08 January 2021: Clinical Research
Respiratory Variations in Peak Peripheral Artery Velocities and Waveforms for Rapid Assessment of Fluid Responsiveness in Traumatic Shock Patients
Qian Zhang1E, Xiu-Rong Shi2E, Yi Shan3D, Jian Wan1AG*, Xuan Ju1B, Xi Song4C, Conghui Fan4C, Xinyuan Lu4D, Jie Sun4D, Liwei Duan2D, Zhaofen Lin2F, Jinlong Liu5CDOI: 10.12659/MSM.928804
Med Sci Monit 2021; 27:e928804
Abstract
BACKGROUND: This study aimed to assess the correlation between the variability of the end-inspiratory and end-expiratory blood flow waveform and fluid responsiveness (FR) in traumatic shock patients who underwent mechanical ventilation by evaluating peripheral arterial blood flow parameters.
MATERIAL AND METHODS: A cohort of 60 patients with traumatic shock requiring mechanical ventilation-controlled breathing received ultrasound examinations to assess the velocity of carotid artery (CA), femoral artery (FA) and brachial artery (BA). A rehydration test was performed in which of 250 mL of 0.9% saline was administered within 30 min between the first and second measurement of cardiac output by echocardiography. Then, all patients were divided into 2 groups, a responsive group (FR+) and a non-responsive group (FR–). The velocity of end-inspiratory and end-expiratory peripheral arterial blood flow of all patients was ultrasonically measured, and the variability were measured between end-inspiratory and end-expiratory.
RESULTS: The changes in the end-inspiratory and end-expiratory carotid artery blood flow velocity waveforms of the FR+ groups were significantly different from those of the FR– group (P<0.001). A statistically significant difference in ΔVmax (CA), ΔVmax (BA), and ΔVmax (FA) between these 2 groups was found (all P<0.001). The ROC curve showed that DVmax (CA) and ΔVmax (BA) were more sensitive values to predict FR compared to ΔVmax (FA). The sensitivity of ΔVmax (CA), ΔVmax (FA), and ΔVmax (BA) was 70.0%, 86.7%, and 93.3%, respectively.
CONCLUSIONS: The study showed that periodic velocity waveform changes in the end-inspiratory and end-expiratory peripheral arterial blood flow can be used for quick assessment of fluid responsiveness.
Keywords: Blood Volume, Carotid Arteries, Shock, Traumatic, Ultrasonography, Blood Flow Velocity, Carotid-Femoral Pulse Wave Velocity, Femoral Artery, Fluid Therapy, Respiration, Respiration, Artificial
Background
The emergency plan for patients with traumatic shock requires effective resuscitation in the shortest possible time to ensure the function of important organs, thereby improving the success rate of treatment and decreasing morbidity. However, after the initial stage of resuscitation, patients with critical illness have a nearly 50% probability of being in a volume-responsive state. While insufficient volume is harmful, excessive volume expansion (VE) will also cause tissue edema and increase mortality [1,2]. Therefore, it is critical to determine whether a patient has fluid responsiveness (FR) before VE. There are numerous FR indicators. In terms of accuracy, FR should be determined by cardiac output (CO) changes in critically ill patients with unstable hemodynamics. CO or stroke volume (SV) increases of more than 10–15% after VE is regarded as the FR criterion standard [3,4].
CO or SV were often determined by using pulse-induced contour cardiac output (PICCO). However, it was a time-consuming and labor-intensive method which requires surgery and therefore is not suitable for patients who need emergency rescue. Nowadays, the increasing availability of point-of-care ultrasound (POCUS) has greatly affected the critical care field. POCUS, in comparison, has been proved to be a non-invasive and cost-effective method to evaluate hypotension, volume status and FR [5]. The application of POCUS can have a positive impact on rescue results [6]. Arterial pressure waveform and pressure values periodically increase and decrease with intermittent inhalation and exhalation during positive-pressure ventilation due to the presence of cardiopulmonary interaction [7]. Such change is particularly significant when fluid is insufficient [7], and thus changes in the peripheral arterial waveform may be used to assess FR. Peripheral arterial waveform has been commonly used in hemodynamic monitoring [8,9]. Doppler velocity waveforms of peripheral arterial blood flow, such as the carotid artery (CA), femoral artery (FA), and brachial artery (BA), can be obtained by POCUS. Therefore, the present study aimed to assess the correlation between the variability of the end-inspiratory and end-expiratory blood flow waveform and FR in traumatic shock patients who underwent mechanical ventilation by evaluating peripheral arterial blood flow parameters.
Material and Methods
PATIENTS:
A cohort of 60 patients with traumatic hemorrhagic shock (distributive shock that can accompany hypovolemic shock was excluded) requiring mechanical ventilation-controlled breathing was enrolled in this prospective study (Figure 1). Factors leading to injuries included, for example, traffic injuries, fall injuries, mechanical injuries, and fall injuries. All of the patients were admitted to our Emergency Trauma Center between 1 January 2018 and 31 May 2019. Enrolled patients were further divided into 2 groups: a responsive group (FR+) and a non-responsive group (FR−). The FR+ group consisted of 30 patients (20 males and 10 females), with an average age of (51.27±16.07) years and an average Injury Severity Score (ISS) of (20±4.8). The FR− group consisted of 19 males and 11 females, with an average age of (55±16.07) years and an average ISS of (20±5.6). The patients were enrolled according to the following inclusion criteria: (1) all patients met the diagnostic criteria for trauma combined with shock, defined as systolic blood pressure <90 mmHg, pulse pressure difference <20 mmHg, or systolic blood pressure drop from baseline ≥40 mmHg in patients with previous hypertension; (2) presence of pale skin, cold perspiration, weak pulse, shortness of breath, oliguria or anuria, changes in consciousness, and invasive mechanical ventilation were also required; and (3) controlled ventilation mode was used, with a tidal volume ≥8 ml/kg and positive end-expiratory pressure (PEEP) ≤5 mmHg. The exclusion criteria were: (1) poor-quality chest ultrasound image results due to chest trauma and other factors; (2) injury of the explored peripheral arteries, peripheral arterial plaque formation, or vascular stenosis; (3) arrhythmia or severe cardiac dysfunction; (4) underlying heart diseases, such as heart valve diseases; and (5) agitation that prevented the patient from cooperating with the examination. Patients undergoing mechanical ventilation were required to have a PEEP >5 mmHg or a tidal volume <8 ml/kg to be considered.
STUDY PROTOCOL:
All 60 traumatic shock patients received POCUS exams. POCUS was performed using the Mindray M9 Diagnostic Ultrasound System (Mindray Co, Shenzhen, China) equipped with a linear array probe (8–12 Hz). To assess carotid artery velocity, the probe was placed above the right clavicle and gently moved until an obvious cross-section of the carotid artery was found. The patient was asked to assume supine position with the head tilted toward the right side to fully expose the right sternocleidomastoid. Afterwards, the probe was placed 2 cm above the inside of the cubital fossa and gently moved until it reached an obvious cross-section of the brachial artery for brachial artery exploration. Meanwhile, the patient’s right arm was gently externally rotated. To assess the velocity of femoral arterial, the operator placed the probe at the groin and gently moved until it reached an obvious cross-section of the femoral artery when the right thigh of the patient was slightly abducted. The rotating probe was placed on the long axis of the abovementioned peripheral arteries, and the angle between the sampling line and the blood flow was adjusted to <60°. Pulse-wave Doppler (PW) mode was used to collect the end-inspiratory and end-expiratory Vmax and TAMAX for 3 respiratory cycles, and the average values were obtained.
All 60 patients received the first echocardiography to obtain CO. Then, a rehydration test was performed in which 250 mL of 0.9% saline was administered within 10 min. Afterwards, all patients received the second echocardiography for CO assessment. If the CO changes were greater than 15%, patients were grouped into the FR+ group. A less than 15% increase in CO was considered as negative (FR−). Regarding the CO measurement, a phased-array probe (2.5–4 MHz) was used to obtain an apical 5-chamber view of the heart. The specimen container was placed at the aortic valve ring, and the systolic aortic annulus diameter (D) and aortic velocity-time integral (VTI) were measured. The CO measurement parameters included the area of the annulus (AAO)=π×(D/2) 2, the stroke volume (SV)=VTI×AAO, and the product of SV and heart rate. To reduce operator error, both operators were trained and certified by the Chinese Critical Ultrasound Study Group (CCUSG).
Patients with significant periodic inspiratory waveforms and expiratory waveform changes were categorized into the subgroup with waveform changes (WF+), and those with no periodic waveform changes or no significant changes were categorized into the subgroup with no waveform changes (WF−). The determination of the significance of periodic inspiratory waveforms and expiratory waveforms changes were confirmed by 2 doctors with more than 5 years’ experience in POCUS. If any disagreement occurred, a third senior doctor with more than 20 years of experience in POCUS was consulted.
The velocity of end-inspiratory and end-expiratory peripheral arterial blood flow of all patients was ultrasonically measured, and the variability was measured between end-inspiratory and end-expiratory. In addition, the number of cases with a periodic waveform or a high/low level of variability in the FR+ group and the significance of the effect of ΔVmax on the FR evaluation of the subgroup with waveform change were determined.
DATA ANALYSIS:
The SPSS 17.0 statistical software package (SPSS Inc, Chicago, IL, USA) was used. The end-inspiratory and end-expiratory peak flow variability in the peripheral artery (ΔVmax) was compared between the FR+ group and FR− group.
A normal distribution test of measurement data indicated that the measurement data of all groups in this study were normally distributed. A paired comparison was performed using a paired
Results
BASELINE CHARACTERISTICS OF ENROLLED PATIENTS:
There were no statistically significant differences in sex, age, body weight, cause of injury, and ISS score between the patients in the FR+ group and those in the FR− group (all P values >0.05) (Table 1).
CORRELATION ANALYSIS OF WAVEFORM MORPHOLOGY CHANGES IN THE FR+ AND FR− GROUPS:
In the CA–FR+ group, 18 patients (accounting for 60% of the CA–FR+ group) showed significant periodic end-inspiratory and end-expiratory velocity waveform morphology changes or high/low waveform changes (Figure 2). Twelve patients were categorized in the WF− group. In the CA–FR− group, 4 patients were categorized in the WF+ group, while 26 patients presented no waveform changes or morphological changes (Figure 3). There was a statistically significant difference between the 2 groups (P<0.001) (Table 2).
Eight patients, accounting for 26.7% of the FA–FR+ group, showed significant end-inspiratory and end-expiratory velocity waveform morphology changes or high/low waveform changes, and there were no significant velocity waveform changes in 22 patients. Five patients in the FA–FR− group showed significant end-inspiratory and end-expiratory velocity waveform changes, while 25 patients showed no waveform changes or morphological changes. However, there was no statistically significant difference between these 2 groups (P>0.05) (Table 2).
Nine patients (accounting for 30% of the BA–FR+ group) were categorized in the WF+ group, while 21 patients showed no significant velocity waveform changes. In the BA–FR− group, 2 patients showed significant end-inspiratory and end-expiratory velocity waveform changes, while 28 patients showed no waveform changes or morphological changes. A significant difference was found between these 2 groups (P<0.05) (Table 2). These results suggest that the velocity waveforms of the CA and BA showed periodic morphology or high/low changes that can be used for the assessment of FR, while that of the FA cannot be used to assess FR.
VARIATIONS OF PERIPHERAL ARTERIAL MAXIMUM FLOW VELOCITY AND CORRELATION ANALYSIS WITH FR:
Table 3 presents the maximum flow velocity of CA, BA, and FA of patients in the FR+ group and FR− group. The ΔVmax (CA) of patients in the FR+ group and FR− group was 17.01±11.15 and 4.12±13.27, respectively. A statistically significant difference in ΔVmax (CA) between these 2 groups was found (P<0.001). Similarly, significant differences in ΔVmax (BA) and ΔVmax (FA) between these 2 groups were also found (both P <0.001). The ΔVmax (BA) of patients in the FR+ group and FR− group was 12.86±6.26, and 6.35±6.56, respectively. According to the ROC analysis, ΔVmax (CA) and ΔVmax (BA) were more sensitive values to predict FR compared to ΔVmax (FA) (Figure 4). The AUC of ΔVmax (CA), ΔVmax (FA), and ΔVmax (BA) was 0.803 (95% CI: 0.692–0.914), 0.788 (95% CI: 0.670–0.906), and 0.822 (95% CI: 0.709–0.935), respectively (Table 4).
In the FR+ Group, we further compared the variability in the maximum velocity between the WF+ Subgroup and WF− Subgroup. Both ΔVmax (CA) and ΔVmax (BA) showed the capability to predict FR between WF+ Subgroup and WF− Subgroup (both P values <0.05) (Table 5). ΔVmax (CA) in the WF+ Subgroup and WF− Subgroup was 19.28±10.29, and 13.60±11.96, respectively (P<0.05). When ΔVmax (CA) in the WF+ Subgroup was greater than 12.57%, the velocity waveform or morphology of the CA had significant periodic changes, with a sensitivity of 77.8% and specificity of 66.7%. ΔVmax (BA) in the WF+ Subgroup and WF− Subgroup was 18.03±6.31, and 10.65±4.87, respectively (P<0.05). In contrast, ΔVmax (FA) was not able to be used to predict FR between WF+ Subgroup and WF− Subgroup (P<0.05) (Table 4).
Discussion
In the field of intensive care, POCUS has become a rapid diagnostic technology that must be mastered. With the popularization and development of POCUS, its scope of application has also expanded rapidly. Ultrasound has even gradually replaced the pulmonary artery catheter and was considered the most effective diagnostic tool for understanding hemodynamic instability and the causes of shock [10]. Although PICCO has been applied for evaluating CO, the limitations of being invasive and time-consuming were also obvious [11]. In clinical practice, echocardiography was favored for CO assessment regarding FR and achieved almost same level of accuracy as that of PICCO [10]. However, it also has limitations [12,13]. For example, to reduce errors, skilled personnel must conduct testing, and such personnel are not easy to secure in the emergency room. Therefore, it is necessary to find a simple and accurate evaluation method that can reflect the change in CO or SV. CO and SV, together with left ventricular end-diastolic area, aortic artery peak velocity variability, peripheral arterial peak velocity variability, inferior vena cava diameter and collapse index, and PPV/SVV, were commonly-used ultrasound indicators for assessing FR. Among these indicators, monitoring of peripheral arteries might be the easiest method [2,14]. McGregor et al. [15] found that carotid artery monitoring was the most easily accepted of several FR assessment methods in the emergency room. The feasibility of carotid artery monitoring was 87.4%. Antiperovitch et al. have used variations of carotid peak velocity to observe the FR of patients, and found that it can to some extent reflect FR [16]. Other FR indicators, such as the passive leg-raising test, are simple and unaffected by spontaneous breathing, and there is no risk of fluid overload. However, the application of these methods is limited in trauma patients due to the impact of injury.
The presence of cardiopulmonary interaction results in arterial pressure waveform and pressure values that periodically increase and decrease with intermittent inhalation and expiration during positive-pressure ventilation, and this change is particularly significant when volume is insufficient. This is the “abnormal phenomenon of reverse pulse” [7]. Desgranges et al. [17] found that when the aortic artery peak velocity variability (ΔVpeak AO) in patients undergoing mechanical ventilation was 13.5%, the sensitivity to predict FR was 84.0%, and the specificity was 72.7%. Morparia et al. [18] suggested that if ΔVpeak AO was ≥12.3%, the patient may have positive FR. The acquisition of ΔVpeak AO requires an accurate and clear assessment of aortic spectra through transthoracic ultrasound, which is largely dependent on more precise operations and equipment. Compared with examinations of the aorta, the use of a linear array probe for peripheral arterial blood flow velocity assessment and waveform detection is simpler, and data are easy to obtain.
For patients with traumatic shock, it is vital to shorten the evaluation time. The present study showed that there was a good correlation between the velocity waveform variability in end-inspiratory and end-expiratory peripheral arterial blood flow and FR (all
Some limitations of our study should be noted. We performed a rapid carotid artery scan in patients with traumatic shock who were undergoing mechanical ventilation. The waveform changes can be used to determine whether a patient has volume responsiveness. However, this was a preliminary study, and the results were limited to patients with mechanical ventilation-controlled breathing. In addition, a multicenter investigation with larger samples should be conducted to confirm the results.
Conclusions
In patients with traumatic shock undergoing mechanical ventilation, significant periodic velocity waveform changes in the end-inspiratory and end-expiratory peripheral arterial blood flow can be used for a quick assessment of fluid responsiveness, especially in the carotid artery and brachial artery.
Figures
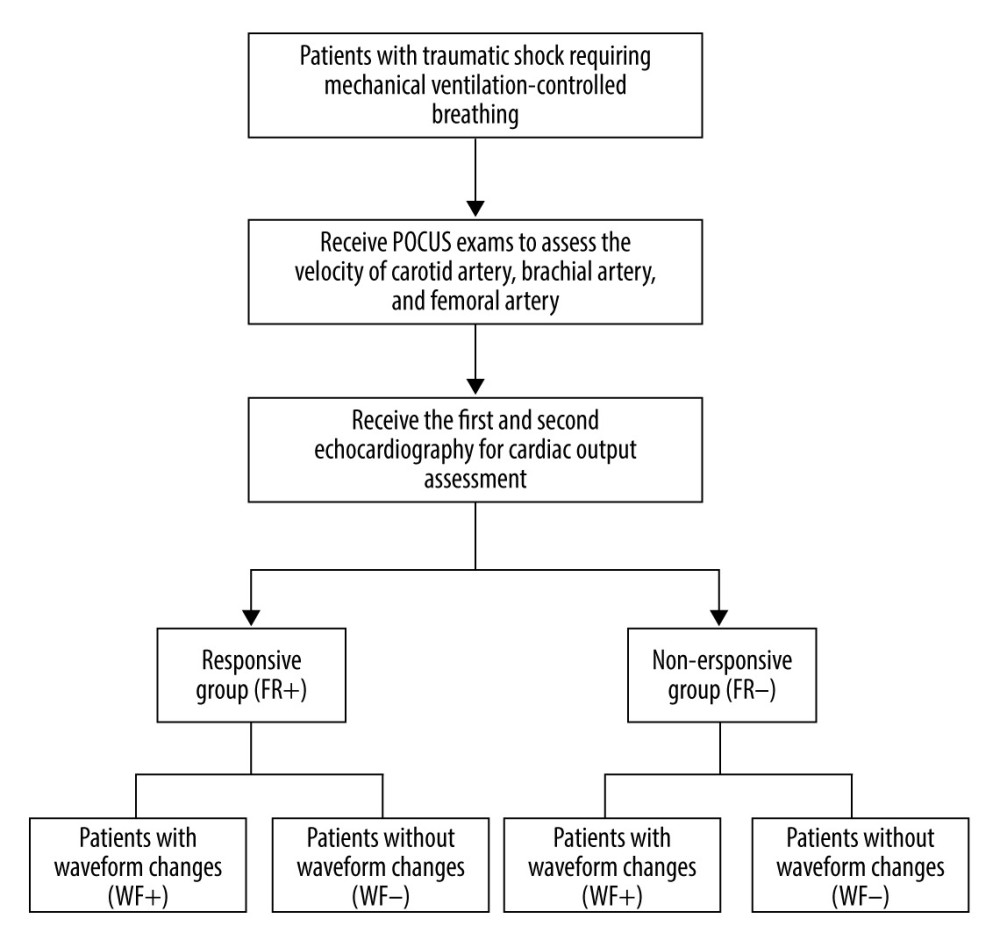 Figure 1. Selection of study population.
Figure 1. Selection of study population. 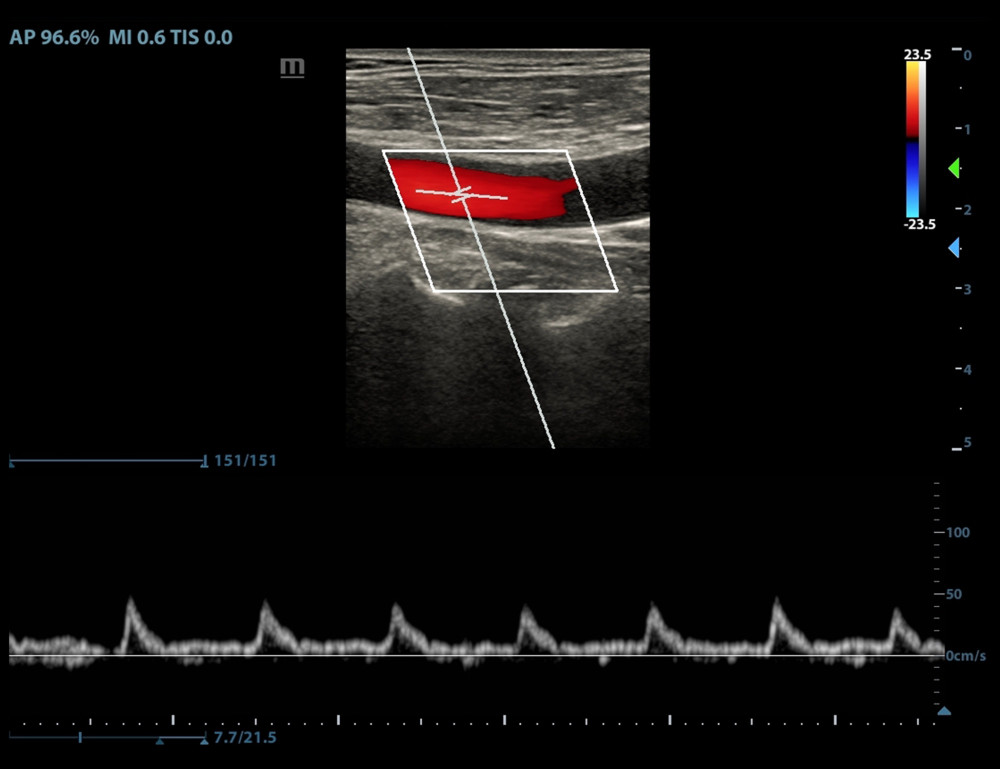 Figure 2. Periodic changes in velocity waveform in carotid artery blood flow with respiration in the fluid-responsive group (FR+ Group).
Figure 2. Periodic changes in velocity waveform in carotid artery blood flow with respiration in the fluid-responsive group (FR+ Group). 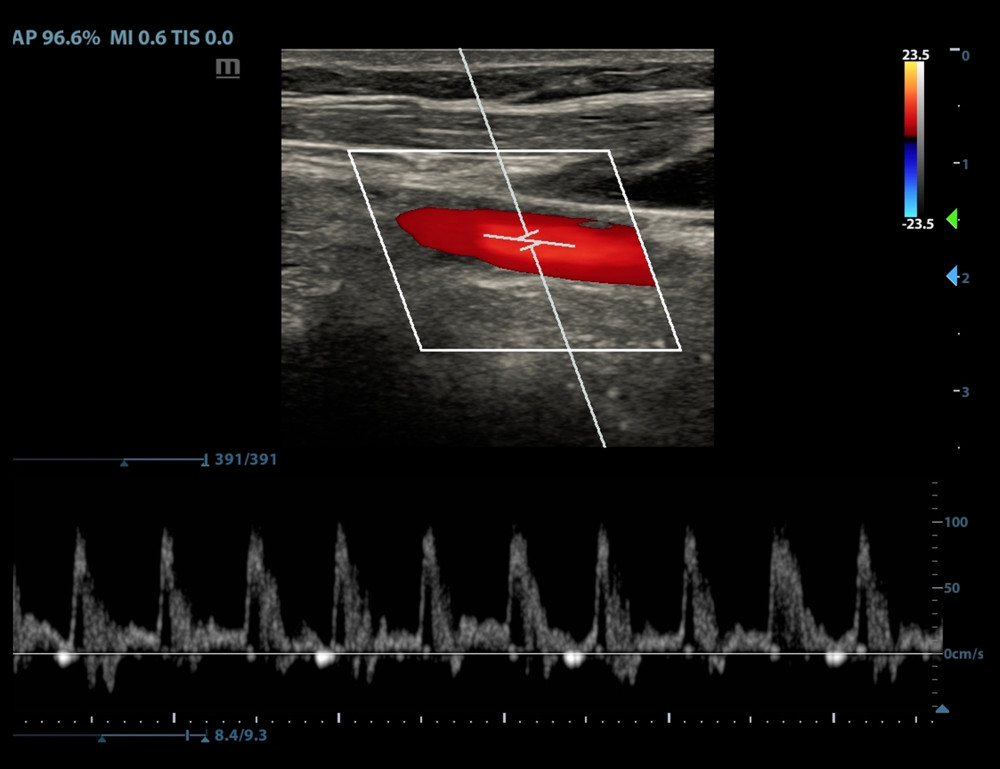 Figure 3. No periodic waveform changes or no significant changes in the velocity waveform in the carotid artery blood flow with respiration in the non-responsive group (FR− Group).
Figure 3. No periodic waveform changes or no significant changes in the velocity waveform in the carotid artery blood flow with respiration in the non-responsive group (FR− Group). 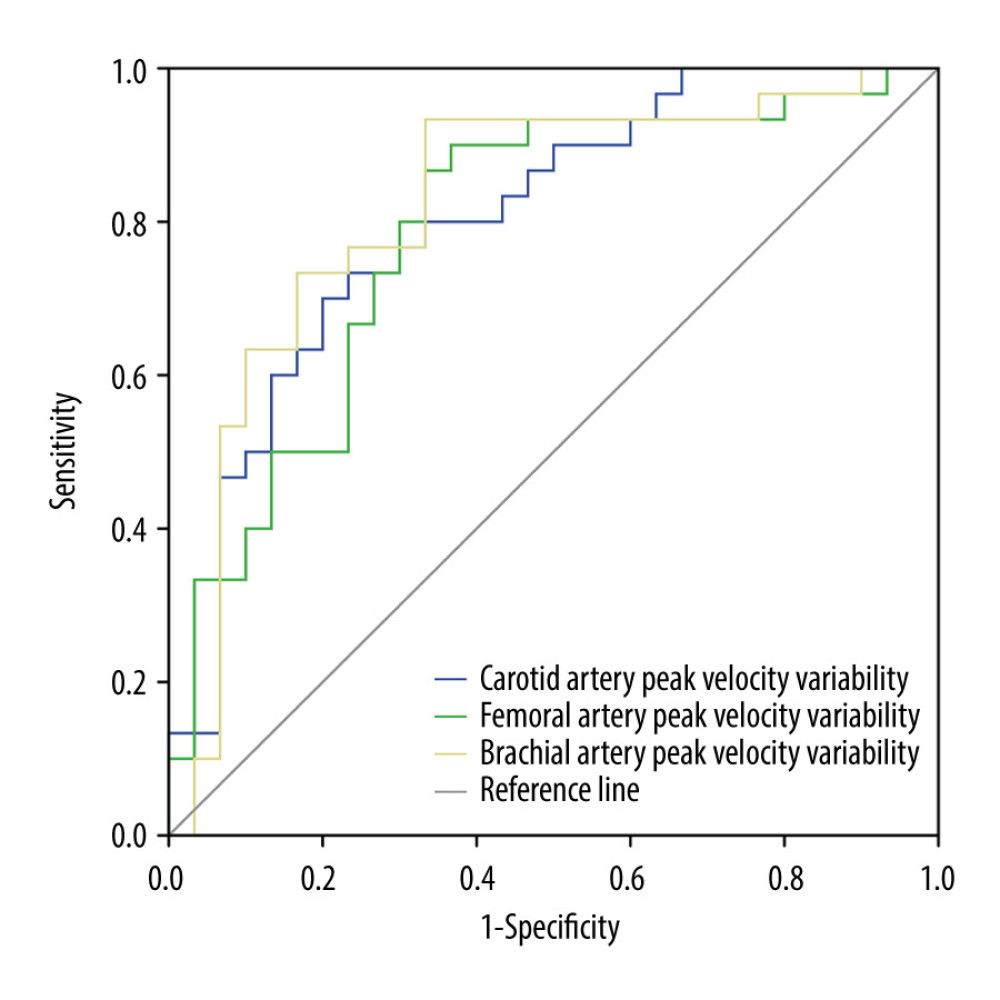 Figure 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries to assess the fluid responsiveness.
Figure 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries to assess the fluid responsiveness. Tables
Table 1. Baseline characteristics compared between patients in the FR+ Group and the FR− Group before and after a rehydration test.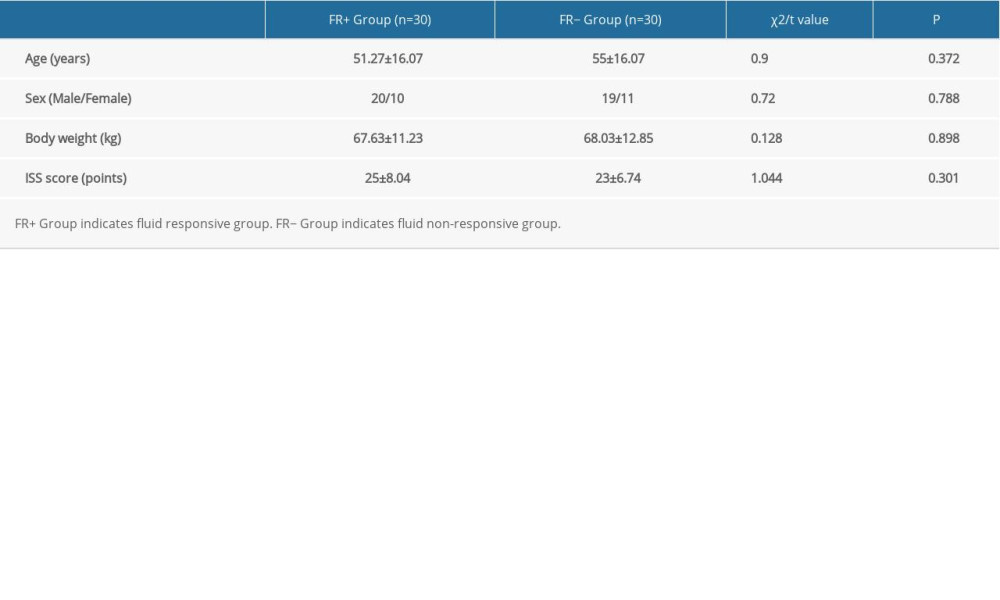 Table 2. Comparison of the number of cases with blood flow velocity waveform changes in peripheral arteries in each group.
Table 2. Comparison of the number of cases with blood flow velocity waveform changes in peripheral arteries in each group.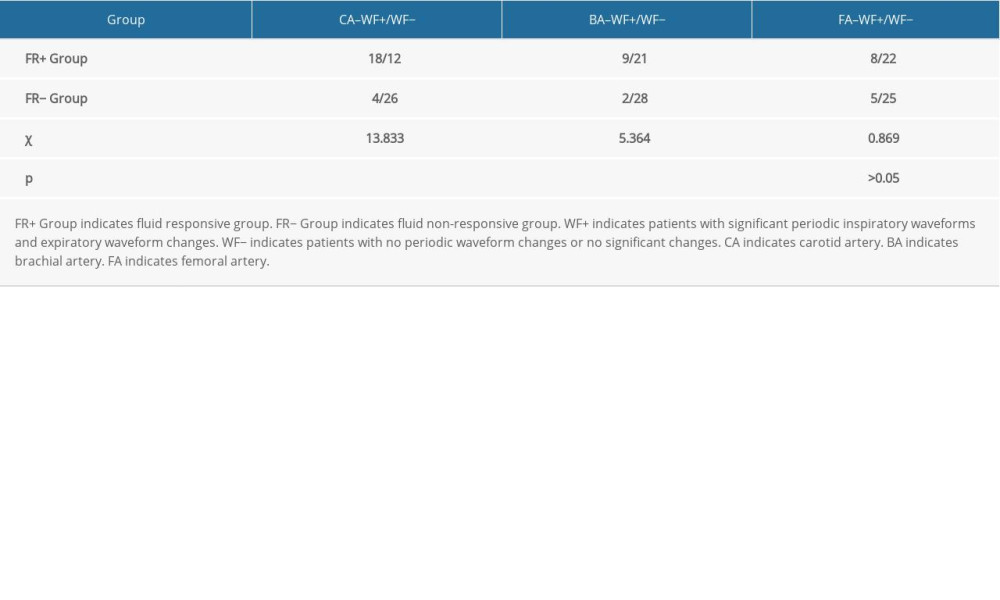 Table 3. Comparison of variability in maximum blood flow velocity in peripheral arteries between the FR+ group and FR− group.
Table 3. Comparison of variability in maximum blood flow velocity in peripheral arteries between the FR+ group and FR− group.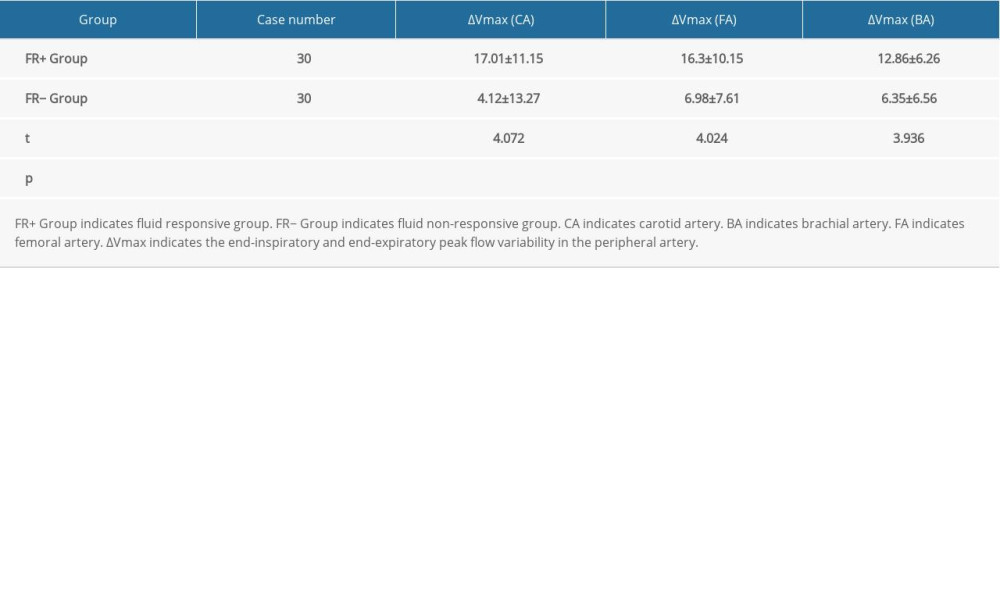 Table 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries.
Table 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries.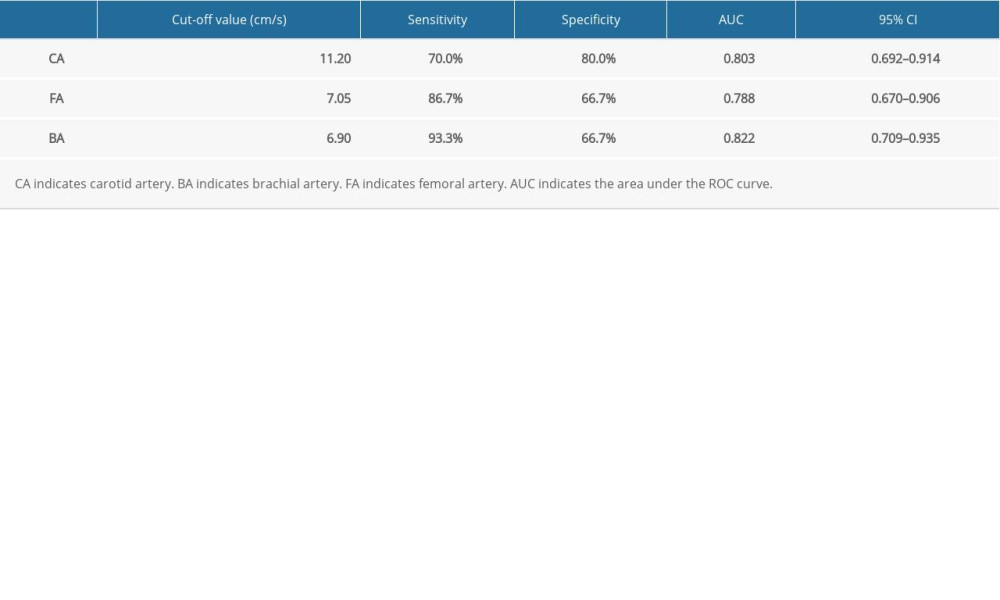 Table 5. Comparison of variability in maximum velocity between the waveform change subgroup (WF+ Subgroup) and the non-waveform change subgroup (WF− Subgroup) within the FR+ group.
Table 5. Comparison of variability in maximum velocity between the waveform change subgroup (WF+ Subgroup) and the non-waveform change subgroup (WF− Subgroup) within the FR+ group.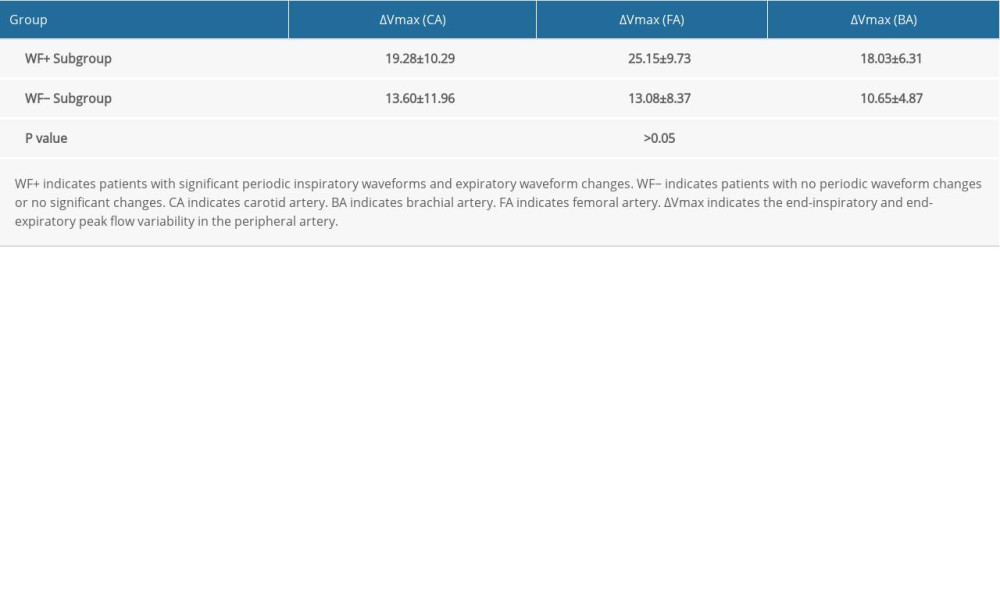
References
1. Boyd JH, Sirounis D, Assessment of adequacy of volume resuscitation: Curr Opin Crit Care, 2016; 22; 424-27
2. Millington SJ, Ultrasound assessment of the inferior vena cava for fluid responsiveness: Easy, fun, but unlikely to be helpful: Can J Anaesth, 2019; 66; 633-38
3. Gupta K, Sondergaard S, Parkin G, Applying mean systemic filling pressure to assess the response to fluid boluses in cardiac post-surgical patients: Intensive Care Med, 2015; 41; 265-72
4. Seckel MA, Ahrens T, Challenges in sepsis care: New sepsis definitions and fluid resuscitation beyond the central venous pressure: Crit Care Nurs Clin North Am, 2016; 28; 513-32
5. Stowell JR, Kessler R, Lewiss RE, Critical care ultrasound: A national survey across specialties: J Clin Ultrasound, 2018; 46; 167-77
6. Vieillard-Baron A, Millington SJ, Sanfilippo F, A decade of progress in critical care echocardiography: A narrative review: Intensive Care Med, 2019; 45; 770-88
7. Liu D: Practice of critical care medicine, 2017, Beijing, People’s Health Publishing House
8. Romagnoli S, Bevilacqua S, Lazzeri C, Most care: A minimally invasive system for hemodynamic monitoring powered by the Pressure Recording Analytical Method (PRAM): HSR Proc Intensive Care Cardiovasc Anesth, 2009; 1; 20-27
9. Chew MS, Aneman A, Haemodynamic monitoring using arterial waveform analysis: Curr Opin Crit Care, 2013; 19; 234-41
10. Cholley B, Echocardiography in the intensive care unit: Beyond “eyeballing”. A plea for the broader use of the aortic velocity-time integral measurement: Intensive Care Med, 2019; 45; 898-901
11. Litton E, Morgan M, The PiCCO monitor: A review: Anaesth Intensive Care, 2012; 40; 393-409
12. Wetterslev M, Møller-Sørensen H, Johansen RR, Perner A: Intensive Care Med, 2016; 42(8); 1223-33
13. Moller-Sorensen H, Graeser K, Hansen KL, Measurements of cardiac output obtained with transesophageal echocardiography and pulmonary artery thermodilution are not interchangeable: Acta Anaesthesiol Scand, 2014; 58(1); 80-88
14. Kaydu A, Gokcek E, Preoperative and postoperative assessment of ultrasonographic measurement of inferior vena cava: A prospective, observational study: J Clin Med, 2018; 7; E145
15. McGregor D, Sharma S, Gupta S, Emergency Department non-invasive cardiac output study (EDNICO): A feasibility and repeatability study: Scand J Trauma Resusc Emerg Med, 2019; 27; 30
16. Antiperovitch P, Iliescu E, Chan B, Carotid systolic flow time with passive leg raise correlates with fluid status changes in patients undergoing dialysis: J Crit Care, 2017; 39; 83-86
17. Desgranges FP, Desebbe O, Pereira de Souza Neto E, Respiratory variation in aortic blood flow peak velocity to predict fluid responsiveness in mechanically ventilated children: A systematic review and meta-analysis: Paediatr Anaesth, 2016; 26; 37-47
18. Morparia KG, Reddy SK, Olivieri LJ, Respiratory variation in peak aortic velocity accurately predicts fluid responsiveness in children undergoing neurosurgery under general anesthesia: J Clin Monit Comput, 2018; 32; 221-26
19. Jozwiak M, Mercado P, Teboul JL, What is the lowest change in cardiac output that transthoracic echocardiography can detect?: Crit Care, 2019; 23; 116
20. Schneider M, Ran H, Aschauer S, Binder C, Visual assessment of right ventricular function by echocardiography: How good are we?: Int J Cardiovasc Imaging, 2019; 35; 2001-8
21. Schneider M, Aschauer S, Mascherbauer J, Echocardiographic assessment of right ventricular function: current clinical practice: Int J Cardiovasc Imaging, 2019; 35; 49-56
22. Doctor M, Siadecki SD, Cooper D, Reliability, laterality and the effect of respiration on the measured corrected flow time of the carotid arteries: J Emerg Med, 2017; 53; 91-97
Figures
 Figure 1. Selection of study population.
Figure 1. Selection of study population. Figure 2. Periodic changes in velocity waveform in carotid artery blood flow with respiration in the fluid-responsive group (FR+ Group).
Figure 2. Periodic changes in velocity waveform in carotid artery blood flow with respiration in the fluid-responsive group (FR+ Group). Figure 3. No periodic waveform changes or no significant changes in the velocity waveform in the carotid artery blood flow with respiration in the non-responsive group (FR− Group).
Figure 3. No periodic waveform changes or no significant changes in the velocity waveform in the carotid artery blood flow with respiration in the non-responsive group (FR− Group). Figure 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries to assess the fluid responsiveness.
Figure 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries to assess the fluid responsiveness. Tables
 Table 1. Baseline characteristics compared between patients in the FR+ Group and the FR− Group before and after a rehydration test.
Table 1. Baseline characteristics compared between patients in the FR+ Group and the FR− Group before and after a rehydration test. Table 2. Comparison of the number of cases with blood flow velocity waveform changes in peripheral arteries in each group.
Table 2. Comparison of the number of cases with blood flow velocity waveform changes in peripheral arteries in each group. Table 3. Comparison of variability in maximum blood flow velocity in peripheral arteries between the FR+ group and FR− group.
Table 3. Comparison of variability in maximum blood flow velocity in peripheral arteries between the FR+ group and FR− group. Table 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries.
Table 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries. Table 5. Comparison of variability in maximum velocity between the waveform change subgroup (WF+ Subgroup) and the non-waveform change subgroup (WF− Subgroup) within the FR+ group.
Table 5. Comparison of variability in maximum velocity between the waveform change subgroup (WF+ Subgroup) and the non-waveform change subgroup (WF− Subgroup) within the FR+ group. Table 1. Baseline characteristics compared between patients in the FR+ Group and the FR− Group before and after a rehydration test.
Table 1. Baseline characteristics compared between patients in the FR+ Group and the FR− Group before and after a rehydration test. Table 2. Comparison of the number of cases with blood flow velocity waveform changes in peripheral arteries in each group.
Table 2. Comparison of the number of cases with blood flow velocity waveform changes in peripheral arteries in each group. Table 3. Comparison of variability in maximum blood flow velocity in peripheral arteries between the FR+ group and FR− group.
Table 3. Comparison of variability in maximum blood flow velocity in peripheral arteries between the FR+ group and FR− group. Table 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries.
Table 4. Receiver operating characteristic (ROC) curves of the end-inspiratory and end-expiratory peak flow variability in the peripheral arteries. Table 5. Comparison of variability in maximum velocity between the waveform change subgroup (WF+ Subgroup) and the non-waveform change subgroup (WF− Subgroup) within the FR+ group.
Table 5. Comparison of variability in maximum velocity between the waveform change subgroup (WF+ Subgroup) and the non-waveform change subgroup (WF− Subgroup) within the FR+ group. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








