25 March 2021: Clinical Research
Correlations of Silent Information Regulator of Transcription 1 (SIRT1) Expression, Inflammatory Factors, and Oxidative Stress with Pulmonary Function in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD)
Xin Zhao1ACEG*, Yueqin Wu1ABDFDOI: 10.12659/MSM.929046
Med Sci Monit 2021; 27:e929046
Abstract
BACKGROUND: The aim of this study was to investigate the correlations of silent information regulator of transcription 1 (SIRT1) expression, inflammatory factors, and oxidative stress with pulmonary function in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
MATERIAL AND METHODS: Bronchoalveolar lavage fluid (BALF) was collected from 188 patients with COPD (83 in stable phase and 105 in acute exacerbation phase) and 56 healthy controls. Subsequently, the SIRT1 expression levels, the IL-6 and IL-8 levels (the representatives of inflammatory factors), and the MDA and SOD levels (indicative of oxidative stress) were detected via enzyme-linked immunosorbent assay. Correlations of SIRT1 expression, inflammatory factors, and oxidative stress with pulmonary function parameters [forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) and FEV1] were measured via Spearman’s correlation analysis.
RESULTS: The levels of inflammatory factors and oxidative stress were elevated and SIRT1 expression remarkably declined in patients with AECOPD compared with those in healthy controls and stable COPD patients (P<0.05). Spearman’s correlation analysis revealed that SIRT1 expression, interleukin (IL)-6, and IL-8 were strongly associated with pulmonary function parameters (FEV1/FVC and FEV1) in patients with AECOPD (P<0.001), while no such obvious correlation was observed in stable COPD patients.
CONCLUSIONS: Oxidative stress and expression levels of inflammatory factors are evidently elevated and SIRT1 expression declines in patients with AECOPD. Moreover, SIRT1 expression is positively associated with pulmonary function parameters, while IL-6 and IL-8 exhibit negative correlations with pulmonary function parameters.
Keywords: Chronic Disease, Lung Diseases, Obstructive, Bronchoalveolar Lavage Fluid, Case-Control Studies, Disease Progression, Forced Expiratory Volume, Interleukin-6, Interleukin-8, Lung, Malondialdehyde, Pulmonary Disease, Chronic Obstructive, Respiratory Function Tests, Sirtuin 1, Vital Capacity
Background
Chronic obstructive pulmonary disease (COPD) is clinically characterized by respiratory symptoms and airflow limitation, which is greatly affected by environmental factors such as exposure to deleterious particles or gases [1]. Acute exacerbation of COPD (AECOPD) is the leading cause of the poor outcome of COPD, which has a high mortality rate and results in a tremendous health and socioeconomic burden [1]. Current treatments can ameliorate respiratory symptoms but cannot reverse lung injury and restore pulmonary function completely [2,3]. It is reported that oxidative stress and inflammation can affect COPD progression [2]. Currently, lipopolysaccharide and cigarette smoke (CS) are mostly utilized to induce COPD [3].
Superoxide dismutase (SOD) reduces oxidative stress and tissue damage through removing superoxide, and lipid peroxidation product malondialdehyde (MDA) and SOD are commonly used to evaluate oxidative damage and oxidative stress [4]. Inflammation and oxidative stress in the lungs during COPD are related to systemic inflammation and the disease [5]. The decrease of antioxidants and overexpression of pro-inflammatory molecules observed in the lungs and peripheral blood is associated with pulmonary function parameters reflecting airway obstruction and its severity, such as forced expiratory volume in one second (FEV1)/forced vital capacity (FVC), and FEV1 [6–8]. As a well-known longevity gene, silent information regulator of transcription 1 (SIRT1) regulates stress resistance and inflammation through deacetylation of intracellular signaling molecules and histones, and it also can regulate age-related changes through different mechanisms, including elevating mitochondrial production by regulating PGC-1α deacetylation [9,10]. Moreover, highly expressed SIRT1 is capable of promoting the release of SIRT1 activators, thus dramatically reducing CS-induced oxidative stress [11]. Owing to its essential role in regulating the oxidative stress response, SIRT1 dysfunction is correlated with aging-related diseases such as COPD [12]. SIRT1 is a negative regulator of matrix metalloproteinase-9 (MMP9) expression, inappropriate elevation of which was involved in the pathogenesis of COPD [13]. It was reported that the SIRT1 level was decreased in macrophages and lungs of patients with COPD [14]. The aim of the present study was to investigate the correlations of SIRT1 expression, inflammatory factors, and oxidative stress with pulmonary function in patients with acute exacerbation of AECOPD.
Material and Methods
EXPERIMENTAL MATERIALS:
Human interleukin (IL)-6 and IL-8 enzyme-linked immunosorbent assay (ELISA) kits and MDA and SOD assay kits were purchased from Beyotime (Shanghai, China), complementary deoxyribonucleic acid (cDNA) reverse transcription kits were bought from Roche (Basel, Switzerland), and SYBR Green quantitative polymerase chain reaction (qPCR) reagents were provided by TaKaRa (Tokyo, Japan).
RESEARCH SUBJECTS:
This study was approved by the Ethics Committee of Tianjin First Central Hospital (No. TH14121004). Signed written informed consents were obtained from all participants before the study. COPD patients and health people enrolled were divided into a Healthy Control group, a Stable COPD group, and an AECOPD group.
ENROLLMENT OF COPD PATIENTS:
From April 2015 to May 2017, 188 patients with COPD (83 in stable phase and 105 in acute exacerbation phase) were admitted to our hospital, and 56 healthy control patients with normal lung function were included. The sample size was not calculated at the beginning of study for some limitations. Eligible cases were included in the study as much as possible. Inclusion criteria were: COPD in stable phase, diagnosed with moderate to severe COPD, presence of a stable condition without infection or exacerbation for more than 4 weeks after hospital discharge, COPD in acute exacerbation phase, and meet the AECOPD diagnostic criteria according to GOLD-guidelines (2015). Exclusion criteria were: pulmonary bulla, pneumothorax, severe and unstable cardiac disease, orthopedic disease, mental disorder, or malignant tumor. Subsequently, 5 mL of blood was collected and centrifuged to obtain serum samples, followed by labeling and storing.
EVALUATION OF PULMONARY FUNCTION:
Pulmonary function testing was performed in all subjects using a standard spirometer in accordance with the standards stipulated by the American Thoracic Society.
DETERMINATION OF SERUM IL-6 AND IL-8 LEVELS IN COPD PATIENTS:
Serum samples were added to 96-well microtiter plates, and reacted at room temperature for 30 min. Then, they were washed 5 times, dried with paper towels, 100 mL of secondary antibody was added with in each well, and reacted for 30 min, followed by termination using TMB. Finally, the absorbance value of each well was measured.
MEASUREMENT OF OXIDATIVE STRESS MARKER CONTENT IN BRONCHOALVEOLAR LAVAGE FLUID (BALF):
The reagents were tested by MDA and SOD assay kits. After incubation, alterations of absorbance at the wavelengths of 550 nm and 532 nm represented SOD and MDA activity changes, respectively.
DETECTION OF MESSENGER RNA (MRNA) EXPRESSION AND SIRT1 ACTIVITY IN SERUM OF COPD PATIENTS:
About 1 mL of TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was added into 500 mL of serum samples to extract total RNA. Next, cDNA was synthesized from total RNA using LightCycler 1.5 and SYBR Green qRCR Mix, followed by real-time PCR (RT-PCR). The value of the target gene was standardized via the value of the β-actin protein.
SIRT1 activity was detected via SIRT1/Sir2 deacetylase fluorescence assay. The values are expressed in terms of the relative fluorescence/μg of protein (AU).
STATISTICAL ANALYSIS:
GraphPad Prism 7 (La Jolla, CA, USA) was utilized to analyze experimental data. The
Results
GROUPING FEATURES:
The data and clinical characteristics (including sex, age, and pulmonary function) of all samples collected in this experiment were statistically analyzed. There were 56 cases in the Healthy Control group, 83 cases in the Stable COPD group, and 105 cases in the AECOPD group, and no differences were observed in sex and age among the 3 groups (P=0.67, P=0.55). Unexpectedly, FEV1% and FEV1/FVC were remarkably lower in COPD patients compared to those in the Heathy Control group (Table 1).
SIRT1 EXPRESSIONS IN HEALTHY CONTROL GROUP, STABLE COPD GROUP, AND AECOPD GROUP:
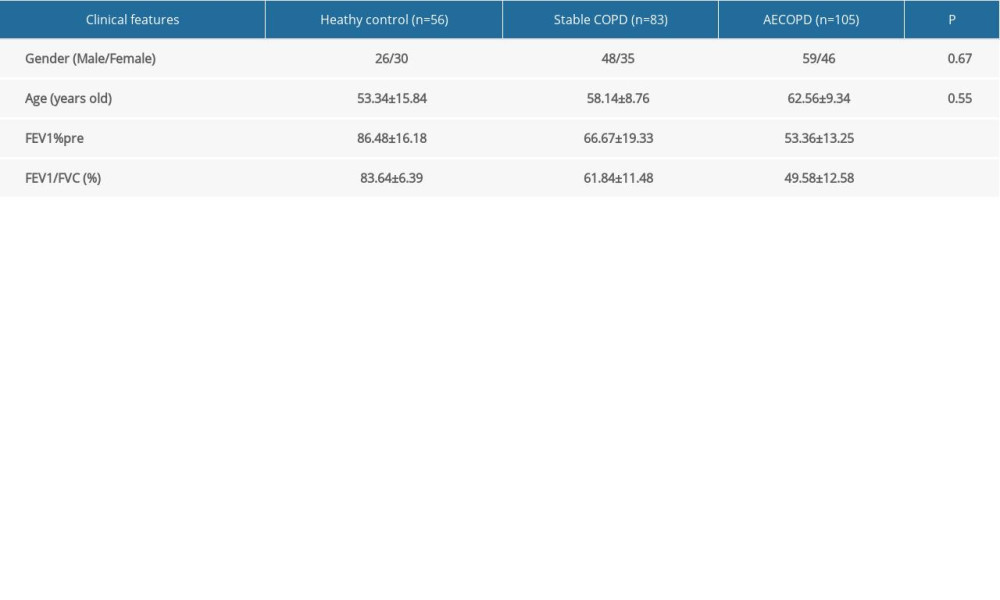
SIRT1 expression in serum of COPD patients was analyzed in this study, and real-time fluorescence quantitative analysis of the transcription results of the SIRT1 gene indicated that the SIRT1 mRNA expression was markedly lower in the AECOPD group in comparison with that in the Stable COPD group (P<0.05), while it was also notably lower in the Stable COPD group than that in Healthy Control group (P<0.05); compared with that the Healthy Control group, it was evidently lower in the AECOPD group (P<0.001) (Figure 1).
CHANGES IN EXPRESSIONS OF PRO-INFLAMMATORY FACTORS (IL-6 AND IL-8):
The results of ELISA indicated that the serum pro-inflammatory factors IL-6 levels and IL-8 were higher in the AECOPD group compare to those in the Stable COPD group (P<0.05), which significantly differed from those in the Healthy Control group (P<0.01), suggesting that the pulmonary inflammation of AECOPD may be alleviated through reducing the expressions of pro-inflammatory factors IL-6 and IL-8 (Figure 2).
CHANGES IN LEVELS OXIDATIVE STRESS MARKERS LEVELS IN BALF IN HEATHY CONTROL GROUP, STABLE COPD GROUP, AND AECOPD GROUP:
SOD expression level was decreased in BALF supernatant in the Stable COPD group and AECOPD group compared with that in the Healthy Control group (P<0.05), while the level of MDA was visibly increased in COPD groups (P<0.05). These results show that the level of oxidative stress in lung tissues of COPD patients is significantly elevated (Figure 3).
CORRELATIONS OF SIRT1 EXPRESSION AND INFLAMMATORY FACTORS WITH PULMONARY FUNCTION IN PATIENTS WITH AECOPD:
Firstly, the relationship between SIRT1 activity and pulmonary function parameters was analyzed, indicating that there were significant linear positive correlations of SIRT1 expression with FEV1 and FEV1/FVC in AECOPD patients (P<0.001, r2=0.75, P<0.001, r2=0.74), and no such obvious correlations were observed in the Stable COPD group (r2=0.40, r2=0.21) (Figure 4A, 4B). The results revealed that changes in SIRT1 expression in AECOPD patients exert a guiding role in predicting disease. The relationship of IL-6 and IL-8 with pulmonary function parameters was analyzed, exhibiting that levels of IL-6 and IL-8 had remarkable linear negative associations with FEV1 and FEV1/FVC in AECOPD patients (IL-6: P<0.001, r2=0.83, P<0.001, r2=0.81, IL-8: P<0.001, r2=0.86, P<0.001, r2=0.80), and no such notable associations were found in the Stable COPD group (IL-6: r2=0.34, r2=0.22, IL-8: r2=0.14, r2=0.47) (Figure 4C–4F).
Discussion
Chronic inflammation in COPD is induced by genetic, environmental, and epigenetic factors; regardless of clinical manifestations, the progressive decline in pulmonary function is a common clinical presentation [15]. This study showed that SIRT1 expression and activity significantly declined. In contrast, pro-inflammatory factors and oxidative stress markers were dramatically overexpressed in COPD patients. Meanwhile, the above indicators were compared in different periods of COPD, which suggested that SIRT1 expression and activity were visibly decreased and pro-inflammatory factors and oxidative stress markers were also distinctly overexpressed in the acute exacerbation stage compared with those in the stable stage, showing that SIRT1 is differently expressed in the different stages of COPD.
It was reported that inflammation and oxidative stress in COPD lung tissues can affect the treatment and progression of the disease [16]. The lung tissues injured by COPD recruit inflammatory cells in the respiratory tract, and in this process, a large amount of reactive oxygen species will be produced, thus leading to oxidative stress [17]. IL-6 and IL-8 play essential roles in the pathogenesis of stable and aggravated COPD [18]. SOD is an anti-inflammatory enzyme and a major antioxidant that reduces oxidative stress and tissue damage through removing superoxide, and MDA is a by-product of the peroxidation of polyunsaturated fatty acids, which is a reliable marker of COPD oxidative stress [19,20]. It was found in this study that IL-6 and IL-8 levels were remarkably higher in BALF of AECOPD patients than those of Healthy Control and Stable COPD patients, and the expression levels were negatively related to FEV1 and FEV1/FVC. Persistent inflammatory reaction destroyed the balance of pro-inflammatory and anti-inflammatory factors in lung tissue of COPD, which is the main cause of lung injury. Additionally, the SOD level was decreased while the MDA level was increased in BALF in AECOPD patients, demonstrating that the reduction in SOD and the increase in MDA is associated with a more oxidized environment in AECOPD patients, which may be caused by the reduced efficiency of anti-aging molecules regulating the cellular redox state and oxidative stress response. The evaluated oxidative stress was associated with increased inflammation and airway remodeling in COPD.
SIRT1 has multiple biological effects, such as oxidative stress and cellular metabolism [21], which have powerful anti-oxidative stress and cardiac anti-apoptotic effects [22]. SIRT1 is a longevity-related protein that is of great significance in maintaining mitochondrial functions [23]. A previous study revealed that the expressions of IL-8 and IL-6 in cells pretreated with SIRT1 activators were weakened, and knockout of SIRT1 exacerbates the pro-inflammatory effect [24]. In addition to inhibiting IL-6 and IL-8 expressions, SIRT1 is able to exert an effect in oxidative stress by inducing the expression of antioxidant enzymes (such as SOD and catalase) [25,26]. In this study, the results of qRT-PCR experiments indicated that SIRT1 expression was markedly decreased in AECOPD patients, and the correlations of SIRT1 expression with pulmonary function parameters showed that SIRT1 expression in the serum of AECOPD patients was positively associated with airway obstruction indicators and its severity. Moreover, the results also revealed that the expression level of SIRT1 was not only associated with COPD, but also with AECOPD. Therefore, it may be utilized as a biomarker to judge the progression of COPD. It is key point to control the inflammatory response and regulate oxidative stress imbalance for treatment of COPD. Our study indicated that the decrease of SIRT1 leads to the intensification of inflammatory and oxidative stress. Thus, SIRT1 may be a therapeutic target in COPD.
For AECOPD patients, the acute onset and high fatality rate are the main problems in treatment. In this study, it was found that there were correlations of SIRT1 gene and inflammatory factors with COPD, but the changes in the levels of inflammatory factors IL-6 and IL-8 are found in various diseases and are not specific. In most AECOPD patients, SIRT1 expression level was significantly reduced, which means that the detection of SIRT1 expression in patients can improve the prediction of AECOPD.
Conclusions
In conclusion, this study indicates that SIRT1 expression is a potential biomarker to reflect the severity of COPD, especially in patients of AECOPD; it is easily measured by peripheral blood sampling and has potential to be utilized as a therapeutic target.
Figures
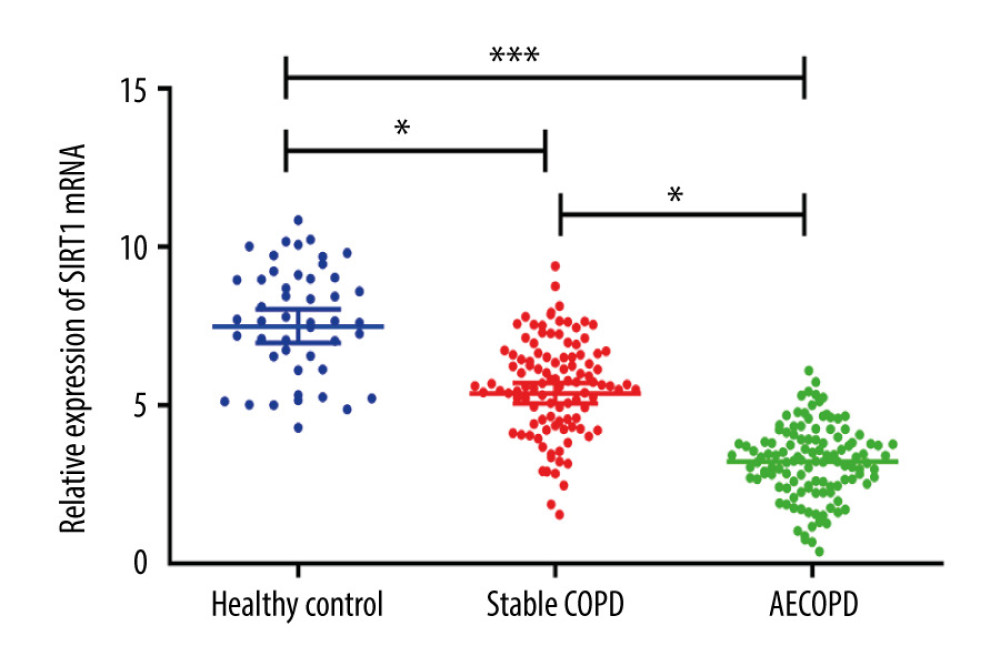 Figure 1. SIRT1 expressions in the Healthy Control group, Stable COPD group, and AECOPD group. The mRNA expression of SIRT1 was markedly lower in the AECOPD group and Stable COPD group in comparison with that in the Control group, while compared with that in the Stable COPD group, SIRT1 activity notably declined in serum of patients in the AECOPD group (P<0.05).
Figure 1. SIRT1 expressions in the Healthy Control group, Stable COPD group, and AECOPD group. The mRNA expression of SIRT1 was markedly lower in the AECOPD group and Stable COPD group in comparison with that in the Control group, while compared with that in the Stable COPD group, SIRT1 activity notably declined in serum of patients in the AECOPD group (P<0.05). 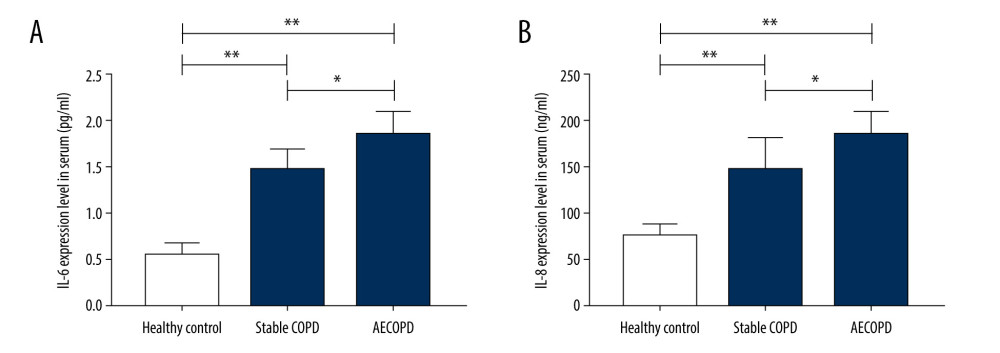 Figure 2. Expressions of IL-6 and IL-8 in the Heathy Control group, Stable COPD group, and AECOPD group. (A, B) Detection of serum levels of IL-6 and IL-8 in the 3 groups via ELISA. Serum levels of IL-6 and IL-8 were obviously higher in the AECOPD group in comparison with those in the Stable COPD group and Healthy Control group (P<0.05).
Figure 2. Expressions of IL-6 and IL-8 in the Heathy Control group, Stable COPD group, and AECOPD group. (A, B) Detection of serum levels of IL-6 and IL-8 in the 3 groups via ELISA. Serum levels of IL-6 and IL-8 were obviously higher in the AECOPD group in comparison with those in the Stable COPD group and Healthy Control group (P<0.05). 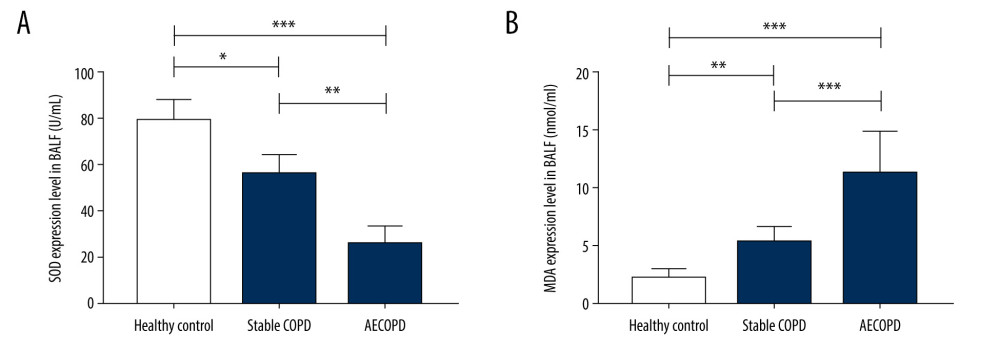 Figure 3. Changes in levels of SOD and MDA in BALF of the Heathy Control group, Stable COPD group, and AECOPD group. (A, B) SOD level was decreased while MDA level was increased in BALF in the AECOPD group compared with that in the Stable COPD group (P<0.05).
Figure 3. Changes in levels of SOD and MDA in BALF of the Heathy Control group, Stable COPD group, and AECOPD group. (A, B) SOD level was decreased while MDA level was increased in BALF in the AECOPD group compared with that in the Stable COPD group (P<0.05). 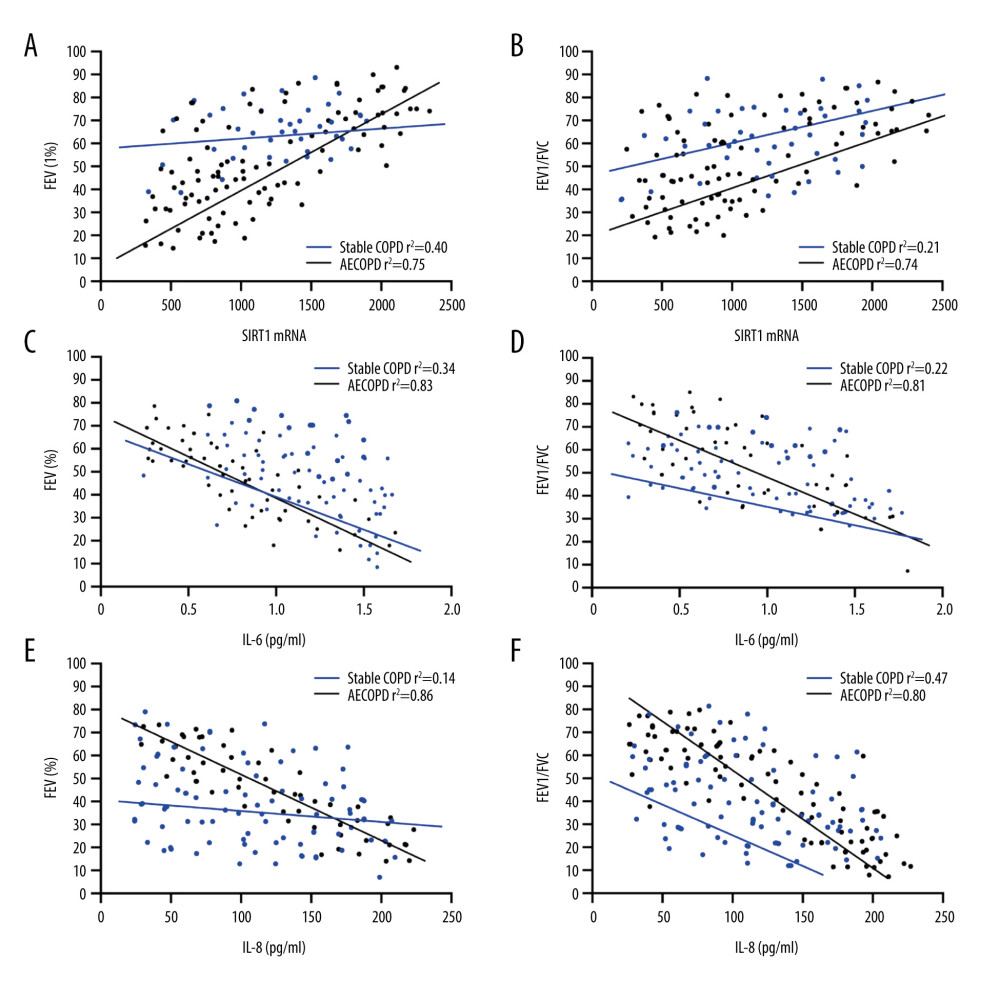 Figure 4. Correlations of SIRT1 expression, IL-6 and IL-8 with FEV1 and FEV1/FVC in AECOPD patients. (A) Correlation between SIRT1 expression and FEV1 in the serum of AECOPD patients. A significant linear positive correlation was observed between SIRT1 activity and FEV1 in AECOPD patients (r2=0.75, P<0.001). (B) Correlation between SIRT1 expression and FEV1/FVC in the serum of AECOPD patients. A marked linear positive correlation was found between SIRT1 activity and FEV1/FVC in AECOPD patients (r2=0.74, P<0.001). (C) Relation between IL-6 and FEV1 in AECOPD patients. IL-6 had a remarkable linear negative relation with FEV1 in AECOPD patients (r2=0.83, P<0.001). (D) Relation between IL-6 and FEV1/FVC in AECOPD patients. IL-6 had a notable linear negative relation with FEV1/FVC in AECOPD patients (r2=0.81, P<0.001). (E) Association between IL-8 and FEV1 in AECOPD patients. There was an obvious linear negative association between IL-8 and FEV1 in AECOPD patients (r2=0.86, P<0.001). (F) Association between IL-8 and FEV1/FVC in AECOPD patients. There was an evident linear negative association between IL-8 and FEV1/FVC in AECOPD patients (r2=0.80, P<0.001).
Figure 4. Correlations of SIRT1 expression, IL-6 and IL-8 with FEV1 and FEV1/FVC in AECOPD patients. (A) Correlation between SIRT1 expression and FEV1 in the serum of AECOPD patients. A significant linear positive correlation was observed between SIRT1 activity and FEV1 in AECOPD patients (r2=0.75, P<0.001). (B) Correlation between SIRT1 expression and FEV1/FVC in the serum of AECOPD patients. A marked linear positive correlation was found between SIRT1 activity and FEV1/FVC in AECOPD patients (r2=0.74, P<0.001). (C) Relation between IL-6 and FEV1 in AECOPD patients. IL-6 had a remarkable linear negative relation with FEV1 in AECOPD patients (r2=0.83, P<0.001). (D) Relation between IL-6 and FEV1/FVC in AECOPD patients. IL-6 had a notable linear negative relation with FEV1/FVC in AECOPD patients (r2=0.81, P<0.001). (E) Association between IL-8 and FEV1 in AECOPD patients. There was an obvious linear negative association between IL-8 and FEV1 in AECOPD patients (r2=0.86, P<0.001). (F) Association between IL-8 and FEV1/FVC in AECOPD patients. There was an evident linear negative association between IL-8 and FEV1/FVC in AECOPD patients (r2=0.80, P<0.001). References
1. Vogelmeier CF, Criner GJ, Martinez FJ, Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary: Am J Respir Crit Care Med, 2017; 195; 557-82
2. Nardini S, Camiciottoli G, Locicero S, COPD: Maximization of bronchodilation: Multidiscip Respir Med, 2014; 9; 50
3. Pelaia G, Vatrella A, Gallelli L, Biological targets for therapeutic interventions in COPD: Clinical potential: Int J Chron Obstruct Pulmon Dis, 2006; 1; 321-34
4. Milevoj KL, Domijan AM, Posavac K, Systemic redox imbalance in stable chronic obstructive pulmonary disease: Biomarkers, 2016; 21; 692-98
5. Su B, Liu T, Fan H, Inflammatory markers and the risk of chronic obstructive pulmonary disease: A systematic review and meta-analysis: PLoS One, 2016; 11; e150586
6. Stanojkovic I, Kotur-Stevuljevic J, Milenkovic B, Pulmonary function, oxidative stress and inflammatory markers in severe COPD exacerbation: Respir Med, 2011; 105(Suppl 1); S31-37
7. Shen Y, Yang T, Guo S, Increased serum ox-LDL levels correlated with lung function, inflammation, and oxidative stress in COPD: Mediators Inflamm, 2013; 2013 972347
8. Kirkham PA, Barnes PJ, Oxidative stress in COPD: Chest, 2013; 144; 266-73
9. Rahman I, Kinnula VL, Gorbunova V, Yao H, SIRT1 as a therapeutic target in inflammaging of the pulmonary disease: Prev Med, 2012; 54(Suppl); S20-28
10. Lavu S, Boss O, Elliott PJ, Lambert PD, Sirtuins – novel therapeutic targets to treat age-associated diseases: Nat Rev Drug Discov, 2008; 7; 841-53
11. Yao H, Sundar IK, Ahmad T, SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism: Am J Physiol Lung Cell Mol Physiol, 2014; 306; L816-28
12. Russomanno G, Corbi G, Manzo V, The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation: Immun Ageing, 2017; 14; 7
13. Nakamaru Y, Vuppusetty C, Wada H, A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9: FASEB J, 2009; 23(9); 2810-19
14. Saravanan R, Se-Ran Y, Vuokko LK, SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease: Am J Respir Crit Care Med, 2008; 177(8); 861-70
15. Barnes PJ, Burney PG, Silverman EK, Chronic obstructive pulmonary disease: Nat Rev Dis Primers, 2015; 1; 15076
16. Tuder RM, Petrache I, Pathogenesis of chronic obstructive pulmonary disease: J Clin Invest, 2012; 122; 2749-55
17. Wood LG, Wark PA, Garg ML, Antioxidant and anti-inflammatory effects of resveratrol in airway disease: Antioxid Redox Signal, 2010; 13; 1535-48
18. Knobloch J, Sibbing B, Jungck D, Resveratrol impairs the release of steroid-resistant inflammatory cytokines from human airway smooth muscle cells in chronic obstructive pulmonary disease: J Pharmacol Exp Ther, 2010; 335; 788-98
19. Mates JM, Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology: Toxicology, 2000; 153; 83-104
20. Antus B, Harnasi G, Drozdovszky O, Barta I, Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde: Respirology, 2014; 19; 74-79
21. Chong ZZ, Shang YC, Wang S, Maiese K, SIRT1: New avenues of discovery for disorders of oxidative stress: Expert Opin Ther Targets, 2012; 16; 167-78
22. Hsu CP, Odewale I, Alcendor RR, Sadoshima J, Sirt1 protects the heart from aging and stress: Biol Chem, 2008; 389; 221-31
23. Sant M, Allemani C, Santaquilani M, EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary: Eur J Cancer, 2009; 45; 931-91
24. Cao L, Liu C, Wang F, Wang H, SIRT1 negatively regulates amyloid-beta-induced inflammation via the NF-kappaB pathway: Braz J Med Biol Res, 2013; 46; 659-69
25. Grabowska W, Sikora E, Bielak-Zmijewska A, Sirtuins, a promising target in slowing down the ageing process: Biogerontology, 2017; 18; 447-76
26. Conti V, Forte M, Corbi G, Sirtuins: Possible clinical implications in cardio and cerebrovascular diseases: Curr Drug Targets, 2017; 18; 473-84
Figures
 Figure 1. SIRT1 expressions in the Healthy Control group, Stable COPD group, and AECOPD group. The mRNA expression of SIRT1 was markedly lower in the AECOPD group and Stable COPD group in comparison with that in the Control group, while compared with that in the Stable COPD group, SIRT1 activity notably declined in serum of patients in the AECOPD group (P<0.05).
Figure 1. SIRT1 expressions in the Healthy Control group, Stable COPD group, and AECOPD group. The mRNA expression of SIRT1 was markedly lower in the AECOPD group and Stable COPD group in comparison with that in the Control group, while compared with that in the Stable COPD group, SIRT1 activity notably declined in serum of patients in the AECOPD group (P<0.05). Figure 2. Expressions of IL-6 and IL-8 in the Heathy Control group, Stable COPD group, and AECOPD group. (A, B) Detection of serum levels of IL-6 and IL-8 in the 3 groups via ELISA. Serum levels of IL-6 and IL-8 were obviously higher in the AECOPD group in comparison with those in the Stable COPD group and Healthy Control group (P<0.05).
Figure 2. Expressions of IL-6 and IL-8 in the Heathy Control group, Stable COPD group, and AECOPD group. (A, B) Detection of serum levels of IL-6 and IL-8 in the 3 groups via ELISA. Serum levels of IL-6 and IL-8 were obviously higher in the AECOPD group in comparison with those in the Stable COPD group and Healthy Control group (P<0.05). Figure 3. Changes in levels of SOD and MDA in BALF of the Heathy Control group, Stable COPD group, and AECOPD group. (A, B) SOD level was decreased while MDA level was increased in BALF in the AECOPD group compared with that in the Stable COPD group (P<0.05).
Figure 3. Changes in levels of SOD and MDA in BALF of the Heathy Control group, Stable COPD group, and AECOPD group. (A, B) SOD level was decreased while MDA level was increased in BALF in the AECOPD group compared with that in the Stable COPD group (P<0.05). Figure 4. Correlations of SIRT1 expression, IL-6 and IL-8 with FEV1 and FEV1/FVC in AECOPD patients. (A) Correlation between SIRT1 expression and FEV1 in the serum of AECOPD patients. A significant linear positive correlation was observed between SIRT1 activity and FEV1 in AECOPD patients (r2=0.75, P<0.001). (B) Correlation between SIRT1 expression and FEV1/FVC in the serum of AECOPD patients. A marked linear positive correlation was found between SIRT1 activity and FEV1/FVC in AECOPD patients (r2=0.74, P<0.001). (C) Relation between IL-6 and FEV1 in AECOPD patients. IL-6 had a remarkable linear negative relation with FEV1 in AECOPD patients (r2=0.83, P<0.001). (D) Relation between IL-6 and FEV1/FVC in AECOPD patients. IL-6 had a notable linear negative relation with FEV1/FVC in AECOPD patients (r2=0.81, P<0.001). (E) Association between IL-8 and FEV1 in AECOPD patients. There was an obvious linear negative association between IL-8 and FEV1 in AECOPD patients (r2=0.86, P<0.001). (F) Association between IL-8 and FEV1/FVC in AECOPD patients. There was an evident linear negative association between IL-8 and FEV1/FVC in AECOPD patients (r2=0.80, P<0.001).
Figure 4. Correlations of SIRT1 expression, IL-6 and IL-8 with FEV1 and FEV1/FVC in AECOPD patients. (A) Correlation between SIRT1 expression and FEV1 in the serum of AECOPD patients. A significant linear positive correlation was observed between SIRT1 activity and FEV1 in AECOPD patients (r2=0.75, P<0.001). (B) Correlation between SIRT1 expression and FEV1/FVC in the serum of AECOPD patients. A marked linear positive correlation was found between SIRT1 activity and FEV1/FVC in AECOPD patients (r2=0.74, P<0.001). (C) Relation between IL-6 and FEV1 in AECOPD patients. IL-6 had a remarkable linear negative relation with FEV1 in AECOPD patients (r2=0.83, P<0.001). (D) Relation between IL-6 and FEV1/FVC in AECOPD patients. IL-6 had a notable linear negative relation with FEV1/FVC in AECOPD patients (r2=0.81, P<0.001). (E) Association between IL-8 and FEV1 in AECOPD patients. There was an obvious linear negative association between IL-8 and FEV1 in AECOPD patients (r2=0.86, P<0.001). (F) Association between IL-8 and FEV1/FVC in AECOPD patients. There was an evident linear negative association between IL-8 and FEV1/FVC in AECOPD patients (r2=0.80, P<0.001). In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952