17 November 2020: Animal Study
Upregulation of Chemokines in the Paraventricular Nucleus of the Hypothalamus in Rats with Stress-Induced Hypertension
Qin Wu1BCDG, Yuping Chen2BDG, Wenying Zhang3B, Siyuan Song3B, Ziyang Xu3B, Hong Zhang4B, Liping Liu5D, Jihu Sun3ADEFG*DOI: 10.12659/MSM.926807
Med Sci Monit 2020; 26:e926807
Abstract
BACKGROUND: The neuroinflammation of paraventricular nucleus (PVN) of the hypothalamus has been implicated in the development of hypertension. The promoted invasion of peripheral immune cells into PVN may be attributed to the upregulation of chemokines, then exacerbating neuroinflammation. We studied the expressions of chemokines, activation of microglial cells, and inflammatory mediators in PVN of rats with stress-induced hypertension (SIH).
MATERIAL AND METHODS: SIH was induced by electrical foot shock combined with noise for 2 h twice a day, at an interval of 4 h for 14 consecutive days. At the end of the 14th day, fresh PVN tissues were collected to measure the expressions of chemokines using the RayBiotech antibody array.
RESULTS: We are the first to report that the expression of CXCL7 was extremely high in PVN of control rats, and was significantly lower in SIH rats. The expressions of CCL2 and CX3CL1 in PVN of SIH rats significantly exceeded those of control rats. The numbers of CX3CR1 (receptor of CX3CL1)-immunostained cells and oxycocin-42 (OX-42, marker of microglia)-positive cells increased in PVN of the SIH rats. The stress enhanced the protein expressions of proinflammatory cytokines IL-6 and IL-17 and reduced those of anti-inflammatory cytokines TGF-β and IL-10 in PVN.
CONCLUSIONS: In PVN of SIH rats, chronic stress induced neuroinflammation characterized by the activated microglia and upregulated proinflammatory cytokines. Expressions of chemokines CXCL7, CX3CL1, and CCL2 were altered. The causal link of chemokines to PVN neuroinflammation and hypertension remain to be determined.
Keywords: Chemokines, Hypertension, Neuroimmunomodulation, Paraventricular Hypothalamic Nucleus, Stress, Physiological, Blood Pressure, Heart Rate, Inflammation Mediators, Microglia, Stress, Psychological
Background
Hypertension is one of the most common chronic diseases. It has high prevalence, disability, and mortality rates and has become a major risk factor for the development of heart, brain, and kidney diseases. According to an updated summary of “Chinese Cardiovascular Disease Report 2018”, there were 245 million hypertensive people in China [1]. The soaring prevalence rate of hypertension seriously threatens people’s health and causes huge social and family burdens.
The fast-paced, competitive lifestyle, long-term excessive workload, sustained tension, long-term emotional or mental pressure, and other psychological stresses can result in stress-induced hypertension (SIH) [2,3]. Accumulating evidence has proven that hypertension has the characteristics of neuroinflammation and sympathetic overactivity [4]. The neuroinflammation of paraventricular nucleus (PVN) of the hypothalamus plays an essential role in the development of hypertension. The corticotrophin-releasing hormone neurons in PVN project to either the rostral ventrolateral medulla or the sympathetic intermediolateral nucleus of the spinal cord, and thus function as a critical cardiovascular and autonomic center controlling sympathetic activity. Microinjection of exogenous cytokines into PVN can elevate the sympathetic outflow and blood pressure [5,6]. In SIH rodents, the serum norepinephrine level or renal sympathetic discharge was increased, accompanied by the upregulation of proinflammatory cytokines [7–9]. Alleviating the neuroinflammation of PVN in hypertensive rats attenuated hypertension by decreasing the serum norepinephrine level or sympathetic discharge [7,10,11]. The upregulation of proinflammatory cytokines such as TNF-α in PVN can activate local neurons through the receptors expressed therein [12], and blocking the receptors can suppress sympathetic overexcitation and reduce blood pressure [13]. Collectively, there is a direct causal relationship between the neuroinflammation of PVN and the augmentation of sympathetic activity or blood pressure.
Our previous study demonstrated that the activation of microglia in PVN of SIH rats contributed greatly to neuroinflammation [7]. The innate microglia of the central nervous system (CNS) respond quickly to infection or inflammation. It is generally accepted that activated microglia can release proinflammatory or anti-inflammatory cytokines, depending on the nature of the signals received. In SIH rats, the activated microglia in PVN mainly produce proinflammatory cytokines that promote inflammation and SIH [7]. In addition to resident microglia, the peripheral immune cells infiltrating into the brain are also involved in neuroinflammation and the development of hypertension [14]. Moreover, we have proven that the permeability of the blood-brain barrier in SIH rats increased and Th17 cells invaded the parenchyma of PVN to secrete IL-17, which facilitated neuroinflammation and the development of hypertension [6].
Chemokines play crucial chemotactic roles in inflammatory response, and induce immune cells to infiltrate and to accumulate in specific tissues [14]. When CNS is damaged, chemokines are released to bind chemokine receptors on the surface of microglia, inducing the aggregation and activation of microglial cells at the damaged site. Meanwhile, activated microglia also release chemokines and recruit white blood cells in the peripheral blood to the damaged site, thereby further aggravating the inflammatory response of CNS. Therefore, whether the infiltration of peripheral immune cells or the activation and aggregation of resident microglia in the brain can be attributed, at least in part, to chemokines remains elusive. In this study, we used RayBiotech GSR-CAA-67 to detect cytokines in PVN of SIH rats, focusing on the analysis of chemokines involved in the neuroinflammation of PVN.
Material and Methods
EXPERIMENTAL ANIMALS AND ESTABLISHMENT OF SIH MODEL:
Eighteen male Sprague-Dawley rats weighing 200–250 g were purchased from Shanghai Jiesijie Experimental Animal Co., Ltd. [Animal Production License: SCXK (Shanghai)-2018-0004, China]. Rat feeding and experimental methods have been previously described [6]. The rats were randomly divided into a control group (Sham) and a stress group (Stress) (n=9). All animal experiments were approved by the Animal Experiment Ethics Committee of Jiangsu Vocational College of Medicine and performed in strict accordance with the guidelines for the care and use of experimental animals. The animals were acclimated to the housing facility for 3 days, followed by stress treatment as previously described [7]. The stress group was subjected to electric foot shock combined with buzzer noise for 2 h twice a day at a 4-h interval for 14 consecutive days. Briefly, each rat was placed in an individual cage (22×22×28 cm) with a grid floor. Intermittent electric shocks (35–80 V, duration of 50–100 ms) delivered every 2–30 s and/or noises (90–100 dB) produced by a buzzer were randomly controlled by a computer. The control group was placed in individual cages without receiving stressors. The systolic blood pressure (SBP) was measured every 2 days using the tail-cuff method while the rats were conscious. Each SBP measurement was repeated 3 times to obtain the average (Non-Invasive Blood Pressure System, CODA, USA). On the 14th day of modeling, all rats were anesthetized with chloral hydrate (0.3 ml of 10% solution per 100 g of body weight, intraperitoneally). Afterwards, the anesthetized rats were decapitated to obtain the brain tissues for western blot or RayBiotech antibody array. The anesthetized rats were transcardially perfused with 4% paraformaldehyde to collect brains for slicing and immunohistochemical (IHC) staining.
ANTIBODY ARRAY ASSAY:
At the end of modeling, the rats were killed by 10% chloral hydrate injection and decapitated, then the brains were quickly taken out, snap-frozen in liquid nitrogen, and stored at −80°C. PVN tissue was taken out from the brain as previously described [7]. Rat cytokine antibody array GS67 (GSR-CAA-67, RayBiotech, USA) was used to detect cytokines in the protein extracts of PVN according to the manufacturer’s instructions. Briefly, protein extracts were diluted to 500 μg/ml with blocking buffer, added to the array pools printed with 75 corresponding anti-cytokine antibodies, and incubated overnight. After washing, a biotin-conjugated anti-cytokine mix was incubated with the pools for 2 h. Finally, Cy3-conjugated streptavidin was used for glass series arrays. Afterwards, the glass slides were scanned to detect the fluorescent signals of microarrays using InnoScan 300 microarray scanner (Innopsys, France). The membrane arrays were exposed to HRP-catalyzing chemiluminescent solution using an Image Quant LAS4000 scanner (GE Healthcare, Waukesha, WI, USA). The signal values were read and normalized using an internal positive control by a RayBiotech analysis tool specifically designed to analyze the data of rat cytokine antibody arrays.
WESTERN BLOT:
PVN tissue was obtained as described in subsection 1.2, and the protocol of western blot was the same as that previously reported [7]. Briefly, 200 μl of pre-cooled extraction reagent was added to the rat PVN sample. The extraction reagent was pre-added to 2 μl of protease inhibitor, 10% PMSF, and phosphatase inhibitor. The sample was homogenized and centrifuged in an ice bath, and the extracted protein solution was transferred into a new centrifuge tube. After protein concentration was measured by the BCA method, the solution was added to 5× SDS-PAGE protein loading buffer and boiled at 100°C for 10 min. Different samples of the same group were prepared into different loading volumes according to equal total protein amount, and appropriate buffer was finally added to make the sample volumes in different wells identical. Then, 10% separation gel and 60 V constant voltage were first used for electrophoresis. When bromophenol blue entered the separation gel from the concentration gel, 120 V constant voltage was used for electrophoresis until the color reached the bottom of the separation gel. Subsequently, proteins were transferred onto a PVDF membrane that was washed with TBST solution 3 times, 5 min each time. The membrane was then blocked with 5% milk in PBS-Tween 20 for 1 h, and incubated overnight at 4°C with mouse anti-IL-6 (ab9324, Abcam, UK; 1: 1000), rabbit anti-IL-17 (PA5-79470, Invitrogen, USA;1: 500), rabbit anti-IL-10 (ab9969, Abcam, UK; 1: 1000), and rabbit anti-TGF-β (ab66043, Abcam, UK; 1: 500) antibodies, respectively. Then, the membrane was washed 3 times (10 min each time), incubated with anti-rabbit or anti-mouse secondary antibodies at room temperature for 60 min, washed, immersed in ECL solution for about 1 min, and photographed with a gel imaging system. The bands were quantified by ImageJ software.
IHC STAINING:
The experimental method was the same as that in our previous report [7]. After modeling, left ventricular perfusion was performed with 150–200 ml of cold normal saline and then with 150–200 ml of 4% paraformaldehyde (PFA). Straight limbs and cocked tail indicated good perfusion. At the end of perfusion, the brain was quickly removed and placed in 4% PFA for 48 h. After dehydration with 20% and 30% sucrose solutions, 18-μm-thick continuous coronal slices were prepared with a freezing microtome (Leica CM 1850, Germany). Starting from the first slice with a PVN section (before and after the bregma: −1.80 mm), 1 slice was taken over 3 in a head-to-tail order, placed in 0.01 mol/L PBS containing 30% glycerol (pH 7.4), and stored at 4°C prior to use. The IHC staining process has been described previously [15]. In brief, the sections were immersed in 30% H2O2/methanol (1: 50) solution to inactivate endogenous peroxidase, and incubated with rabbit anti-CX3CR1 (ab7201, Abcam, UK; 1: 100) and mouse anti-Integrin αM (OX-42) (sc-53086, Santa Cruz, USA; 1: 50) antibodies overnight at 4°C, followed by incubation with biotin-labeled rabbit immunoglobulin G secondary antibody and streptavidin-biotin complex (SA1028, Wuhan Boster Biological Technology, Ltd., China) at room temperature for 30 min. The sections were visualized by 3,3′-diaminobenzidine, mounted with neutral resin, observed under an Olympus BX51 microscope (Japan), and photographed with a digital camera. Immunoreactive cells were counted using ImageJ software. The positive cells in 6 slices were counted for each rat brain, and the average was recorded.
STATISTICAL ANALYSIS:
All experimental data were statistically analyzed with GraphPad Prism 8.0 software and expressed as mean±standard deviation (χ̄±SD), with n representing the number of rats. Univariate analysis between the 2 groups was conducted using the
Results
STRESS ELEVATED BLOOD PRESSURE AND HEART RATE AND ACTIVATED MICROGLIA IN PVN:
The stress group rats had significantly higher blood pressure (Figure 1A, n=9, P<0.01) and heart rate (Figure 1B, n=9, P<0.05) than those of the sham group. The microglia in PVN of SIH rats were activated, as evidenced by the increased number of OX-42-positive cells in comparison with the sham group (Figure 1C, 1D, n=3, P<0.01). In addition, the cell body was larger and round, and the cellular processes were shortened (Figure 1C).
STRESS CHANGED CHEMOKINE EXPRESSIONS IN PVN:
To evaluate the influence of stress on chemokines, the RayBiotech antibody array GSR-CAA-67 was used to detect proteins in the PVN tissues from SIH and control rats. Among the 67 detected proteins, 11 chemokines with Protein IDs of MCP-1 (CCL2), MIP-1a (CCL3), RANTES (CCL5), Eotaxin (CCL11), CTACK (CCL27), CINC-1 (CXCL1), CINC-3 (CXCL2), CINC-2 (CXCL3), LIX (CXCL5), TCK-1 (CXCL7), and Fractalkine (CX3CL1) were analyzed. As shown in Figure 2, the expression of CXCL7 was extremely high in PVN of control rats, which was significantly lower in SIH rats (n=3, P<0.01). The CCL2 expression in SIH rats was significantly higher than in control rats (n=3, P<0.05). Additionally, the expression of CX3CL1 was significantly higher in SIH rats compared with control rats (n=3, P<0.01).
STRESS INDUCED CHANGES OF INFLAMMATORY CYTOKINES IN PVN:
The protein expressions of proinflammatory cytokines IL-6 and IL-17 and anti-inflammatory cytokines IL-10 and TGF-β in PVN were detected by western blot. As shown in Figure 3A and 3B, the expressions of IL-6 and IL-17 in PVN of SIH rats were significantly higher but those of IL-10 and TGF-β were significantly lower than those of the control group (Sham) (n=9, P<0.05; P<0.01). Furthermore, IHC staining of PVN showed that the expression of CX3CL1 receptor CX3CR1 in SIH rats was significantly higher than in control rats (Figure 3C, 3D; n=3, P<0.05). CX3CR1-positive cells are small and gracile (Figure 3C) and CX3CR1 is a selective marker for microglia in the central nervous system. Therefore, CX3CR1-positive cells in PVN are considered as microglia.
Discussion
In this study, stimulation using plantar shock combined with noise for 14 days raised the blood pressure and heart rate in rats. Also, chronic stress induced activation of microglia in PVN, upregulation of proinflammatory cytokines IL-6 and IL-17, and decrease of anti-inflammatory cytokines IL-10 and TGF-β, indicating a neuroinflammatory state. We have previously reported that cytokines such as IL-6 and IL-10 were double-labeled with a microglia marker Iba1 in PVN of SIH rats [7], suggesting that the cytokines detected by western blot are secreted from activated microglia. We found for the first time that the expression of CXCL7 was extremely high in PVN of control rats, which decreased significantly in SIH rats. The expressions of CCL2 and CX3CL1 in PVN of SIH rats significantly exceeded those of control rats.
The upregulation of proinflammatory mediators in PVN is closely associated with hypertension in humans and rodents through the regulation of sympathetic outflow by changing the activity of neurons [16]. We have previously used minocycline to inhibit the activation of microglia in PVN, which reduced the levels of proinflammatory cytokines and prevented the progression of SIH [7]. The hypothalamic PVN neuroinflammation enhanced the activities of the sympathetic-adrenal medulla system and hypothalamus-pituitary-adrenal (HPA) axis, which contributed to the progression of SIH in rats [15]. SIH progresses essentially through neuroinflammation caused by the activation of microglia in PVN [7]. However, the factors resulting in the upregulation of proinflammatory cytokines in PVN that control cardiovascular activity remain unclear.
Neuroinflammation refers to chronic inflammation that occurs in the CNS. Under pathological conditions such as injury, ischemia, hypoxia, stress and immune abnormalities, the expressions of proinflammatory cytokines, including chemokines in damaged brain regions, increases, forming an inflammatory environment which activates resident microglia [16]. In addition to resident microglia, the infiltrating peripheral immune cells including adaptive immune T lymphocytes and B lymphocytes, and macrophages in the brain can also release proinflammatory cytokines [17], thus aggravating inflammation of the brain and inducing chronic neuroinflammation. We previously found in SIH rats that Th17 cells invaded PVN and released IL-17, which induced elevation of blood pressure when injected to PVN [6]. The adaptive immune cells in distinct brain regions were recruited by chemokines [14]. To assess the contribution of chemokines to neuroinflammation in PVN of SIH rats, we performed cytokine antibody array assay for PVN tissues. Of the 67 detected cytokines, 11 chemokines were expressed in PVN. Among them, CXCL7 had the highest expression, followed by CCL2 and then CX3CL1. In contrast, chronic stress significantly decreased CXCL7 expression but increased expression of CX3CL1 and CCL2 in PVN of SIH rats. Our results demonstrated that stress changed the expressions of chemokines in the brain, consistent with previous reports that chronic stress promoted chemokine expressions in CNS [18]. In the brain, chemokines are constitutively expressed in vascular endothelial cells, microglia, astrocytes, and neurons in physiological conditions, which, however, are altered in pathological conditions [19,20]. It has been well documented that chronic stress induces the activation of microglia and upregulation of proinflammatory cytokines in rodent brain regions, including PVN, as presented in this study and previous reports [7,18], which could promote expression of chemokines in the central nervous system [20,21]. Moreover, the upregulation of chemokines in the brain is partly attributed to stress-induced overactivation of the HPA axis and sympathetic nervous system [22], in turn exacerbating these stress responses. However, the cellular expression of chemokines in PVN needs to be explored and the causal relationship between chemokines and modulation of blood pressure remains unclear.
Microglia are resident immune cells of the CNS. In the resting state, microglia are branched, with small cell body, concentrated cytoplasm, and long or slender protrusions. In the activated state, microglial cells are amoeba-shaped, with enlarged round cell body and thick or short protrusions, as in PVN of SIH rats in the present study, suggesting that microglia are activated under chronic stress. The microglia can express receptors for a variety of cytokines, including chemokines. In the brain, CX3CR1 is almost exclusively present on microglia [23]. In this study, CX3CL1 was upregulated and microglia were activated. In PVN of the SIH rats, CX3CL1 was upregulated. To investigate whether the activation of microglia was mediated by enhanced CX3CL1/CX3CR1 signaling, we measured the CX3CR1 expression on microglia in PVN. IHC staining showed that the microglial expression of CX3CR1 increased. Since microglia are the main source of proinflammatory cytokines in PVN of SIH rats [7], PVN neuroinflammation may be largely ascribed to enhanced CX3CL1/CX3CR1 signaling due to the upregulation of CX3CL1 and the increased microglial expression of CX3CR1. Ho et al. demonstrated that intracerebroventricular administration of CX3CR1 inhibitor AZD8797 inhibited CX3CR1-microglia-mediated neuroinflammation in the nucleus tractus solitarii of fructose-induced hypertensive rats and attenuated the elevation of blood pressure [24], implying the involvement of microglia CX3CL1/CX3CR1 signaling in blood pressure regulation. This signaling was also involved in the microglia activation and neuroinflammation of stress-induced depressed rats. The expressions of CX3CL1 and CX3CR1 increased in the hippocampus and prefrontal cortex of the adult rats exposed to a chronic mild stress paradigm for 2 weeks [25]. Bollinger et al. found that chronic restraint stress induced microglial activation by elevating CX3CL1-CX3CR1 expression in the medial prefrontal cortex of male rats [26]. In stress-induced depressed mice, CX3CR1 gene knockout reduced the number of hippocampal microglia activated by stress, and significantly ameliorated depression [27]. The lack of CX3CR1 in the microglia of mice prevented the impairment of brain function and social behavior induced by chronic stress [28]. In short, the CX3CL1/CX3CR1 axis is a key signaling pathway that activates microglia and regulates the microglial release of inflammatory cytokines in nervous system diseases. Although the CX3CL1/CX3CR1 signaling system is well-established to cause the dysfunction of CNS by activating microglia, it can also protect brain function. The upregulated CX3CL1/CX3CR1 signaling protects neurons by releasing adenosine from activated microglia and regulating synaptic transmission [29]. Ragozzino et al. demonstrated that rapid administration of CX3CL1 reduced the excitatory postsynaptic potential [30]. The different effects of CX3CL1 may be ascribed to the difference between its concentrations. Low-concentration CX3CL1 may work as a neuromodulator, whereas high-concentration CX3CL1 may play a proinflammatory role [31]. Therefore, the effects of CX3CL1/CX3CR1 signaling depend on changes in the local microenvironment of the nervous system [32].
Chemokine CCL2, also known as MCP-1, increased in PVN of SIH rats in the present study, in agreement with previous studies on the upregulation of CCL2 in hypertensive rats [33]. In addition, Wang et al. found that selective blockade of CCL2 receptor CCR2 reduced the blood pressure in rodent models of hypertension, further supporting the role of CCL2 in development of hypertension [34]. The upregulation of CCL2 has also been linked to recruitment of peripheral immune cells into PVN of hypertensive rats [35]. Ataka et al. reported that chronic psychological stress stimulated the expression of CCL2 in the neurons of PVN and induced the infiltration of bone marrow-derived microglia into PVN [36]. Moreover, CCL2-induced infiltration of bone marrow cells into PVN and differentiation into bone marrow-derived microglia have been demonstrated to contribute to hypertension [33]. Hence, the increase of CCL2 in PVN of SIH rats may induce the chemoattraction of bone marrow-derived cells that expressed higher level of CCR2 than resident microglia and triggered an inflammatory cascade in PVN, thereby elevating the blood pressure. The repeated social defeat stress that induced anxiety selectively increased the CCL2 mRNA expression in mouse microglia [37], and microglial CCL2 may mediate the recruitment of inflammatory monocytes to the brain [37]. Accordingly, the upregulation of CCL2 in PVN may facilitate Th17 cell infiltration into PVN [6], but this requires further investigation.
CXCL7 was once thought to be released from platelet granules upon platelet activation, but has been found to be secreted by other cells. In the early stage of rheumatoid arthritis, synovial macrophages transiently produce CXCL7 [38]. Pillai et al. reported that CXCL7 was expressed by monocytes cocultured with stromal cells [39]. CXCL7 has mostly been found to be expressed in tumor cells to promote their proliferation or migration [40]. CXCL7 released by vascular endothelial cells has been identified as a chemoattractant for human neural stem cells, with its level rising in the cerebrospinal fluid in bacterial meningitis and neurosyphilis [41]. To the best of our knowledge, this is the first study to show that CXCL7 has an extremely high expression in PVN of rats, although such expression decreased upon SIH. By binding the cognate receptor CXCR2, CXCL7 shows strong neutrophil chemotactic activity and orchestrates movement of neutrophils to the injured site, thus essentially regulating inflammation [42]. Recent studies revealed that chronic stress stimuli caused increase in the hippocampal CXCR2 expression of depressed mice [18]. Overall, the upregulation of CXCL7 in PVN may recruit Th17 cells to PVN of SIH rats, as we previously reported [6] or directly excite PVN neurons that constitutively express CXCR2 [43], which consequently aggravates neuroinflammation, heightens sympathetic tone, and increases blood pressure. Nevertheless, further in-depth studies are required to confirm these effects of CXCL7/CXCR2 signaling.
Several limitations exist in this study. First, the cellular expression of chemokines CX3CL1, CCL2, and CXCL7 in PVN of SIH rats needs to be examined by co-staining the specific chemokine with Iba1 to reveal microglial sources of chemokines. Furthermore, the microglial release of chemokines needs investigation by culturing microglia with proinflammatory cytokines
Conclusions
We present data showing that chronic stress resulted in the activation of microglia-expressed CX3CR1, the upregulation of chemokines CCL2 and CX3CL1 and proinflammatory cytokines IL-6 and IL-17, and downregulation of chemokine CXCL7 and anti-inflammatory cytokines IL-10 and TGF-β in PVN of the SIH rats. The activated microglia and upregulation of proinflammatory cytokines IL-6 and IL-17 indicated the neuroinflammation state of PVN.
Figures
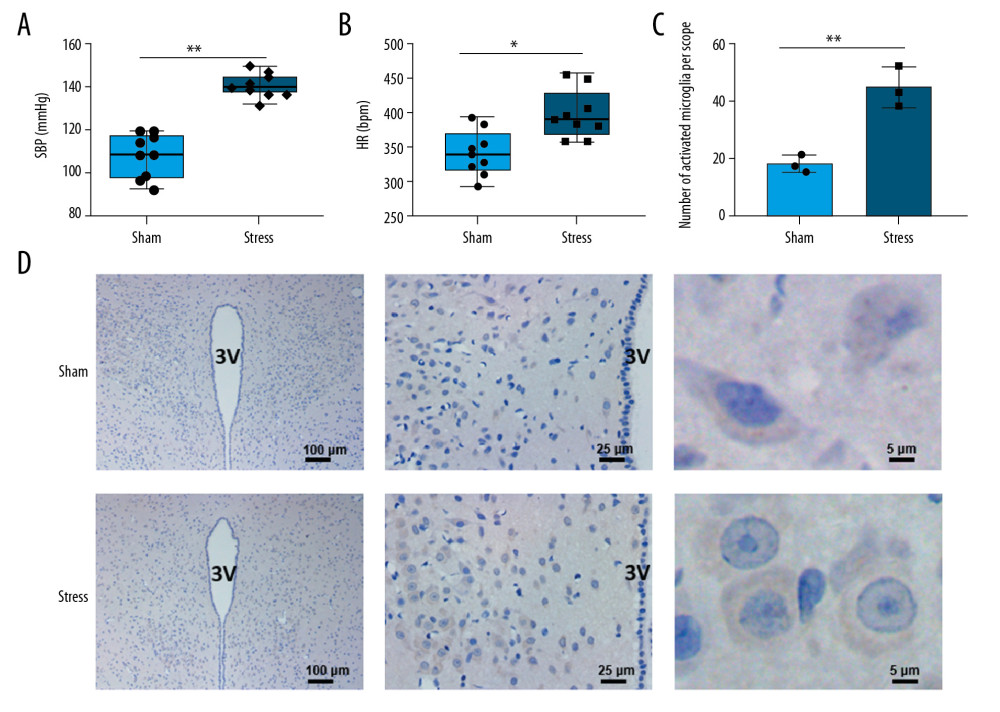 Figure 1. Changes in blood pressure, heart rate, and microglia in PVN. (A) Chronic stress increased blood pressure (n=9), ** P<0.01; (B) Chronic stress increased heart rate (n=9), * P<0.05; (C) Morphological changes of microglial cells in PVN (3V, third ventricle); (D) Statistical analysis of OX-42-positive cells (n=3), ** P<0.01.
Figure 1. Changes in blood pressure, heart rate, and microglia in PVN. (A) Chronic stress increased blood pressure (n=9), ** P<0.01; (B) Chronic stress increased heart rate (n=9), * P<0.05; (C) Morphological changes of microglial cells in PVN (3V, third ventricle); (D) Statistical analysis of OX-42-positive cells (n=3), ** P<0.01. 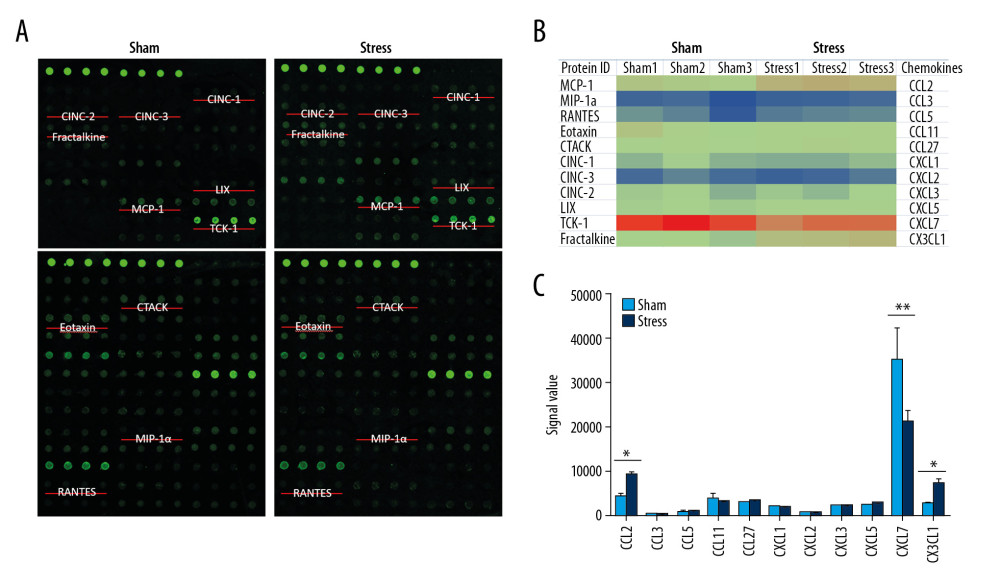 Figure 2. Expressions of chemokines in PVN. (A) Positions of 11 chemokines on the antibody array; (B) Heat map shows changes of chemokines; (C) Statistical analysis of expressions of chemokines (n=3). * P<0.05; ** P<0.01.
Figure 2. Expressions of chemokines in PVN. (A) Positions of 11 chemokines on the antibody array; (B) Heat map shows changes of chemokines; (C) Statistical analysis of expressions of chemokines (n=3). * P<0.05; ** P<0.01. 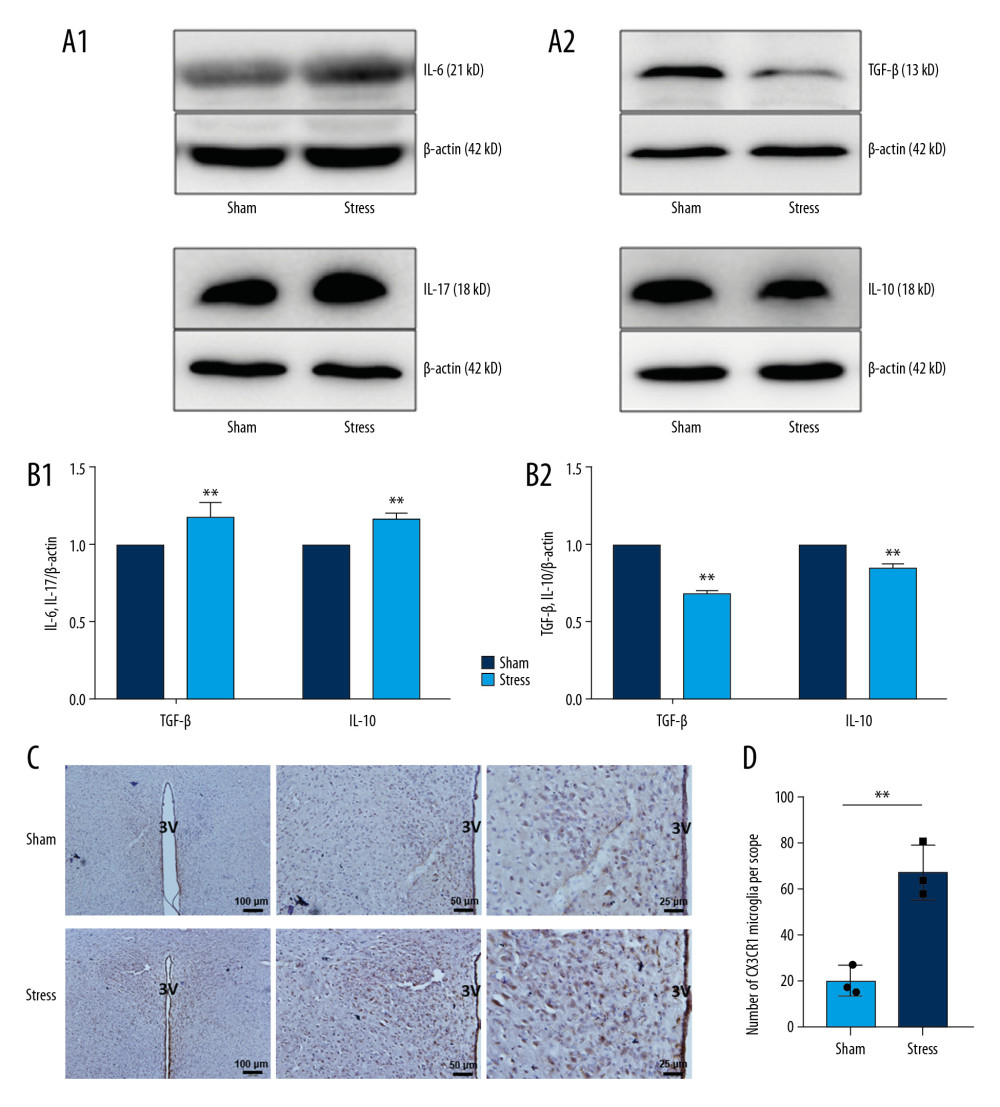 Figure 3. Changes of inflammatory cytokines in PVN. (A, B) Expressions and statistical analysis of proinflammatory and anti-inflammatory cytokines (n=9, * P<0.05; ** P<0.01); (C, D) Morphology and statistical analysis of chemokine CX3CL1 receptor CX3CR1(n=3, * P<0.05).
Figure 3. Changes of inflammatory cytokines in PVN. (A, B) Expressions and statistical analysis of proinflammatory and anti-inflammatory cytokines (n=9, * P<0.05; ** P<0.01); (C, D) Morphology and statistical analysis of chemokine CX3CL1 receptor CX3CR1(n=3, * P<0.05). References
1. Ma L, Chen W, Gao R, China cardiovascular diseases report 2018: An updated summary: J Geriatr Cardiol, 2020; 17(1); 1-8
2. Liu MY, Li N, Li WA, Association between psychosocial stress and hypertension: A systematic review and meta-analysis: Neurol Res, 2017; 39(6); 573-80
3. Johnson AK, Xue B, Central nervous system neuroplasticity and the sensitization of hypertension: Nat Rev Nephrol, 2018; 14(12); 750-66
4. Li Y, Wei B, Liu X, Microglia, autonomic nervous system, immunity and hypertension: Is there a link?: Pharmacol Res, 2020; 155; 104451
5. Shi Z, Gan XB, Fan ZD, Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats: Acta Physiol (Oxf), 2011; 203(2); 289-97
6. Wu Q, Mi Y, Cheng W, Infiltrating T helper 17 cells in the paraventricular nucleus are pathogenic for stress-induced hypertension: Biochem Biophys Res Commun, 2019; 515(1); 169-75
7. Mi Y, Wu Q, Yuan W, Role of microglia M1/M2 polarisation in the paraventricular nucleus: New insight into the development of stress-induced hypertension in rats: Auton Neurosci, 2018; 213; 71-80
8. Hu L, Zhang S, Ooi K, Microglia-derived NLRP3 activation mediates the pressor effect of prorenin in the rostral ventrolateral medulla of stress-induced hypertensive Rats: Neurosci Bull, 2020; 36(5); 475-92
9. Zhang S, Hu L, Jiang J, HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia: J Neuroinflammation, 2020; 17(1); 15
10. Li T, Chen Y, Gua C, Elevated oxidative stress and inflammation in hypothalamic paraventricular nucleus are associated with sympathetic excitation and hypertension in rats exposed to chronic intermittent hypoxia: Front Physiol, 2018; 9; 840
11. Zhang DD, Liang YF, Qi J, Carbon monoxide attenuates high salt-induced hypertension while reducing pro-inflammatory cytokines and oxidative stress in the paraventricular nucleus: Cardiovasc Toxicol, 2019; 19(5); 451-64
12. Glass MJ, Chan J, Pickel VM, Ultrastructural characterization of tumor necrosis factor alpha receptor type 1 distribution in the hypothalamic paraventricular nucleus of the mouse: Neuroscience, 2017; 352; 262-72
13. Korim WS, Elsaafien K, Basser JR, In renovascular hypertension, TNF-α type-1 receptors in the area postrema mediate increases in cardiac and renal sympathetic nerve activity and blood pressure: Cardiovasc Res, 2019; 115(6); 1092-101
14. Elsaafien K, Korim WS, Setiadi A, Chemoattraction and recruitment of activated immune cells, central autonomic control, and blood pressure regulation: Front Physiol, 2019; 10; 984
15. Wu Q, Xu Z, Song S, Gut microbiota modulates stress-induced hypertension through the HPA axis: Brain Res Bull, 2020; 162; 49-58
16. Shen XZ, Li Y, Li L, Microglia participate in neurogenic regulation of hypertension: Hypertension, 2015; 66(2); 309-16
17. Garden GA, Epigenetics and the modulation of neuroinflammation: Neurotherapeutics, 2013; 10(4); 782-88
18. Chai HH, Fu XC, Ma L, The chemokine CXCL1 and its receptor CXCR2 contribute to chronic stress-induced depression in mice: FASEB J, 2019; 33(8); 8853-64
19. Bajetto A, Bonavia R, Barbero S, Chemokines and their receptors in the central nervous system: Front Neuroendocrinol, 2001; 22(3); 147-84
20. Le Thuc O, Blondeau N, Nahon JL, The complex contribution of chemokines to neuroinflammation: Switching from beneficial to detrimental effects: Ann NY Acad Sci, 2015; 1351; 127-40
21. Trettel F, Di Castro MA, Limatola C, Chemokines: Key molecules that orchestrate communication among neurons, microglia and astrocytes to preserve brain function: Neuroscience, 2020; 439; 230-40
22. Westfall S, Iqbal U, Sebastian M, Gut microbiota mediated allostasis prevents stress-induced neuroinflammatory risk factors of Alzheimer’s disease: Prog Mol Biol Transl Sci, 2019; 168; 147-81
23. Harrison JK, Jiang Y, Chen S, Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia: Proc Natl Acad Sci USA, 1998; 95(18); 10896-901
24. Ho CY, Lin YT, Chen HH, CX3CR1-microglia mediates neuroinflammation and blood pressure regulation in the nucleus tractus solitarii of fructose-induced hypertensive rats: J Neuroinflammation, 2020; 17(1); 185
25. Rossetti AC, Papp M, Gruca P, Stress-induced anhedonia is associated with the activation of the inflammatory system in the rat brain: Restorative effect of pharmacological intervention: Pharmacol Res, 2016; 103; 1-12
26. Bollinger JL, Bergeon Burns CM, Wellman CL, Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex: Brain Behav Immun, 2016; 52; 88-97
27. Hellwig S, Brioschi S, Dieni S, Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice: Brain Behav Immun, 2016; 55; 126-37
28. Milior G, Lecours C, Samson L, Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress: Brain Behav Immun, 2016; 55; 114-25
29. Lauro C, Cipriani R, Catalano M, Adenosine A1 receptors and microglial cells mediate CX3CL1-induced protection of hippocampal neurons against Glu-induced death: Neuropsychopharmacology, 2010; 35(7); 1550-59
30. Ragozzino D, Di Angelantonio S, Trettel F, Chemokine fractalkine/CX3CL1 negatively modulates active glutamatergic synapses in rat hippocampal neurons: J Neurosci, 2006; 26(41); 10488-98
31. Maciejewski-Lenoir D, Chen S, Feng L, Characterization of fractalkine in rat brain cells: Migratory and activation signals for CX3CR-1-expressing microglia: J Immunol, 1999; 163(3); 1628-35
32. Luo P, Chu S, Zhang Z, Fractalkine/CX3CR1 is involved in the cross-talk between neuron and glia in neurological diseases: Brain Res Bull, 2019; 146; 12-21
33. Santisteban MM, Ahmari N, Carvajal JM, Involvement of bone marrow cells and neuroinflammation in hypertension: Circ Res, 2015; 117(2); 178-91
34. Wang Y, Zhu M, Xu H, Role of the monocyte chemoattractant protein-1/C-C chemokine receptor 2 signaling pathway in transient receptor potential vanilloid type 1 ablation-induced renal injury in salt-sensitive hypertension: Exp Biol Med (Maywood), 2015; 240(9); 1223-34
35. Wang M, Kang Y, Li X, Central blockade of NLRP3 reduces blood pressure via regulating inflammation microenvironment and neurohormonal excitation in salt-induced prehypertensive rats: J Neuroinflammation, 2018; 15(1); 95
36. Ataka K, Asakawa A, Nagaishi K, Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice: PLoS One, 2013; 8(11); e81744
37. McKim DB, Weber MD, Niraula A, Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety: Mol Psychiatry, 2018; 23(6); 1421-31
38. Yeo L, Adlard N, Biehl M, Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis: Ann Rheum Dis, 2016; 75(4); 763-71
39. Pillai MM, Iwata M, Awaya N, Monocyte-derived CXCL7 peptides in the marrow microenvironment: Blood, 2006; 107(9); 3520-26
40. Macgregor HL, Garcia-Batres C, Sayad A, Tumor cell expression of B7-H4 correlates with higher frequencies of tumor-infiltrating APCs and higher CXCL17 expression in human epithelial ovarian cancer: Oncoimmunology, 2019; 8(12); e1665460
41. Li XX, Zhang J, Wang ZY, Increased CCL24 and CXCL7 levels in the cerebrospinal fluid of patients with neurosyphilis: J Clin Lab Anal, 2020 [Online ahead of print]
42. Ghasemzadeh M, Kaplan ZS, Alwis I, The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi: Blood, 2013; 121(22); 4555-66
43. Semple BD, Kossmann T, Morganti-Kossmann MC, Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks: J Cereb Blood Flow Metab, 2010; 30(3); 459-73
Figures
 Figure 1. Changes in blood pressure, heart rate, and microglia in PVN. (A) Chronic stress increased blood pressure (n=9), ** P<0.01; (B) Chronic stress increased heart rate (n=9), * P<0.05; (C) Morphological changes of microglial cells in PVN (3V, third ventricle); (D) Statistical analysis of OX-42-positive cells (n=3), ** P<0.01.
Figure 1. Changes in blood pressure, heart rate, and microglia in PVN. (A) Chronic stress increased blood pressure (n=9), ** P<0.01; (B) Chronic stress increased heart rate (n=9), * P<0.05; (C) Morphological changes of microglial cells in PVN (3V, third ventricle); (D) Statistical analysis of OX-42-positive cells (n=3), ** P<0.01. Figure 2. Expressions of chemokines in PVN. (A) Positions of 11 chemokines on the antibody array; (B) Heat map shows changes of chemokines; (C) Statistical analysis of expressions of chemokines (n=3). * P<0.05; ** P<0.01.
Figure 2. Expressions of chemokines in PVN. (A) Positions of 11 chemokines on the antibody array; (B) Heat map shows changes of chemokines; (C) Statistical analysis of expressions of chemokines (n=3). * P<0.05; ** P<0.01. Figure 3. Changes of inflammatory cytokines in PVN. (A, B) Expressions and statistical analysis of proinflammatory and anti-inflammatory cytokines (n=9, * P<0.05; ** P<0.01); (C, D) Morphology and statistical analysis of chemokine CX3CL1 receptor CX3CR1(n=3, * P<0.05).
Figure 3. Changes of inflammatory cytokines in PVN. (A, B) Expressions and statistical analysis of proinflammatory and anti-inflammatory cytokines (n=9, * P<0.05; ** P<0.01); (C, D) Morphology and statistical analysis of chemokine CX3CL1 receptor CX3CR1(n=3, * P<0.05). In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








