21 November 2020: Clinical Research
The Role of Salivary Pepsin in the Diagnosis of Gastroesophageal Reflux Disease (GERD) Evaluated Using High-Resolution Manometry and 24-Hour Multichannel Intraluminal Impedance-pH Monitoring
Zihao GuoABCDEF, Yanhong WuAB, Jing ChenC, Shutian ZhangAFG, Chuan ZhangBDGDOI: 10.12659/MSM.927381
Med Sci Monit 2020; 26:e927381
Abstract
BACKGROUND: The Lyon Consensus classification confirms or rules out gastroesophageal reflux disease (GERD). The refractory symptoms of patients with GERD taking proton pump inhibitors (PPIs) are challenging in clinical practice. Salivary pepsin concentration was proposed as a diagnostic biomarker for GERD. We aimed to determine the diagnostic value of salivary pepsin concentration for patients with conclusive GERD, based on the Lyon classification, and the correlation of salivary pepsin concentration with parameters of high-resolution manometry and 24-h multichannel intraluminal impedance-pH in patients with PPI-refractory symptoms.
MATERIAL AND METHODS: Saliva samples obtained from 130 patients who were suspicious for GERD and had PPI-refractory symptoms were used for pepsin determination using the enzyme-linked immunosorbent assay. All patients underwent upper gastrointestinal endoscopy, high-resolution manometry, 24-h multichannel intraluminal impedance, and pH recording and were classified as conclusive GERD, inconclusive GERD, and evidence against GERD groups according to Lyon classification.
RESULTS: Salivary pepsin concentration was 8.2 ng/mL (3.8–17.8 ng/mL), 4.0 ng/mL (2.3–6.1 ng/mL), and 2.4 ng/mL (2.2–3.1 ng/mL) in conclusive GERD, inconclusive GERD, and evidence against GERD groups, respectively (P<0.001), and had a negative correlation with distal mean nocturnal baseline impedance and positive correlations with acid exposure time, total number of reflux events, and esophagogastric junction type. The area under the ROC curve of salivary pepsin for conclusive GERD was 0.76 (0.68–0.84), with a sensitivity of 76.36% and a specificity of 63.41% for conclusive GERD diagnosis at a cut-off value of 4.21 ng/mL.
CONCLUSIONS: Salivary pepsin test had moderate diagnostic value for conclusive GERD by Lyon classification in patients with PPI-refractory symptoms.
Keywords: Diagnosis, esophageal pH monitoring, Gastroesophageal Reflux, Pepsin A, Saliva, Electric Impedance, Manometry, ROC Curve
Background
Gastroesophageal reflux disease (GERD) is a common esophageal disease with increasing prevalence worldwide [1,2]. It is defined by the Montreal Definition and Classification of GERD as a digestive disorder or complication caused by the reflux of the stomach contents into the esophagus [3]. The first-line treatment for GERD is a proton pump inhibitor (PPI). However, the symptoms of up to 40% of patients are not well controlled by PPIs [4]. When patients have persistent troublesome GERD symptoms and objective evidence of GERD after optimized PPI therapy, it is defined as PPI-refractory GERD, which is a challenging problem in clinical practice [5–7]. Although PPI-refractory GERD is a benign disease, like inflammatory bowel disease, it significantly decreases the quality of life of patients [8].
GERD is diagnosed based on esophageal tests including high-resolution manometry (HRM), esophagogastroduodenoscopy, and ambulatory reflux monitoring according to the practical procedure guidelines provided by the Lyon Consensus. In comparison with other guidelines for GERD, the Lyon Consensus clearly defines parameters that conclusively establish the presence of GERD and characteristics that rule out GERD. The presence of severe esophagitis, Barrett’s esophagus, peptic strictures, and acid exposure time (AET) >6% indicate conclusive evidence of GERD. The combination of normal endoscopy and distal AET <4% and reflux episodes <40 per 24 h off-PPI pH-impedance monitoring provides sufficient evidence refuting GERD. In addition, the Lyon Consensus includes novel parameters, such as the post-reflux swallow-induced peristaltic wave index (PSPWI), mean nocturnal baseline impedance (MNBI), esophagogastric junction (EGJ) type, and esophagogastric junction contractile integral (length and vigor) (EGJ-CI) [9]. However, esophagogastroduodenoscopy, HRM, and ambulatory reflux monitoring are all invasive and relatively expensive procedures and are not considered ideal diagnostic tools.
Pepsin is a proteolytic enzyme whose precursor pepsinogen is produced only in the stomach. Therefore, an increased pepsin level in saliva is considered a specific biomarker for gastric reflux or laryngopharyngeal reflux [10–16]. Some studies have used the salivary pepsin level as a useful diagnostic marker for GERD [13–16]. Detecting salivary pepsin using the fibrinogen digestion method, western blot analysis, enzyme-linked immunosorbent assay (ELISA), and Peptest, a rapid lateral flow test, is considered to be convenient and noninvasive [13–17]. Wang et al. validated the efficacy of Peptest for GERD in a multicenter study in China of 1032 patients with suspected gastroesophageal reflux, including 488 patients with non-erosive reflux disease (NERD), 221 patients with erosive esophagitis, and 323 healthy controls and found that Peptest had a specificity of 60% and a sensitivity of 85%, 86%, and 84% for GERD, NERD, and erosive esophagitis, respectively [18]. Hayat et al. used the salivary pepsin level determined by using Peptest to discriminate patients with reflux-related symptoms (58 patients with GERD and 26 patients with hypersensitive esophagus) from patients with functional heartburn (FH, n=27) and healthy controls (n=100) and found that Peptest showed a sensitivity of 78.6% and specificity of 64.9% in the diagnosis of GERD and hypersensitive esophagus [12].
However, no studies have explored the association of salivary pepsin concentration with GERD classification based on the Lyon Consensus in patients with PPI-resistant symptoms and the relationship of salivary pepsin concentration with MNBI, PSPW, EGJ type, and EGJ-CI. The present study aimed to (1) assess the diagnostic value of salivary pepsin concentration for conclusive GERD by the Lyon Consensus in patients with PPI-refractory symptoms and (2) explore the relationships of salivary pepsin concentration with GERD classification by the Lyon Consensus in patients with PPI-refractory symptoms, as well as with MNBI, PSPW, EGJ type, and EGJ-CI.
Material and Methods
SUBJECTS:
A total of 130 adult patients with PPI-refractory symptoms of suspicious GERD who were admitted to the Beijing Tongren Hospital were enrolled in the study. Patients who had at least a 2-week history of symptoms of heartburn with or without regurgitation were eligible for enrollment as patients suspicious for GERD. Those patients who did not respond to twice-daily PPI therapy for at least 8 weeks were diagnosed with PPI-refractory symptoms [4–6,19]. As HRM and impedance-pH/manometry are valuable tools in clinical and esophageal investigation [20], all patients underwent a salivary pepsin test, endoscopy, HRM and 24-h multichannel intraluminal impedance and pH recording (24-h MII-pH). Patients who had histories of thoracic, esophageal, or gastric surgery, eosinophilic or infectious esophagitis, and severe esophageal motility disorders, such as achalasia and scleroderma, or were pregnant were excluded. The study was approved by the Beijing Tongren Hospital Medical Ethics Committee (No. TRECKY2015-114). All participants signed the informed consent prior to participating the study.
SALIVA COLLECTION AND PEPSIN ASSAY:
Salivary samples were collected when patients awoke (around 7 AM) and 2 h after breakfast (around 10 AM). Patients spit at least 2 mL of saliva into testing tubes containing 0.5 mL of 0.01 M citric acid (pH 2.5), which were then stored at −80°C before analysis. For pepsin measurement, salivary samples were centrifuged at 1000 g for 15 min at 4°C. The supernatants were then collected and used to measure pepsin levels as described previously [21] using an ELISA kit (Cloud-Clone, Houston, TX, USA) with a minimum detectable level of <0.93 ng/mL.
ENDOSCOPIC EXAMINATION:
To assess mucosal injury, all patients underwent upper gastrointestinal endoscopy using a GIF-260 upper gastrointestinal endoscope (Olympus, Japan). Esophagitis was defined as macroscopically visible erosion within the distal esophagus during endoscopic examination and was graded according to the Los Angeles (LA) Classification [22].
MII-PH RECORDING:
All patients were instructed to stop treatment with PPIs, H2 receptor antagonists, and prokinetics at least 7 days prior to reflux monitoring. The position of the lower esophageal sphincter was measured using HRM, and MII-pH was recorded for at least 23 h after an overnight fast. The pH-impedance catheter with 8 impedance rings and 1 pH ring (Ref. No 261A, Given Imaging, Los Angeles, CA, USA) was placed in the nose to ensure that the distal esophageal pH sensor was 5 cm proximal to the lower esophageal sphincter (LES) and impedance sensors were positioned 3, 5, 7, 9, 15, and 17 cm above the LES. During data acquisition, patients recorded their meals and activities and logged their symptoms electronically. After 24 h, the patients returned to the hospital for catheter removal. At this time, the symptom diaries were reviewed. The total reflux events and total AET were recorded. The mean value of 3 measurements of 10 min taken during the nighttime recumbent period was calculated to obtain the MNBI. The mean value of the distal 4 channels was calculated as distal MNBI [23]. The PSPW was defined as previously described [24]. The PSPWI was calculated manually as the ratio of the number of PSPWs to the number of total reflux events [24]. Data from the pH-impedance monitor were downloaded from an ambulatory pH measurement system (Ohmega, Medical Measurement Systems, Inc, Williston, VT, USA) and analyzed using the MMS database software v9.3 (Medical Measurement Systems). All data were initially identified by the software and subsequently verified and calculated by 2 of the authors (GZH and WYH) for accuracy confirmation.
HRM RECORDING:
HRM was performed after an overnight fast using a 22-channel transnasal multilumen polyvinyl catheter (diameter, 3.6 mm; Medical Measurement Systems), which was perfused continuously with distilled water at a rate of 0.15 mL/min by a low-compliance pneumohydraulic capillary infusion system (Solar GI; Medical Measurement Systems). Esophageal motility was assessed by 10 water swallows (5 mL/swallow) at 30 s intervals in a recumbent position. HRM recordings were analyzed manually using database software v9.3 (Medical Measurement Systems). Manometry data were collected and analyzed according to the Chicago Classification v3.0 [25]. The EGJ morphology was classified into 3 subtypes according to the relative localization of the LES and crural diaphragm as described in the Chicago Classification. EGJ-CI was evaluated as previously described [26,27]. Fragmented peristalsis and ineffective esophageal motility were defined as hypomotility in our study.
GROUP DEFINITIONS:
Patients were classified into 3 groups on the basis of upper endoscopic findings, 24-h pH-MII monitoring, and HRM results based on the Lyon Consensus as follows: (1) conclusive evidence for GERD (conclusive GERD): presence of erosive esophagitis LA grade C&D, long-segment Barrett’s esophagus or peptic strictures and/or AET >6%; (2) evidence against pathologic reflux (evidence against GERD): without erosive esophagitis, AET <4%, and <40 reflux episodes while the patients were off PPIs; (3) inconclusive evidence for GERD (inconclusive GERD): LA grade A&B erosive esophagitis, and/or AET between 4% and 6%, and/or 40 to 80 reflux episodes. Factors such as positive reflux-symptom association, which was defined as the symptom index > 50%, the symptom association probability >95%, reflux episode >80, MNBI <2292 ohms, PSPWI <61%, hiatus hernia, esophageal hypomotility, or increased confidence for presence of pathological reflux when evidence was otherwise borderline or inconclusive [9,28].
STATISTICAL ANALYSIS:
Data were analyzed using SPSS v23.0 (SPSS Inc, Chicago, IL, USA) and Prism software v6.0 (GraphPad Software Inc, La Jolla, CA, USA). Categorical data were expressed as numbers or percentages. Normality of data was analyzed with the Shapiro-Wilk test [29]. Continuous data with a non-normal distribution were presented as median (interquartile range [IQR]). Continuous data with a normal distribution were presented as mean±standard deviation. Categorical variables were analyzed using Pearson’s chi-squared test, and continuous variables were analyzed using the Mann-Whitney U test with Bonferroni correction. One-way analysis of variance was used to compare continuous variables among groups. Spearman’s or Wilcoxon rank-sum tests were used to assess the correlations between pepsin concentration and reflux/HRM variables. To determine the diagnostic value of the salivary pepsin concentration of GERD, receiver operator characteristic (ROC) curves were constructed, which offered a summary of sensitivity and specificity across a range of cut-off points. By maximizing Youden’s index, the optimal cut-off point was obtained [30]. A value of P<0.05 was taken to indicate statistical significance.
Results
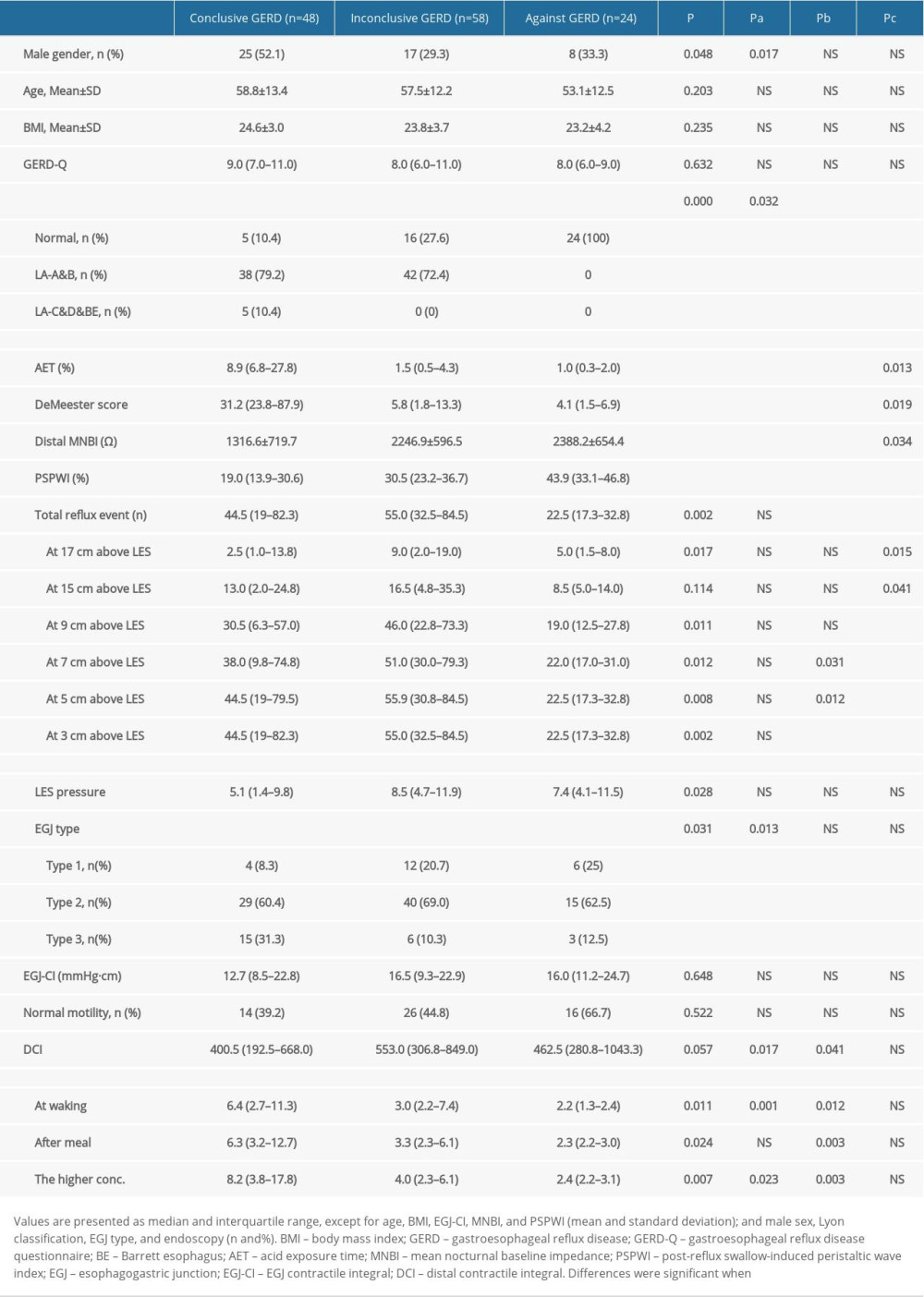
THE DEMOGRAPHIC CHARACTERISTICS OF PATIENTS AND THE RESULTS OF ENDOSCOPY, 24-H PH-MII MONITORING, AND HRM:
A total of 130 patients with refractory GERD symptoms were included. As shown in Figure 1, 48 patients had conclusive GERD, 58 patients had inconclusive GERD, and 24 patients had evidence against GERD based on the Lyon Consensus and the results of upper endoscopic findings and 24-h pH-MII monitoring. Of the 48 patients with conclusive GERD, 4 had LA grade C&D erosive esophagitis, 1 had long-segment Barrett’s mucosa, 1 had peptic strictures on endoscopy, and 44 had AET >6%. Of the 58 patients with inconclusive GERD, 42 had LA grade A&B erosive esophagitis, 24 had an AET between 4% and 6%, 22 had positive reflux-symptom association, 16 had reflux episode >80, 33 had low MNBI (<2292 ohms), and 58 had low PSPWI (PSPWI <61%). Of the 24 patients with evidence against GERD, 10 had reflux hypersensitivity (RH), and 14 had FH. The Table 1 shows the results of endoscopy, 24-h pH-MII, and HRM of patients in the 3 different groups. There were significant differences among patients with conclusive GERD, inconclusive GERD, and evidence against GERD in sex, endoscopic results, 24-h pH-MII parameters (AET, Distal MNBI, PSPWI, and numbers of reflux events) and some HRM parameters (LES pressure and EGJ type). The Shapiro-Wilk test showed that age (P=0.061), BMI (P=0.125), and distal MNBI (P=0.047) had a normal distribution and the other parameters had a non-normal distribution (P<0.05).
Among patients with evidence against GERD, there were no significant differences between the RH and FH groups in age (53.2±14.2 years
SALIVARY PEPSIN CONCENTRATION:
The salivary pepsin concentrations at waking and after breakfast are shown in the Table 1 and Figure 2A, 2B. The salivary pepsin concentrations at waking and after breakfast were not significantly different for the same patients (P=0.854); therefore, the higher value was used for further analysis and is listed in the Table 1. Overall, salivary pepsin concentrations were significantly different among patients with conclusive GERD, inconclusive GERD, and evidence against GERD (P<0.001) (Table 1, Figure 2C). Pepsin concentration was the highest at 8.2 ng/mL (3.8–17.8 ng/mL) for patients with conclusive GERD, followed in turn by 4.0 ng/mL (2.3–6.1 ng/mL) for patients with inconclusive GERD and 2.4 ng/mL (2.2–3.1 ng/mL) for patients with evidence against GERD. In addition, among patients with evidence against GERD, the salivary pepsin concentration was not significantly different between the RH and FH groups (2.3 ng/mL [2.1–8.8 ng/mL] vs. 2.4 ng/mL [2.2–3.0 ng/mL]; P=0.335].
The diagnostic value of salivary pepsin concentration was calculated using the ROC curve to differentiate patients with conclusive GERD from patients with inconclusive GERD (Figure 3). The AUC area of salivary pepsin concentration was 0.76 (0.68–0.84) for diagnosis of conclusive GERD. At the best cut-off salivary pepsin concentration of 4.21 ng/mL, its sensitivity and specificity were 76.36% and 63.41%, respectively.
CORRELATION ANALYSES BETWEEN SALIVARY PEPSIN CONCENTRATION, HRM AND 24-H PH-MII PARAMETERS:
Spearman’s correlation analysis was conducted to evaluate the potential correlations between salivary pepsin concentration, HRM, and 24-h pH-MII parameters. The results showed that salivary pepsin concentration was negatively correlated with distal MNBI (rs=−0.365, P<0.001) (Figure 4B) and positively correlated with AET (rs=0.480, P<0.001), total number of reflux events (rs=0.203, P=0.02), number of reflux events at 17 cm above LES (rs=0.184, P=0.036), and EGJ type (rs=0.268, P=0.002) (Figure 4A, 4C). However, salivary pepsin concentration had no significant correlation with sex (P=0.806), age (P=0.262), BMI (P=0.358), GERD-Q (P=0.224), number of reflux events at 15 cm above LES (P=0.076), number of reflux events at 9 cm above LES (P=0.289), number of reflux events at 7 cm above LES (P=0.066), number of reflux events at 5 cm above LES (P=0.050), PSPWI (P=0.06), EGJ-CI (P=0.064), LES pressure (P=0.310), hypomotility (P=0.603), and DCI (P=0.231).
Discussion
Pepsin is an enzyme activated from pepsinogen in the stomach. Therefore, its detection in saliva can be explained only by an episode of reflux. Salivary pepsin detection has been regarded as a noninvasive diagnostic method for GERD and laryngopharyngeal reflux (LPR). Du et al. showed that the AUC area was 0.868 for GERD. The patients with GERD in that study were defined as having reflux esophagitis (LA grades A to D), abnormal pH, or AET ≥4.2% [13]. In contrast, Race et al. found that salivary pepsin is not a reliable tool for the diagnosis of GERD [31]. A meta-analysis showed that the AUC area of salivary pepsin for LPR/GERD diagnosis was 0.71 (95% CI: 0.67–0.75), showing a moderate diagnostic value [32]. However, the patients with GERD in the previously published studies were not diagnosed according to the Lyon Consensus classification. The Lyon classification has strict standards for diagnosing or ruling out GERD. Only patients with high-grade esophagitis (LA grades C or D), peptic structuring, Barrett’s esophagus, or AET >6% were considered confirmatory evidence for GERD [9]. To the best of our knowledge, the salivary pepsin concentration in patients with PPI-refractory symptoms has not been previously measured, and ours is the first study showing that among suspicious GERD patients with PPI-refractory symptoms, patients with conclusive evidence of GERD had the highest salivary pepsin concentration, followed in turn by patients with inconclusive or borderline evidence of GERD and patients with evidence against GERD (
Previous studies have shown that salivary pepsin concentration is positively correlated with AET and number of reflux episodes [12,13]. Likewise, our study showed positive correlations with the total number of reflux events (rs=0.203,
The Lyon Consensus proposes that >80 reflux episodes per 24 h are definitively abnormal, while a number <40 is physiological, and intermediate values are inconclusive [9]. In our study, the total reflux number was highest in the inconclusive GERD group (mostly non-acid and weakly acid reflux episodes), which might lead to a higher number of episodes of proximal reflux. Though the proximal and total reflux number was highest in the inconclusive GERD group, the pepsin concentration was highest in the conclusive GERD group. We thought the reason might be that the pepsin concentration in saliva was associated not only with the frequency but also with the duration of the reflux. It was unknown whether a long duration of reflux can bring more pepsin to saliva than a short duration of reflux, and this requires further study.
Du et al. showed that salivary pepsin concentration had a low negative correlation with LES pressure [13]. The PSPWI is a reflection of the integrity of primary peristalsis after reflux episodes [9,24]. The present study showed that salivary pepsin concentration had no significant correlation with PSPWI, EGJ-CI, LES pressure, hypomotility, and DCI, whereas it had a weak positive correlation with EGJ type, suggesting that salivary pepsin concentration is not correlated with esophageal motility, which is partially reflected by DCI and PSPWI. But salivary pepsin concentration was positively correlated with EGJ barrier function, which is partially reflected by EGJ type and LES pressure.
MNBI was measured by the average of baseline impedance from 24-h pH-MII monitoring during sleep over 3 periods of 10 min. MNBI reflects the integrity of mucosa and is associated with intercellular space and tight junctions. Previous studies showed that MNBI was the lowest for erosive esophagitis, followed by true NERD and then RH, and was normal in FH and healthy controls (≥2292 ohms) [33–35]. A low MNBI (<2292 Ω) might be a predictor of a good response to anti-reflux therapy [23]. It is interesting that salivary pepsin concentration was negatively correlated with distal MNBI. Several studies have shown that pepsin could directly damage the esophageal and laryngeal mucosa, subsequently resulting in inflammation. In non-acidic and weakly acidic reflux, pepsin might be a major cause of mucosa damage [36–38]. A study has shown that although laryngeal tissues can tolerate a pH of 4.0, in the presence of pepsin, laryngeal tissues will be damaged by that pH level [39]. The relationship of MNBI with pepsin concentration might indicate the pepsin could damage mucosal integrity.
Our study has several strengths. First, every patient with refractory symptoms of GERD underwent endoscopy, HRM, and 24-h MII-pH monitoring to rule out cancer, other causes of esophagitis, and severe esophageal motility disorders. Second, patients were accurately classified based on the Lyon Consensus. Third, our study is the first showing that salivary pepsin concentration was negatively correlated with MNBI, which indicates that pepsin might contribute to impaired mucosal integrity. Nevertheless, our study is the first to show the relationship of salivary pepsin concentration with the Lyon Consensus classification, as well as MNBI, PSPWI, and EGJ type in patients with PPI-refractory symptoms. Our study showed that among patients with PPI-refractory symptoms, salivary pepsin concentration was significantly higher in patients with conclusive GERD than in patients with inconclusive GERD and evidence against GERD. The elevated salivary pepsin concentration might support the diagnosis of conclusive GERD in patients with PPI-refractory symptoms. Also, our study is the first to demonstrate that salivary pepsin concentration had a negative correlation with distal MNBI and positive correlations with AET, total number of reflux events, number of proximal reflux episodes, and EGJ type.
However, our study also has some limitations. First, it was a single-center study and therefore may have selection bias. Second, some patients underwent endoscopy after PPI therapy, which may have led to a lower grade of endoscopic LA classification of patients in the initial disease period. Third, no follow-up data were obtained to assess the predictive value of salivary pepsin concentration for clinical outcomes. Finally, salivary pepsin concentration was not measured in duplicate during the ELISA procedure, which might hinder the pepsin measurement accuracy.
Conclusions
Overall, the salivary pepsin test had a moderate diagnostic value for conclusive GERD by the Lyon classification in patients with PPI-refractory symptoms.
Figures
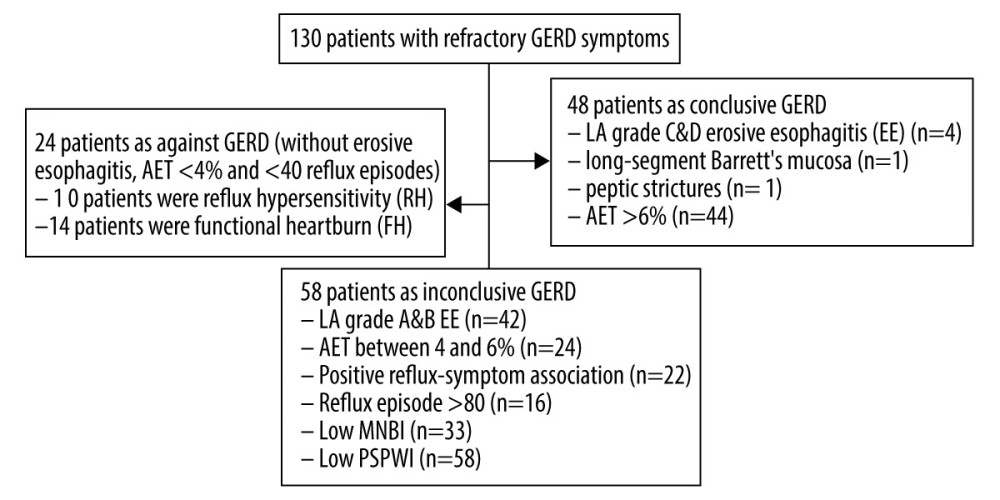 Figure 1. Flowchart showing participant recruitment and patient classification.
Figure 1. Flowchart showing participant recruitment and patient classification. 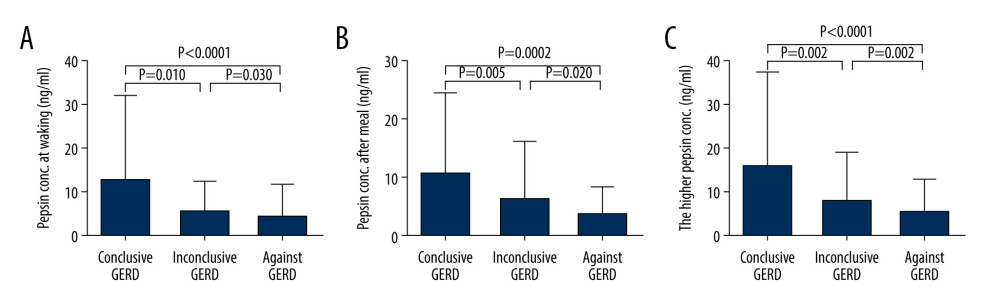 Figure 2. (A) The concentrations of pepsin upon waking in different groups. (B) The concentrations of pepsin after breakfast in different groups. (C) The higher concentrations of pepsin for each patient (out of the 2 samples) in different groups. Abbreviation: GERD, gastroesophageal reflux disease.
Figure 2. (A) The concentrations of pepsin upon waking in different groups. (B) The concentrations of pepsin after breakfast in different groups. (C) The higher concentrations of pepsin for each patient (out of the 2 samples) in different groups. Abbreviation: GERD, gastroesophageal reflux disease. 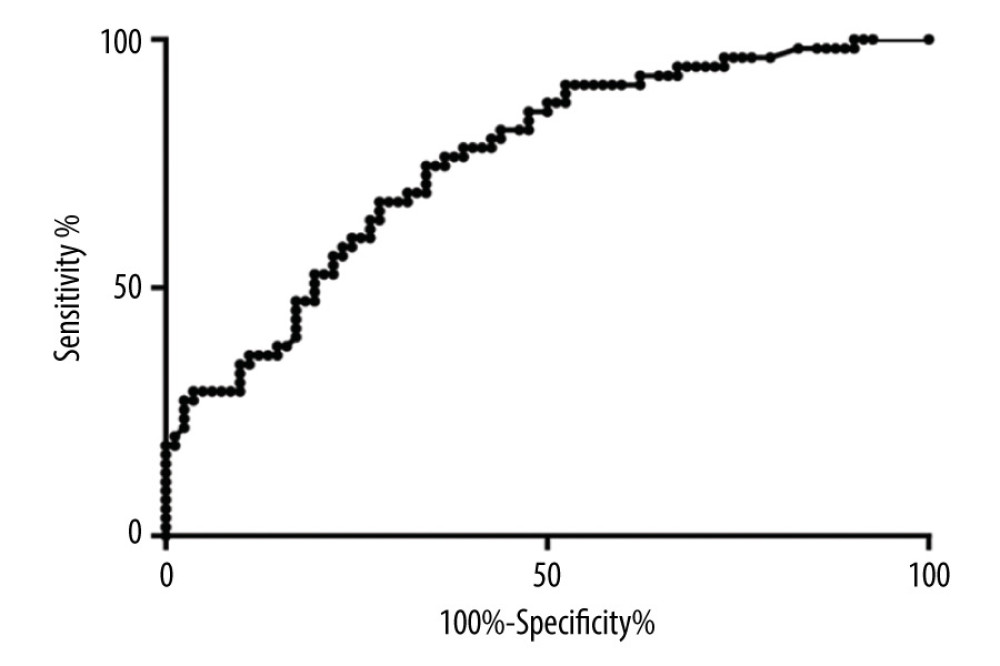 Figure 3. Receiver operating characteristic curve analysis for diagnostic value of salivary pepsin for conclusive gastro-esophageal reflux disease (GERD).
Figure 3. Receiver operating characteristic curve analysis for diagnostic value of salivary pepsin for conclusive gastro-esophageal reflux disease (GERD). 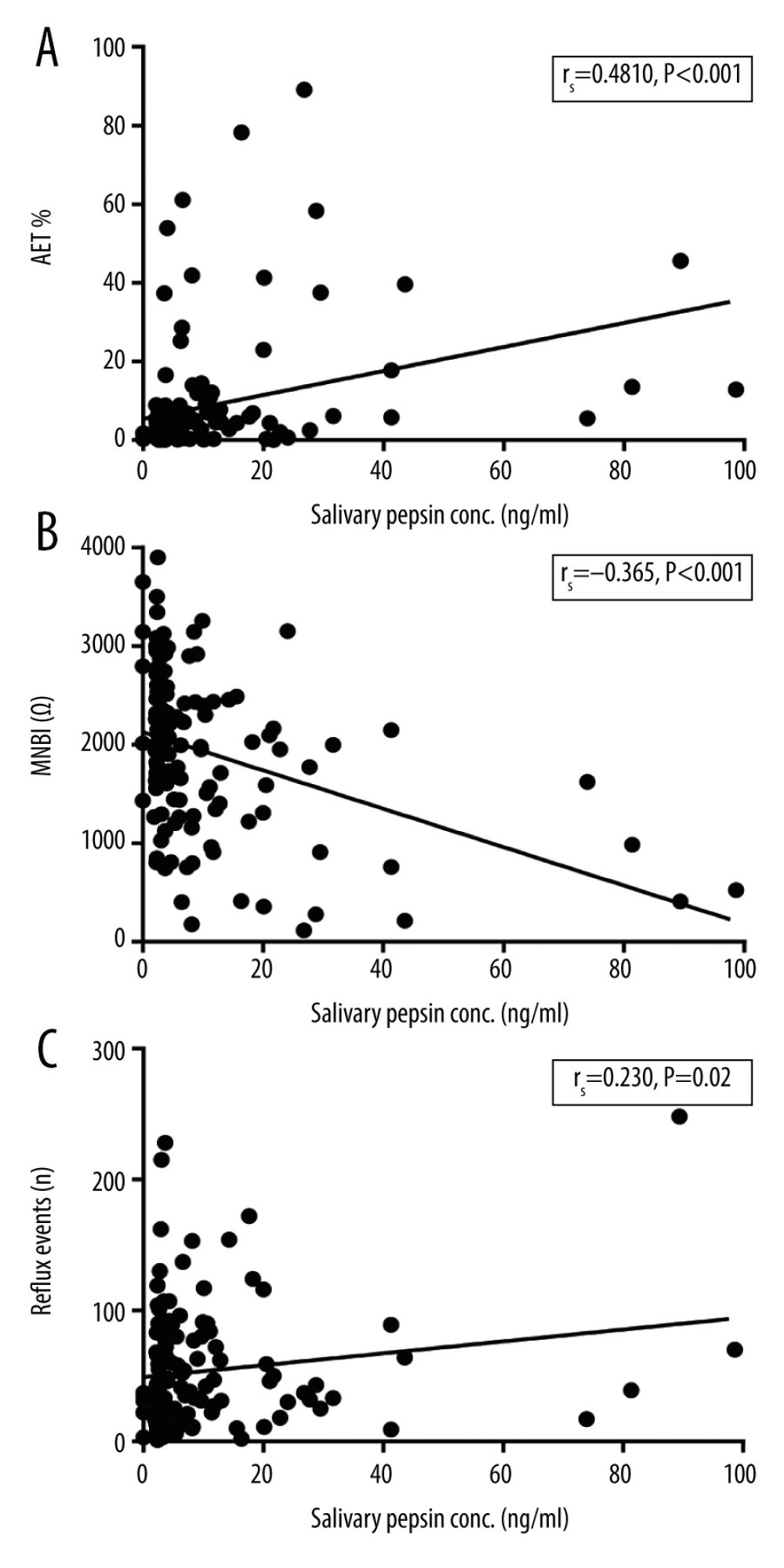 Figure 4. Correlation analyses between salivary pepsin concentration and (A) acid exposure time (AET); (B) mean nocturnal baseline impedance (MNBI); and (C) total reflux events.
Figure 4. Correlation analyses between salivary pepsin concentration and (A) acid exposure time (AET); (B) mean nocturnal baseline impedance (MNBI); and (C) total reflux events. References
1. Richter JE, Rubenstein JH, Presentation and epidemiology of gastroesophageal reflux disease: Gastroenterology, 2018; 154(2); 267-76
2. Eusebi LH, Ratnakumaran R, Yuan Y, Global prevalence of, and risk factors for, gastrooesophageal reflux symptoms: A meta-analysis: Gut, 2018; 67(3); 430-40
3. Vakil N, van Zanten SV, Kahrilas P, The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus: Am J Gastroenterol, 2006; 101(8); 1900-20 quiz 1943
4. Fass R, Sifrim D, Management of heartburn not responding to proton pump inhibitors: Gut, 2009; 58; 295-309
5. Yadlapati R, DeLay K, Proton pump inhibitor-refractory gastroesophageal reflux disease: Med Clin North Am, 2019; 103(1); 15-27
6. Yadlapati R, Vaezi MF, Vela MF, Management options for patients with GERD and persistent symptoms on proton pump inhibitors: Recommendations from an expert panel: Am J Gastroenterol, 2018; 113(7); 980-86
7. Ates F, Francis DO, Vaezi MF, Refractory gastroesophageal reflux disease: Advances and treatment: Expert Rev Gastroenterol Hepatol, 2014; 8(6); 657-67
8. Peery AF, Dellon ES, Lund J, Burden of gastrointestinal disease in the United States: 2012 update: Gastroenterology, 2012; 143(5); 1179-87
9. Gyawali CP, Kahrilas PJ, Savarino E, Modern diagnosis of GERD: The Lyon Consensus: Gut, 2018; 67(7); 1351-62
10. Doğru M, Kuran G, Haytoğlu S, Role of laryngopharyngeal reflux in the pathogenesis of otitis media with effusion: J Int Adv Otol, 2015; 11(1); 66-71
11. Iannella G, Di NG, Plateroti R, Investigation of pepsin in tears of children with laryngopharyngeal reflux disease: Int J Pediatr Otorhinolaryngol, 2015; 79(12); 2312-15
12. Hayat JO, Gabieta-Somnez S, Yazaki E, Pepsin in saliva for the diagnosis of gastrooesophageal reflux disease: Gut, 2015; 64(3); 373-80
13. Du X, Wang F, Hu Z, The Diagnostic value of pepsin detection in saliva for gastro-esophageal reflux disease: A preliminary study from China: BMC Gastroenterol, 2017; 17(1); 107
14. Potluri S, Friedenberg F, Parkman HP, Comparison of a salivary/sputum pepsin assay with 24-hour esophageal pH monitoring for detection of gastric reflux into the proximal esophagus, oropharynx, and lung: Dig Dis Sci, 2003; 48(9); 1813-17
15. Kim TH, Lee KJ, Yeo M, Pepsin detection in the sputum/saliva for the diagnosis of gastroesophageal reflux disease in patients with clinically suspected atypical gastroesophageal reflux disease symptoms: Digestion, 2008; 77(3–4); 201-6
16. Saritas Yuksel E, Hong SK, Rapid salivary pepsin test: Blinded assessment of test performance in gastroesophageal reflux disease: Laryngoscope, 2012; 122(6); 1312-16
17. Hayat JO, Yazaki E, Moore AT, Objective detection of esophagopharyngeal reflux in patients with hoarseness and endoscopic signs of laryngeal inflammation: J Clin Gastroenterol, 2014; 48(4); 318-27
18. Wang YF, Yang CQ, Chen YX, Validation in China of a non-invasive salivary pepsin biomarker containing two unique human pepsin monoclonal antibodies to diagnose gastroesophageal reflux disease: J Dig Dis, 2019; 20(6); 278-87
19. Fock KM, Talley N, Goh KL, Asia-Pacific consensus on the management of gastrooesophageal reflux disease: An update focusing on refractory reflux disease and Barrett’s oesophagus: Gut, 2016; 65(9); 1402-15
20. Kahrilas PJ, Sifrim D, High-resolution manometry and impedance-pH/manometry: Valuable tools in clinical and investigational esophagology: Gastroenterology, 2008; 135(3); 756-69
21. Jung AR, Kwon OE, Park JM, Association between pepsin in the saliva and the subjective symptoms in patients with laryngopharyngeal reflux: J Voice, 2019; 33(2); 150-54
22. Lundell LR, Dent J, Bennett JR, Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification: Gut, 1999; 45; 172-80
23. Patel A, Wang D, Sainani N, Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastrooesophageal reflux disease: Aliment Pharmacol Ther, 2016; 44; 890-98
24. Frazzoni M, Manta R, Mirante VG, Esophageal chemical clearance is impaired in gastroesophageal reflux disease – A 24h impedance-pH monitoring assessment: Neurogastroenterol Motil, 2013; 25; 399-406
25. Kahrilas PJ, Bredenoord AJ, Fox M, The Chicago classification of esophageal motility disorders, v3.0: Neurogastroenterol Motil, 2015; 27; 160-74
26. Rengarajan A, Bolkhir A, Gor P, Esophagogastric junction and esophageal body contraction metrics on high resolution manometry predict esophageal acid burden: Neurogastroenterol Motil, 2018; 30(5); e13267
27. Gor P, Li Y, Munigala S, Interrogation of esophagogastric junction barrier function using the esophagogastric junction contractile integral: An observational cohort study: Dis Esophagus, 2016; 29; 820-28
28. Matteo G, Brigida B, Vincenzo S, The Lyon Consensus: Does it differ from the previous ones?: J Neurogastroenterol Motil, 2020; 26(3); 311-21
29. Henderson AR, Testing experimental data for univariate normality: Clin Chim Acta, 2006; 366(1–2); 112-29
30. Steyerberg EW, Harrell FE, Borsboom GJ, Internal validation of predictive models: J Clin Epidemiol, 2001; 54(8); 774-81
31. Race C, Chowdry J, Russell JM, Studies of salivary pepsin in patients with gastro-oesophageal reflux disease: Aliment Pharmacol Ther, 2019; 49(9); 1173-80
32. Wang J, Zhao Y, Ren J, Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: A meta-analysis: Eur Arch Otorhinolaryngol, 2018; 275(3); 671-78
33. Ya Mei S, Yan G, Feng G, Role of esophageal mean nocturnal baseline impedance and post-reflux swallow-induced peristaltic wave index in discriminating Chinese patients with heartburn: J Neurogastroenterol Motil, 2019; 25(4); 515-20
34. De Bortoli N, Martinucci I, Savarino E, Association between baseline impedance values and response proton pump inhibitors in patients with heartburn: Clin Gastroenterol Hepatol, 2015; 13; 1082-88
35. Frazzoni M, Savarino E, de Bortoli N, Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease: Clin Gastroenterol Hepatol, 2016; 14; 40-46
36. Johnston N, Yan JC, Hoekzema CR, Pepsin promotes proliferation of laryngeal and pharyngeal epithelial cells: Laryngoscope, 2012; 122(6); 1317-25
37. Adhami T, Goldblum JR, Richter JE, The role of gastric and duodenal agents in laryngeal injury: An experimental canine model: Am J Gastroenterol, 2004; 99(11); 2098-106
38. Hirschowitz BI, Pepsin and the esophagus: Yale J Biol Med, 1999; 72(2–3); 133-43
39. Bulmer DM, Ali MS, Brownlee IA, Laryngeal mucosa: Its susceptibility to damage by acid and pepsin: Laryngoscope, 2010; 120(4); 777-82
Figures
 Figure 1. Flowchart showing participant recruitment and patient classification.
Figure 1. Flowchart showing participant recruitment and patient classification. Figure 2. (A) The concentrations of pepsin upon waking in different groups. (B) The concentrations of pepsin after breakfast in different groups. (C) The higher concentrations of pepsin for each patient (out of the 2 samples) in different groups. Abbreviation: GERD, gastroesophageal reflux disease.
Figure 2. (A) The concentrations of pepsin upon waking in different groups. (B) The concentrations of pepsin after breakfast in different groups. (C) The higher concentrations of pepsin for each patient (out of the 2 samples) in different groups. Abbreviation: GERD, gastroesophageal reflux disease. Figure 3. Receiver operating characteristic curve analysis for diagnostic value of salivary pepsin for conclusive gastro-esophageal reflux disease (GERD).
Figure 3. Receiver operating characteristic curve analysis for diagnostic value of salivary pepsin for conclusive gastro-esophageal reflux disease (GERD). Figure 4. Correlation analyses between salivary pepsin concentration and (A) acid exposure time (AET); (B) mean nocturnal baseline impedance (MNBI); and (C) total reflux events.
Figure 4. Correlation analyses between salivary pepsin concentration and (A) acid exposure time (AET); (B) mean nocturnal baseline impedance (MNBI); and (C) total reflux events. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952