28 June 2021: Clinical Research
Transforming Growth Factor Beta 1 and p44/42 Expression in Cardinal Ligament Tissues of Patients with Pelvic Organ Prolapse
Ying Zhao1ABCE, Zhijun Xia1ADF*, Te Lin1BCF, Meiying Qin1BCDOI: 10.12659/MSM.930433
Med Sci Monit 2021; 27:e930433
Abstract
BACKGROUND: Pelvic organ prolapse (POP) is a disease associated with collagen loss and decreased fibroblast proliferation. Transforming growth factor beta 1 (TGF-β1) controls collagen synthesis and degradation in pelvic connective tissue. Although the p44/42 MAPK pathway has been implicated in collagen production and extracellular matrix disorders, its expression in POP remains unknown. This study aimed to investigate TGF-β1 and p44/42 expression in cardinal ligament tissues in patients with POP.
MATERIAL AND METHODS: Cardinal ligament tissues were obtained from 30 patients with POP (POP group) and 30 patients with benign gynecological disorders who had undergone total hysterectomy (control group). The clinical characteristics of the 2 groups were summarized. Immunohistochemical staining and western blotting analysis were performed to measure the expression of TGF-β1, p44/42, phospho-p44/42, MMP9, TIMP1, caspase 3, collagen I, and collagen III in the cardinal ligament tissues.
RESULTS: Patients with POP had significantly lower TGF-β1 and phospho-p44/42 levels than did control patients (P<0.05). The expression of TIMP1, collagen I, and collagen III was significantly lower, and the expression of MMP9 and caspase 3 was significantly higher in the POP group than in the control group (P<0.05). Moreover, the expression of phospho-p44/42 was positively correlated with the expression of TGF-β1, collagen I, and collagen III.
CONCLUSIONS: The expression levels of phospho-p44/42 and TGF-β1 were decreased in patients with POP and were positively correlated with collagen expression. Low levels of TGF-β1 and phospho-p44/42 expression in patients with POP may be associated with the occurrence of POP.
Keywords: Mitogen-Activated Protein Kinases, pelvic organ prolapse, Transforming Growth Factor beta1, Collagen, Extracellular Matrix, Ligaments
Background
Pelvic organ prolapse (POP) is characterized by the weakening of pelvic supportive tissue in women, leading to prolapse of the uterus, bladder, and rectum outside the pelvis through the vagina [1]. POP, which is more common in older women, affects approximately 9% of women worldwide and greatly impacts their quality of life [2,3]. An epidemiological survey of 78.5 million person-years in a US population found that 76 in 100 000 women underwent surgery for POP between 2005 and 2010 [4].
The pelvic organs are mainly supported by connective tissues, of which the cardinal ligament of the uterus is an important part. POP occurs when connective tissues are disrupted and weakened [5,6]. The pelvic connective tissue comprises an extracellular matrix (ECM) and relatively few cells. Elastin and collagens produced by fibroblastic cells are major components of the ECM, maintaining its integrity, strength, and flexibility [7]. Previous studies have shown that the pelvic supportive tissues of patients with POP have significantly lower contents of collagen I and III [8–10]. These findings suggest that alterations in collagen metabolism and fibroblast proliferation play significant roles in POP development.
Transforming growth factor beta (TGF-β) is a family of multifunctional cytokines that is important for ECM metabolism and fibroblast proliferation [11]. TGF-β1 controls collagen synthesis and degradation in pelvic connective tissue by stimulating the synthesis of tissue inhibitors of metalloproteinases (TIMPs) and inhibiting the activity of matrix metalloproteinases (MMPs) [12,13]. MMP9, which is inhibited by TIMP1, is involved in fibrosis, ECM degradation, and tissue destruction [14]. TGF-β1 signaling occurs via the Smad-dependent and -independent pathways [15,16]. Liu et al [17] reported that TGF-β1 expression is associated with the severity of POP and that the TGF-β1/Smad3 signaling pathway participates in its occurrence. Indeed, treatment of human uterosacral ligamental fibroblasts with TGF-β1 attenuates ECM loss via the TGF-β1/Smad3 signaling pathway [18].
The Smad-independent signaling pathway is initiated by the activation of mitogen-activated protein kinases (MAPKs) such as the p44/42 (ERK1/2) MAPK pathway [19], which is a critical intracellular mediator of MMP9 expression induced by matrix fragments [20]. Importantly, p44/42 signaling mediates TGF-β-regulated collagen production and degradation in the development of kidney fibrosis [21]. Previous studies have implicated p44/42 MAPK signaling in ECM metabolism disorder [22] and the modulation of collagen I expression in human dermal fibroblasts [23]. Moreover, p44/42 MAPK activation is widely associated with anti-apoptotic functions [24,25]. Collectively, these findings suggest that the p44/42 MAPK pathway is associated with fibroblast growth and collagen synthesis. However, whether the p44/42 MAPK pathway participates in the TGF-β1-dependent regulation of pelvic ligament collagen synthesis remains unknown.
In this study, we aimed to identify differences in expression levels of TGF-β1, phospho-p44/42, collagen type I, and collage type III in cardinal ligament tissue in patients with POP and patients without POP. To this aim, we assessed the expression of TGF-β1, p44/42, phospho-p44/42, collagen I, collagen III, TIMP1, MMP9, and the apoptotic protease caspase 3 in cardinal ligament tissue of patients with and without POP. In addition, we examined whether phospho-p44/42 protein levels correlated with those of TGF-β1, collagen I, and collagen III in these patients.
Material and Methods
PATIENTS AND SPECIMENS:
This study was conducted at the Shengjing Hospital of the China Medical University (Shenyang, China) and was approved by the ethics committee of the hospital (No. 2018PS68K). Thirty patients who had undergone surgery for POP were recruited as the POP group. Additionally, we selected 30 patients with benign gynecological disorders who had undergone total hysterectomy as the control group, including 8 patients with cervical intraepithelial neoplasia and 22 patients with uterine fibroids. Patients with estrogen-related diseases or a history of hormone therapy within 3 months prior to the study were excluded. All procedures followed the principles of the 1975 Declaration of Helsinki, and all patients provided written informed consent.
A cardinal ligament tissue sample from each patient was obtained during surgery. The tissue sample was stored at −80°C for western blotting analysis or fixed in 4% paraformaldehyde for 48 h for immunohistochemical (IHC) staining.
IHC STAINING:
Cardinal ligament tissue was fixed in 4% paraformaldehyde for 48 h, and a 4-μm-thick paraffin-embedded section was prepared. The protein expression of collagen I, collagen III, TGF-β1, p44/42, and phospho-p44/42 was examined using IHC staining. Briefly, sections were de-waxed in xylene and dehydrated in ethanol. After antigen retrieval with citrate buffer (Beijing Golden Bridge Biotechnology, Beijing, China), the sections were incubated in 3% H2O2 for 20 min and then blocked with 10% goat serum for 20 min. Subsequently, the sections were incubated overnight at 4°C with primary antibodies against the following: collagen I (1: 600, Abcam, Cambridge, United Kingdom), collagen III (1: 400, Abcam), TGF-β1 (1: 200, Abcam), p44/42 (1: 400, Cell Signaling Technology, USA), phospho-p44/42 (1: 400, Cell Signaling Technology), MMP9 (1: 200, Proteintech Group, USA), TIMP1 (1: 100, Affinity Biosciences, USA), and apoptosis-associated protein caspase3 (1: 300, Proteintech). Thereafter, goat anti-rabbit secondary antibody (1: 5000, Merck, Darmstadt, Germany) or goat anti-mouse secondary antibody (1: 5000, Merck) was added, and the formation of antigen-antibody complexes was visualized using a diaminobenzidine detection kit (Solarbio Life Sciences, China). To observe the results, a Nikon microscope was used at 200× and 400× magnification (Nikon Optical, Japan). Image-pro plus 6.0 software (Media Cybernetics, USA) was used to analyze the staining results. The optical density value of the positive part was detected by Image-pro plus software, and the mean density was calculated by the ratio of optical density value to the distribution area.
WESTERN BLOTTING:
Cardinal ligament tissue (1 g) was first ground in liquid nitrogen and then underwent lysis with radioimmunoprecipitation assay buffer (Beyotime), followed by centrifugation at 5000
STATISTICAL ANALYSIS:
Data were analyzed using SPSS software version 19.0 (IBM, USA), and GraphPad Prism 8.0 (GraphPad Software, USA) was used for data processing. Data are presented as the mean±standard deviation. Independent sample 2-sided
Results
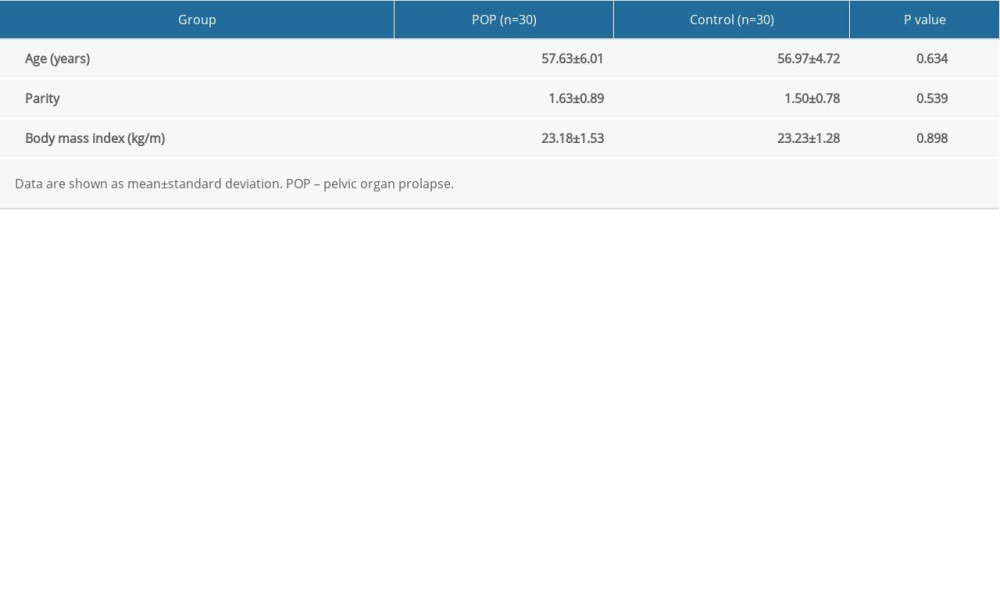
The clinical characteristics of the 60 patients enrolled in the study are summarized in Table 1. We calculated the average age, parity, and body mass index of the 2 groups, and these characteristics were summarized and compared between the 2 groups (
IHC staining showed that the expression of collagen I and III was significantly lower in the POP group than in the control group (0.131±0.025 vs 0.181±0.037 and 0.109±0.024 vs 0.169±0.038, respectively,
The POP group had significantly lower TGF-β1 expression than the control group, (0.138±0.025 vs 0.191±0.041,
In addition, phospho-p44/42 protein levels were significantly positively correlated with TGF-β1 (r=0.054,
The expression of MMP9 was significantly higher (
Discussion
POP is a manifestation of the aging of pelvic supportive tissue in women. Loss of collagen and decreased fibroblast proliferation contribute to the development of POP [8–11]. Previous studies have shown that patients with POP have significantly lower contents of collagen in the pelvic supportive tissues [8–10], suggesting that alterations in collagen metabolism and fibroblast proliferation play significant roles in POP development. TGF-β1 controls collagen synthesis and degradation in pelvic connective tissue by stimulating TIMP synthesis and inhibiting MMP activity [12,13]. The TGF-β1/Smad3 signaling pathway participates in the occurrence of POP, and TGF-β1 expression is associated with its severity [18]. The Smad-independent TGF-β1 signaling pathway activates MAPKs such as p44/42 [19], which is a critical intracellular mediator of MMP9 expression [20]. Previous studies have implicated p44/42 signaling in TGF-β-regulated collagen production and degradation during the development of kidney fibrosis [21], ECM metabolism disorder [22], and the modulation of collagen I expression in human dermal fibroblasts [23]. Although these findings suggest that the p44/42 MAPK pathway is associated with fibroblast growth and collagen synthesis, it remains unknown whether the expression of p44/42 is different in prolapse. The aim of this study was to investigate TGF-β1 and p44/42 expression in cardinal ligament tissues of patients with POP.
In this study, we confirmed that the expression of collagen I and III is significantly downregulated in the cardinal ligaments of patients with POP. Moreover, we demonstrated that the protein expression of TGF-β1 and phospho-p44/42 is significantly lower in the cardinal ligaments of patients with POP than in those of control patients. In addition, phospho-p44/42 protein levels were significantly positively correlated with TGF-β1, collagen I, and collagen III levels in the POP and control groups. Additionally, we found that MMP9 and caspase 3 expression was significantly higher, whereas TIMP1 expression was significantly lower, in the POP group compared with the control group.
The balance between MMPs and TIMPs significantly contributes to ECM remodeling. MMP9, which is inhibited by TIMP1 and is involved in fibrosis, ECM degradation, and tissue destruction [14]. These findings support our present observations on MMP9 and TIMP1 expression. The increased expression of MMP9 and the lower expression of TIMP1 in patients with POP may increase ECM degradation and decrease collagen expression. Alarab et al [26] reported that the dysregulation of MMP/TIMP complexes causes connective tissue defects, which result in weakened vaginal wall support and POP development. Moreover, the anti-apoptotic functions of p44/42, which are exerted by controlling cell proliferation and differentiation, have previously been demonstrated [24,25]. In the present study, we found that the expression of the apoptotic protease caspase 3 was significantly increased in the tissues of patients with POP, which may have been due to the lower expression of phospho-p44/42 in these patients.
Collectively, our results suggest that the occurrence of POP may be due to the lower expression of TGF-β1 and p44/42, followed by the collagen reduction and enhanced apoptosis in the cardinal ligament. Thus, TGF-β1-dependent p44/42 signaling represents a promising therapeutic pathway for POP prevention.
However, this study has some limitations. First, the sample size was small and should be increased to further verify the expression of p44/42 in prolapse cases. In addition, the role of p44/42 in collagen metabolism should be further demonstrated in vitro experiments by examining the regulation of TGF-β1 and p44/42 in cells. Therefore, further studies to address these issues are needed.
Conclusions
The cardinal ligaments of patients with POP exhibited significantly lower expression of TGF-β1 and phospho-p44/42 than those of patients without POP. TGF-β1 and phospho-p44/42 were positively correlated with collagen expression. TGF-β1 and phospho-p44/42 may affect collagen metabolism and be associated with the occurrence of POP, but further studies are needed for confirmation.
Figures
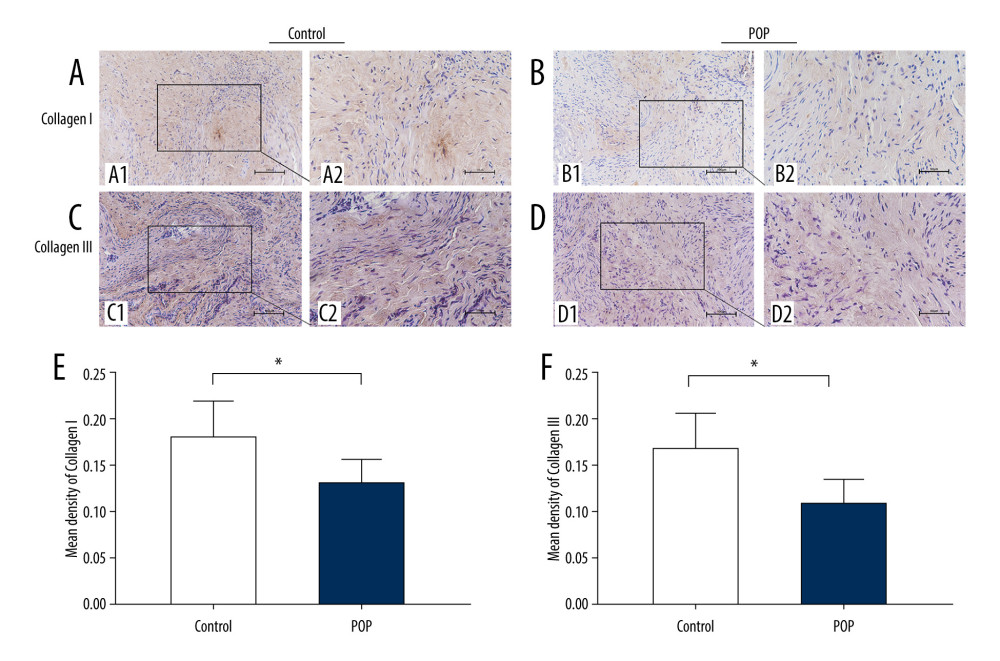 Figure 1. Immunohistochemical staining for collagen I and collagen III in the cardinal ligament. (A–D) Representative images showing that the expression of collagen I and III in the control group was statistically significantly higher than that in the POP group. (E, F) Quantitative analysis. * P<0.05. Magnification: 200× (A1–D1) and 400× (A2–D2). POP – pelvic organ prolapse.
Figure 1. Immunohistochemical staining for collagen I and collagen III in the cardinal ligament. (A–D) Representative images showing that the expression of collagen I and III in the control group was statistically significantly higher than that in the POP group. (E, F) Quantitative analysis. * P<0.05. Magnification: 200× (A1–D1) and 400× (A2–D2). POP – pelvic organ prolapse. 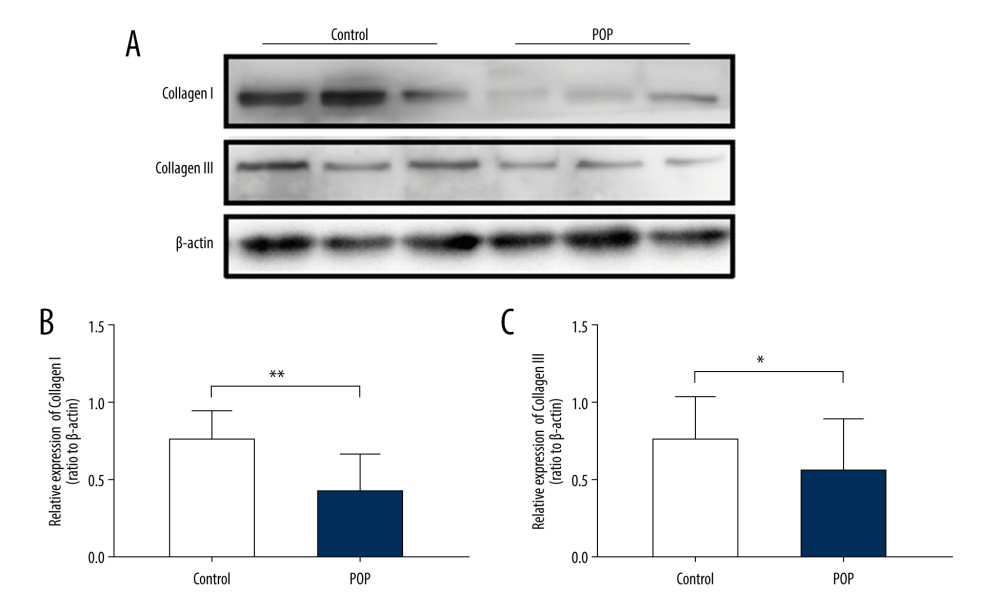 Figure 2. Western blot for collagen I and collagen III in the cardinal ligament. (A) Western blot analysis of collagen I and collagen III expression. (B, C) Relative densitometry analysis. * P<0.05, ** P<0.01.
Figure 2. Western blot for collagen I and collagen III in the cardinal ligament. (A) Western blot analysis of collagen I and collagen III expression. (B, C) Relative densitometry analysis. * P<0.05, ** P<0.01. 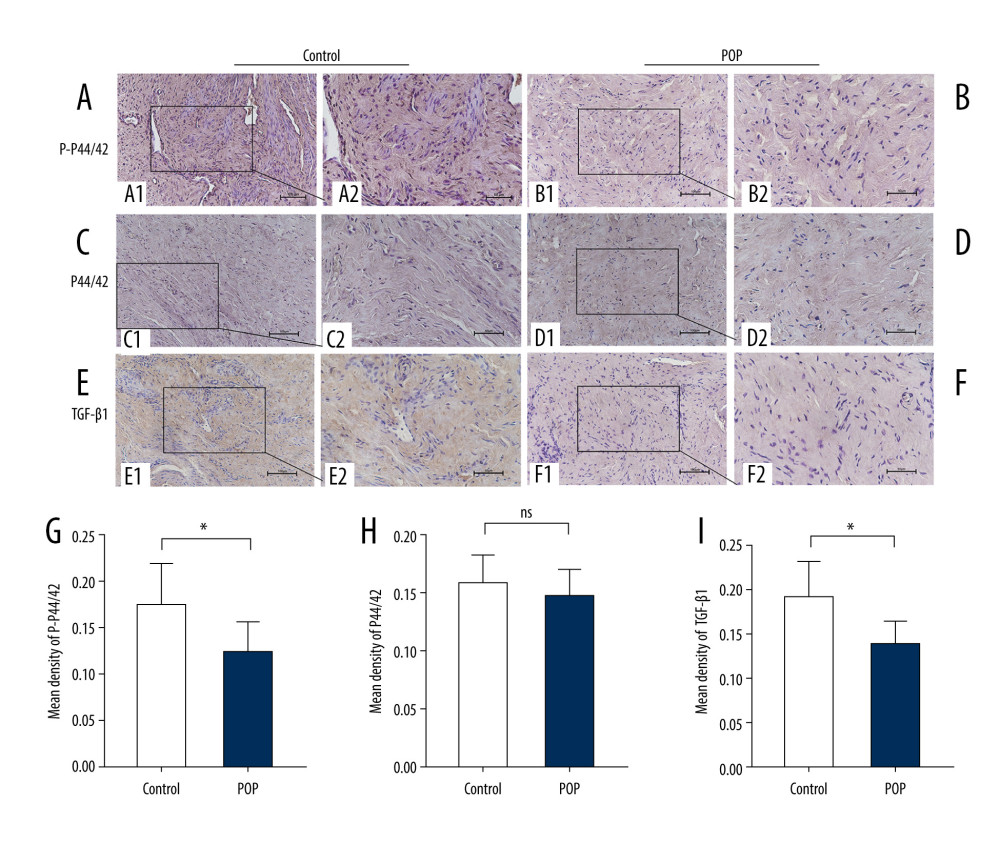 Figure 3. Immunohistochemical staining for TGF-β1, p44/42, and phospho-p44/42 in the cardinal ligament. (A–F) Representative images showing that the expression of TGF-β1 and phospho-p44/42 in the control group was statistically significantly higher than that in the POP group. In contrast, the difference in p44/42 expression between the groups was not significant. (G–I) Quantitative analysis. *P<0.05; ns non-significant. Magnification: 200× (A1–F1) and 400× (A2–F2). POP – pelvic organ prolapse; TGF-β1 – transforming growth factor beta 1; P-P44/42 – phospho-p44/42.
Figure 3. Immunohistochemical staining for TGF-β1, p44/42, and phospho-p44/42 in the cardinal ligament. (A–F) Representative images showing that the expression of TGF-β1 and phospho-p44/42 in the control group was statistically significantly higher than that in the POP group. In contrast, the difference in p44/42 expression between the groups was not significant. (G–I) Quantitative analysis. *P<0.05; ns non-significant. Magnification: 200× (A1–F1) and 400× (A2–F2). POP – pelvic organ prolapse; TGF-β1 – transforming growth factor beta 1; P-P44/42 – phospho-p44/42. 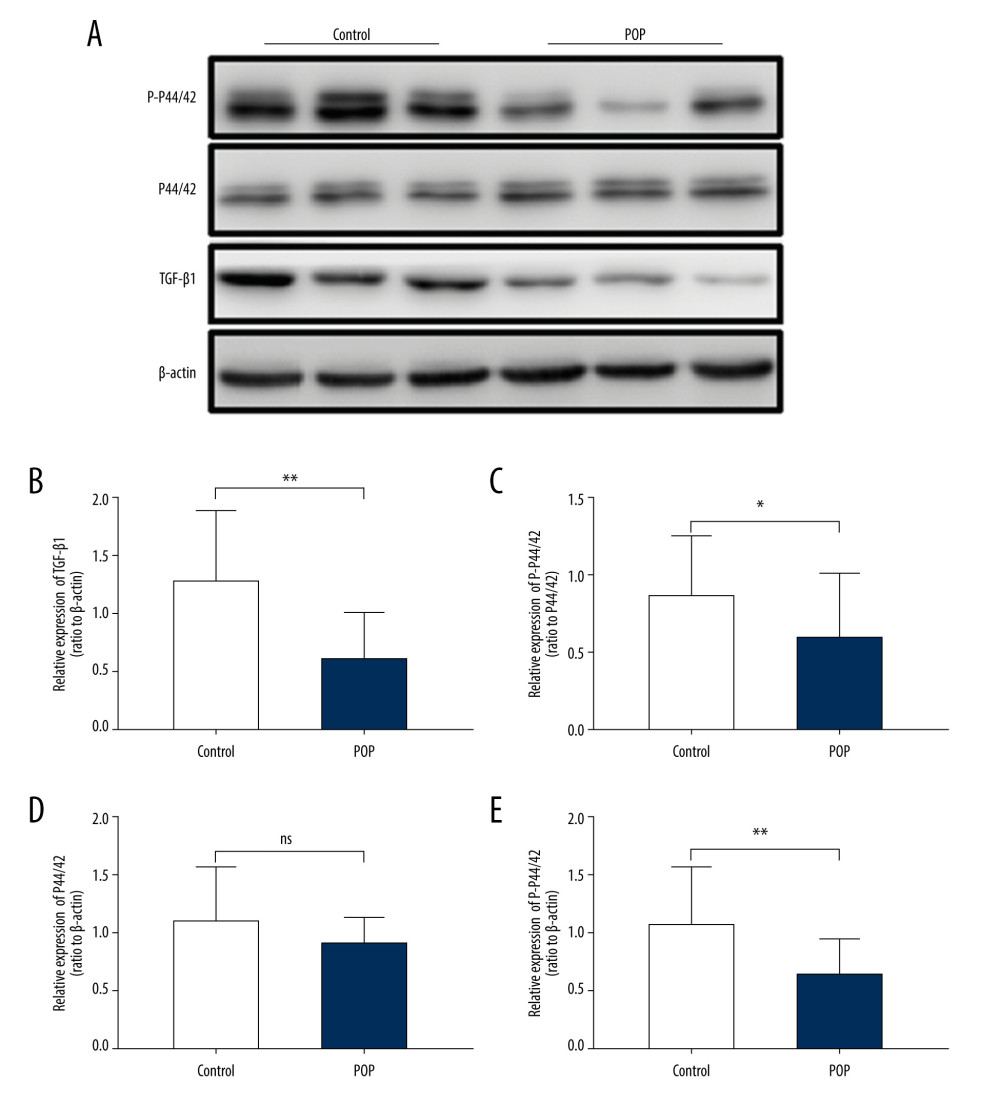 Figure 4. Western blot for TGF-β1, P44/42, and phospho-p44/42 in the cardinal ligament. (A) Western blot analysis of TGF-β1, P44/42, and phospho-p44/42 expression. (B–E) Relative densitometry analysis. * P<0.05; ** P<0.01; ns – non-significant.
Figure 4. Western blot for TGF-β1, P44/42, and phospho-p44/42 in the cardinal ligament. (A) Western blot analysis of TGF-β1, P44/42, and phospho-p44/42 expression. (B–E) Relative densitometry analysis. * P<0.05; ** P<0.01; ns – non-significant. 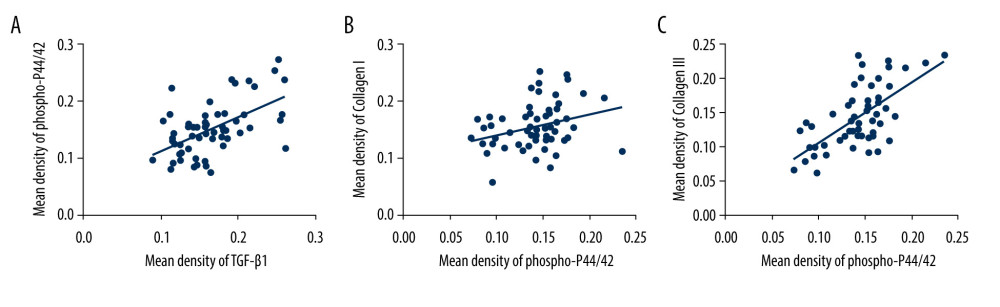 Figure 5. The correlation between phospho-p44/42 and TGF, collagen I, and collagen III. Phospho-p44/42 levels were significantly positively correlated with TGF-β1, collagen I, and collagen III. P-P44/42, phospho-p44/42 (A–C).
Figure 5. The correlation between phospho-p44/42 and TGF, collagen I, and collagen III. Phospho-p44/42 levels were significantly positively correlated with TGF-β1, collagen I, and collagen III. P-P44/42, phospho-p44/42 (A–C). 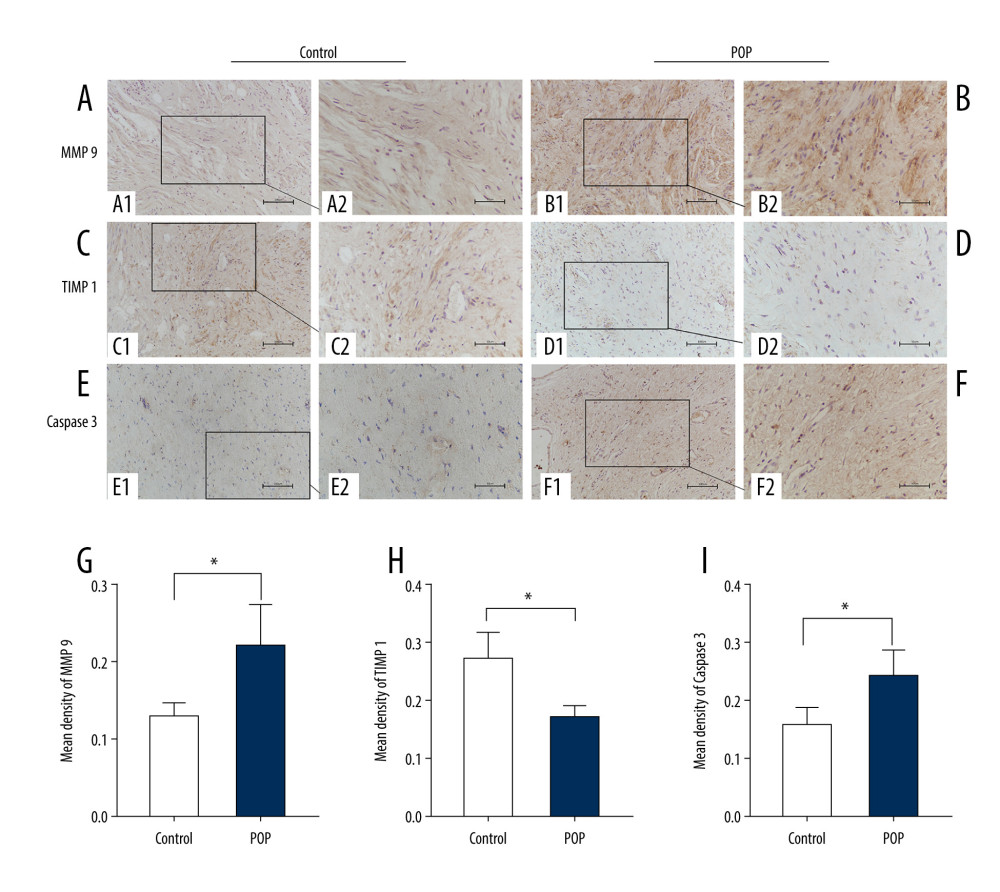 Figure 6. Immunohistochemical staining for MMP9, TIMP1, and caspase 3 in the cardinal ligament. (A–F) Representative images showing that compared with the control group, the expression of MMP9 and caspase 3 was significantly higher and that of TIMP1 was significantly lower in the POP group. Quantitative analysis (G–I). * P<0.05. Magnification: 200× (A1–F1) and 400× (A2–F2). POP – pelvic organ prolapse; TGF-β1 – transforming growth factor beta 1.
Figure 6. Immunohistochemical staining for MMP9, TIMP1, and caspase 3 in the cardinal ligament. (A–F) Representative images showing that compared with the control group, the expression of MMP9 and caspase 3 was significantly higher and that of TIMP1 was significantly lower in the POP group. Quantitative analysis (G–I). * P<0.05. Magnification: 200× (A1–F1) and 400× (A2–F2). POP – pelvic organ prolapse; TGF-β1 – transforming growth factor beta 1. 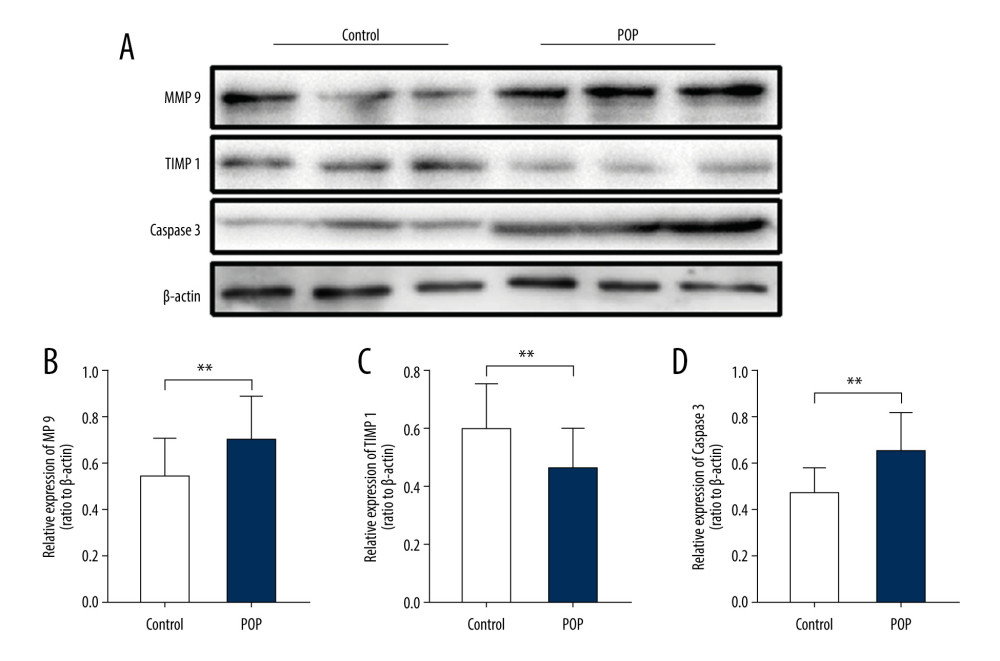 Figure 7. Western blot for MMP9, TIMP1, and caspase 3 in the cardinal ligament. (A) Western blot analysis of MMP9, TIMP1, and caspase 3 expression. (B–D) Relative densitometry analysis. * P<0.05, **P<0.01.
Figure 7. Western blot for MMP9, TIMP1, and caspase 3 in the cardinal ligament. (A) Western blot analysis of MMP9, TIMP1, and caspase 3 expression. (B–D) Relative densitometry analysis. * P<0.05, **P<0.01. References
1. Chung SH, Kim WB, Various approaches and treatments for pelvic organ prolapse in women: J Menopausal Med, 2018; 24; 155-62
2. Vos T, Flaxman AD, Naghavi M, Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010: Lancet, 2012; 380(9859); 2163-96
3. Mattsson NK, Karjalainen PK, Tolppanen AM, Pelvic organ prolapse surgery and quality of life-a nationwide cohort study: Am J Obstet Gynecol, 2020; 222(6); 588.e1-e10
4. Jonsson FM, Edenfield AL, Pate V, Trends in use of surgical mesh for pelvic organ prolapse: Am J Obstet Gynecol, 2013; 208; 71-79
5. De Landsheere L, Munaut C, Nusgens B, Histology of the vaginal wall in women with pelvic organ prolapse: a literature review: Int Urogynecol J, 2013; 24(12); 2011-20
6. Chen B, Yeh J, Alterations in connective tissue metabolism in stress incontinence and prolapse: J Urol, 2011; 186(5); 1768-72
7. Ruiz-Zapata AM, Kerkhof MH, Ghazanfari S, Vaginal fibroblastic cells from women with pelvic organ prolapse produce matrices with increased stiffness and collagen content: Sci Rep, 2016; 6; 22971
8. Budatha M, Roshanravan S, Zheng Q, Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans: J Clin Invest, 2011; 121(5); 2048-59
9. Han L, Wang L, Wang Q, Association between pelvic organ prolapse and stress urinary incontinence with collagen: Exp Ther Med, 2014; 7(5); 1337-41
10. Hu Y, Wu R, Li H, Expression and significance of metalloproteinase and collagen in vaginal wall tissues of patients with pelvic organ prolapse: Ann Clin Lab Sci, 2017; 47(6); 698-705
11. Iwahashi M, Muragaki Y, Decreased type III collagen expression in human uterine cervix of prolapse uteri: Exp Ther Med, 2011; 2(2); 271-74
12. Ghavami S, Yeganeh B, Zeki AA, Autophagy and the unfolded protein response promote profibrotic effects of TGF-β1 in human lung fibroblasts: Am J Physiol Lung Cell Mol Physiol, 2018; 314(3); L493-504
13. Zhang Q, Liu C, Hong S, Excess mechanical stress and hydrogen peroxide remodel extracellular matrix of cultured human uterosacral ligament fibroblasts by disturbing the balance of MMPs/TIMPs via the regulation of TGF b1 signaling pathway: Mol Med Rep, 2017; 15(1); 423-30
14. Bałkowiec M, Maksym RB, Włodarski PK, The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (review): Mol Med Rep, 2018; 18(3); 3123-36
15. Mikuła-Pietrasik J, Sosińska P, Janus J, Bystander senescence in human peritoneal mesothelium and fibroblasts is related to thrombospondin-1-dependent activation of transforming growth factor-β1: Int J Biochem Cell Biol, 2013; 45(9); 2087-96
16. Tominaga K, Suzuki HI, TGF-β signaling in cellular senescence and aging-related pathology: Int J Mol Sci, 2019; 20(20); 5002
17. Liu C, Wang Y, Li BS, Role of transforming growth factor β 1 in the pathogenesis of pelvic organ prolapse: A potential therapeutic target: Int J Mol Med, 2017; 40(2); 347-56
18. Jabłońska-Trypuć A, Matejczyk M, Rosochacki S, Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs: J Enzyme Inhib Med Chem, 2016; 31; 177-83
19. Mulder KM, Role of Ras and Mapks in TGFbeta signaling: Cytokine Growth Factor Rev, 2000; 11(1–2); 23-35
20. Zhang F, Banker G, Liu X, The novel function of advanced glycation end products in regulation of MMP-9 production: J Surg Res, 2011; 171(2); 871-76
21. Cheng X, Gao W, Dang Y, Both ERK/MAPK and TGF-Beta/Smad signaling pathways play a role in the kidney fibrosis of diabetic mice accelerated by blood glucose fluctuation: J Diabetes Res, 2013; 2013; 463740
22. Yue J, Jin S, Gu S, High concentration magnesium inhibits extracellular matrix calcification and protects articular cartilage via Erk/autophagy pathway: J Cell Physiol, 2019; 234(12); 23190-201
23. Kim YI, Kim KS, Ahn HJ, Reduced matrix metalloproteinase and collagen transcription mediated by the TGF-β/Smad pathway in passaged normal human dermal fibroblasts: J Cosmet Dermatol, 2020; 19(5); 1211-18
24. Yue J, López JM, Understanding MAPK signaling pathways in apoptosis: Int J Mol Sci, 2020; 21(7); 2346
25. Chou YT, Chen LY, Tsai SL, Ribose-5-phosphate isomerase A overexpression promotes liver cancer development in transgenic zebrafish via activation of ERK and β-catenin pathways: Carcinogenesis, 2019; 40(3); 461-73
26. Alarab M, Kufaishi H, Lye S, Expression of extracellular matrix-remodeling proteins is altered in vaginal tissue of premenopausal women with severe pelvic organ prolapse: Reprod Sci, 2014; 21(6); 704-15
Figures
 Figure 1. Immunohistochemical staining for collagen I and collagen III in the cardinal ligament. (A–D) Representative images showing that the expression of collagen I and III in the control group was statistically significantly higher than that in the POP group. (E, F) Quantitative analysis. * P<0.05. Magnification: 200× (A1–D1) and 400× (A2–D2). POP – pelvic organ prolapse.
Figure 1. Immunohistochemical staining for collagen I and collagen III in the cardinal ligament. (A–D) Representative images showing that the expression of collagen I and III in the control group was statistically significantly higher than that in the POP group. (E, F) Quantitative analysis. * P<0.05. Magnification: 200× (A1–D1) and 400× (A2–D2). POP – pelvic organ prolapse. Figure 2. Western blot for collagen I and collagen III in the cardinal ligament. (A) Western blot analysis of collagen I and collagen III expression. (B, C) Relative densitometry analysis. * P<0.05, ** P<0.01.
Figure 2. Western blot for collagen I and collagen III in the cardinal ligament. (A) Western blot analysis of collagen I and collagen III expression. (B, C) Relative densitometry analysis. * P<0.05, ** P<0.01. Figure 3. Immunohistochemical staining for TGF-β1, p44/42, and phospho-p44/42 in the cardinal ligament. (A–F) Representative images showing that the expression of TGF-β1 and phospho-p44/42 in the control group was statistically significantly higher than that in the POP group. In contrast, the difference in p44/42 expression between the groups was not significant. (G–I) Quantitative analysis. *P<0.05; ns non-significant. Magnification: 200× (A1–F1) and 400× (A2–F2). POP – pelvic organ prolapse; TGF-β1 – transforming growth factor beta 1; P-P44/42 – phospho-p44/42.
Figure 3. Immunohistochemical staining for TGF-β1, p44/42, and phospho-p44/42 in the cardinal ligament. (A–F) Representative images showing that the expression of TGF-β1 and phospho-p44/42 in the control group was statistically significantly higher than that in the POP group. In contrast, the difference in p44/42 expression between the groups was not significant. (G–I) Quantitative analysis. *P<0.05; ns non-significant. Magnification: 200× (A1–F1) and 400× (A2–F2). POP – pelvic organ prolapse; TGF-β1 – transforming growth factor beta 1; P-P44/42 – phospho-p44/42. Figure 4. Western blot for TGF-β1, P44/42, and phospho-p44/42 in the cardinal ligament. (A) Western blot analysis of TGF-β1, P44/42, and phospho-p44/42 expression. (B–E) Relative densitometry analysis. * P<0.05; ** P<0.01; ns – non-significant.
Figure 4. Western blot for TGF-β1, P44/42, and phospho-p44/42 in the cardinal ligament. (A) Western blot analysis of TGF-β1, P44/42, and phospho-p44/42 expression. (B–E) Relative densitometry analysis. * P<0.05; ** P<0.01; ns – non-significant. Figure 5. The correlation between phospho-p44/42 and TGF, collagen I, and collagen III. Phospho-p44/42 levels were significantly positively correlated with TGF-β1, collagen I, and collagen III. P-P44/42, phospho-p44/42 (A–C).
Figure 5. The correlation between phospho-p44/42 and TGF, collagen I, and collagen III. Phospho-p44/42 levels were significantly positively correlated with TGF-β1, collagen I, and collagen III. P-P44/42, phospho-p44/42 (A–C). Figure 6. Immunohistochemical staining for MMP9, TIMP1, and caspase 3 in the cardinal ligament. (A–F) Representative images showing that compared with the control group, the expression of MMP9 and caspase 3 was significantly higher and that of TIMP1 was significantly lower in the POP group. Quantitative analysis (G–I). * P<0.05. Magnification: 200× (A1–F1) and 400× (A2–F2). POP – pelvic organ prolapse; TGF-β1 – transforming growth factor beta 1.
Figure 6. Immunohistochemical staining for MMP9, TIMP1, and caspase 3 in the cardinal ligament. (A–F) Representative images showing that compared with the control group, the expression of MMP9 and caspase 3 was significantly higher and that of TIMP1 was significantly lower in the POP group. Quantitative analysis (G–I). * P<0.05. Magnification: 200× (A1–F1) and 400× (A2–F2). POP – pelvic organ prolapse; TGF-β1 – transforming growth factor beta 1. Figure 7. Western blot for MMP9, TIMP1, and caspase 3 in the cardinal ligament. (A) Western blot analysis of MMP9, TIMP1, and caspase 3 expression. (B–D) Relative densitometry analysis. * P<0.05, **P<0.01.
Figure 7. Western blot for MMP9, TIMP1, and caspase 3 in the cardinal ligament. (A) Western blot analysis of MMP9, TIMP1, and caspase 3 expression. (B–D) Relative densitometry analysis. * P<0.05, **P<0.01. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952