05 December 2020: Clinical Research
Correlation Between Speech Repetition Function and the Arcuate Fasciculus in the Dominant Hemisphere Detected by Diffusion Tensor Imaging Tractography in Stroke Patients with Aphasia
Hong Wang12ACDEG*, Shuqing Li3BCE, Yanhong Dai1BCD, Qiwei Yu4BEDOI: 10.12659/MSM.928702
Med Sci Monit 2020; 26:e928702
Abstract
BACKGROUND: Repetition disorder can be used as an important criterion for aphasia classification, and damaged arcuate fasciculus in the dominate hemisphere has been reported to be closely related to repetition disorder, but the underlying neurological mechanism remains unclear.
MATERIAL AND METHODS: Fifteen stroke patients with poststroke aphasia and 9 healthy controls were included in the study. The value of fractional anisotropy (FA) in the dominate arcuate fasciculus in stroke patients and healthy controls were measured using DTI. We also assessed their repetition dysfunction with the Aphasia Battery of Chinese (ABC) assessment and calculated the correlation between the FA values in the dominate arcuate fasciculus and ABC scores of word repetition and sentence repetition.
RESULTS: There was a moderate correlation between the total score of repetition evaluation and the FA value of injured arcuate fasciculus in the dominant hemisphere (r=0.551, P=0.033). We found no correlation between the score of word repetition and the FA value of injured arcuate fasciculus in the dominant hemisphere (r=0.330, P=0.230), but there was a strong correlation between the score of sentence repetition and the FA value of injured arcuate fasciculus in the dominant hemisphere (r=0.795, P≤0.001).
CONCLUSIONS: We found that unintegrated left arcuate fasciculus might be related to the repetition dysfunction after stroke, especially sentence repetition deficit, which suggests that sentence repetition evaluation could be used to indicate the integrity of the arcuate fasciculus in the dominant hemisphere after stroke.
Keywords: Aphasia, diffusion tensor imaging, Stroke, Cerebrum, Speech, white matter
Background
About 21–38% of acute stroke patients and 10–18% of chronic stroke patients are reported to have aphasia [1–3]. Repetition disorder is a common symptom of aphasia, such as Wernicke aphasia, Broca aphasia, conduction aphasia, and complete aphasia, and may have various degrees of repetition deficit. Moreover, a repetition disorder could be used as an important criterion for aphasia classification. However, the neurological mechanism underlying repetition disorder in stroke patients with aphasia remains unclear.
Arcuate fasciculus (AF) is a white-matter bundle connecting the frontal, temporal, and parietal cortical areas [4]. The direct pathway (known as the classic pathway, which connects the temporal cortex to the prefrontal cortex) has been linked to phonetics production [5], whereas the indirect pathway (including the anterior segment connecting the inferior parietal cortex and Broca’s area and a posterior segment connecting temporal and parietal regions) has been linked to verbal comprehension (semantic/phonological transcoding, complex syntactic processing) [6]. Most previous research has shown that individuals with impaired repetition have underlying damaged AF in the dominate hemisphere [7–9]. Lichtheim et al. [10] believed that a broken connection between the auditory speech center and the spoken expression center was the physiological basis of the repetition dysfunction and that the main structure connecting the 2 language centers is the AF in the dominant hemisphere. Other researches have shown that preservation of the left AF is associated with positive language outcome [11,12]. However, occasional case reports show that this association between the AF and repetition performance has not been consistently upheld. Selnes et al. reported a patient with dominant hemisphere AF lesion presenting normal repetition performance by diffusion tensor imaging (DTI) [13]. Also, repetition is a complex process in which word repetition and sentence repetition involve different pathways. For example, after speech therapy, a stroke patient with aphasia had significant word repetition function improvement, while sentence repetition improvement was not obvious [14]. Thus, whether repetition deficits are related to a lesion of the AF remains an open question. Furthermore, if there is a correlation between these, which subtypes of the repetition performance (word repetition or sentence repetition) are more easily affected?
DTI, as an effective neuroimaging technique for identification of white-matter fibers in the living brain, can be used to evaluate the integrity of AF in the dominant hemisphere [15]. In this study, we attempted to explore the relationship between the integrity of AF in the dominant hemisphere and repetition function (not only the whole repetition performance, but also including the word repetition and sentence repetition) using DTI technology in poststroke aphasia patients. We also aimed to identify clinical predictors for aphasia stroke patients.
Material and Methods
SUBJECTS:
The stroke patients with poststroke aphasia (the aphasia group) were recruited from the Department of Rehabilitation and the Department of Neurology in the First Affiliated Hospital of Jinan University between 2015 and 2016.
Inclusion criteria were: 1) stroke, as defined by the classification and diagnosis of cerebrovascular diseases in the Fourth National Conference in China on Cerebrovascular Diseases (1995) [16], with DTI examination clearly indicating infarction or bleeding lesions in the AF in the dominant hemisphere (left hemisphere), but not in the nondominant hemisphere; 2) patients diagnosed with aphasia with repetition dysfunction based on the Aphasia Battery of Chinese (ABC) assessment [17]; 3) first-ever stroke(s) within 6 months of onset; 4) between 40 and 75 years old, with education of more than 6 years; 5) all patients were right-handed, as assessed by the Edinburgh Handedness Questionnaire [18]; 6) patients who were able to complete the ABC assessment and MRI image scanning.
Nine healthy right-handed subjects with age and years of education matched with the patient group participated in this study as the healthy control (HC) group (3 females, 6 males; mean age, 53.4±9.3 years; demographic information shown in Table 1), who could complete the MRI image scanning and had no brain organic disease, history of psychotropic drug abuse, speech disorders, or cognitive impairments.
Our study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Jinan University (No. 2014-022), and informed consent was obtained from all subjects or their families.
REPETITION FUNCTION ASSESSMENT:
The ABC was used to evaluate the language function of 15 patients in our study, which is based on the cultural specificity of the Chinese and the Western Aphasia Battery (WAB) [19]. The reliability and validity testing were well performed in the Chinese population [20]. The ABC includes 9 subdomains for investigating the speech abilities involved in question and answer, comprehension, repetition, naming, reading, writing, structure and space, ability for use, and calculation. The repetition sub-item includes 2 parts: word repetition, and sentence repetition. The scores of total repetition performance (100 points), word repetition performance (24 points), and sentence repetition performance (76 points) were used as observation indicators in our study. All assessments were completed by the same leading speech therapist in our hospital.
DTI DATA ACQUISITION AND PROCESSING:
DTI was acquired on a 3.0 T Magnetic Resonance Imaging System (General Electric Discovery 750), as well as T1-weighted images and T2-weighted images. Scanning parameters were as follow: T1-weighted images (echo time, 30 ms; rotation angle, 15°; flip time, 4500 ms; slice thickness, 1 mm; voxel, 0.93×0.93×1 mm3; number of slices, 164), T2-weighted images (repetition time, 8000 ms; echo time, 165 ms; slice thickness/slice spacing, 1 mm/1.5 mm); DTI (repetition time/echo time, 5000 ms/68.0 ms; reconstructed to matrix, 128×128; field of view, 25.6×25.6 cm2; number of excitations, 1; b=1000 s/mm2; 25 dispersion-sensitive gradients; slice thickness/slice spacing=3 mm/0 mm).
Functool software (version 9.4.05a, General Electric Medical System) was used for DTI data processing, including three-dimensional reconstruction and the fractional anisotropy (FA) value measurement of the AF on the left hemisphere. The measurement site was placed underneath the inferior limbic gyrus (the position of the AF corner) (Figure 1A). Two regions of interest (ROIs) were selected to track left AF, ROI 1 on the posterior temporal lobe (Figure 1B), and ROI 2 on the posterior parietal lobe in the superior longitudinal fasciculus (Figure 1C) [21]. The area of ROI is 32 mm2. The whole process was performed by the same advanced radiologist. The FA values of the AF in each subject were measured 3 times, and the average was used in the analyses.
STATISTICAL ANALYSIS:
The data were statistically analyzed using SPSS 20.0 software. The continuous variables of 2 groups (the age, educational years, and FA value of AF in the dominant hemisphere) were measured by two-sample
Results
Fifteen stroke patients with aphasia (2 females and 13 males; mean age, 52.73±11.71 years) and 9 health subjects (3 females and 6 males; mean age, 53.44±9.38 years) were recruited to the aphasia group and control groups, respectively. There were no significant differences in age (
For the aphasia group, the speech repetition evaluation of ABC assessment (including word repetition scores, sentence repetition scores, and total repetition scores), the stroke types and injured brain regions, and the FA values of the AF in dominate hemisphere of 15 aphasia patients are shown in Table 2. There was a moderate correlation between the total score of repetition evaluation and the FA value of injured AF in the dominant hemisphere (r=0.551,
Discussion
LIMITATIONS:
This study has a few limitations. The sample size was relatively small due to a high examination fee for the DTI data collection. Moreover, there was heterogeneity among stroke patients with aphasia recruited in the patient group. Finally, it is hard to completely rule out the effect of eventual physiological improvement on speech performances. Future studies need to recruit more subjects to the patient group and perform hierarchical statistical analysis based on the type of aphasia or degree of repetition deficit.
Conclusions
We found that injured AF in the dominant hemisphere might be related to repetition dysfunction, especially sentence repetition deficit. Our results suggest that facilitation of the AF in the dominant hemisphere could be an important strategy in neuro-rehabilitation for stroke patients with aphasia, so that the patients with the sentence repetition deficit might benefit more than the patients with word repetition deficit.
Figures
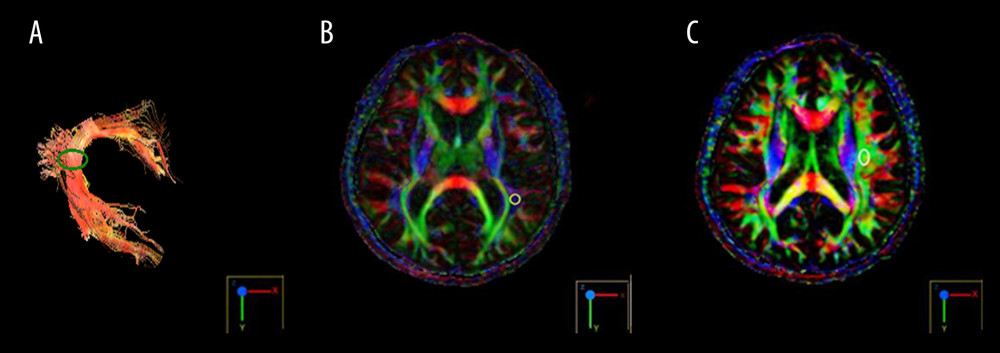 Figure 1. Diffusion tensor imaging of the arcuate fasciculus in the normal subject. (A) The FA value measurement point of the arcuate fasciculus (green circle); (B) The ROI 1 position (white circle); (C) The ROI 2 position (white circle). FA – fractional anisotropy; ROI – region of interest.
Figure 1. Diffusion tensor imaging of the arcuate fasciculus in the normal subject. (A) The FA value measurement point of the arcuate fasciculus (green circle); (B) The ROI 1 position (white circle); (C) The ROI 2 position (white circle). FA – fractional anisotropy; ROI – region of interest. 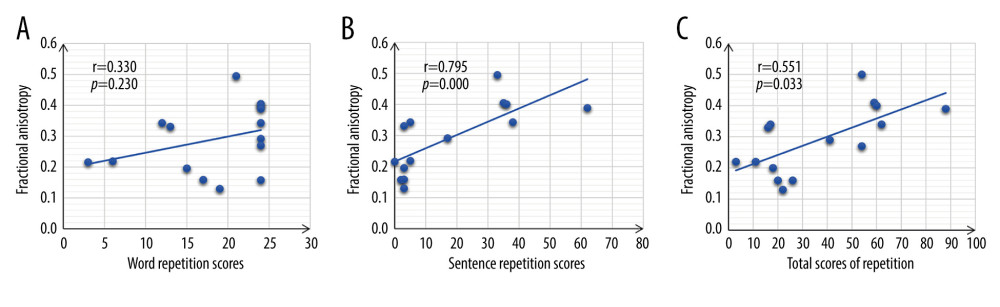 Figure 2. Correlation of DTI FA values of arcuate fasciculus and repetition outcomes. (A) The correlation of DTI FA values of the arcuate fasciculus and the word repetition scores. (B) shows the correlation of DTI FA values of the arcuate fasciculus in the dominant hemisphere and the sentence repetition scores. (C) The correlation of DTI FA values of arcuate fasciculus and the total scores of repetitions. R values (r) and p-values of the regression model are shown in the figures. DTI – diffusion tensor imaging; FA – fractional anisotropy.
Figure 2. Correlation of DTI FA values of arcuate fasciculus and repetition outcomes. (A) The correlation of DTI FA values of the arcuate fasciculus and the word repetition scores. (B) shows the correlation of DTI FA values of the arcuate fasciculus in the dominant hemisphere and the sentence repetition scores. (C) The correlation of DTI FA values of arcuate fasciculus and the total scores of repetitions. R values (r) and p-values of the regression model are shown in the figures. DTI – diffusion tensor imaging; FA – fractional anisotropy. References
1. Berthier ML, Poststroke aphasia: Epidemiology, pathophysiology and treatment: Drugs Aging, 2005; 22(2); 163-82
2. Engelter ST, Gostynski M, Papa S, Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis: Stroke, 2006; 37(6); 1379-84
3. Elman RJ, Bernstein-Ellis E, The efficacy of group communication treatment in adults with chronic aphasia: J Speech Lang Hear Res, 1999; 42(2); 411-19
4. Sierpowska J, Gabarrós A, Fernandez-Coello A, Words are not enough: Nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection: J Neurosurg, 2016; 126(2); 435-45
5. Catani M, Jones DK, Ffytche DH, Perisylvian language networks of the human brain: Ann Neurol, 2005; 57(1); 8-16
6. Friederici AD, Gierhan SME, The language network: Curr Opin Neurobiol, 2013; 23(2); 250-54
7. Berthier ML, Starkstein SE, Leiguarda R, Transcortical aphasia. Importance of the nonspeech dominant hemisphere in language repetition: Brain, 1991; 114(Pt 3); 1409-27
8. De-Torres I, Dávila G, Berthier ML, Repeating with the right hemisphere: Reduced interactions between phonological and lexical-semantic systems in crossed aphasia?: Front Hum Neurosci, 2013; 7(6); 675
9. Chernoff BL, Teghipco A, Garcea FE, Reorganized language network connectivity after left arcuate fasciculus resection: A case study: Cortex, 2020; 123; 173-84
10. Lichtheim L, On aphasia: Brain, 1885; 7(7); 433-84
11. Yu QW, Wang H, Li S, Dai Y, Predictive role of subcomponents of the left arcuate fasciculus in prognosis of aphasia after stroke A retrospective observational study: Medicine, 2019; 98(23); e15775
12. Caverzasi E, Hervey-Jumper SL, Jordan KM, Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas: J Neurosurg, 2016; 125(1); 33-45
13. Selnes OA, Van Zijl PCM, Barker PB, MR diffusion tensor imaging documented arcuate fasciculus lesion in a patient with normal repetition performance: Aphasiology, 2002; 16(9); 897-902
14. Li SQ, Wang H, Chen ZM, Dai YH, A case report of aphasia patient with repetition restoration: J Rehabilitation Medicine, 2017; 27(2); 49
15. Alexander AL, Lee JE, Lazar M, Field AS, Diffusion tensor imaging of the brain: Neurotherapeutics, 2007; 4(3); 316-29
16. The 4 National Conference on Cerebrovascular Diseases of the Chinese Medical Association, Diagnostic points of various cerebrovascular diseases: Chinese Journal of Neurology, 1996; 29(6); 2
17. Liu L, Luo X-G, Dy C-L, Characteristics of language impairment in Parkinson’s disease and its influencing factors: Transl Neurodegener, 2015; 4(1); 2
18. Oldfield RC, The assessment and analysis of handedness: The Edinburgh inventory: Neuropsychologia, 1971; 9(1); 97-113
19. Shewan CM, Kertesz A, Reliability and validity characteristics of the Western Aphasia Battery (WAB): J Speech Hear Disord, 1980; 45(3); 308-24
20. Gao SR, Zhao SL, A standardization research of the aphasia battery of Chinese: Chinese Mental Health Journal, 1992; 6; 125-28
21. Henning Stieglitz L, Seidel K, Wiest R, Localization of primary language areas by arcuate fascicle fiber tracking: Neurosurgery, 2012; 70(1); 56-64 discussion 64–65
22. Tábuas-Pereira Miguel, Beato-Coelho J, Ribeiro J, Single word repetition predicts long-term outcome of aphasia caused by an ischemic stroke: J Stroke Cerebrovasc Dis, 2020; 29(2); 104566
23. Abo M, Senoo A, Watanabe S, Language-related brain function during word repetition in post-stroke aphasics: Neuroreport, 2004; 15(12); 1891-94
24. Dehaene-Lambertz G, Dehaenne S, Anton J-L, Functional segregation of cortical language areas by sentence repetition: Hum Brain Mapp, 2006; 27(5); 360-71
25. Klem M, Melby-Lervåg M, Hagtvet B, Sentence repetition is a measure of children’s language skills rather than working memory limitations: Dev Sci, 2015; 18(1); 146-54
26. Wang Y, Relations between the sides of linguistic cerebral dominance and manuality in Chinese aphasics: Chin Med J (Engl), 1996; 109(7); 572-75
27. Marshall CR, Hardy CJD, Volkmer A, Primary progressive aphasia: A clinical approach: J Neurol, 2018; 265(6); 1474-90
28. Niogi SN, Mukherjee P, Diffusion tensor imaging of mild traumatic brain injury: J Head Trauma Rehabil, 2010; 25(4); 241-55
29. Tak HJ, Jang SH, Relation between aphasia and arcuate fasciculus in chronic stroke patients: BMC Neurol, 2014; 14; 46
30. Kim SH, Lee DG, You H, The clinical application of the arcuate fasciculus for stroke patients with aphasia: A diffusion tensor tractography study: Neurorehabilitation, 2011; 29(3); 305-10
31. Koay CG, Chang LC, Carew JD, A unifying theoretical and algorithmic framework for least squares methods of estimation in diffusion tensor imaging: J Magn Reson, 2006; 182(1); 115-25
32. Gullick MM, Booth JR, The direct segment of the arcuate fasciculus is predictive of longitudinal reading change: Dev Cogn Neurosci, 2015; 13; 68-74
33. Turkeltaub PE, Messing S, Norise C, Hamilton RH, Are networks for residual language function and recovery consistent across aphasic patients?: Neurology, 2011; 76(20); 1726-34
34. Heiss WD, Kessler J, Thiel A, Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia: Ann Neurol, 1999; 45(4); 430-38
35. Li SQ, Wang H, Chen ZM, Dai YH, A Case report of aphasia repetition function recovery: Rehabilitation Medicine, 2017; 27(2); 49-52
36. Cao Y, The characteristics of the working memory in patients with Chinese aphasia: Journal of Audiology and Speech Pathology, 2017; 25(2); 143-48
37. Li X, Jiao J, Jibiki I, Cerebral activation areas during repetition tests with functional MRI in normal adults: Journal of China-Japan Friendship Hospital, 2013; 27(5); 263-65
38. Wang X, Zhao R, Zevin JD, Yang J, The neural correlates of the interaction between semantic and phonological processing for Chinese character r: Front Psychol, 2016; 7; 947
Figures
 Figure 1. Diffusion tensor imaging of the arcuate fasciculus in the normal subject. (A) The FA value measurement point of the arcuate fasciculus (green circle); (B) The ROI 1 position (white circle); (C) The ROI 2 position (white circle). FA – fractional anisotropy; ROI – region of interest.
Figure 1. Diffusion tensor imaging of the arcuate fasciculus in the normal subject. (A) The FA value measurement point of the arcuate fasciculus (green circle); (B) The ROI 1 position (white circle); (C) The ROI 2 position (white circle). FA – fractional anisotropy; ROI – region of interest. Figure 2. Correlation of DTI FA values of arcuate fasciculus and repetition outcomes. (A) The correlation of DTI FA values of the arcuate fasciculus and the word repetition scores. (B) shows the correlation of DTI FA values of the arcuate fasciculus in the dominant hemisphere and the sentence repetition scores. (C) The correlation of DTI FA values of arcuate fasciculus and the total scores of repetitions. R values (r) and p-values of the regression model are shown in the figures. DTI – diffusion tensor imaging; FA – fractional anisotropy.
Figure 2. Correlation of DTI FA values of arcuate fasciculus and repetition outcomes. (A) The correlation of DTI FA values of the arcuate fasciculus and the word repetition scores. (B) shows the correlation of DTI FA values of the arcuate fasciculus in the dominant hemisphere and the sentence repetition scores. (C) The correlation of DTI FA values of arcuate fasciculus and the total scores of repetitions. R values (r) and p-values of the regression model are shown in the figures. DTI – diffusion tensor imaging; FA – fractional anisotropy. Tables
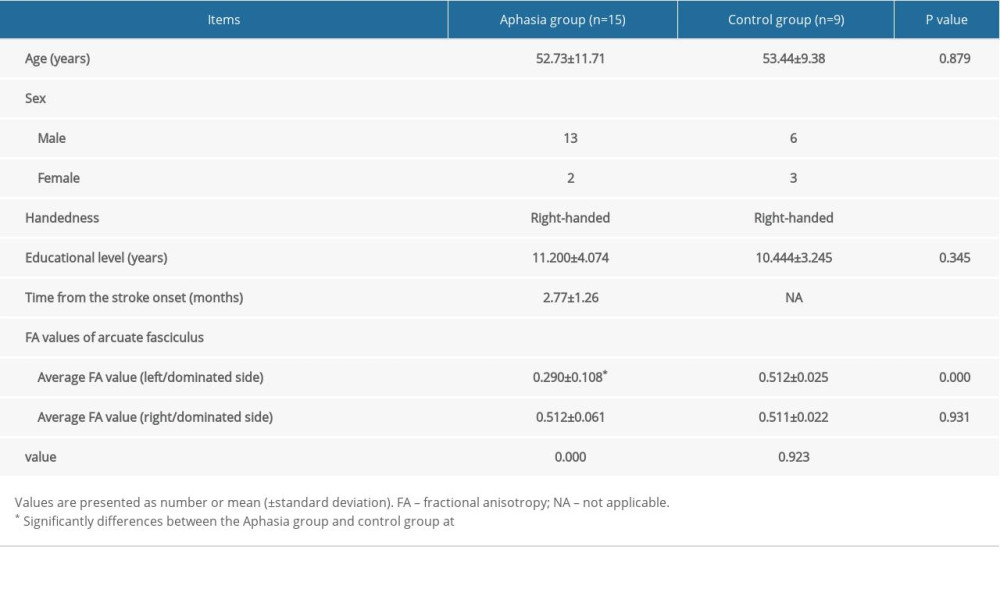 Table 1. Subject demographics and diffusion tensor imaging data of the arcuate fasciculus.
Table 1. Subject demographics and diffusion tensor imaging data of the arcuate fasciculus.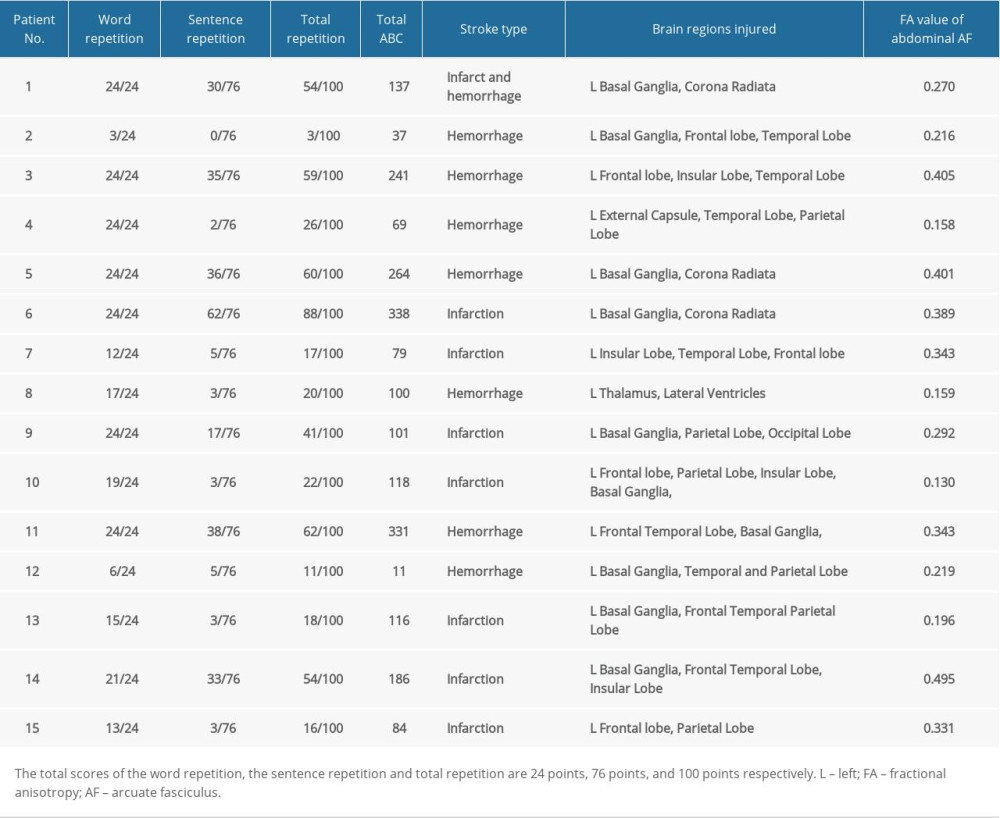 Table 2. Repetition evaluation (the subtest score of the Aphasia Battery in Chinese) and diffusion tensor imaging data of the arcuate fasciculus.
Table 2. Repetition evaluation (the subtest score of the Aphasia Battery in Chinese) and diffusion tensor imaging data of the arcuate fasciculus. Table 1. Subject demographics and diffusion tensor imaging data of the arcuate fasciculus.
Table 1. Subject demographics and diffusion tensor imaging data of the arcuate fasciculus. Table 2. Repetition evaluation (the subtest score of the Aphasia Battery in Chinese) and diffusion tensor imaging data of the arcuate fasciculus.
Table 2. Repetition evaluation (the subtest score of the Aphasia Battery in Chinese) and diffusion tensor imaging data of the arcuate fasciculus. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








