09 May 2021: Clinical Research
Epidemiology of Influenza Viruses and Viruses Causing Influenza-Like Illness in Children Under 14 Years Old in the 2018–2019 Epidemic Season in Poland
Katarzyna Kondratiuk1ACDEF*, Ewelina Hallmann1CDEF, Katarzyna Łuniewska1CDEF, Karol Szymański1BCDEF, Lidia Brydak1AEFGDOI: 10.12659/MSM.929303
Med Sci Monit 2021; 27:e929303
Abstract
BACKGROUND: This study aimed to investigate the epidemiology of influenza viruses and viruses that caused influenza-like disease in children under 14 years of age in the 2018-2019 epidemic season in Poland, and to identify the public health lessons that can be learned.
MATERIAL AND METHODS: Nose and throat swabs were used to obtain samples. The samples were analyzed in the National Influenza Center, Department of Influenza Research at the National Institute of Public Health-National Institute of Hygiene as well as in 16 Voivodship Sanitary Epidemiological Stations across the country. Methods of RNA isolation depended on the laboratory where the isolation was performed. In all laboratories, quantitative polymerase chain reactions were used to determine the influenza virus type as well as the subtype.
RESULTS: The study group was confirmed to be infected with influenza A and B, with influenza A/H1N1/pdm09 as the dominant subtype. Among the age group of children up to 14 years of age, cases of infection with viruses that cause influenza-like disease were also reported. It was noticeable that the largest number of confirmed cases of infection was recorded in the group of the youngest children (0-4 years). In addition, several different variants of co-infection were registered.
CONCLUSIONS: This population study showed that in the 2018-2019 epidemic season in Poland children aged under 14 years were at risk of influenza virus infection and its complications. The presented data support increasing the percentage of children being vaccinated in Poland.
Keywords: Epidemiology, Influenza, Human, Respiratory Syncytial Virus, Human, Influenza A virus, Influenza B virus, Adolescent, Age Distribution, Child, Child, Preschool, coinfection, epidemics, Infant, Infant, Newborn, Influenza A Virus, H1N1 Subtype, Poland, Seasons
Background
Influenza is an acute disease of viral etiology, characterized by very high contagiousness. It poses a threat to societies owing to its global spread, ability to change its antigenic properties, and severity of post-influenza complications, which often require hospitalization and may even lead to death.
People of all ages are at risk of contracting influenza virus. Children are particularly at risk owing to the immaturity of their immune system. For the same reason, they are also at risk of complications from influenza; this applies especially to children with impaired immunity, those with chronic diseases, and children under 2 years of age. Influenza can be severe even in a previously healthy child who does not have risk factors for a complicated course of the disease. For many years, it has been thought that only young children with comorbidities could be severely affected by influenza. However, severe cases of this disease also occur among healthy children. In a study conducted in the United States from October 2004 to September 2012, a total of 830 deaths of children and adolescents caused by influenza complications were reported, and 43% of the children did not have comorbidities [1].
Influenza infections usually occur in children because of the ease of transmission of influenza viruses within groups of children and the lack of immunological memory of influenza viruses circulating in the population in earlier epidemic seasons.
Infection in children is spread mainly by droplets through mucus aerosol containing viruses or indirectly through objects contaminated with secretions from the respiratory system of a sick person. During direct contact, the virus is transmitted by inhalation of microscopic secretions from the respiratory tract of the infected person, and it is the most infectious in the symptomatic period of infection. A child can transmit the virus for longer than 10 days, while young children can transmit the virus for up to 6 days before the symptoms appear. The onset of influenza is sudden and acute. The characteristic symptoms in children include a high fever, even above 39°C, chills, headache, muscle aches, cough, general weakness, and malaise. The course of influenza infection in children is also characterized by gastrointestinal symptoms, including abdominal pain, vomiting, and diarrhea. Otitis media may also be present [2]. It is worth emphasizing that the above-mentioned symptoms are not specific only to influenza infection, but also to infections caused by other respiratory viruses that cause influenza-like disease, including parainfluenza 1–3, human respiratory syncytial virus (RSV), human corona viruses 229E/NL63 and OC42, human rhinovirus, human adenovirus, and human metapneumovirus, which makes it difficult for a doctor to make an accurate diagnosis [3].
Influenza complications can be very serious; they include pneumonia and bronchitis, secondary bacterial pneumonia and bronchiolitis, streptococcal pharyngitis, exacerbation of chronic diseases (asthma, cystic fibrosis, diabetes, chronic kidney failure), muscle pain, myocarditis, myocarditis and pericarditis, meningitis or encephalitis, otitis media, auditory receptor dysfunction, partial hearing loss or deafness, neurological complications including Guillain-Barré syndrome, transverse myelitis, worsening of seizures, and graft rejection [2].
Various types of influenza vaccine are used to prevent influenza. In Poland, since the last epidemic season of 2019–2020, inactivated vaccines, known as split or split virion vaccines, and subunit vaccines, containing isolated surface antigens and known as subunit and virion-weakened live vaccines (intranasal), were used. Split influenza vaccines are administered intramuscularly to every child from 6 months of age, provided there are no medical contraindications. Subunit vaccines, also administered intramuscularly, are recommended for children from the age of 3 years. In November 2019, it became possible to vaccinate children and adolescents against influenza with live attenuated intranasal vaccines. The vaccine is intended for the active immunization of children and adolescents from 24 months to 17 years of age. The preparation is in the form of nasal spray [4,5]. Children play a major role in spreading influenza, so increasing the number of vaccinations among them helps to cut the transmission of the disease and reduce its spread among household members. Therefore, vaccinating children against influenza protects not only those who are vaccinated, but may also indirectly protect their relatives, such as younger siblings, parents, and grandparents. Unfortunately, flu vaccination in children in Poland is used far too rarely. Children from 2 to 5 years of age are the most vaccinated group, and infants are least frequently vaccinated, despite it being known that the youngest children are most at risk for developing complications from influenza.
Therefore, this study aimed to investigate the epidemiology of influenza viruses and viruses that caused influenza-like illness in children under 14 years of age in the 2018–2019 epidemic season in Poland, and to identify the public health lessons that can be learned.
Material and Methods
ISOLATION OF RNA:
RNA was isolated from the nasal and pharyngeal swabs collected from the patients, which were suspended in 1 mL of saline. For RNA isolation, the Maxwell 16 Total Viral Nucleic Acid Purification kit (Promega Corporation, Madison, WI, USA) was used to isolate the genetic material at the Department of Influenza Research of the NIPH-NIH. The isolation was performed from a 200-μL sample suspended in saline, following the manufacturer’s instructions. Elution of the RNA was performed using 50 μL of RNase-free water.
REAL-TIME POLYMERASE CHAIN REACTION:
Quantitative polymerase chain reaction was used to determine the influenza virus type and subtype. At the Department of Influenza Research of the NIPH-NIH, the reaction was performed using Rotor-Gene Q (Qiagen) and the SuperScript Platinum III kit (Invitrogen). Primer and probe kits (influenza A, influenza A/H3N2/, influenza A/H1N1/pdm09, and influenza B) obtained from the International Reagent Resource (IRR) of the Centers for Disease Control and Prevention were used. The sequences of the primers and probes from IRR were not publicly available. RNA was subjected to reverse transcription (50°C for 30 min). After initiation (1 cycle at 95°C for 2 min), the DNA was subjected to 45 cycles of amplification: denaturation (95°C for 15 s), annealing (55°C for 30 s), and elongation (72°C for 20 s). The positive controls of the reactions were viruses derived from the vaccine for the 2018–2019 epidemic season (A/Michigan/45/2015, A/H1N1/pdm09, A/Singapore/INFIMH-16-0019/2016 (H3N2), and B/Phuket/3073/2013). The negative control was the RNase-free water provided in the kit. RNA of vaccine viruses selected by the World Health Organization were used as positive controls. The research was also carried out in the VSESs. The methods used are presented in Table 1.
STATISTICAL ANALYSIS:
We performed statistical tests to assess whether there were differences between age groups in relation to their vulnerability to various types of viruses. Treating an influenza infection as “statistical success” and a non-influenza infection as “statistical failure”, we were able to perform 2-sample Z-tests on the data.
Results
In the 2018–2019 epidemic season from October 2018 until the end of September 2019, a total of 994 samples from children up to 14 years of age were tested, and 230 samples (23.1%) came from the sentinel program. Confirmed cases accounted for 50.6% (503 samples) of all tested samples. There was a clear dominance of influenza A (447 cases) over influenza B (5 cases) among the infected patients. Among influenza A viruses, the majority were samples with influenza A/H1N1/pdm09 (308 samples, 68.9% of all the cases of influenza A). Only 3 cases of infection caused by influenza A/H3N2/virus (0.7%) were confirmed. This may corroborate the thesis that this virus subtype is most common in people over 65 years of age [6]. Among influenza A-positive cases, the remaining were influenza A viruses not subject to subtyping (136 samples, 30.4% of influenza A confirmations) (Figure 1).
In line with the innovation in influenza surveillance introduced by the NIPH-NIH, the results were also analyzed in the 3 smaller age groups (0–4 years, 5–9 years, and 10–14 years) [7]. The highest number of infections among all 3 age groups was in the youngest children (0–4 years) for both influenza A (253 patients) and influenza B (4 patients). In children aged 5 to 9 years, 154 infections with influenza A and 1 infection with influenza B were recorded. In children aged 10 to 14 years, 40 infections were confirmed to be due to influenza A and no infections due to influenza B were found (Figure 2).
In the children up to 14 years of age, cases of infection with viruses that cause influenza-like disease were also reported. Additional analysis was carried out in the 3 smaller age groups (0–4 years, 5–9 years, 10–14 years). The results showed that the largest number of confirmed cases of infection with viruses that cause influenza-like disease was recorded in the group of the youngest children (0–4 years). There were 43 cases (89.6%) of confirmed infections with viruses that cause influenza-like disease in children aged 0 to 4 years, while there were 2 cases (4.2%) in children aged 5 to 9 years, and there were 3 cases (6.2%) in children aged 10 to 14 years. RSV was the most common of the viruses causing influenza-like disease (27 cases). The highest number of infections with this virus was recorded in the age group 0–4 years. In this group, single cases of infections with other viruses that cause influenza-like disease, including 6 cases of human rhinovirus and 3 cases of parainfluenza 3, were confirmed (Figure 3).
In children under 14 years of age, in the 2018–2019 epidemic season, co-infections, defined as simultaneous infection with 2 or more respiratory viruses, were detected. These co-infections were influenza viruses and viruses that cause influenza-like disease. The registered co-infections are presented in Table 2.
Discussion
LIMITATIONS OF THE STUDY:
This work is based solely on the analyses of samples that were reported to the sentinel influenza surveillance system. Not all tested samples are reported to the system; therefore, the number of patients studied in Poland in given seasons could be much higher.
Conclusions
Influenza poses a severe threat to people of all age groups, including children. In every epidemic season, a large number of children and teenagers become ill or die from influenza or from post-influenza complications. We believe the most effective method of preventing this disease is vaccination. The presented data support that the rate of vaccination in Poland should be increased in children from 6 months to 14 years of age.
Figures
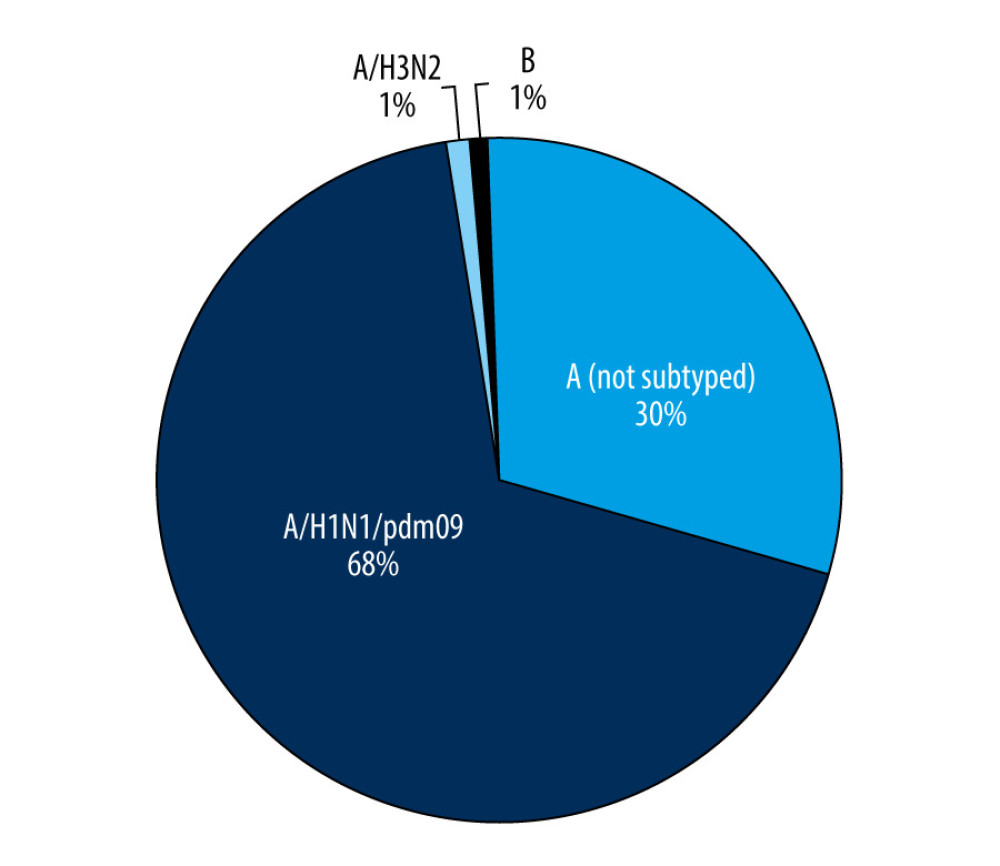 Figure 1. The number of confirmed cases of influenza in children aged under 14 years in the 2018–2019 influenza season in Poland.
Figure 1. The number of confirmed cases of influenza in children aged under 14 years in the 2018–2019 influenza season in Poland. 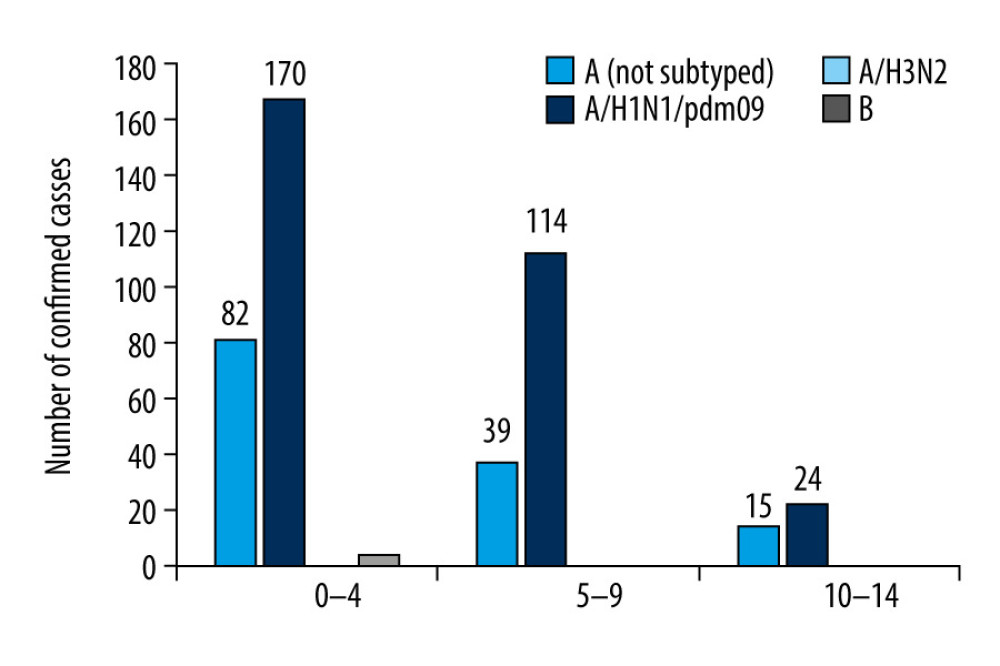 Figure 2. Participation of individual types and sub types of influenza viruses in children aged under 14 years in the 2018–2019 influenza season in Poland.
Figure 2. Participation of individual types and sub types of influenza viruses in children aged under 14 years in the 2018–2019 influenza season in Poland. 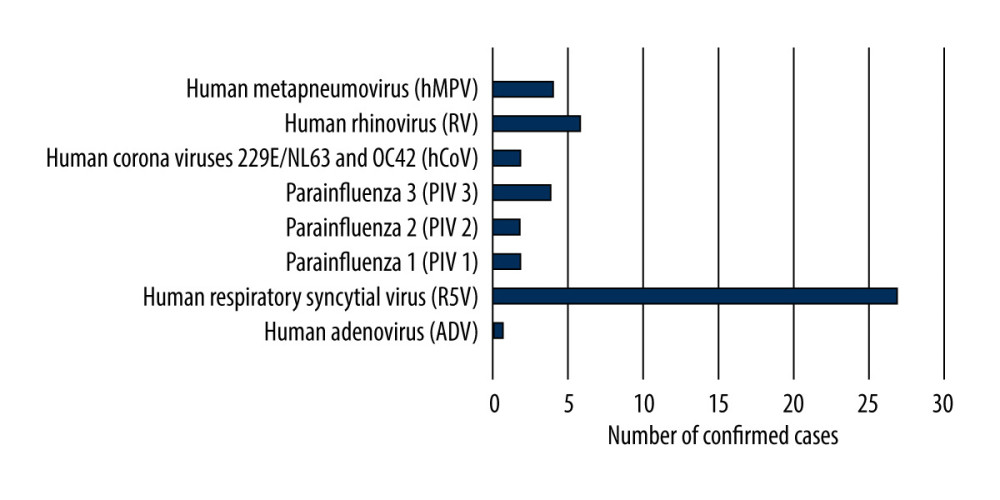 Figure 3. Number of viruses causing influenza-like illness detected in children aged under 14 years in the 2018–2019 epidemic season in Poland.
Figure 3. Number of viruses causing influenza-like illness detected in children aged under 14 years in the 2018–2019 epidemic season in Poland. 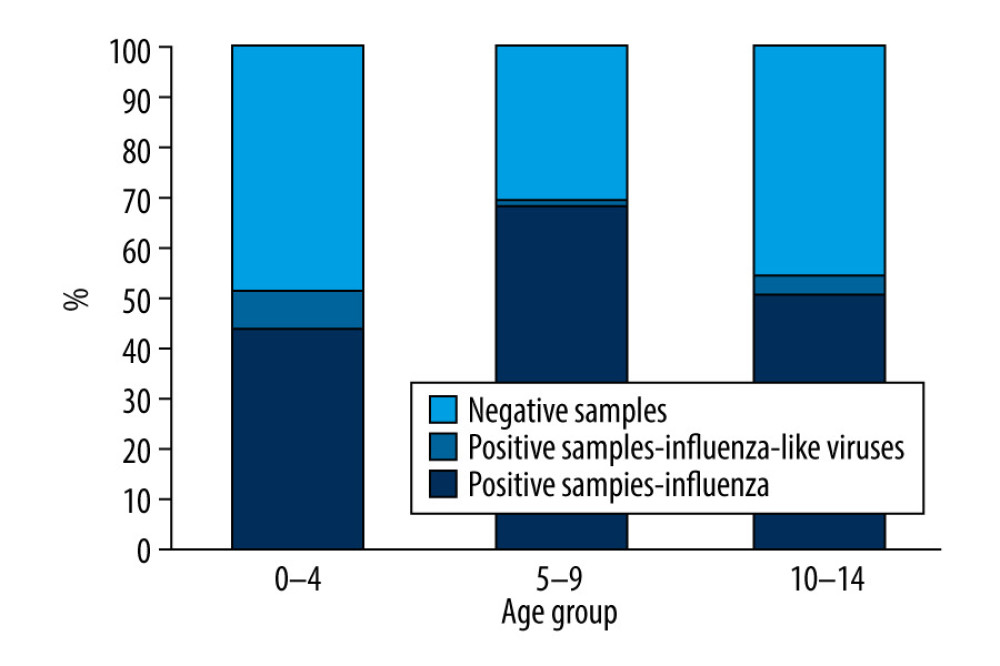 Figure 4. Percentage of samples positive in relation to all tested samples in detected in children aged under 14 years in the 2018–2019 epidemic season in Poland.
Figure 4. Percentage of samples positive in relation to all tested samples in detected in children aged under 14 years in the 2018–2019 epidemic season in Poland. References
1. Principi N, Esposito S, Protection of children against influenza: Emerging problems: Hum Vaccin Immunother, 2018; 14(3); 750-57
2. Brydak LB, 2008; 101-23, Warsaw, Rytm [in Polish]
3. Brydak LB, 2008; 125-4023, Warsaw, Rytm [in Polish]
4. Grohskopf LA, Alyanak E, Broder KR, Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2019–20 influenza season: MMWR Recomm Rep, 2019; 68(3); 1-21
5. American Academy of Pediatrics, Committee on Infectious Diseases, Recommendations for prevention and control of influenza in children, 2019–2020: Pediatrics, 2019; 144(4); e20192478
6. Adlhoch C, Snacken R, Melidou Athe European Influenza Surveillance Network, Dominant influenza A(H3N2) and B/Yamagata virus circulation in EU/EEA, 2016/17 and 2017/18 seasons, respectively: Euro Surveill, 2018; 23(13); 18-00146
7. Bednarska K, Hallmann-Szelińska E, Kondratiuk KInnovations in influenza surveillance: Probl Hig Epidemiol, 2016; 97(2); 101-5 [in Polish]
8. Flu News Europe: Weekly influenza surveillance – Bulletin Archives [cited 2020 Sept 12]https://flunewseurope.org/Archives
9. : FluNet Home Page 2010–2020 [cited 2020 Sept 12]http://ais.paho.org/phip/viz/ed_flu.asp
10. Łuniewska K, Szymański K, Hallmann-Szelińska E, Infections caused by influenza viruses among children in Poland during the 2017/18 epidemic season: Adv Exp Med Biol, 2019; 1211; 97-102
11. Cieślak K, Kowalczyk D, Szymański K, Influenza and influenza-like viruses: Frequent infections in children under 14 years of age during the 2016/2017 epidemic season: Adv Exp Med Biol, 2018; 1114; 83-87
12. Brydak LBInfluenza – prevention and treatment in children and youth: Stand Med Pediatr, 2019; 2(16); 162-71 [in Polish]
13. NIZP-PZH [cited 2020 Sept 15][in Polish]http://wwwold.pzh.gov.pl/oldpage/epimeld/grypa/index.htm
14. Buchan SA, Chung H, Campitelli MA, Vaccine effectiveness against laboratory-confirmed influenza hospitalizations among young children during the 2010–11 to 2013–14 influenza seasons in Ontario, Canada: PLoS One, 2017; 12(11); e0187834
15. Hallmann-Szelińska E, Bednarska K, Kondratiuk K, Viral infections in children in the 2014/2015 epidemic season in Poland: Adv Exp Med Biol, 2016; 1211; 97-102
16. Cieślak K, Szymański K, Kowalczyk D, Brydak LB, Influenza and influenza-like viruses in children in the epidemic season 2015/2016 in Poland: Adv Exp Med Biol, 2016; 968; 13-18
17. Chrobak E, Machura E, Wrzask MThe course of RSV infections in hospitalized newborns and young children: Przegl Lek, 2011; 68; 63-67
18. Cegielska K, Pogonowska M, Kalicki BAnalysis of respiratory syncytial virus infections in children up to 24 months old, hospitalized in the Department of Pediatrics, Pediatric Nephrology and Allergology of the Military Institute of Medicine between 2016 and 2017: Pediatr Med Rodz, 2018; 14(1); 69-77 [in Polish]
Figures
 Figure 1. The number of confirmed cases of influenza in children aged under 14 years in the 2018–2019 influenza season in Poland.
Figure 1. The number of confirmed cases of influenza in children aged under 14 years in the 2018–2019 influenza season in Poland. Figure 2. Participation of individual types and sub types of influenza viruses in children aged under 14 years in the 2018–2019 influenza season in Poland.
Figure 2. Participation of individual types and sub types of influenza viruses in children aged under 14 years in the 2018–2019 influenza season in Poland. Figure 3. Number of viruses causing influenza-like illness detected in children aged under 14 years in the 2018–2019 epidemic season in Poland.
Figure 3. Number of viruses causing influenza-like illness detected in children aged under 14 years in the 2018–2019 epidemic season in Poland. Figure 4. Percentage of samples positive in relation to all tested samples in detected in children aged under 14 years in the 2018–2019 epidemic season in Poland.
Figure 4. Percentage of samples positive in relation to all tested samples in detected in children aged under 14 years in the 2018–2019 epidemic season in Poland. Tables
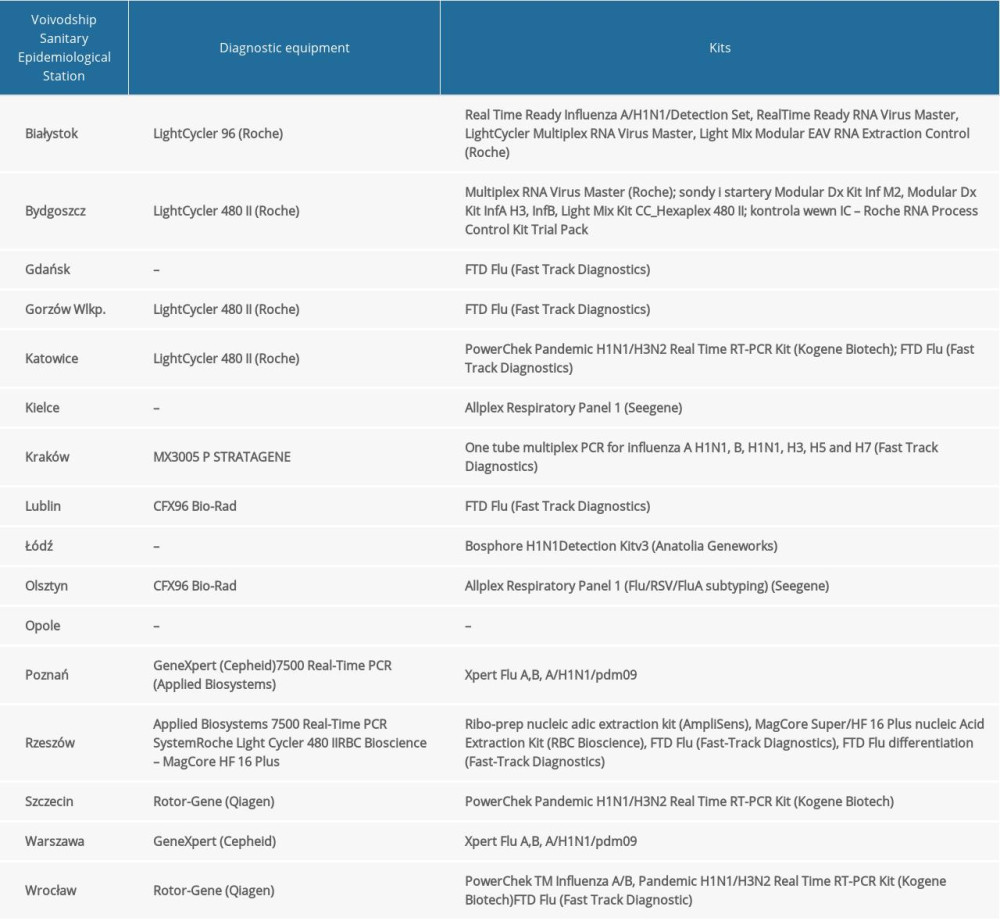 Table 1. The diagnostic methods used in 16 Voivodship Sanitary Epidemiological Stations in the 2018–2019 epidemic season in Poland.
Table 1. The diagnostic methods used in 16 Voivodship Sanitary Epidemiological Stations in the 2018–2019 epidemic season in Poland.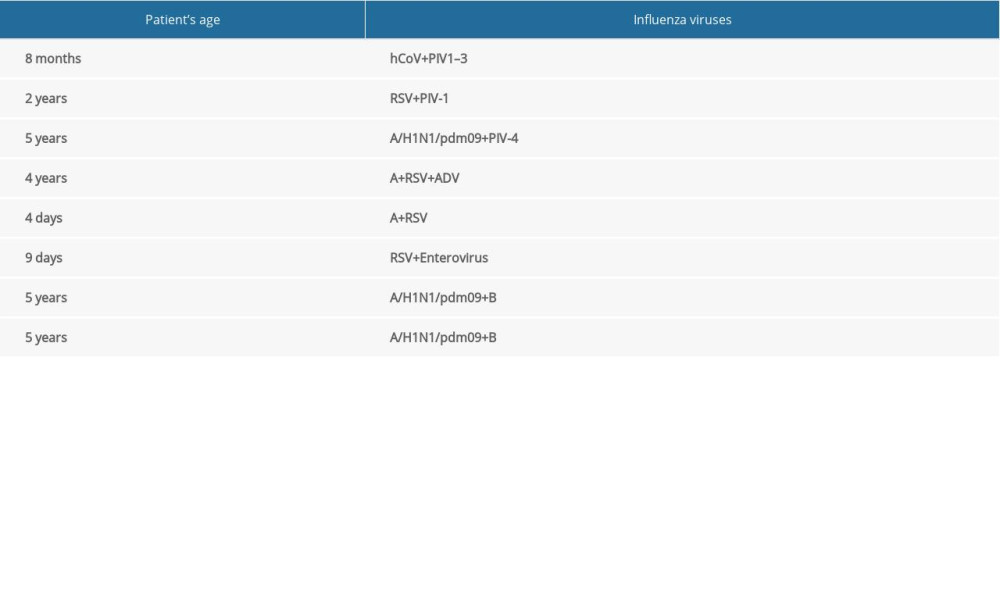 Table 2. Co-infections of respiratory viruses in the 2018–2019 epidemic season.
Table 2. Co-infections of respiratory viruses in the 2018–2019 epidemic season. Table 1. The diagnostic methods used in 16 Voivodship Sanitary Epidemiological Stations in the 2018–2019 epidemic season in Poland.
Table 1. The diagnostic methods used in 16 Voivodship Sanitary Epidemiological Stations in the 2018–2019 epidemic season in Poland. Table 2. Co-infections of respiratory viruses in the 2018–2019 epidemic season.
Table 2. Co-infections of respiratory viruses in the 2018–2019 epidemic season. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








