19 December 2021: Hypothesis
Tissue-Engineered Skin Regenerative Units for Epidermal Keratinocytes Expansion and Wound Healing
Xinjian Zhang1CE, Wen Xu2EF*, Xinlei Hu3ADOI: 10.12659/MSM.932978
Med Sci Monit 2021; 27:e932978
Abstract
ABSTRACT: Chronic wounds have become an increasing medical and economic problem of aging societies because they are difficult to manage. Tissue engineering provides new perspectives for the clinically applicable skin substitutes. Epidermal keratinocytes play an important role in wound epithelization and construction of tissue-engineered skin substitutes. How to obtain a large number of autologous epidermal keratinocytes in a short time is the main problem that limits the application of tissue-engineered skin and epidermal cell membranes. Developing an appropriate method for reproducing the biological potential of cell–cell interactions and simulating the three-dimensional structure between cells has great significance for epidermal keratinocytes expansion and full-thickness skin regeneration. In this article, we propose the concept of tissue-engineered skin regeneration units (TESRUs) as the smallest unit with complete full-thickness skin regeneration ability. First, autologous dermal fibroblasts are cultured in biodegradable macroporous microcarriers to provide the mesenchyme support. Second, autologous epidermal keratinocytes and autologous melanocytes are incubated with the fibroblasts-loaded microcarriers and expand in vitro. Incorporating the above co-culture method into the macroporous microcarriers is reasonable for maintaining cell–cell interactions in spatial and temporal context and providing a suitable growth niche for epidermal keratinocytes. Moreover, TESRUs are composed of fibroblasts, keratinocytes, and melanocytes and have complete full-thickness skin regeneration ability. We suggest that TESRUs could be a promising strategy to repair full-thickness skin defects for clinical applications if the hypothesis proves to be practical.
Keywords: Dermis, regenerative medicine, Tissue Engineering, Wound Healing, Epidermis, Humans, Keratinocytes, Models, Biological, Regeneration
Background
Full-thickness skin defects caused by trauma, burns, and non-healing ulcers are very common in the clinical setting, and represent a major burden on world health care systems. There are several strategies for wound healing, including split thickness grafts, tissue-engineered scaffolds, and cultured epidermal keratinocyte autografts [1,2]. Because skin grafting has limits, multiple approaches for engineering skin tissue products have attracted attention. However, skin substitutes with scaffolds have encountered a problem with vascularization after transplantation. At the same time, tissue-engineered skin products need a large number of epidermal keratinocytes, which are difficult to culture. Using an allogeneic epidermal keratinocyte suspension seems to be an alternative strategy but has problems caused by the lack of scaffolds and immunogenicity. Therefore, researchers have turned to directly administering skin cell suspensions into wounds [3,4]. Clinical trials to directly apply skin epidermal keratinocytes suspended in concentrated thrombocytes to wounds have demonstrated a significant improvement in the healing process. However, this method leads to the appearance of micro-cracks in the tissues underlying the epidermis and dermis that are populated by single skin cells, which require surgical re-intervention [4].
Wound healing is a complex physiological process that involves numerous cell types. Its mechanism is strictly organized via cell interactions. Recently, studies have focused on developing tissue-engineered skin substitutes that use synthetic biomaterials to provide structural support and incorporate human skin cell types to regenerate the damaged skin [5]. Developing an appropriate method for reproducing the biological potential of cell–cell interactions, simulating the three-dimensional structure between cells, has great significance for epidermal keratinocytes expansion and full-thickness skin regeneration.
Hypothesis
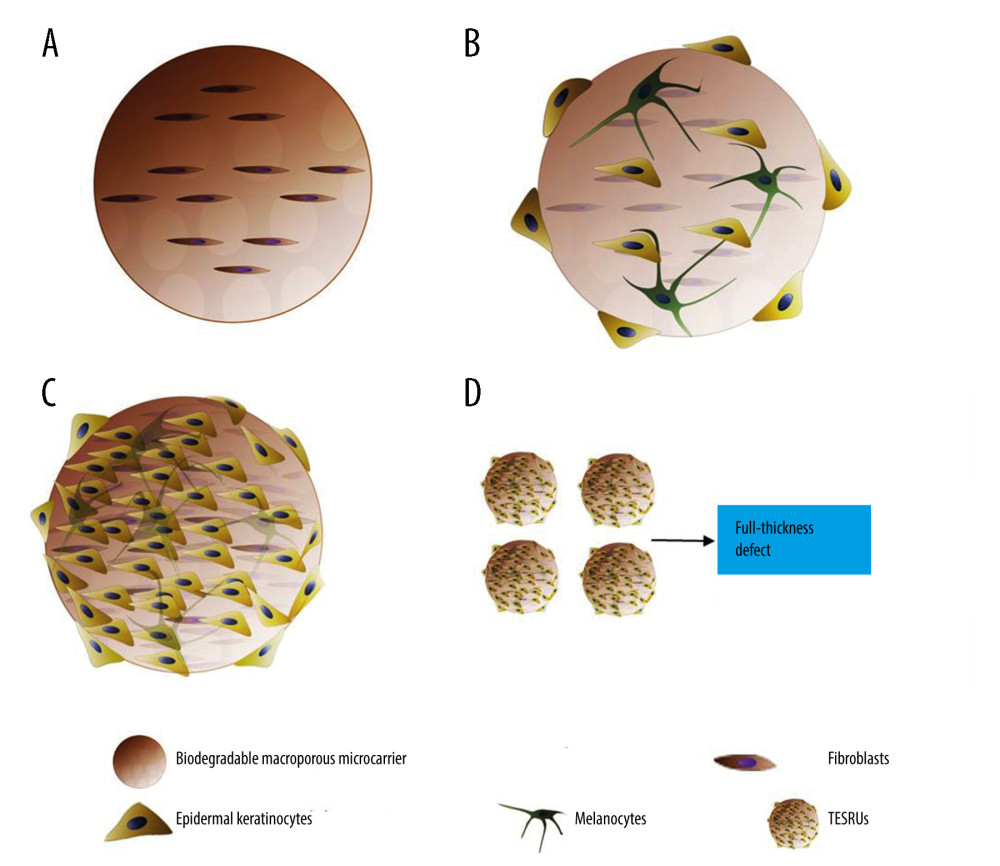
A hypothesis is proposed for a method to reproduce fibroblast–epidermal keratinocyte–melanocyte interactions and provides a temporary three-dimensional structure for living cells to construct skin substitutes with full-thickness regeneration ability. In this method, degradable macroporous microcarriers are introduced as a temporary three-dimensional microstructure for cell adhesion and expansion. Autologous fibroblasts are first planted on the degradable macroporous microcarriers. They will migrate to the inner space of the microcarriers through pores on the surface and provide the mesenchyme support for cell adhesion and proliferation. Second, autologous epidermal keratinocytes and autologous melanocytes are incubated with the fibroblast-loaded microcarriers, such as bioreactor, and expanded in vitro. This will provide a suitable growth niche that mimics cell–cell interactions in vivo for epidermal keratinocytes expansion, and potentially form a mature skin microstructure simultaneously before final grafting. Based on these sequential steps, the concept of tissue-engineered skin regeneration units (TESRUs) as the smallest unit with complete full-thickness skin regeneration ability is proposed (Figure 1).
Evaluation of Hypothesis
Epidermal keratinocytes, which comprise ~95% of the cells within the epidermis, have been recognized as a powerful tool in skin regeneration. Epidermal keratinocytes play an important role in wound epithelization and would healing. However, it is still difficult to obtain adequate numbers of epidermal keratinocytes that retain their differentiation potential. How to obtain a large number of autologous epidermal keratinocytes in a short time is the main problem that limits the application of tissue-engineered skin and epidermal cell membrane. It is known that culture confluence can induce commitment to terminal differentiation by expression of suprabasal keratin 1 (K1) and 10 (K10) genes [6]. The keratinocytes were always expanded and isolated by enzymatic detachment from culture flasks and directly engrafted onto patients. However, the use of enzymes and lack of three-dimensional structure limited its application. It is known that epidermal keratinocytes are regulated by their niches to maintain tissue homeostasis and repair. Other studies that cultured human keratinocytes on a fibrin indicated that it facilitated the formation of dermo-epidermal junction and favored the regeneration of normal epidermis [7]. Co-culture methods establish and mimic the synthetic interactions between cell populations in vivo. A theory of obtaining a large volume of autologous epidermal keratinocytes in suspension through multiple cell interactions seems to be reasonable. Rheinwald et al developed a method for the long-term expansion of primary human epidermal keratinocytes by co-culture with 3T3 mouse embryonic fibroblasts. Fibroblasts provide a supportive in vitro environment for the expansion of human stratified epidermal keratinocyte cells [8]. This shows that the co-culture of fibroblasts and epidermal keratinocytes promotes the proliferation of epidermal keratinocytes, maintains their stemness, and delays their differentiation [9]. In addition, fibroblasts can facilitate re-epithelialization in wounded human skin equivalents [10]. Moreover, evidence shows that cultured epidermal keratinocytes on collagen implanted with fibroblasts for a period of time can form an artificial dermis and epidermis. This has already been used for venous ulcer and diabetic foot treatment, with Apligraf as a well-known example [11].
Melanocytes are another cell type that can be included to mimic the cell–cell interactions in vivo and improve the regeneration ability of a tissue engineering product. Melanocytes synthesize the melanin that produces the skin’s color, and thus fill a cosmetic need. Liu et al [12] developed a tissue engineering skin substitute composed of human fibroblasts, melanocytes, and keratinocytes in a type I collagen gel. The results showed proper integration and morphology and successful repair of skin defects in athymic mice, and black skins were observed by 6 weeks after grafting. In addition, melanocytes protect epidermal keratinocytes from ultraviolet radiation-induced changes in their DNA structure [13,14]. Melanocytes in autologous engineered skin substitutes can restore photoprotection after grafting to full-thickness skin wound, regardless of whether light or dark pigmentation phototype melanocytes were used [15].
A three-dimensional structure is required to deliver adequate numbers of epidermal keratinocytes to a wound, which is necessary for epithelialization and wound healing. Biodegradable microcarriers provide a temporary three-dimensional structure for cell adhesion and proliferation and help to form a three-dimensional skin substitute after delivery to a poorly vascularized wound. A biodegradable microcarrier-based suspension culture containing various cell types has also been reported to be a good alternative for effective ex vivo expansion [16]. It was found that isolated human epidermal keratinocytes cultured on CultiSpher-G microcarriers multiplied by 44.9-fold in a microcarrier-bioreactor culture in 17 days, whereas two-dimensional cultures reached confluence in 9 days and only expanded by 7.4-fold. Moreover, microcarrier-expanded epidermal keratinocytes retained their capacity to form an epidermis and exhibited a morphology similar to that of native skin [17]. In addition, it is possible to promote the proliferation and maturation of epidermal keratinocytes and melanocytes by adjusting the culture medium, such as through serum, epidermal growth factor (EGF), and fibroblast growth factor-basic (bFGF) supplements [18]. In this way, the fibroblasts in the microcarriers are inhibited by competition from other cell populations and provide a supportive in vitro environment for the expansion of epidermal keratinocytes and melanocytes [10]. It is speculated that the crosstalk between the fibroblasts, epidermal keratinocytes, and melanocyte in the microcarriers could facilitate epithelialization and have potential for full-thickness skin regeneration.
It seems that fibroblasts and the biodegradable microcarriers, as well as melanocytes, might provide a suitable growth niche for epidermal keratinocytes. One possible mechanism is the crosstalk between the fibroblasts, epidermal keratinocytes, and melanocytes via secreted factors [19–21], and via extracellular matrix (ECM) deposition [22]. The feeder cell culture system is an example that demonstrated that the epidermal keratinocyte stem cell phenotype depends on the interaction with fibroblasts. The fibroblasts are inhibited by competition from the surrounding epidermal keratinocytes and melanocytes, and serve as a source of growth factors and cytokines to support the functions of these epidermal keratinocytes and melanocytes, including transforming growth factor beta (TGF-β), keratinocyte growth factor (KGF), EGF, bFGF, and tumor necrosis factor-α (TNF-α), which bind to receptors and modulate intracellular signaling cascades related to the epidermal keratinocyte and melanocyte proliferation and functions [19–21]. Such cascades include Wnt/β-catenin, PI3K/Akt, and the MAPK/ERK signaling pathway [19,23]. In addition, there is a stronger increased secretion of IL-6 (Interleukin-6) [24], TGF-β [25], collagenases, MMP-3 (matrix metallopeptidase 3), and TIMPs (tissue inhibitors of metalloproteinases) [26] in epidermal keratinocyte–fibroblast co-cultures in comparison to monocultures, which has been shown to stimulate epidermal keratinocyte proliferation, differentiation, and ECM deposition [22]. Another possible mechanism is the crosstalk between fibroblasts, epidermal keratinocytes, and melanocytes via extracellular vesicles (EVs) from those cells. EVs have been implicated in many mechanisms, such as cell–stroma interactions and angiogenesis [27,28]. It is reported that fibroblasts can produce EVs that can stimulate mesenchyme growth when cultured with serum or plasma [29]. On the other hand, exosomes derived from keratinocytes can not only stimulate fibroblast migration and promote wound healing [30], but also modulate pigmentation in melanocytes [31].
Conclusions
We present the novel concept of TESRUs as the smallest unit with complete full-thickness skin regeneration ability. TESRUs can potentially form and simultaneously deliver to the wound a mature skin microstructure to regenerate full-thickness skin, independent of scaffolds and vascularization. It is believed that TESRUs will provide a promising strategy in skin tissue engineering if a thorough understanding can be gained of the intimate interactions between the fibroblasts, epidermal keratinocytes, and melanocytes. However, further in vivo and clinical trials are still necessary.
References
1. Bhardwaj N, Chouhan D, Mandal BB, Tissue engineered skin and wound healing: Current strategies and future directions: Curr Pharm Des, 2017; 23(24); 3455-82
2. Ho J, Walsh C, Yue D, Current advancements and strategies in tissue engineering for wound healing: A comprehensive review: Adv Wound Care (New Rochelle), 2017; 6(6); 191-209
3. Velander P, Theopold C, Bleiziffer O, Cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model: J Surg Res, 2009; 157(1); 14-20
4. Guerid S, Darwiche SE, Berger MM, Autologous keratinocyte suspension in platelet concentrate accelerates and enhances wound healing – a prospective randomized clinical trial on skin graft donor sites: Platelet concentrate and keratinocytes on donor sites: Fibrogenesis Tissue Repair, 2013; 6(1); 8
5. Sierra-Sanchez A, Kim KH, Blasco-Morente G, Arias-Santiago S, Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries: NPJ Regen Med, 2021; 6(1); 35
6. Poumay Y, Pittelkow MR, Cell density and culture factors regulate keratinocyte commitment to differentiation and expression of suprabasal K1/K10 keratins: J Invest Dermatol, 1995; 104(2); 271-76
7. Ronfard V, Rives JM, Neveux Y, Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix: Transplantation, 2000; 70(11); 1588-98
8. Rheinwald JG, Green H, Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells: Cell, 1975; 6(3); 331-43
9. Hynds RE, Bonfanti P, Janes SM, Regenerating human epithelia with cultured stem cells: Feeder cells, organoids and beyond: EMBO Mol Med, 2018; 10(2); 139-50
10. Lombardi B, Casale C, Imparato G, Spatiotemporal evolution of the wound repairing process in a 3D human dermis equivalent: Adv Healthc Mater, 2017; 6(13); 201601722
11. Zaulyanov L, Kirsner RS, A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers: Clin Interv Aging, 2007; 2(1); 93-98
12. Liu Y, Suwa F, Wang X, Reconstruction of a tissue-engineered skin containing melanocytes: Cell Biol Int, 2007; 31(9); 985-90
13. Costin GE, Hearing VJ, Human skin pigmentation: melanocytes modulate skin color in response to stress: FASEB J, 2007; 21(4); 976-94
14. Bannasch H, Fohn M, Unterberg T, Skin tissue engineering: Clin Plast Surg, 2003; 30(4); 573-79
15. Goyer B, Pereira U, Magne B, Impact of ultraviolet radiation on dermal and epidermal DNA damage in a human pigmented bilayered skin substitute: J Tissue Eng Regen Med, 2019; 13(12); 2300-11
16. Sun LY, Lin SZ, Li YS, Functional cells cultured on microcarriers for use in regenerative medicine research: Cell Transplant, 2011; 20(1); 49-62
17. Borg DJ, Dawson RA, Leavesley DI, Functional and phenotypic characterization of human keratinocytes expanded in microcarrier culture: J Biomed Mater Res A, 2009; 88(1); 184-94
18. Blaimauer K, Watzinger E, Erovic BM, Effects of epidermal growth factor and keratinocyte growth factor on the growth of oropharyngeal keratinocytes in coculture with autologous fibroblasts in a three-dimensional matrix: Cells Tissues Organs, 2006; 182(2); 98-105
19. Werner S, Krieg T, Smola H, Keratinocyte-fibroblast interactions in wound healing: J Invest Dermatol, 2007; 127(5); 998-1008
20. Peura M, Siltanen A, Saarinen I, Paracrine factors from fibroblast aggregates in a fibrin-matrix carrier enhance keratinocyte viability and migration: J Biomed Mater Res A, 2010; 95(2); 658-64
21. Stunova A, Vistejnova L, Dermal fibroblasts-A heterogeneous population with regulatory function in wound healing: Cytokine Growth Factor Rev, 2018; 39; 137-50
22. Benny P, Badowski C, Lane EB, Raghunath M, Making more matrix: Enhancing the deposition of dermal-epidermal junction components in vitro and accelerating organotypic skin culture development, using macromolecular crowding: Tissue Eng Part A, 2015; 21(1–2); 183-92
23. Qiang L, Yang S, Cui YH, He YY, Keratinocyte autophagy enables the activation of keratinocytes and fibroblasts and facilitates wound healing: Autophagy, 2020 [Online ahead of print]
24. Lichtman MK, Otero-Vinas M, Falanga V, Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis: Wound Repair Regen, 2016; 24(2); 215-22
25. Maas-Szabowski N, Starker A, Fusenig NE, Epidermal tissue regeneration and stromal interaction in HaCaT cells is initiated by TGF-alpha: J Cell Sci, 2003; 116(Pt 14); 2937-48
26. Sawicki G, Marcoux Y, Sarkhosh K, Tredget EE, Ghahary A, Interaction of keratinocytes and fibroblasts modulates the expression of matrix metalloproteinases-2 and -9 and their inhibitors: Mol Cell Biochem, 2005; 269(1–2); 209-16
27. Rackov G, Garcia-Romero N, Esteban-Rubio S, Vesicle-mediated control of cell function: The role of extracellular matrix and microenvironment: Front Physiol, 2018; 9; 651
28. Carrasco E, Soto-Heredero G, Mittelbrunn M, The role of extracellular vesicles in cutaneous remodeling and hair follicle dynamics: Int J Mol Sci, 2019; 20(11); 2758
29. Moulin VJ, Mayrand D, Messier H, Shedding of microparticles by myofibroblasts as mediator of cellular cross-talk during normal wound healing: J Cell Physiol, 2010; 225(3); 734-40
30. Sjoqvist S, Kasai Y, Shimura D, Oral keratinocyte-derived exosomes regulate proliferation of fibroblasts and epithelial cells: Biochem Biophys Res Commun, 2019; 514(3); 706-12
31. Lo Cicero A, Delevoye C, Gilles-Marsens F, Exosomes released by keratinocytes modulate melanocyte pigmentation: Nat Commun, 2015; 6; 7506
In Press
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952