC nodules larger than 5 cm are quite different from stage A1 HCC, in terms of biological behavior and prognosis. As such, it would be proper to separate single large HCC tumors from early-stage HCC.
There were several limitations in this study regarding interpretation of the results. First, selection bias was unavoidable due to its retrospective nature. In addition, all the patients were followed up only for long-term OS; recurrence-free survival and time-to-progression were not available in the current study. Given that all of the patients were included between 2008 and 2012, patients who received molecular targeted therapies in recent years were not included in the current cohort. As such, more studies are needed to verify our results and take into consideration patients receiving targeted therapies.
Conclusions
In conclusion, in the present study, patients with a single HCC nodule larger than 5 cm had worse survival than patients with BCLC stage A1 HCC, but similar OS to patients with BCLC stage B HCC. Multivariable analysis showed that BCLC stage A2+B
Figures
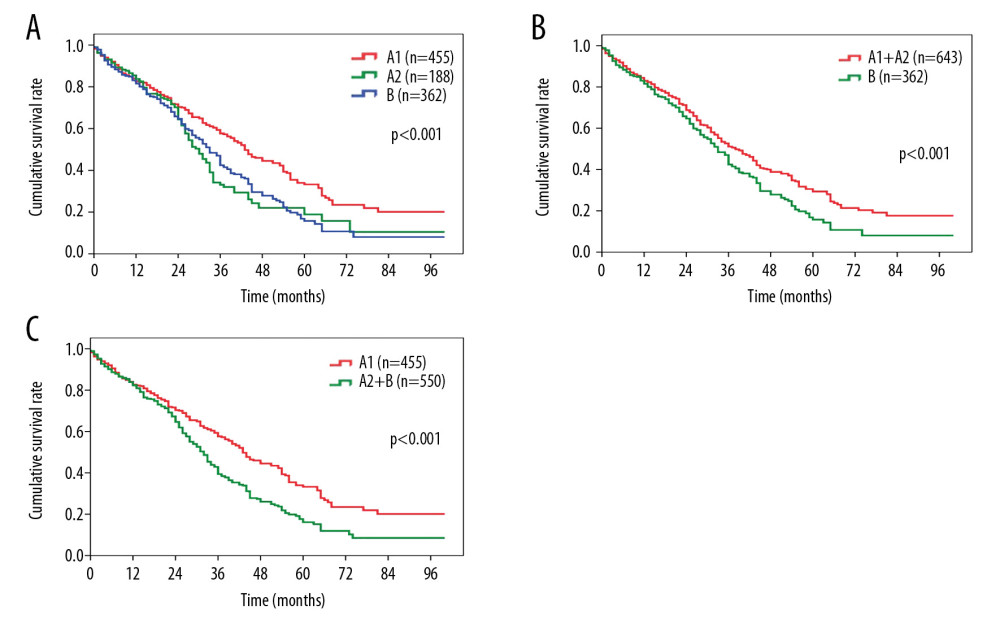 Figure 1. Comparison of overall survival among patients with HCC in (A) BCLC stage A1 or stage A2 vs. BCLC stage B; (B) BCLC stage A1+A2 vs. BCLC stage B; (C) BCLC stage A1 vs. BCLC stage A2+B. HCC – hepatocellular carcinoma; BCLC – Barcelona Clinic Liver Cancer.
Figure 1. Comparison of overall survival among patients with HCC in (A) BCLC stage A1 or stage A2 vs. BCLC stage B; (B) BCLC stage A1+A2 vs. BCLC stage B; (C) BCLC stage A1 vs. BCLC stage A2+B. HCC – hepatocellular carcinoma; BCLC – Barcelona Clinic Liver Cancer. 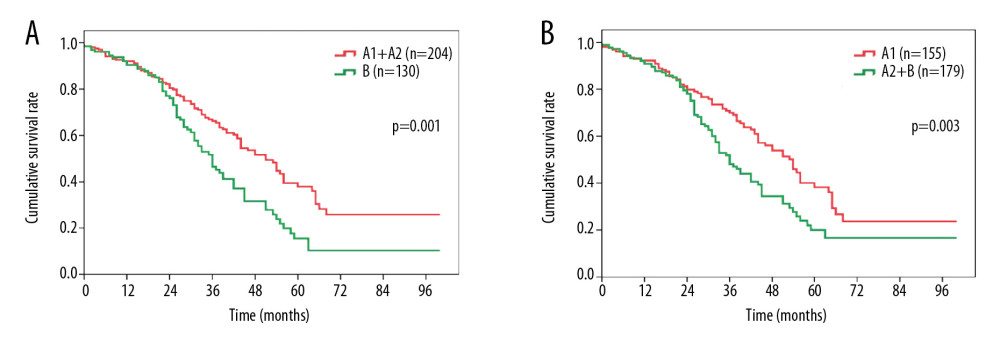 Figure 2. Overall survival comparison among patients who underwent surgical resection for hepatocellular carcinoma in (A) BCLC stage A1+A2 vs. BCLC stage B, and (B) BCLC stage A1 vs. BCLC stage A2+B. BCLC – Barcelona Clinic Liver Cancer.
Figure 2. Overall survival comparison among patients who underwent surgical resection for hepatocellular carcinoma in (A) BCLC stage A1+A2 vs. BCLC stage B, and (B) BCLC stage A1 vs. BCLC stage A2+B. BCLC – Barcelona Clinic Liver Cancer. 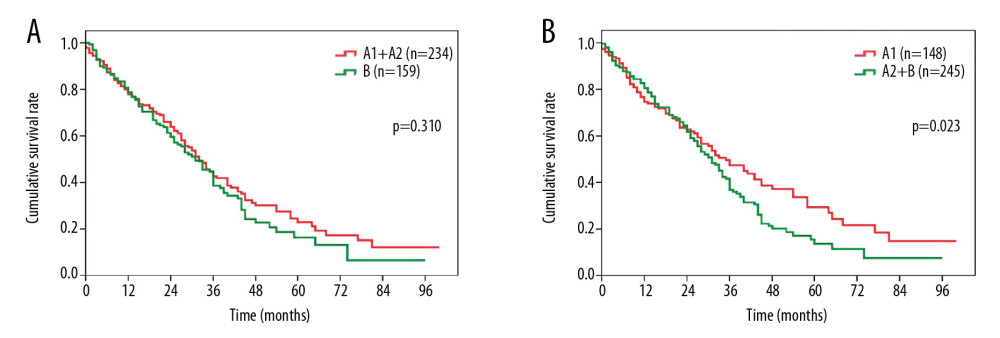 Figure 3. Overall survival comparison among patients who underwent transarterial chemoembolization for hepatocellular carcinoma in (A) BCLC stage A1+A2 vs. BCLC stage B, and (B) BCLC stage A1 vs. BCLC stage A2+B. BCLC – Barcelona Clinic Liver Cancer.
Figure 3. Overall survival comparison among patients who underwent transarterial chemoembolization for hepatocellular carcinoma in (A) BCLC stage A1+A2 vs. BCLC stage B, and (B) BCLC stage A1 vs. BCLC stage A2+B. BCLC – Barcelona Clinic Liver Cancer. 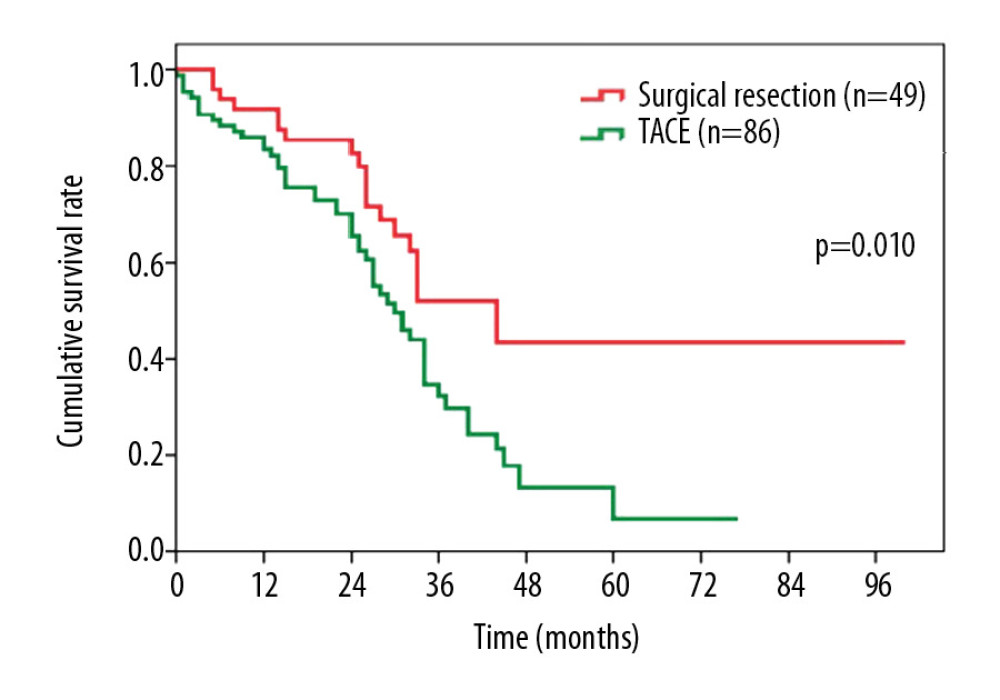 Figure 4. Overall survival comparison among patients with hepatocellular carcinoma in BCLC stage A2 who underwent surgical resection vs. transarterial chemoembolization. BCLC – Barcelona Clinic Liver Cancer.
Figure 4. Overall survival comparison among patients with hepatocellular carcinoma in BCLC stage A2 who underwent surgical resection vs. transarterial chemoembolization. BCLC – Barcelona Clinic Liver Cancer. References
1. Alavi M, Janjua NZ, Chong M, Trends in hepatocellular carcinoma incidence and survival among people with hepatitis C: An international study: J Viral Hepat, 2018; 25; 473-81
2. Baecker A, Liu X, La Vecchia C, Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors: Eur J Cancer Prev, 2018; 27; 205-12
3. Ganne-Carrie N, Chaffaut C, Bourcier V, Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis: J Hepatol, 2018; 69; 1274-83
4. Chan AWH, Zhong J, Berhane S, Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection: J Hepatol, 2018; 69; 1284-93
5. Gan W, Huang JL, Zhang MX, New nomogram predicts the recurrence of hepatocellular carcinoma in patients with negative preoperative serum AFP subjected to curative resection: J Surg Oncol, 2018; 117; 1540-47
6. Pravisani R, Baccarani U, Isola M, Impact of surgical complications on the risk of hepatocellular carcinoma recurrence after hepatic resection: Updates Surg, 2018; 70; 57-66
7. European Association for Study of Liver, European Organisation for Research and Treatment of Cancer, EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma: Eur J Cancer, 2012; 48; 599-641
8. Llovet JM, Bru C, Bruix J, Prognosis of hepatocellular carcinoma: The BCLC staging classification: Semin Liver Dis, 1999; 19; 329-38
9. Bruix J, Sherman MAmerican Association for the Study of Liver Disease, Management of hepatocellular carcinoma: An update: Hepatology, 2011; 53; 1020-22
10. Wu MY, Qiao Q, Wang K, Development and validation of pre- and post-operative models to predict recurrence after resection of solitary hepatocellular carcinoma: A multi-institutional study: Cancer Manag Res, 2020; 12; 3503-12
11. Gao SX, Liao R, Wang HQ, A nomogram predicting microvascular invasion risk in BCLC 0/A hepatocellular carcinoma after curative resection: Biomed Res Int, 2019; 2019; 9264137
12. Forner A, Reig ME, de Lope CR, Current strategy for staging and treatment: The BCLC update and future prospects: Semin Liver Dis, 2010; 30; 61-74
13. Mazzaferro V, Roayaie S, Poon R, Dissecting EASL/AASLD recommendations with a more careful knife: A comment on “surgical misinterpretation” of the BCLC staging system: Ann Surg, 2015; 262; e17-18
14. Bruix J, Llovet JM, Prognostic prediction and treatment strategy in hepatocellular carcinoma: Hepatology, 2002; 35; 519-24
15. Torzilli G, Belghiti J, Kokudo N, A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: An observational study of the HCC East-West study group: Ann Surg, 2013; 257; 929-37
16. Gao Q, Wang XY, Zhou J, Heterogeneity of intermediate-stage HCC necessitates personalized management including surgery: Nat Rev Clin Oncol, 2015; 12; 10
17. Cho Y, Sinn DH, Yu SJ, Survival analysis of single large (>5 cm) hepatocellular carcinoma patients: BCLC A versus B: PLoS One, 2016; 11; e0165722
18. Jung YK, Jung CH, Seo YS, BCLC stage B is a better designation for single large hepatocellular carcinoma than BCLC stage A: J Gastroenterol Hepatol, 2016; 31; 467-74
19. Bruix J, Fuster J, A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West Study Group: Ann Surg, 2015; 262; e30
20. Cucchetti A, Djulbegovic B, Tsalatsanis A, When to perform hepatic resection for intermediate-stage hepatocellular carcinoma: Hepatology, 2015; 61; 905-14
21. Liu PH, Su CW, Hsu CY, Solitary large hepatocellular carcinoma: Staging and treatment strategy: PLoS One, 2016; 11; e0155588
22. Kokudo N, Takemura N, Hasegawa K: Hepatol Res, 2019; 49; 1109-13
23. Korean Liver Cancer Association, National Cancer Center, 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma: Gut Liver, 2019; 13; 227-99
24. Vogel A, Cervantes A, Chau I, Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up: Ann Oncol, 2019; 30; 871-73
25. Pawlik TM, Delman KA, Vauthey JN, Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma: Liver Transpl, 2005; 11; 1086-92
26. Liu YW, Lin CC, Yong CC, Prognosis after resection of single large hepatocellular carcinoma: Results from an Asian high-volume liver surgery center: PLoS One, 2020; 15; e0230897
27. Labgaa I, Demartines N, Melloul E, Surgical resection versus transarterial chemoembolization for intermediate stage hepatocellular carcinoma (BCLC-B): An unsolved question: Hepatology, 2019; 69; 923
28. Zhaohui Z, Shunli S, Bin C, Hepatic resection provides survival benefit for selected intermediate-stage (BCLC-B) hepatocellular carcinoma patients: Cancer Res Treat, 2019; 51; 65-72
29. Bruix J, Takayama T, Mazzaferro V, Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial: Lancet Oncol, 2015; 16; 1344-54
30. Liu S, Li H, Guo L, Tumor size affects efficacy of adjuvant transarterial chemoembolization in patients with hepatocellular carcinoma and microvascular invasion: Oncologist, 2019; 24; 513-20
31. Zakaria HM, Macshut M, Gaballa NK, Total tumor volume as a prognostic value for survival following liver resection in patients with hepatocellular carcinoma. Retrospective cohort study: Ann Med Surg (Lond), 2020; 54; 47-53
32. Rhee H, Chung T, Yoo JE, Gross type of hepatocellular carcinoma reflects the tumor hypoxia, fibrosis, and stemness-related marker expression: Hepatol Int, 2020; 14; 239-48
Figures
 Figure 1. Comparison of overall survival among patients with HCC in (A) BCLC stage A1 or stage A2 vs. BCLC stage B; (B) BCLC stage A1+A2 vs. BCLC stage B; (C) BCLC stage A1 vs. BCLC stage A2+B. HCC – hepatocellular carcinoma; BCLC – Barcelona Clinic Liver Cancer.
Figure 1. Comparison of overall survival among patients with HCC in (A) BCLC stage A1 or stage A2 vs. BCLC stage B; (B) BCLC stage A1+A2 vs. BCLC stage B; (C) BCLC stage A1 vs. BCLC stage A2+B. HCC – hepatocellular carcinoma; BCLC – Barcelona Clinic Liver Cancer. Figure 2. Overall survival comparison among patients who underwent surgical resection for hepatocellular carcinoma in (A) BCLC stage A1+A2 vs. BCLC stage B, and (B) BCLC stage A1 vs. BCLC stage A2+B. BCLC – Barcelona Clinic Liver Cancer.
Figure 2. Overall survival comparison among patients who underwent surgical resection for hepatocellular carcinoma in (A) BCLC stage A1+A2 vs. BCLC stage B, and (B) BCLC stage A1 vs. BCLC stage A2+B. BCLC – Barcelona Clinic Liver Cancer. Figure 3. Overall survival comparison among patients who underwent transarterial chemoembolization for hepatocellular carcinoma in (A) BCLC stage A1+A2 vs. BCLC stage B, and (B) BCLC stage A1 vs. BCLC stage A2+B. BCLC – Barcelona Clinic Liver Cancer.
Figure 3. Overall survival comparison among patients who underwent transarterial chemoembolization for hepatocellular carcinoma in (A) BCLC stage A1+A2 vs. BCLC stage B, and (B) BCLC stage A1 vs. BCLC stage A2+B. BCLC – Barcelona Clinic Liver Cancer. Figure 4. Overall survival comparison among patients with hepatocellular carcinoma in BCLC stage A2 who underwent surgical resection vs. transarterial chemoembolization. BCLC – Barcelona Clinic Liver Cancer.
Figure 4. Overall survival comparison among patients with hepatocellular carcinoma in BCLC stage A2 who underwent surgical resection vs. transarterial chemoembolization. BCLC – Barcelona Clinic Liver Cancer. Tables
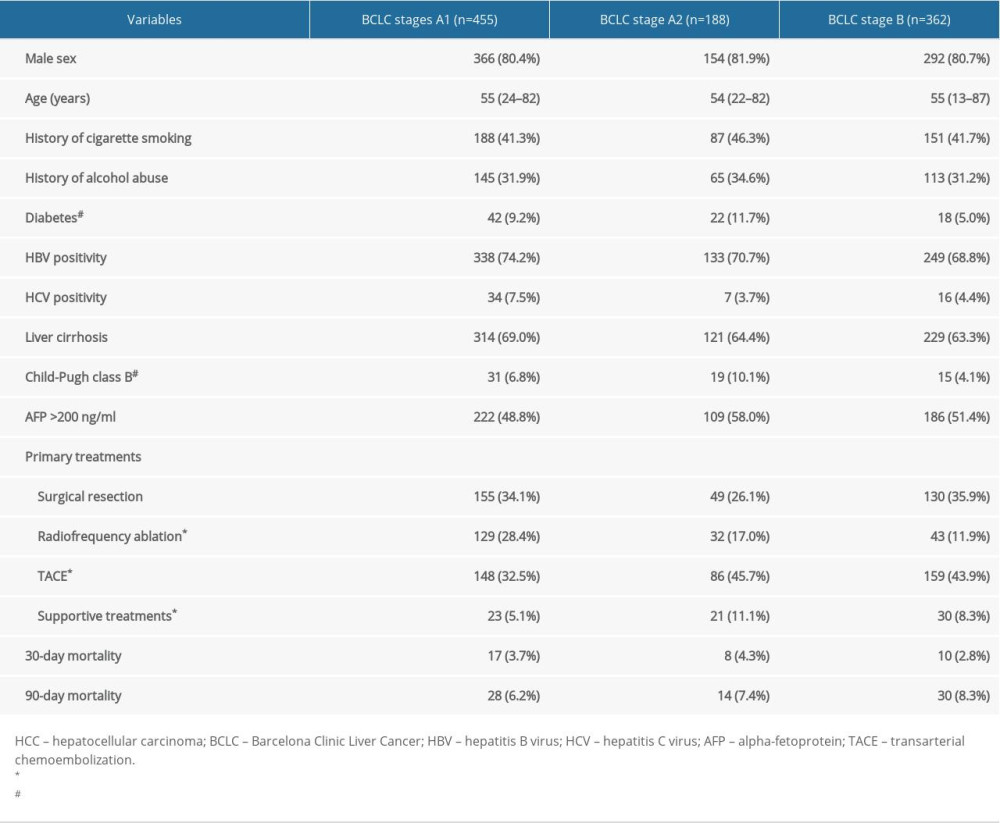 Table 1. Characteristics of the patients with hepatocellular carcinoma in BCLC stages A1, A2, and B.
Table 1. Characteristics of the patients with hepatocellular carcinoma in BCLC stages A1, A2, and B.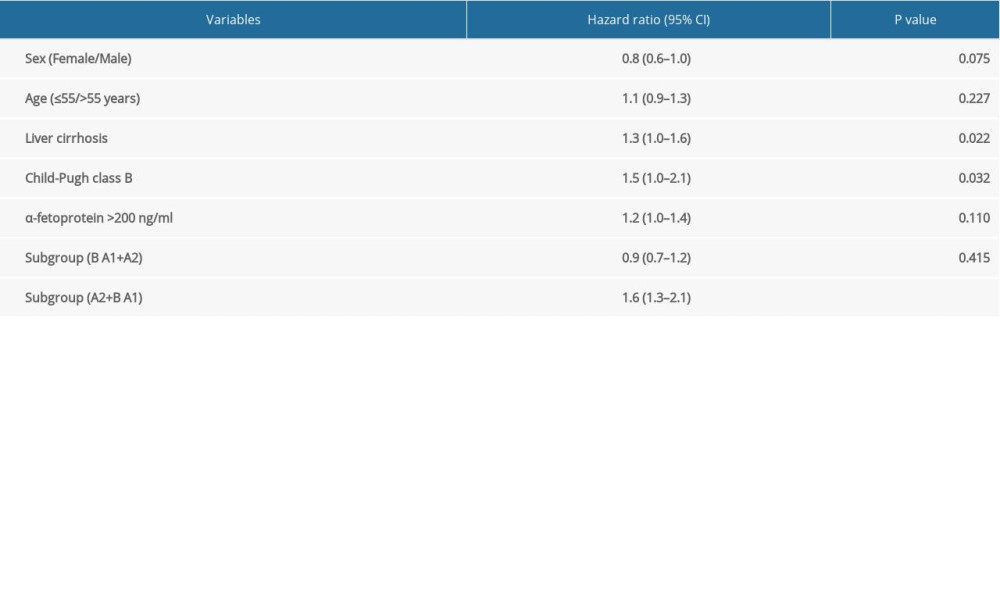 Table 2. Multivariable analysis by Cox proportional hazard regression analysis for overall survival.
Table 2. Multivariable analysis by Cox proportional hazard regression analysis for overall survival. Table 1. Characteristics of the patients with hepatocellular carcinoma in BCLC stages A1, A2, and B.
Table 1. Characteristics of the patients with hepatocellular carcinoma in BCLC stages A1, A2, and B. Table 2. Multivariable analysis by Cox proportional hazard regression analysis for overall survival.
Table 2. Multivariable analysis by Cox proportional hazard regression analysis for overall survival. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








