12 July 2021: Clinical Research
Characteristics and Related Factors of High-Risk Human Papillomavirus Infection in Pregnant Women
Yingying Chen12ABCDEF, Jie Dong2BF, Boliang Chu2BD, Xiaoxing Zhang2BC, Xiaofang Ru2B, Yun Chen2B, Yunyan Chen2B, Xiaodong Cheng1A*DOI: 10.12659/MSM.929100
Med Sci Monit 2021; 27:e929100
Abstract
BACKGROUND: Cervical cancer is a risk for women worldwide. The aim of this study was to examine the occurrence of high-risk human papillomavirus (HR-HPV) infection and its related factors in pregnant women and provide a scientific basis for the targeted prevention and treatment of cervical cancer in pregnant women.
MATERIAL AND METHODS: A total of 1774 pregnant women were included, and 1774 non-pregnant women were selected as controls. Cervical exfoliated cells were collected from all women for HR-HPV (AptimaE6, E7mRNA) and ThinPrep cytologic testing, and the vaginal discharge of all pregnant women was tested for pH level and routine pathogenic microorganisms.
RESULTS: The HPV-16-positive and HPV-16/18/45-positive rates in pregnant women were higher than those of non-pregnant women (P<0.05). There was a statistically significant difference in HR-HPV-positive rate, HPV-16-positive rate, and non-HPV-16/18/45-positive rate among pregnant women of different ages (P<0.05). There was a statistically significant difference in HR-HPV-positive rate and non-HPV-16/18/45-positive rate in the first, second, and third trimester (P<0.05). The HR-HPV-positive rate, HPV-16-positive rate, HPV-18/45-positive rate, and non-HPV-16/18/45-positive rate of pregnant women with concurrent infection were higher than those in women without concurrent infection (P<0.05). The HR-HPV-positive and HPV-16/18/45-positive rates in pregnant women were associated with cytologic examination results (P<0.05).
CONCLUSIONS: The overall infection rates of HR-HPV-16 and HR-HPV-18/45 in pregnant women were higher than those in non-pregnant women. The gestation period was found to be a susceptible period for infection with HR-HPV, and we recommend the implementation of cervical cancer screening based on HR-HPV testing in pregnant women.
Keywords: Human papillomavirus 16, Human papillomavirus 18, Pregnancy, Vaginal Discharge, Early Detection of Cancer, Papillomavirus Infections, Pregnant Women
Background
Cervical cancer ranks fourth in cancers of women for both incidence and mortality across the world [1]. According to World Health Organization statistical data, about 75 000 new cases of cervical cancer occur in China every year [2]. Studies have confirmed that human papillomavirus (HPV) plays a key role in the development of cervical cancer. It is the most common sexually transmitted virus in the world, with more than 150 subtypes [3]. HPV genital tract infection is subclassified into low-risk subtypes (6, 11, 42, and 43) and high-risk subtypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59), according to their potential risk of causing malignant diseases. High-risk HPV (HR-HPV) subtypes such as HPV subtype 16 (HPV-16) and HPV-18 are considered the main viruses associated with malignant diseases such as cervical cancer, vaginal cancer, vulvar cancer, anal cancer, and head and neck cancer [4], and HPV-16 is the most common subtype in women who have HPV, followed by HPV-18 [5]. The HPV-16 and HPV-18/45 subtype test (Aptima HPV-GT) is a qualitative E6/E7 oncogene messenger RNA test that can detect HPV-16, HPV-18, and HPV-45.
Young women (<30 years of age) are at the greatest risk of HPV infection, although most of them have no clinical symptoms and can clear the virus through their strong immune systems. However, in developing countries, the majority of pregnancies occur in women under the age of 30 years; therefore, these women are suitable for cervical cancer screening. There are few cervical cancer screenings performed for pregnant women in China at present for reasons including China’s national conditions, patients’ insufficient knowledge of cervical cancer and precancer, and pregnant women’s fear of miscarriage and refusal to have vaginal examination. In addition, there are few targeted studies on the occurrence and influencing factors of HPV infection in this population of women. The purpose of this study was to target pregnant women who receive antenatal care in the hospital, analyze the occurrence and related factors of HR-HPV infection in pregnant women, and provide a scientific basis for an appropriate prevention strategy of cervical cancer in these women.
Material and Methods
STUDY POPULATION:
Pregnant women who received antenatal care at the Huzhou Maternal & Child Health Care Hospital in Zhejiang Province from January 2018 to January 2020 and met the following conditions were enrolled into the study: no history of cervical cancer or precancer or history of other malignant tumors; no history of cervical surgical treatment; not in the active period of autoimmune diseases or taking long-term oral immunosuppressor medication; aged between 20 and 44 years; no liquid-based thin-layer cytology (TCT) or HPV test within the past year; no pathological gestation such as placenta previa or vasa previa; and agreed to participate in this study voluntarily and signed informed consent. During this period, a total of 22 133 pregnant women received antenatal care in our hospital, of which 1774 met the above conditions and were included in the study.
Non-pregnant women who received health examinations in our hospital during the same period were selected as controls when they met the following criteria: no history of cervical cancer or precancer or history of other malignant tumors; no history of cervical surgical treatment; not in the active period of autoimmune diseases or taking long-term oral immunosuppressor medication; not pregnant; and agreed to participate in this study voluntarily. At a 1: 1 ratio, 1774 age-matched non-pregnant women were selected randomly as controls, according to a random numbers table of the clinic visiting order. Figure 1 shows the flow chart of the test.
EXPERIMENTAL ETHICS:
The study was approved by the Ethics Committee of Huzhou Maternal & Child Health Care Hospital (Ethics Committee No. 2019-018; Chairperson, Pingya He), and written informed consent was provided by all patients before enrollment in the study.
TEST REAGENT AND METHOD:
For the detection of HR-HPV, cervical exfoliated cells were collected, and Aptima HPV and Aptima HPV-GT test kits (Hologic Corporation, USA) were used to test for the 14 high-risk HPV subtypes specified by the International Agency for Research on Cancer, according to the manufacturer’s instructions. The following were detected: HPV subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. For specimens that tested positive by the Aptima HPV test, the Aptima HPV-GT test kit was used to further detect HPV subtyping (16,18/45). For specimens that tested negative by the Aptima HPV test, the Aptima HPV-GT test was also regarded as negative.
For the cytological examination, cervical exfoliated cells were collected, and 1 mL of cervical exfoliated cell preservation solution was used for the Aptima HPV test. The fully automatic ThinPrep 2000 liquid-based cytology processor (Hologic Corporation, USA) was used to make liquid-based thin-layer cell sheets out of the remaining preserved solution and to conduct the cytologic examinations, according to the instruction manual. The cytological diagnoses were made according to the Bethesda system [6], proposed in 2014.
Next, vaginal discharge pH levels and common pathogenic microorganisms were detected. The physiological saline containing vaginal discharge was evenly spread on a sterile glass slide. A Nissan Olympus optical microscope was used to confirm the view field under 10× magnification and to observe 10 to 15 fields. Using the microscope under 40× magnification, the hyphae, spores of Candida albicans, trichomoniasis, and clue cells were found and observed. Precision pH test strips were used to determine the pH value of vaginal discharge. The diagnostic criteria strictly followed the third edition of the National Guide to Clinical Laboratory Procedures [7].
STATISTICAL METHOD:
SPSS version 22.0 was used for statistical analysis. The independent
Results
THE RATE OF HR-HPV INFECTION IN PREGNANT WOMEN AND NON-PREGNANT WOMEN:
The average age of the 1774 women in the pregnant group was 27.9±5.3 years, and the average age of the 1774 women in the non-pregnant group was 28.3±5.3 years. There was no significant difference in age between the 2 groups. Figure 2 shows the positive rates of HR-HPV and HPV-16/18/45 in the 2 groups, while Figure 3 illustrates the statistics for each age group comparison between pregnant women and non-pregnant women.
There were statistically significant differences in HR-HPV-positive rate, HPV-16-positive rate, and non-HPV-16/18/45 positive rate among pregnant women in all age groups (P<0.05). Figure 4 shows the comparison of each age group. There was no significant difference in the HPV-18/45-positive rate among pregnant women of all ages.
TCT RESULTS OF PREGNANT WOMEN AND NON-PREGNANT WOMEN OF DIFFERENT AGES:
Table 1 shows the rates and the statistically significant differences in abnormal cytologic examination results in pregnant women and non-pregnant women. Pairwise comparison by age revealed that the abnormal rates of cytologic examination in non-pregnant women aged 25 to 29 years and 40 to 44 years were significantly higher than those of pregnant women in the same age groups (P<0.05), and there was no statistically significant difference in other age groups. There was no statistically significant difference in the abnormal rate of cytologic examination between pregnant women of all ages.
THE RESULTS OF HR-HPV AND CYTOLOGY EXAMINATION OF PREGNANT WOMEN AT DIFFERENT GESTATIONAL WEEKS:
Among the 1774 pregnant women, 594 (33.5%) were in the first trimester (<14 weeks gestation), 980 (55.2%) were in the second trimester (14–27 weeks+6 days gestation), and 200 (11.3%) were in the third trimester (≥28 weeks gestation). Table 2 shows the differences in the positive rates of HR-HPV and the differences in non-HPV-16/18/45 among the 3 trimesters. Pairwise comparison showed that the positive rates of HR-HPV in women in the first and second trimesters were higher than those in the third trimester (P<0.017). Also, pairwise comparison showed that the positive rates of non-HPV-16 and non-HPV-18/45 in women in the first and second trimester were higher than those in the third trimester (P<0.017).
Table 3 shows the results of the cervical cytology examination of pregnant women and the difference among the 3 trimesters. There was an overall significant difference among the 3 trimesters (P<0.05) and also between the first and the third trimesters (P=0.017), but there was no statistically significant difference between the first and the second trimesters or the second and the third trimesters.
CORRELATION BETWEEN THE VAGINAL DISCHARGE EXAMINATION AND HR-HPV INFECTION IN PREGNANT WOMEN:
Tables 4 and 5 show the results of the vaginal discharge examination. The positive rate of HR-HPV in pregnant women with a vaginal discharge pH level >4.5 was higher than those with pH ≤4.5 (P<0.05). The positive rate of HPV-16 in pregnant women with pH >4.5 was significantly higher than that of the pregnant women with pH ≤4.5 (P<0.05). There was no significant difference between the HPV-18/45-positive rates in the 2 groups. The HPV-16/18/45-positive rate in pregnant women with a pH level >4.5 was higher than that of the pregnant women with a pH ≤4.5 (P<0.05). The non-HPV-16-positive or non-HPV-18/4-positive rates in pregnant women with a pH >4.5 were significantly higher than in the pregnant women with a pH ≤4.5 (P<0.05). The positive rate of HR-HPV in pregnant women with concurrent vaginal infection was significantly higher than that in pregnant women without concurrent infection (P<0.05). The HPV-16-positive rate, HPV-18/45-positive rate, and non-HPV-16/18/45-positive rate of pregnant women with concurrent vaginal infection were higher than those of pregnant women without concurrent infection (P<0.05).
CORRELATION BETWEEN THE RESULT OF CERVICAL CYTOLOGICAL EXAMINATION AND HR-HPV INFECTION IN PREGNANT WOMEN:
Table 6 shows the HR-HPV test results of women with different cervical cytological diagnoses. Among the 1774 pregnant women, the cytological examination results of 1701 (95.9%) patients were negative for intraepithelial lesion or malignancy (NILM), 46 (2.6%) patients had atypical squamous cells of undetermined significance (ASCUS), 21 (1.2%) patients had low-grade squamous intraepithelial lesions (LSIL), 4 (0.2%) patients had atypical squamous cells, which cannot exclude high-grade squamous intraepithelial lesions (HSIL) (ASC-H), and 2 (0.1%) patients had HSIL. The positive rate of HR-HPV and the total positive rate of HPV-16 and HPV-18/45 increased significantly with cervical cytological diagnosis (P<0.05).
Among the 1774 pregnant women, 390 (22.0%) had vaginal bleeding from the cervix when cervical exfoliation cytology samples were taken. The blood volume was about 3 mL to 7 mL, and bleeding stopped within 5 min after pressure was applied with a cotton swab. There were no cases of miscarriage or infection. A total of 262 of the pregnant women were HR-HPV-positive and/or had an abnormal cytological examination, among which 6 had LSIL and were HR-HPV-16/18/45-positive, and another 6 had ASC-H or worse. All 12 of these patients refused colposcopy examination during pregnancy for fear of miscarriage. Seventy-one women had not delivered at the time of this report, and 58 pregnant women were lost to follow-up for reasons including phone number changes and delivering at local hospitals. Seventy-nine patients were reviewed for HPV and/or cytological examination on the 42nd postpartum day, and the results of those who previously had cytological examination results of ASCUS had all changed to NILM. Sixty-five patients changed to HR-HPV-negative, 10 patients were still non-HPV-16/18/45-positive, 1 patient who had been HPV-16-positive changed to non-HPV-16/18/45-positive, and 3 patients who were non-HPV-16/18/45-positive became HPV-16-positive. Fifty-four patients underwent colposcopy examination at 1 to 3 months postpartum, and a cervical biopsy was performed when necessary. Among them, 38 patients’ colposcopy examinations indicated cervicitis, 9 indicated LSIL, and 8 indicated HSIL. These patients underwent further cervical conization, and the postoperative specimens were consistent with the colposcopy-directed biopsy.
Discussion
The positive rate of HPV varies greatly in many studies due to differences in the included population, detection method, and experimental design. Research shows that the HPV-positive rate in the general population in some areas of China ranges from 13.3% to 18.4% [8], while the reported HPV-positive rate in the reproductive tract in pregnant women is between 5.4% and 37.2% [9]. To date, many observational studies reported HPV infection during pregnancy, but the results are inconsistent. Some studies reported that the infection rate of HPV in pregnant women is higher than that in non-pregnant women [10] because of the mild immunosuppressive state during pregnancy. However, other reports showed no difference in the HPV-positive rate between pregnant and non-pregnant women [11]. The positive rate of HR-HPV in pregnant women in the present study was 13.6%, which is similar to the 12.5% of 1183 pregnant women reported by Takakuwa et al [12], who also showed no significant difference in the HR-HPV-positive rate of 12.8% in non-pregnant women. In the present study, the positive rate of HR-HPV in pregnant women in the age group of 20 to 24 years was higher than that of non-pregnant women in the same age group. The rate of single HPV-16-positive and overall HPV-16/18/45-positive rates in pregnant women were higher than that in non-pregnant women. Schulze et al [13] reported that the positive rate of HR-HPV during pregnancy, especially of HPV-16, was significantly higher than that of non-pregnant women. This is consistent with the results of our study. The frequency of sexual activity during pregnancy is not high, so the reason for the increase of the positive rate of HPV-16 and other HR-HPVs may be the increase in pregnancy-related hormones and the selective activation of these viruses by immune factors, or the changes in the hormone environment and immune response may be beneficial to HPV infection and its persistent existence [14].
Our study showed that there were statistically significant differences in the HR-HPV-positive rate, HPV-16-positive rate, HPV-16/18/45-positive rate, and non-HPV-16/18/45-positive rate among pregnant women in all age groups. Both the HR-HPV-positive rate and HPV-16-single-positive rate were highest in the age group of 20 to 24 years. Some studies found that HPV infection was negatively correlated with age. The highest positivity rate is observed in 20- to 24-year-old women, and the rates decrease with age [9,15]. Takakuwa et al [12] also confirmed that the HPV infection rate of pregnant women <25 years is significantly higher than that of the women aged >25 years, the same phenomenon seen in non-pregnant women, which may be due to more frequent sexual activity or more sexual partners in this age group than in older women. Some studies [5] found that there was a double-peak of HPV infection. The first peak appears in women < 25 years old, and the second peak appears in women ≥44 years old. One reason for the second peak in non-pregnant women may be changes in hormone levels during the perimenopausal period. Another reason may be changes in sexual behaviors of women of this age group and their partners. A woman’s pregnancy occurs during a specific age span. Few women give birth over the age of 45, and not many women given birth over the age of 40. In this study, there were 69 patients in the age group of 40 to 44 years, with a positive rate of HR-HPV of 13%.
Some studies have reported that the positive rates of HPV in the first and second trimesters are not higher than those in the third trimester [11]. Banura et al [16] reported that the positive rates of HPV in the first and second trimester were comparable to those in the third trimester, while Liu et al [9] reported that the infection rates of HPV in the 3 trimesters showed a “V” trend. However, in the present study, the positive rates of HR-HPV were significantly higher in the first and second trimesters than they were in the third trimester, and were the highest in the second trimester. This may be correlated to the decrease of the immune response to HPV infection in the first and second trimesters and the rebound of the immune response to HPV infection and the increased clearance ability in the third trimester. However, the HPV infection rate in each trimester is still controversial, and more studies are needed.
Vaginal microbiota play an important role in protecting women’s health. A healthy vaginal microbiome is mainly composed of
In this study, the rate of cervical cytologic examination abnormality in pregnant women was 4.1%, which is consistent with the 2% to 7% abnormal rate reported by Hunter et al [25]. Cytology abnormality in our study was 4.7% in the first trimester and 4.4% in the second trimester, which are comparable to most studies; however, the 1.0% we found in the third trimester was significantly lower than that found in most studies. There were fewer women in the third trimester included in the present study for various reasons; therefore, this group may not be representative, owing to possible selection bias. Non-pregnant women in our study had a rate of cytology abnormalities of 6.6%, higher than that of women during pregnancy. These results are consistent with the research by Wu et al [26]. The positive rate of HR-HPV and the total positive rate of HPV-16/18/45 in the present study were significantly increased with the upgrading of cytological diagnosis. This result is consistent with the results of most studies in non-pregnant women [27]. However, there are few large-scale studies on combined cytologic examination and HPV screening in pregnant women. Although the increased estrogen level during pregnancy moves the cervical columnar epithelium out to the portio vaginalis of the cervix, the basal cells in the cervical transformation zone can have nuclear enlargement and deep dyeing, and the transformation zone is more likely to change under the stimulation by physical damage, infection, and changes in vaginal pH level. This change is easily confused with cervical glandular epithelial cell atypia and increases the diagnosis rate of cervical intraepithelial neoplasia; however, the cytological examination during pregnancy is equivalent to that in the non-pregnancy period [28]. Brun-Micaleff et al [29] also reported that cervical cytological examination during pregnancy was effective and worth recommending.
Conclusions
Implementing population-based screening programs can significantly reduce cancer mortality and morbidity, especially for breast cancer, cervical cancer, and colorectal cancer [30]. In recent years, the age of occurrence of cervical cancer has become lower. For women who do not participate in a cervical cancer screening program, pregnancy is an opportunity for screening, with the prenatal examination clinics being ideal screening places. Pregnancy is a special physiological state, which is more likely to be affected by HR-HPV; therefore, it is most important to receive a cervical cancer screening consisting of combined cytologic examination and HR-HPV testing during pregnancy. However, this study was a cross-sectional study, and the persistence, clearance rate, and consequences of HPV infection are not fully elucidated. For patients with positive HR-HPV and/or an abnormal TCT, we need to perform further follow-up to guide the treatment of abnormal cervical cancer screening results during and after pregnancy.
Figures
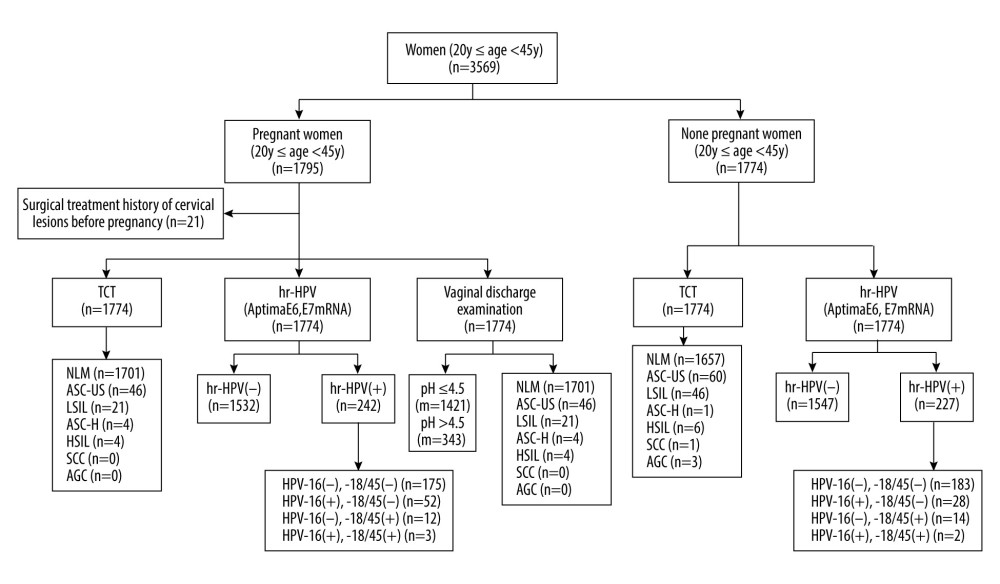 Figure 1. Flow chart of the test. NILM – no intraepithelial lesion or malignancy; ASCUS – abnormal squamous cells of uncertain significance; LSIL – low-grade squamous intraepithelial lesion; ASC-H – atypical squamous cells, cannot exclude HSIL; HSIL – high-grade squamous intraepithelial lesion; SCC – squamous cell carcinoma; AGC – atypical glandular cells; VVC – vulvovaginal candidiasis; BV – bacterial vaginosis; TV – trichomonas vaginalis.
Figure 1. Flow chart of the test. NILM – no intraepithelial lesion or malignancy; ASCUS – abnormal squamous cells of uncertain significance; LSIL – low-grade squamous intraepithelial lesion; ASC-H – atypical squamous cells, cannot exclude HSIL; HSIL – high-grade squamous intraepithelial lesion; SCC – squamous cell carcinoma; AGC – atypical glandular cells; VVC – vulvovaginal candidiasis; BV – bacterial vaginosis; TV – trichomonas vaginalis. 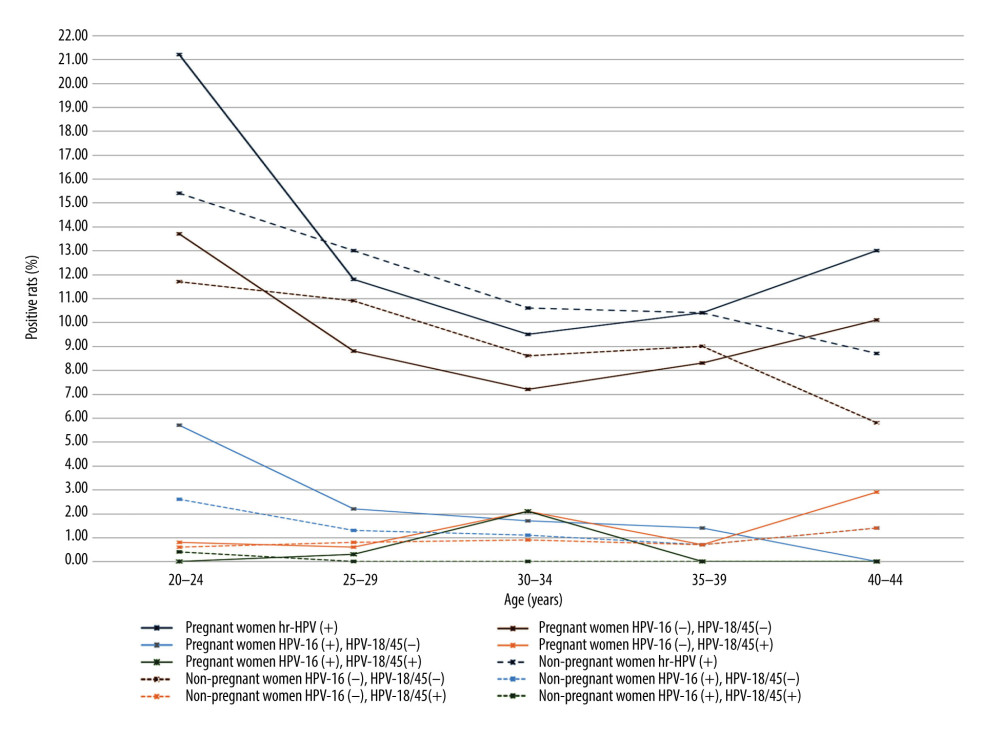 Figure 2. The positive rates of high-risk human papillomavirus (HR-HPV) and HPV subtype 16/18/45 in pregnant and non-pregnant women.
Figure 2. The positive rates of high-risk human papillomavirus (HR-HPV) and HPV subtype 16/18/45 in pregnant and non-pregnant women. 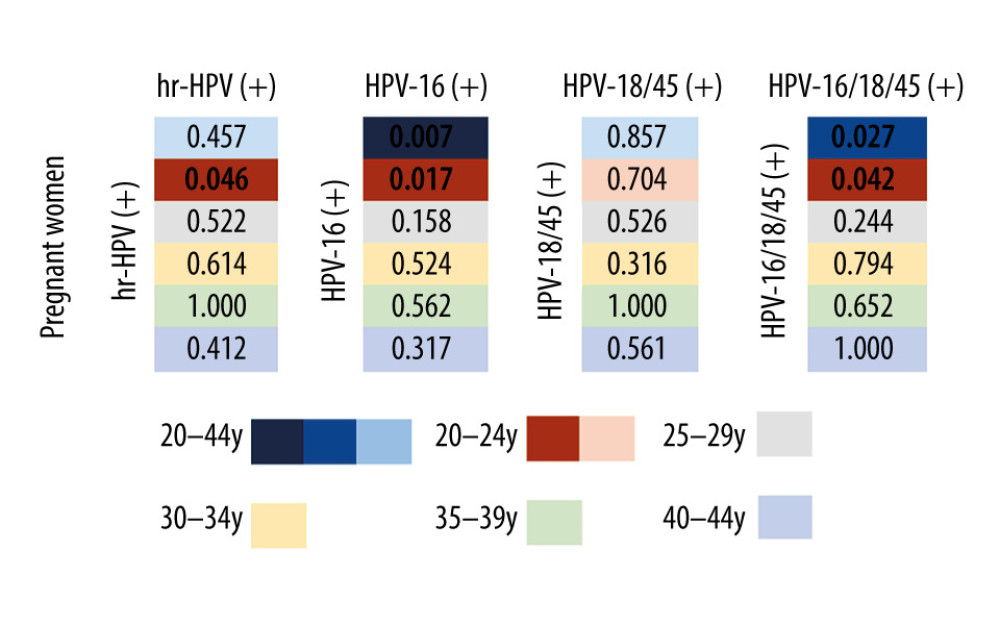 Figure 3. The differences in the positive rates of high-risk human papillomavirus (HR-HPV) and HPV subtype 16/18/45 between pregnant and non-pregnant women in each age group.
Figure 3. The differences in the positive rates of high-risk human papillomavirus (HR-HPV) and HPV subtype 16/18/45 between pregnant and non-pregnant women in each age group. 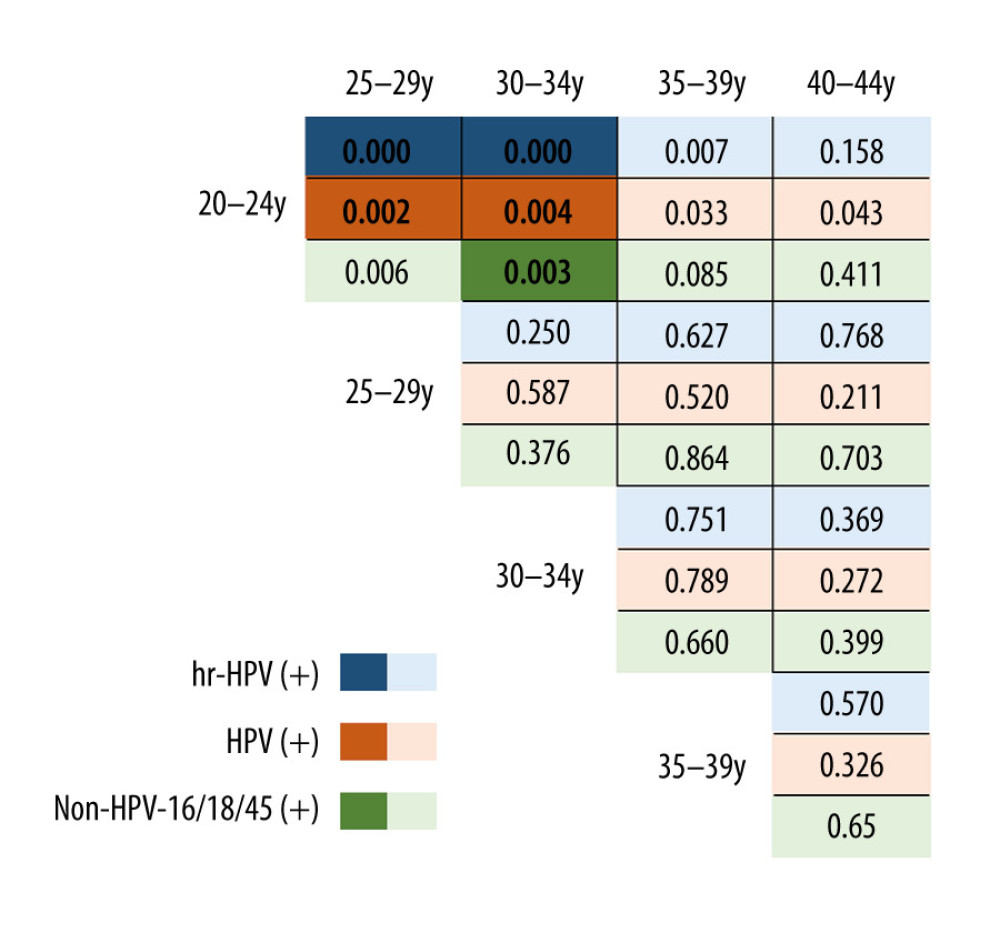 Figure 4. The difference in the positive rate of high-risk human papillomavirus (HR-HPV), HPV subtype 16 (HPV-16) and non-HPV-16/18/45 in pregnant women by age group.
Figure 4. The difference in the positive rate of high-risk human papillomavirus (HR-HPV), HPV subtype 16 (HPV-16) and non-HPV-16/18/45 in pregnant women by age group. Tables
Table 1. Cervical cytology examination results of pregnant women and non-pregnant women by different age groups.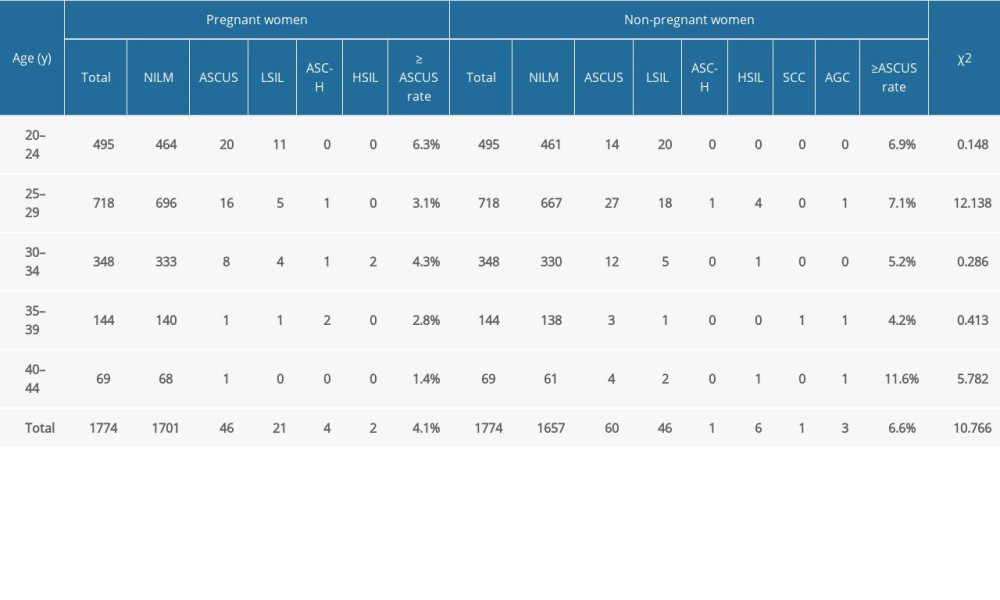 Table 2. Results of high-risk human papillomavirus testing in pregnant women in different gestational weeks.
Table 2. Results of high-risk human papillomavirus testing in pregnant women in different gestational weeks.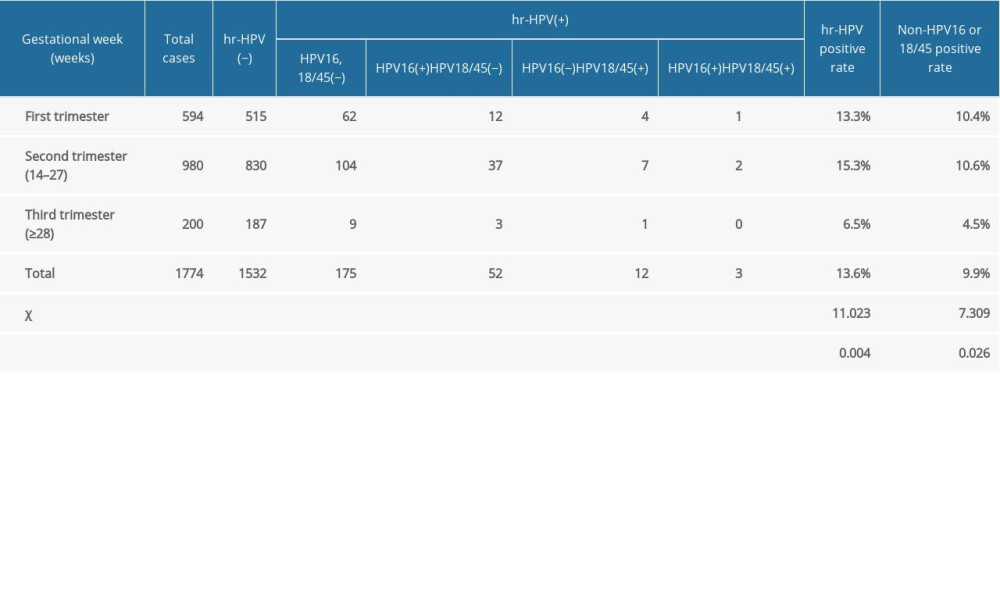 Table 3. Results of cervical cytology examination of pregnant women at different gestational weeks.
Table 3. Results of cervical cytology examination of pregnant women at different gestational weeks.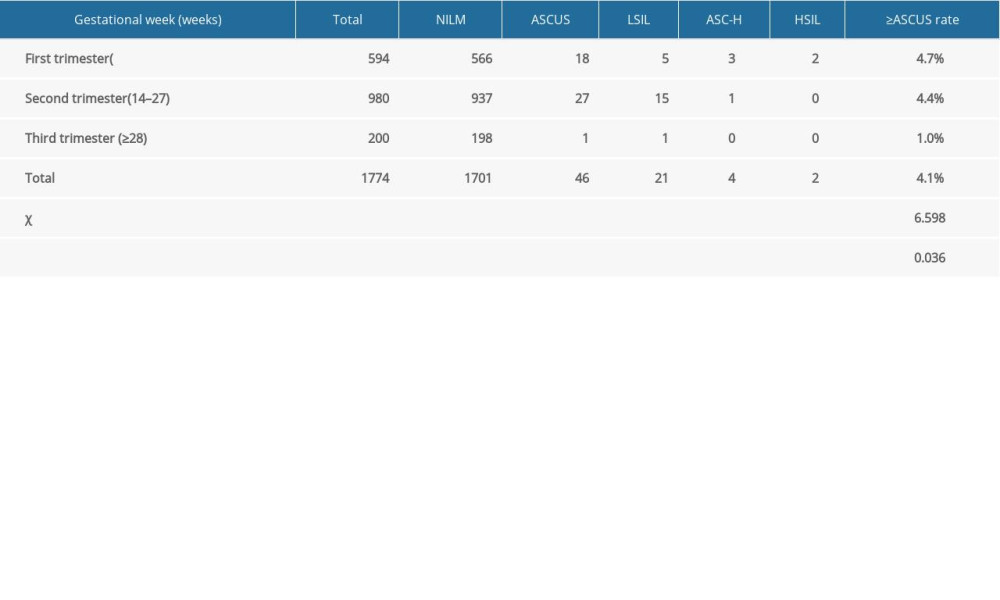 Table 4. The effect of pH value of vaginal secretion on high-risk human papillomavirus infection in pregnant women.
Table 4. The effect of pH value of vaginal secretion on high-risk human papillomavirus infection in pregnant women.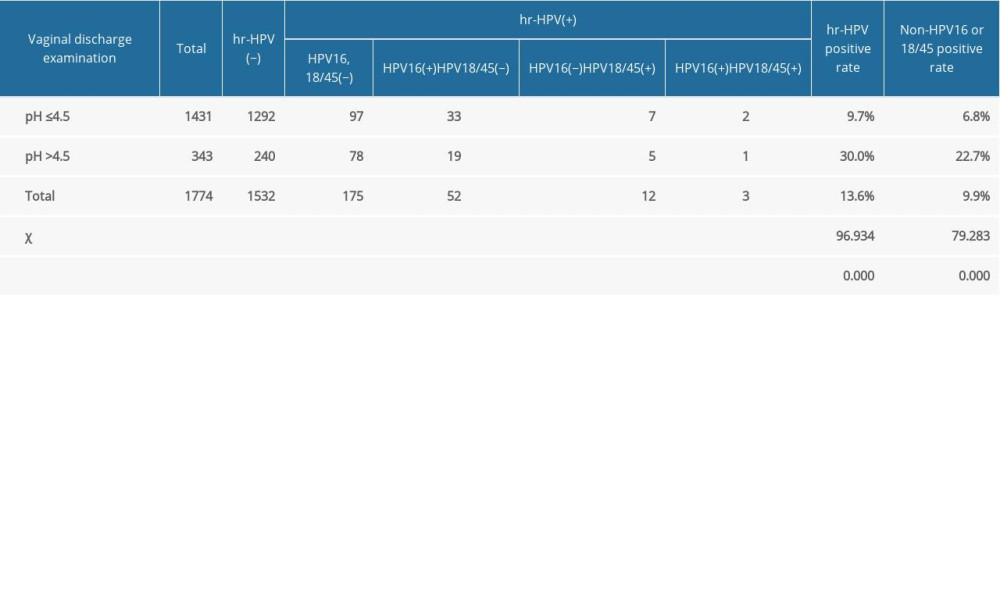 Table 5. The influence of vaginal secretion infection on high-risk human papillomavirus infection in pregnant women.
Table 5. The influence of vaginal secretion infection on high-risk human papillomavirus infection in pregnant women.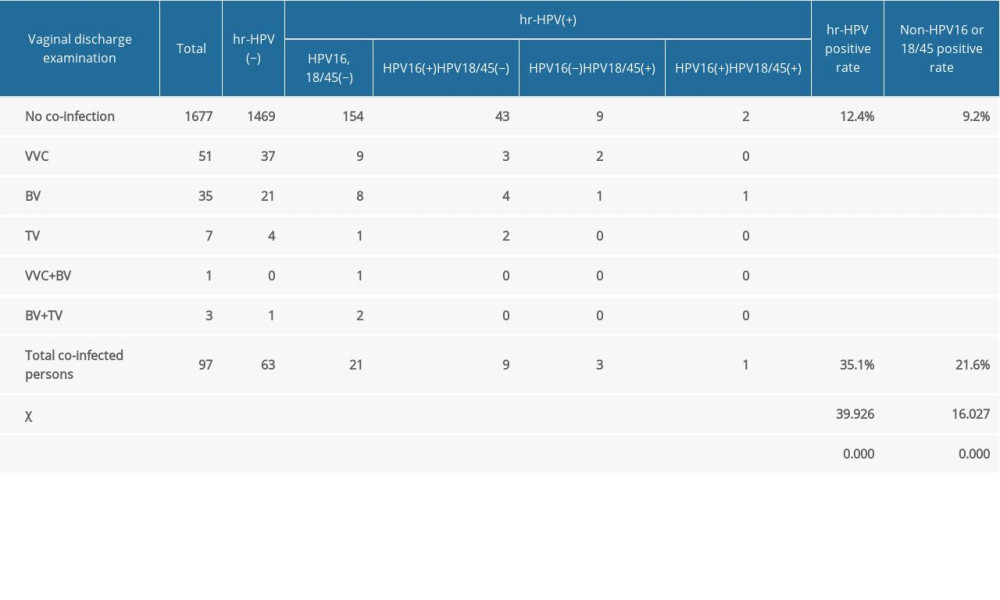 Table 6. High-risk human papillomavirus test results of women with different cervical cytological diagnoses.
Table 6. High-risk human papillomavirus test results of women with different cervical cytological diagnoses.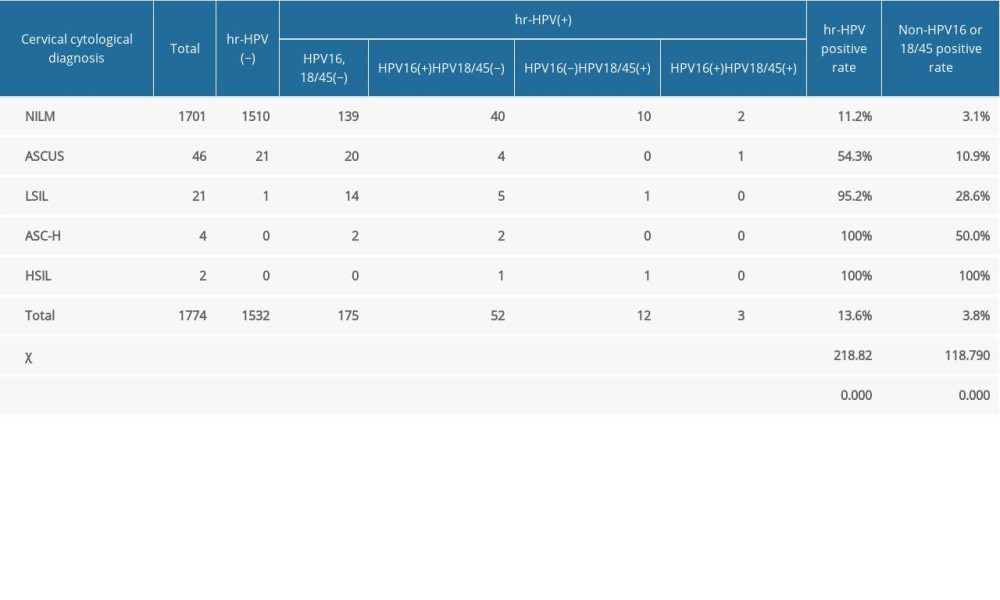
References
1. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68(6); 394-424
2. Li S, Hu T, Lv W, Changes in prevalence and clinical characteristics of cervical cancer in the People’s Republic of China: A study of 10,012 cases from a nationwide working group: Oncologist, 2013; 18(10); 1101-7
3. Kim MA, Han GH, Kim JH, Current status of human papillomavirus infection and introduction of vaccination to the national immunization program in Korea: An Overview: J Korean Med Sci, 2018; 33(52); e331
4. Beachler DC, Gonzales FA, Kobrin SC, HPV vaccination initiation after the routine-recommended ages of 11–12 in the United States: Papillomavirus Res, 2016; 2; 11-16
5. de Sanjosé S, Diaz M, Castellsagué X, Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis: Lancet Infect Dis, 2007; 7(7); 453-59
6. Nayar R, Wilbur DC, The Pap test and Bethesda 2014: Cancer Cytopathol, 2015; 123(5); 271-81
7. Ye Y, Wang Y, Shen Z: National guide to clinical laboratory procedures, 2006, Nanjing, Southeast University Press
8. Baloch Z, Li Y, Yuan T, Epidemiologic characterization of human papillomavirus (HPV) infection in various regions of Yunnan Province of China: BMC Infect Dis, 2016; 16; 228
9. Liu P, Xu L, Sun Y, The prevalence and risk of human papillomavirus infection in pregnant women: Epidemiol Infect, 2014; 142(8); 1567-78
10. Aydin Y, Atis A, Tutuman T, Prevalence of human papilloma virus infection in pregnant Turkish women compared with non-pregnant women: Eur J Gynaecol Oncol, 2010; 31(1); 72-74
11. Schmeink CE, Melchers WJ, Hendriks JC, Human papillomavirus detection in pregnant women: A prospective matched cohort study: J Womens Health (Larchmt), 2012; 21(12); 1295-301
12. Takakuwa K, Mitsui T, Iwashita M, Studies on the prevalence of human papillomavirus in pregnant women in Japan: J Perinat Med, 2006; 34(1); 77-79
13. Schulze MH, Völker FM, Lugert R, High prevalence of human papillomaviruses in Ghanaian pregnant women: Med Microbiol Immunol, 2016; 205(6); 595-602
14. Pandey D, Solleti V, Jain G, Human papillomavirus (HPV) infection in early pregnancy: Prevalence and implications: Infect Dis Obstet Gynecol, 2019; 2019; 4376902
15. Bell MC, Schmidt-Grimminger D, Patrick S, There is a high prevalence of human papillomavirus infection in American Indian women of the Northern Plains: Gynecol Oncol, 2007; 107(2); 236-41
16. Banura C, Franceschi S, van Doorn LJ, Prevalence, incidence and clearance of human papillomavirus infection among young primiparous pregnant women in Kampala, Uganda: Int J Cancer, 2008; 123(9); 2180-87
17. Kero K, Rautava J, Syrjänen K, Association of asymptomatic bacterial vaginosis with persistence of female genital human papillomavirus infection: Eur J Clin Microbiol Infect Dis, 2017; 36(11); 2215-19
18. Guo YL, You K, Qiao J, Bacterial vaginosis is conducive to the persistence of HPV infection: Int J STD AIDS, 2012; 23(8); 581-84
19. Gillet E, Meys JF, Verstraelen H, Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: A meta-analysis: BMC Infect Dis, 2011; 11; 10
20. Chen Y, Hong Z, Wang W, Association between the vaginal microbiome and high-risk human papillomavirus infection in pregnant Chinese women: BMC Infect Dis, 2019; 19(1); 677
21. Nasioudis D, Linhares IM, Ledger WJ, Bacterial vaginosis: A critical analysis of current knowledge: BJOG, 2017; 124(1); 61-69
22. Clarke MA, Rodriguez AC, Gage JC, A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection: BMC Infect Dis, 2012; 12; 3
23. DiGiulio DB, Callahan BJ, McMurdie PJ, Temporal and spatial variation of the human microbiota during pregnancy: Proc Natl Acad Sci USA, 2015; 112(35); 11060-65
24. Dareng EO, Ma B, Famooto AO, Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women: Epidemiol Infect, 2016; 144(1); 123-37
25. Hunter MI, Monk BJ, Tewari KS, Cervical neoplasia in pregnancy. Part 1: Screening and management of preinvasive disease: Am J Obstet Gynecol, 2008; 199(1); 3-9
26. Wu X, Huang XH, Zhang WY, Fluid-based thin-layer method for screening of squamous intraepithelial lesions in pregnant women: Zhonghua Fu Chan Ke Za Zhi, 2006; 41(10); 689-92 [in Chinese]
27. Wang J, Du Y, Dong J, Clinical significance of genotyping for human papillomavirus (HPV) 16 18/45 combined with cytology in cervical exfoliated cells in HPV oncogenic mRNA-positive women: Gynecol Oncol, 2019; 153(1); 34-40
28. Origoni M, Salvatore S, Perino A, Cervical Intraepithelial Neoplasia (CIN) in pregnancy: The state of the art: Eur Rev Med Pharmacol Sci, 2014; 18(6); 851-60
29. Brun-Micaleff E, Coffy A, Rey V, Cervical cancer screening by cytology and human papillomavirus testing during pregnancy in French women with poor adhesion to regular cervical screening: J Med Virol, 2014; 86(3); 536-45
30. Feng RM, Zong YN, Cao SM, Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics: Cancer Commun (Lond), 2019; 39(1); 22
Figures
 Figure 1. Flow chart of the test. NILM – no intraepithelial lesion or malignancy; ASCUS – abnormal squamous cells of uncertain significance; LSIL – low-grade squamous intraepithelial lesion; ASC-H – atypical squamous cells, cannot exclude HSIL; HSIL – high-grade squamous intraepithelial lesion; SCC – squamous cell carcinoma; AGC – atypical glandular cells; VVC – vulvovaginal candidiasis; BV – bacterial vaginosis; TV – trichomonas vaginalis.
Figure 1. Flow chart of the test. NILM – no intraepithelial lesion or malignancy; ASCUS – abnormal squamous cells of uncertain significance; LSIL – low-grade squamous intraepithelial lesion; ASC-H – atypical squamous cells, cannot exclude HSIL; HSIL – high-grade squamous intraepithelial lesion; SCC – squamous cell carcinoma; AGC – atypical glandular cells; VVC – vulvovaginal candidiasis; BV – bacterial vaginosis; TV – trichomonas vaginalis. Figure 2. The positive rates of high-risk human papillomavirus (HR-HPV) and HPV subtype 16/18/45 in pregnant and non-pregnant women.
Figure 2. The positive rates of high-risk human papillomavirus (HR-HPV) and HPV subtype 16/18/45 in pregnant and non-pregnant women. Figure 3. The differences in the positive rates of high-risk human papillomavirus (HR-HPV) and HPV subtype 16/18/45 between pregnant and non-pregnant women in each age group.
Figure 3. The differences in the positive rates of high-risk human papillomavirus (HR-HPV) and HPV subtype 16/18/45 between pregnant and non-pregnant women in each age group. Figure 4. The difference in the positive rate of high-risk human papillomavirus (HR-HPV), HPV subtype 16 (HPV-16) and non-HPV-16/18/45 in pregnant women by age group.
Figure 4. The difference in the positive rate of high-risk human papillomavirus (HR-HPV), HPV subtype 16 (HPV-16) and non-HPV-16/18/45 in pregnant women by age group. Tables
 Table 1. Cervical cytology examination results of pregnant women and non-pregnant women by different age groups.
Table 1. Cervical cytology examination results of pregnant women and non-pregnant women by different age groups. Table 2. Results of high-risk human papillomavirus testing in pregnant women in different gestational weeks.
Table 2. Results of high-risk human papillomavirus testing in pregnant women in different gestational weeks. Table 3. Results of cervical cytology examination of pregnant women at different gestational weeks.
Table 3. Results of cervical cytology examination of pregnant women at different gestational weeks. Table 4. The effect of pH value of vaginal secretion on high-risk human papillomavirus infection in pregnant women.
Table 4. The effect of pH value of vaginal secretion on high-risk human papillomavirus infection in pregnant women. Table 5. The influence of vaginal secretion infection on high-risk human papillomavirus infection in pregnant women.
Table 5. The influence of vaginal secretion infection on high-risk human papillomavirus infection in pregnant women. Table 6. High-risk human papillomavirus test results of women with different cervical cytological diagnoses.
Table 6. High-risk human papillomavirus test results of women with different cervical cytological diagnoses. Table 1. Cervical cytology examination results of pregnant women and non-pregnant women by different age groups.
Table 1. Cervical cytology examination results of pregnant women and non-pregnant women by different age groups. Table 2. Results of high-risk human papillomavirus testing in pregnant women in different gestational weeks.
Table 2. Results of high-risk human papillomavirus testing in pregnant women in different gestational weeks. Table 3. Results of cervical cytology examination of pregnant women at different gestational weeks.
Table 3. Results of cervical cytology examination of pregnant women at different gestational weeks. Table 4. The effect of pH value of vaginal secretion on high-risk human papillomavirus infection in pregnant women.
Table 4. The effect of pH value of vaginal secretion on high-risk human papillomavirus infection in pregnant women. Table 5. The influence of vaginal secretion infection on high-risk human papillomavirus infection in pregnant women.
Table 5. The influence of vaginal secretion infection on high-risk human papillomavirus infection in pregnant women. Table 6. High-risk human papillomavirus test results of women with different cervical cytological diagnoses.
Table 6. High-risk human papillomavirus test results of women with different cervical cytological diagnoses. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








