31 October 2020: Clinical Research
Monitoring the Changes of Upper Limb Water in Breast Cancer Patients by Segmental Multi-Frequency Bioelectrical Impedance Analysis
Lijuan Zhang1ACG, Huizhen Zhang1BDF, Xiaohang Liu1BDE, Qiaoling Zhong1BF, Qinghua Luo1BC, Yiheng Zhang2BC, Ni Gong3AFG*, Huiying Qin4AFG, Anli Yang1CDEDOI: 10.12659/MSM.927804
Med Sci Monit 2020; 26:e927804
Abstract
BACKGROUND: Our study aims to investigate the role of segmental multi-frequency bioelectrical impedance analysis (SMF-BIA) in the monitoring of upper limb water changes of patients with breast cancer before and after surgery to aid in establishing a new approach to preventing lymphedema.
MATERIAL AND METHODS: This study included 442 female patients with breast cancer. We used SMF-BIA to monitor changes in body composition. Data were collected 1 day before surgery and 7 days and 3 months after surgery.
RESULTS: The average body mass index (BMI) of patients was normal but, in 22.8% of patients, the percentage of body fat exceeded the average, which is known as invisible obesity. Moreover, the weight, BMI, basal metabolic rate, inorganic salt content, muscle content, total body water, and extracellular water of patients increased 7 days after surgery (P<0.05), but recovered to preoperative levels within 3 months. In addition, protein content, skeletal muscle content, and intracellular water increased 7 days after surgery, but decreased within 3 months to even lower levels than before surgery (P<0.05). The extracellular water and total body water ratios increased continuously within the 3 months after surgery. Finally, the segmental water ratio of the healthy and affected upper limbs increased, while the bioelectrical impedance value decreased; however, they were still within the normal range.
CONCLUSIONS: SMF-BIA monitoring may provide more detailed information for making individual nursing care plans in patients with breast cancer. Further studies with long-term follow-up are urgently needed to establishment a lymphedema risk predictive model.
Keywords: Breast, Electric Impedance, lymphedema, Nursing Assessment, Adiposity, Body Mass Index, Body Water, Breast Cancer Lymphedema, Breast Neoplasms, Upper Extremity
Background
Breast cancer surgery and radiotherapy can affect the lymphatic system, leading to breast cancer-related lymphedema [1]. Progressively swelling limbs can lead to fatigue, changes in appearance, anxiety, and depression. These lymphedema-related problems negatively affect patient quality of life, limiting participation in daily, family, and social activities. Because the treatment of lymphedema becomes more difficult in the late stage, timely screening and diagnosis are particularly important in delaying its progression and improving or maintaining the quality of life of patients. At present, the early detection of upper limb lymphedema after breast cancer surgery is mostly performed using the arm circumference difference method and the volume difference method. The measurement of arm circumference is simple and fast; however, this method is inaccurate and unreliable because there can be significant differences in measurement results from the use of different tape measures or from different healthcare providers performing the measurements [2]. The results of the volume difference method are accurate but complicated and can easily cause cross-infection in patients with upper limb trauma [3]. Many patients have subjective feelings of edema before any obvious changes in the circumference and volumes of affected limbs are noticed, indicating that the patients may have early lymphedema. However, early lymphedema is difficult to diagnose by clinical signs and measurement of arm circumference volume, which results in clinicians missing the best time to intervene in these early cases.
In recent years, segmented multi-frequency bioelectrical impedance analysis (SMF-BIA) has been widely used in the evaluation of breast cancer-related lymphedema, with the intention of achieving early prevention and treatment [4]. Bioelectrical impedance technology is a simple, safe, and noninvasive method to measure lymphedema. It obtains the electrical impedance of each segment of the body by measuring the frequency current acting on the segments and analyzing the tissue composition [5,6]. When the current frequency is low, the current is mainly being transmitted through extracellular fluid, which allows the measurement of extracellular water (ECW); when the current frequency is high, the current is being transmitted through intracellular and extracellular fluid, which allows the measurement of total body water (TBW) [7]. The impedance value of low-frequency current reflects the changes in extracellular fluid components, which can reflect the status of lymphedema. Compared with the whole body measurement, the SMF-BIA technique can apply multiple currents at different frequency conditions to measure various body parts (right arm, left arm, torso, right leg, and left leg) individually, providing the most accurate analysis of
Invisible obesity in patients with breast cancer can lead to a decline in body function, affecting patient treatment and prognosis. This study aimed to use the BIA technique to monitor the dynamic changes of upper limb water in patients with breast cancer, further analyze the bioelectrical impedance and water content of upper limbs, and identify invisible obesity to provide more detailed information for the creation of a later-stage lymphedema risk prediction model in patients with breast cancer.
Material and Methods
PARTICIPANTS:
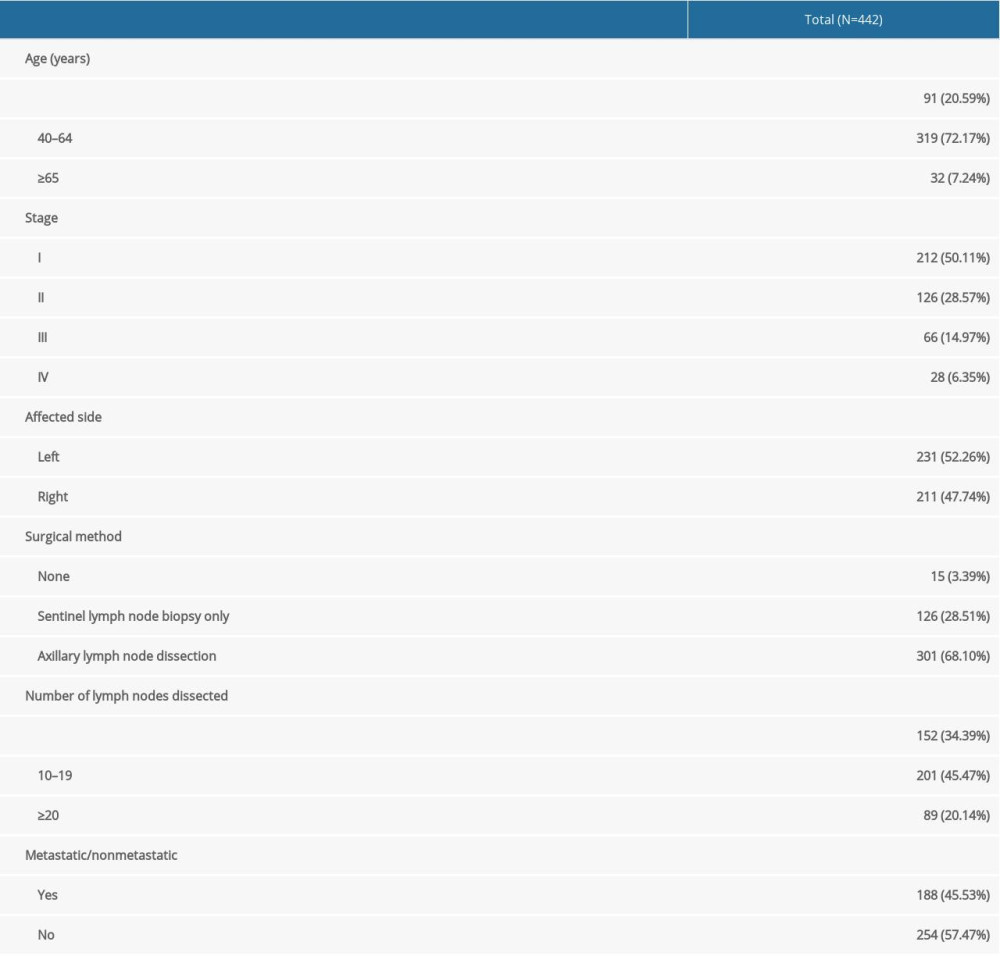
Convenience sampling was used in this study. From January to December 2018, 442 female patients with breast cancer were recruited in the breast department of a grade 3A tumor specialist hospital in Guangzhou. The inclusion criteria were as follows: (1) diagnosis with breast cancer by a pathologist for which surgical treatment was confirmed; (2) age ≥18 years; (3) possession of normal cognitive and communication skills and ability to facilitate investigations; and (4) consent given to participate in this study. The exclusion criteria were as follows: (1) inability to complete questionnaires and other study measurements due to mental or intellectual factors; (2) tumor recurrence, distant metastasis, or metastatic cancer; (3) severe complications of other body systems; (4) pregnancy; (5) severe obesity with BMI >35; and (6) other issues, including severe dehydration, edema, ascites, effusion in the serous cavity, or placement of pacemakers and metals in the body, which could affect the measurement results. The data were derived from a single set of repeated measurements. The sample size was estimated using the table titled “Sample Size Selection in Single Group Repeated Measures Analysis” [9]. With α=0.05 and β=0.2, the sample size should be 165 cases. However, based on an estimated 20% lost to follow-up rate, we needed at least 207 (165/0.8) cases included in our study.
RESEARCH TOOLS:
The same weight scale and height measuring instrument were used for all patients. For the measurement of body composition, the SMF-BIA Inbody S10 (InBody Co., Ltd., Korea) was used according to the manufacturer’s instructions. The measurement indexes included (1) the body composition index, consisting of body weight, BMI, protein, inorganic salt, body fat mass, fat-free mass, bone mineral content, skeletal muscle content, muscle mass, body fat content, percentage of body fat, basal metabolic rate, arm circumference; and (2) the water index, consisting of the TBW, ECW, intracellular water, ECW/TBW ratio (ranges: normal, 0.36–0.39; mild edema, 0.39–0.40; edema, >0.40) segmental water ratio, and bioelectrical impedance values at 1 kHz and 5 kHz.
METHOD OF DATA COLLECTION:
This study was approved by the Ethics Committee of our hospital. All patients signed an informed consent. The study procedures were performed at a room temperature of 20°C to 25°C. Before the measurement appointment, patients were instructed to avoid vigorous exercise, physical activities, and bathing. Patients were asked to fast for 2 h before the measurement and to not drink for 1 h prior. They were further asked to empty their stool and urine and avoid scheduling the measurement during their menstrual period. Patients removed outerwear, shoes, and socks before being measured. They were required to not carry or wear mobile phones, watches, and metal jewelry. The body composition measurements were performed at a fixed period in the morning for all patients.
Before measurements were made, patients rested for 10 min. Then, they stood upright on the bottom plate of the body composition analyzer for approximately 5 min. Patients were required to maintain their position until the measurements were complete, with eyes straight ahead, arms spread at an angle of about 15° from the body, and feet shoulder-width apart. Patient data including sex, age, height, and weight were entered into the analyzer. Before applying the electrodes, the skin was cleaned with an alcohol swab; then, segmental measurements were conducted. Eight-channel contact electrodes were used for the measurement of the thumbs and middle fingers of both hands and the lower back of the internal and external malleolus of both feet. The impedance values of the head, left upper limb, right upper limb, torso, left lower limb, and right lower limb were measured, in turn. The measurement indexes mainly included TBW, intracellular water, ECW, segmental water proteins, inorganic salts, BMI, body fat, percentage of body fat, skeletal muscle content, and ECW/TBW ratio. During the measurements, the patients were asked to maintain their position for a total of 2 min.
STATISTICAL ANALYSIS:
The statistical analysis was conducted using SPSS version 19.0. Data were described as frequency and mean±standard deviation. The single-factor repeated measures analysis of variance was used to compare the differences between the 3 measurement results of the body composition and water indexes of both upper limbs.
Results
PATIENT CHARACTERISTICS:
All patients were women and between 24 and 80 years old (average, 48.10±10.11 years). Of 442 patients, 338 (78.68%) were clinically diagnosed with stage I/II breast cancer, and 52.26% of patients had left-sided breast cancer. Additionally, 301 patients (68.10%) had axillary lymph node dissection and nearly half of the patients had 10 to 19 lymph nodes dissected; 45.53% of patients have lymph node metastasis. Detailed patient information is shown in Table 1.
DYNAMIC CHANGES IN THE WATER CONTENT AND BIOELECTRICAL IMPEDANCE OF AFFECTED UPPER LIMBS:
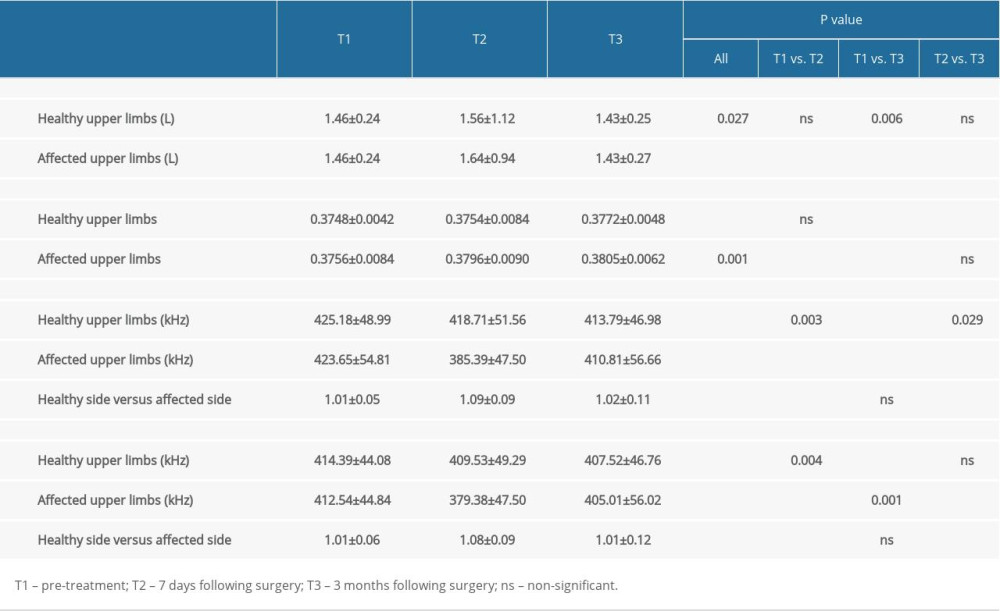
The ECW/TBW ratio of the healthy upper limbs in patients with breast cancer was significantly higher at 3 months following surgery than before surgery. The ECW/TBW ratio of the affected upper limbs was also higher at 7 days and 3 months after surgery compared with before surgery, and the differences were statistically significant. Accordingly, the 1 kHz bioelectrical impedance of the healthy upper limbs decreased, while that of the affected upper limbs was lower than before surgery at 7 days after surgery and then higher than before surgery 3 months after; the difference between each timepoint was statistically significant. The ratio of healthy upper limbs to affected upper limbs under the 1 kHz bioelectrical impedance was higher 7 days after surgery than before surgery, and then was lower than before surgery at 7 days and 3 months after surgery; the differences between each timepoint were statistically significant. Additionally, the 5 kHz bioelectrical impedance of the healthy upper limbs decreased after surgery. However, the 5 kHz bioelectrical impedance of the affected upper limbs was lower at 7 days after surgery than before surgery and was higher at 3 months after surgery than before surgery and at 7 days after surgery; the differences between each timepoint were statistically significant. Under the 5 kHz bioelectrical impedance, the ratios of healthy upper limbs vs. affected upper limbs were higher at 7 days after surgery and then lower at 3 months after surgery, compared with before surgery (P<0.05). The details are shown in Table 2.
DYNAMIC CHANGES IN BODY COMPOSITION IN PATIENTS WITH BREAST CANCER WITHIN 3 MONTHS AFTER SURGERY:
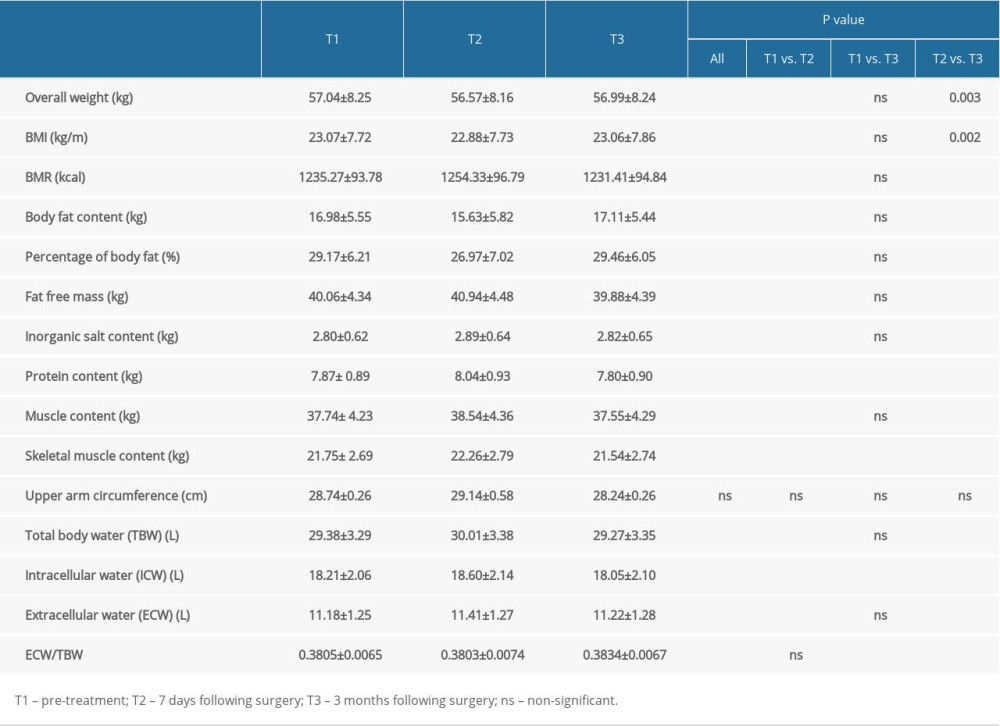
The results showed that the total body weight, BMI, and body fat content of patients with breast cancer were at first lower at 7 days after surgery and then were higher at 3 months after surgery, compared with before surgery; the differences between all timepoints were statistically significant. Basal metabolic rate, inorganic salt content, muscle content, TBW, and ECW were higher at 7 days after surgery and then lower at 3 months after surgery, compared with before surgery; the differences between the values 7 days after surgery and those at the preoperative and 3-month post-surgery timepoints were statistically significant. Protein content, skeletal muscle content, and intracellular water content were at first lower 7 days after surgery than before surgery, and then were lower at 3 months after surgery than before surgery and at 7 days after surgery; the differences between each timepoint were statistically significant. The ECW/TBW ratio showed an increasing trend. The change in the upper limb circumference of the affected side was not statistically significant. Details are shown in Table 3.
Discussion
Breast cancer surgery affects the lymphatic system and the distribution of limb water. There are subtle changes in body composition before the circumference and volume of the affected limb change. The trend of changes can indicate whether the patient is at risk of lymphedema. In addition, trends can specify personalized and precise care plans to avoid lymphedema after surgery and help patients recover quickly.
Our results showed that the BMI level of the patients with breast cancer was normal before surgery and 3 months after surgery, but the percentage of body fat exceeded the average in 22.8% of patients, indicating that they had invisible obesity (data not shown). It is known that obesity is an important factor affecting the treatment and prognosis of lymphedema. To eliminate invisible obesity in these patients, the monitoring of body fat content and other indexes should be increased, health education should be strengthened, maintenance of regular aerobic exercise should be encouraged, impedance exercise should be appropriately increased [10], and the importance of fat loss and muscle gain should be emphasized in subsequent nursing procedures [11].
During this study, we monitored the body composition of our patients 1 day before surgery and 7 days and 3 months after surgery. These values included total weight, BMI, body fat content, basal metabolic rate, inorganic salt content, muscle content, TBW, and ECW. Although most of these values changed within 7 days after surgery, they returned to the preoperative state 3 months after surgery in some patients. These changes resulted partly from the lack of food and water intake on the day of surgery and the release of stress hormones and inflammatory mediators after the surgery [12,13]. Protein content, skeletal muscle content, and intracellular water content rose to the highest levels 7 days after surgery, and then significantly decreased 3 months after surgery, which may be related to the impact of surgery and subsequent chemotherapy or endocrine therapy on the human body. Studies have pointed out that patients with tumors have a relatively insufficient protein intake and reduced protein synthesis rate, which leads to a continuous decrease in skeletal muscle protein [14]. Therefore, more attention should be paid to rational nutritional intake and physical exercise in postoperative patients with breast cancer.
Moreover, reports from other centers have shown that the changes in the ECW/TBW ratio not only estimate the body fluid content of patients but also have important predictive significance for the changes in patients’ conditions [15,16]. In the present study, the ECW/TBW ratio in breast cancer patients continued rising to 3 months after surgery. Therefore, to avoid the occurrence of postoperative lymphedema, it is necessary to monitor patients closely, including conducting risk evaluation.
Additionally, when performing a modified radical mastectomy or sentinel lymph node biopsy, the axillary lymph node dissection results in a lack of normal tissue covering. Being prone to adhesion with the surrounding tissues and scar contracture, the lymph pathway disruption between the affected upper limbs and cervical and thoracic regions will obstruct lymphatic reflux, leading to the leakage of normal lymph fluid from the capillary veins and capillary lymph vessels around the axillary veins. If the drainage time is too long, a lymphatic fistula can easily form, causing a large amount of protein-rich tissue fluid and lymphatic fluid to accumulate abnormally in the tissue space [17]. Although our data showed no significant changes in the upper limb circumference of patients with breast cancer at 3 months following surgery, the noted changes in the water content and bioelectrical impedance of the patients’ upper limbs are still worthy of attention. Compared with the preoperative value, the ECW/TBW ratio of healthy upper limbs of patients with breast cancer did not increase significantly at 7 days after surgery, but did increase significantly by 3 months after surgery. The ECW/TBW ratio of affected upper limbs increased significantly 7 days after surgery and was equivalent to the level 3 months after surgery; that is, the water content of healthy and affected upper limbs of patients with breast cancer increased 3 months after surgery, but the segmental water ratio was still within the normal range, which may be related to the establishment of collateral circulation of lymphedema and the redistribution of lymphatic fluid.
Furthermore, based on the principle that the impedance value of low-frequency currents can reflect the changes in extracellular fluid composition [18], we also examined the changes in bioelectrical impedance values of both upper limbs at low-frequency currents in the current study. The 1 kHz bioelectrical impedance of healthy upper limbs dropped continuously, while that of affected upper limbs increased first and then decreased, but was still lower than the preoperative level. The ratio of the healthy side to the affected side increased first and then decreased, but it was still higher than the preoperative level. The 5 kHz bioelectrical impedance of healthy upper limbs decreased significantly 7 days after surgery and was equivalent to the level 3 months after surgery. The 5 kHz bioelectrical impedance of affected upper limbs decreased first and then increased, but it was still lower than the preoperative level. The ratio of the healthy side to the affected side increased first and then decreased to the preoperative level. The change of bioelectrical impedance measured at the low-frequency and medium-frequency currents corresponds to the results of segmental water ratio, whereby the bioelectrical impedance value of both upper limbs of the patients with breast cancer decreased (i.e., water increased) by 3 months after surgery. The bioelectrical impedance value of the healthy side was higher than that of the affected side, and the bioelectrical impedance difference of both upper limbs was most prominent within 7 days after surgery, which decreased over time. The above data show that the ECW/TBW ratio of the patients with breast cancer increased in the whole body after surgery and the bioelectrical impedance of both upper limbs decreased, while the water content increased more in the affected side than in the healthy side. This may be related to the obstruction of lymphatic fluid reflux in the affected limb after surgery [16].
Since lymphedema after breast cancer surgery is related to the blockage and destruction of the lymphatic network, the 3-level lymphedema prevention system is important. This system includes primary, secondary, and tertiary prevention. Primary prevention is etiological prevention and includes 3 steps: (1) preoperative: a multi-specialist team works out the best treatment plan and enhances patient understanding of and attention to lymphedema (2) intraoperative: as many sentinel and axillary lymph nodes as possible should be reserved to reduce the obstruction of lymphatic fluid reflux; (3) postoperative: evaluate the patients’ knowledge of functional exercise, predict the lymphedema risk, and screen out high-risk patients [19]. Secondary prevention entails early detection, early diagnosis, and early treatment as follows: (1) early detection: actively evaluate the conditions of affected limbs of the patients with breast cancer from the first day following surgery to the discharge from the hospital to screen out patients with early lymphedema; (2) early diagnosis: the patients with potential lymphedema found in early screening should be promptly referred to the lymphedema clinic for further diagnosis; (3) early treatment: once lymphedema is diagnosed, patients should be informed of early treatment. Tertiary prevention is clinical prevention. During the treatment of lymphedema, it is necessary to monitor the therapeutic effect regularly. Patients tend to achieve better results under the supervision of lymphedema therapists in hospitals, while lack of supervision at home and poor compliance may lead to general or fluctuating effects. To improve the therapeutic effect, the follow-up network information platform, which links rehabilitation management between the hospital and a home-based internet platform, has become an important new channel to improve the management of lymphedema patients [20].
There were limitations in our study. The relatively small sample size and short follow-up period are the 2 main limitations. A well-designed multicenter study with larger patient enrollment and long-term follow-up should be performed to further explore the role of SMF-BIA in lymphedema prediction for postoperative patients with breast cancer.
Conclusions
We found that BIA monitoring is important for detecting changes in the body composition of breast cancer patients and we have provided more detailed information for the creation of individual nursing care plans. Further studies with long-term follow-up are urgently needed to establishment a lymphedema risk prediction model.
References
1. Miller KD, Siegel RL, Lin CC, Cancer treatment and survivorship statistics, 2016: Cancer J Clin, 2016; 66(4); 271-89
2. Smoot BJ, Wong JF, Dodd MJ, Comparison of diagnostic accuracy of clinical measures of breast cancer-related lymphedema: Area under the curve: Arch Phys Med Rehabil, 2011; 92(4); 603-10
3. Fu MR, Ridner SH, Armer J, Post-breast cancer. Lymphedema: Part 1: Am J Nurs, 2009; 109(7); 48-54 quiz 55
4. Cornish BH, Chapman M, Hirst C, Early diagnosis of lymphedema using multiple frequency bioimpedance: Lymphology, 2001; 34(1); 2-11
5. Jung M, Jeon JY, Yun GJ, Reference values of bioelectrical impedance analysis for detecting breast cancer-related lymphedema: Medicine (Baltimore), 2018; 97(44); e12945
6. Ward LC, Dylke ES, Kilbreath SL, Measurement of hand volume by bioelectrical impedance spectroscopy: Lymphat Res Biol, 2012; 10(2); 81-86
7. Fu MR, Axelrod D, Cleland CM, Symptom report in detecting breast cancer-related lymphedema: Breast Cancer (Dove Med Press), 2015; 7; 345-52
8. Hung YC, Bauer JD, Horsely P, Body composition following stem cell transplant: Comparison of bioimpedance and air-displacement plethysmography: Nutrition, 2014; 30(9); 1000-6
9. Barcikowski RS, Robey RR, Decisions in single group repeated measures analysis-statistical tests and 3 computer packages: Am Stat, 1984; 38(2); 148-50
10. Berlit S, Brade J, Tuschy B, Whole-body bioelectrical impedance analysis in assessing upper-limb lymphedema after breast cancer therapy: Anticancer Res, 2013; 33(10); 4553-56
11. De Fátima Guerreiro Godoy M, Silva EB, De Godoy JM, Bioimpedance to screen for abdominal fat in patients with breast cancer treatment-related lymphedema: Breast Dis, 2016; 36(2–3); 73-76
12. Milosavljevic SB, Pavlovic AP, Trrkovic SV, Influence of spinal and general anesthesia on the metabolic, hormonal, and hemodynamic response in elective surgical patients: Med Sci Monit, 2014; 20; 1833-40
13. Finnerty CC, Mabvuure NT, Ali A, The surgically induced stress response: J Parenter Enteral Nutr, 2013; 37(5 Suppl); 21s-29s
14. Qi Q, Fei X, Huang XInvestigation and study of body composition and sarcopenia in elderly patients with malignant tumors: Nursing Research, 2018; 32(23); 3682-86 [in Chinese]
15. Kim EJ, Choi MJ, Lee JH, Extracellular fluid/intracellular fluid volume ratio as a novel risk indicator for all-cause mortality and cardiovascular disease in hemodialysis patients: PLoS One, 2017; 12(1); e0170272
16. Fan S, Sayed RH, Davenport A, Extracellular volume expansion in peritoneal dialysis patients: Int J Artif Organs, 2012; 35(5); 338-45
17. Armer JM, Stewart BR, Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months: Lymphology, 2010; 43(3); 118-27
18. Fortin J, Habenbacher W, Heller A, Non-invasive beat-to-beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement: Comput Biol Med, 2006; 36(11); 1185-203
19. Sherman KA, Koelmeyer L, The role of information sources and objective risk status on lymphedema risk-minimization behaviors in women recently diagnosed with breast cancer: Oncol Nurs Forum, 2011; 38(1); E27-36
20. Wu Q, Kue J, Zhu X, Effects of nurse-led support via WeChat, a smartphone application, for breast cancer patients after surgery: A quasi-experimental study: Telemed J E Health, 2020; 26(2); 226-34
In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952