27 July 2021: Clinical Research
The Association Between Plasma α-Synuclein (α-syn) Protein, Urinary Alzheimer-Associated Neuronal Thread Protein (AD7c-NTP), and Apolipoprotein Epsilon 4 (ApoE ɛ4) Alleles and Cognitive Decline in 60 Patients with Alzheimer’s Disease Compared with 28 Age-Matched Normal Individuals
Shuang Lv12BCDEF, Xiao Zhou23B, Yiming Li4BE, Shujuan Zhang12BCD, Yu Wang2F, Shuhong Jia2D, Xiaoqian Niu12B, Lei Wang12B, Dantao Peng123A*DOI: 10.12659/MSM.932998
Med Sci Monit 2021; 27:e932998
Abstract
BACKGROUND: Accumulating evidence has shown that α-synuclein (α-syn) pathology is involved in the pathophysiology of Alzheimer’s disease (AD). This study aimed to investigate the association between the levels of plasma α-syn protein, urinary Alzheimer-associated neuronal thread protein (AD7c-NTP), apolipoprotein epsilon 4 (ApoE ε4) alleles and cognitive decline in 60 AD patients compared with 28 age-matched normal controls (NCs) at a single center.
MATERIAL AND METHODS: All participants underwent α-syn, apolipoprotein E (ApoE), AD7c-NTP, cholesterol (CHO), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides (TGs) analyses, neuropsychological scale assessments and neuroimaging analysis. Moreover, urine and peripheral blood samples were collected from all participants. The levels of plasma α-syn and AD7c-NTP were assayed using an enzyme-linked immunosorbent assay (ELISA) kit. Other test results were obtained from China-Japan Friendship Hospital.
RESULTS: We found that plasma α-syn levels were significantly different between AD patients and NCs (p=0.045). α-Syn levels were also associated with AD7c-NTP (r=0.231, p=0.03) but not ApoE e4 (Z=-0.147, p=0.883) levels. Neither a-syn [CHO (p=0.432), HDL (p=0.484), LDL (p=0.733) or TGs (p=0.253)] nor AD7c-NTP [CHO (p=0.867), HDL (p=0.13), LDL (p=0.57) or TGs (p=0.678)] had a relationship with lipids.
CONCLUSIONS: This study showed that the levels of plasma α-syn protein and urinary AD7c-NTP were significantly increased in AD patients compared with NCs, but not with ApoE alleles or serum lipid levels.
Keywords: AD7c-NTP Protein, Human, alpha-Synuclein, Alzheimer Disease, Apolipoprotein E4, cognitive dysfunction, Nerve Tissue Proteins, Neuropsychological Tests
Background
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease in the central nervous system with clinical features of memory loss and cognitive decline [1]. Amyloid-β (Aβ) and tau are involved in the pathophysiological processes underlying AD [2]. AD is the most common type of dementia, accounting for 3/5–4/5 of all cases [3]. AD is a thorny problem for both society and families. Unfortunately, a recent study showed that 99.6% of clinical trials ended in failure during 2000 and 2012 [4]. Once dementia is apparent, it may be too late to initiate the treatment for AD, which cascades toward inevitable outcomes [5]. Thus, it is important to identify AD biomarkers and define the relationships among them.
Recent studies have shown that approximately 1/3 of AD patients have cerebral α-synuclein (α-syn) depositions [6]. Wang’s study showed that serum α-syn levels were different between AD and Parkinson’s disease dementia through enzyme-linked immunosorbent assay (ELISA) [7]. A study injected α-syn preformed fibrils into transgenic mice with 5 familial Alzheimer’s disease mice (5xFAD). Aβ deposits accelerated α-syn protein in the brain of 5xFAD [8]. The accumulation and spreading of tau was lower in the brains of mice without endogenous α-syn than in those of blank control mice[9]. Aβ42 and α-syn can promote each other’s oligomerization [10,11]. In particular, α-syn stimulates the oligomerization of Aβ42, which leads to its precipitation and the formation of hybrid ring-like structures [12]. Thus, accumulating evidence has shown that α-syn pathology is involved in the pathophysiology of AD.
Another study showed that urinary Alzheimer-associated neuronal thread protein (AD7c-NTP) co-localizes with tau-immunoreactive neurofibrillary tangles [13]. Furthermore, overexpression of AD7c-NTP is related to neurite sprouting and cell death, which is closely associated with the pathophysiological mechanisms underlying AD [14]. The urine AD7c-NTP level is elevated in AD, which has also been identified as a biomarker for AD patients [15]. Similarly, the apolipoprotein E (ApoE) allele is also associated with tau-immunoreactive neurofibrillary tangles and Aβ deposition [16]. Three common alleles of ApoE – ɛ2, ɛ3, and ɛ4 – are defined by 2 single-nucleotide polymorphisms (rs429358 and rs7412) that reside in the coding region of exon 4. They overlap with a well-defined CpG island (CGI). A study showed that normal elderly individuals with ApoE ɛ4 homozygotes (ɛ4/ɛ4) have a very high risk of developing clinical AD [17]. It is known that the risk of AD is increased 3- to 4-fold in individuals with only one ɛ4 allele compared to patients without ɛ4 alleles [18]. Apolipoproteins and α-syn, with their amphipathic helices, insert into lipid membranes and influence their curvature [19]. Wojciech Paslawski’s study [20] showed that in patients with Parkinson’s disease, α-syn interacts with ApoE in human cerebrospinal fluid (CSF). Thus, α-syn may have a relationship with ApoE.
All 3 biomarkers participate in the pathophysiological processes of Aβ and tau. The study also showed that they were higher in AD patients [7,15,17]. However, the relationship among them remains unclear. All these biomarkers also have a relationship with lipids [21,22]. ApoE is a lipid carrier in not only the peripheral nervous system but also the central nervous system [23]. A study showed that α-syn plays a role in the regulation of synaptic vesicle fusion and is dynamic at the membrane interface, which suggests α-syn-lipid interactions [24]. A study of 2180 participants showed that after adjusting for age and sex, there were significant differences in urinary AD7c-NTP levels between blood lipids [21]. We aimed to explore their relationships and provide evidence that they can reflect the risk of AD development. Then, we explored whether cholesterol (CHO), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TGs) were associated with these biomarkers. Combining these biomarkers and examining their relationships may have clinically meaningful benefits.
Therefore, this study aimed to investigate the association between the levels of plasma α-syn protein, urinary AD7c-NTP, and apolipoprotein epsilon 4 (ApoE ɛ4) alleles and cognitive decline in 60 patients with AD compared with 28 age-matched normal individuals at a single center.
Material and Methods
STUDY POPULATION:
The study was reviewed and approved by our ethics committee and institutions (Ethics ID: 2017SY51). All subjects signed an informed consent form at the beginning of the study. The purpose of our study and the principle of voluntary participation were explained to each subject. AD patients must be accompanied by their fiduciaries. The study population included 60 AD patients and 28 normal controls (NCs). All of them were selected from the China-Japan Friendship Hospital from 2018 March to 2021 January.
CLINICAL DIAGNOSTIC CRITERIA:
The subjects were recruited by 3 experienced doctors (Dr. Peng, Dr. Wang, and Dr. Jia) through the Department of Neurology of China-Japan Friendship Hospital. Sixty subjects were diagnosed with AD based on the diagnostic criteria of the National Institute on Aging-Alzheimer’s Association (NIA-AA) [25,26], with the Standardized Mini-Mental State Examination (MMSE) ≤23, Montreal Cognitive Assessment (MoCA) ≤23, Clinical Dementia Rating (CDR) >0.5, Geriatric Depression Scale (GDS) <11, and Hachinski Ischemia Scale (HIS) <4. In addition, all subjects underwent neuroimaging analysis (magnetic resonance imaging; MRI) to rule out other brain diseases leading to cognitive decline. Moreover, we conducted ApoE genotyping, and eligible subjects were invited for the baseline visit. At the same time, we recruited 28 healthy subjects of similar sex and age as those in the AD group for the control group.
INCLUSION CRITERIA:
The inclusion criteria were as follows: (1) men or women; (2) age between 45 and 95 years old; (3) right-handed; (4) native Chinese individuals; (5) AD diagnosis established by NIA-AA criteria or NCs; and (6) AD patients accompanied by their fiduciaries who voluntarily signed the informed consent form.
EXCLUSION CRITERIA:
The exclusion criteria were as follows: (1) severe AD and cognitive impairment caused by cerebral blood disease; (2) a history of stroke, brain tumor, or other nervous system diseases and serious heart, liver, lung, kidney and other systemic diseases; (3) moderate to severe depression and other mental diseases; and (4) occurrence of other neurological diseases or the presence of cardiac pacemakers, coronary stents, dental implants and other metal implants, cochlear implants and other ferromagnetic materials. Participants who failed to follow the study protocol were also excluded.
URINE AD7C-NTP ASSAY:
Each subject collected 10-ml, first, clean-catch, and midstream urine samples in the morning. Then, the urine samples were stored at 4°C. Levels of AD7c-NTP were assayed using an ELISA kit (catalog no.2400416, Anqun Biological Technology Co. Ltd., Shenzhen, China). All experiments were performed according to the manufacturer’s instructions [27]. Then, each plate was washed with phosphate-buffered saline (PBS). Next, urine samples were incubated with biotinylated rabbit anti-AD7c-NTP antibody at 37°C for 1 h. Then, each plate was washed with PBS. Horseradish peroxidase-labeled avidin was added to each plate and incubated at 37°C for 30 min. Each plate was washed with PBS. Then, 50 mL of chromogenic reagents A and B was added to each plate and incubated at 37°C for 15 min. Finally, 50 mL of sulfuric acid was added to stop the reaction. The reaction product was measured at an optical density (OD) value of 450 nm with a multimode reader (Multiskan MK3, Thermo Scientific, Waltham, Massachusetts, United States).
PLASMA α-SYN PROTEIN ASSAY:
Peripheral blood (4 ml) was collected from each subject in the morning. The plasma was centrifuged and separated by centrifugation (CR312, Jouan, Evreux, Eure, France). Then, the plasma samples were stored at -80°C. Plasma concentrations of α-syn protein were measured by ELISA kits with reagents for human detection (catalog no. ab260052, Abcam, Cambridgeshire, United Kingdom). The details of the experiments were as described above. The reaction product was measured at an OD value of 450 nm with a multimode reader (Multiskan MK3, Thermo Scientific, Waltham, Massachusetts, USA).
STATISTICAL ANALYSIS:
We used SPSS Version 20 (Armonk, United States) to perform the statistical analyses in our study. The data were first defined to determine whether the distribution of the data was normal. All the data were non-normally distributed in our study. Medians and interquartile ranges (IQRs) were used for non-Gaussian distributed variables at baseline. Two-group comparisons, such as the analysis of differences in baseline characteristics between the AD and NC groups, were analyzed by the 2 independent-samples Mann-Whitney U test (unpaired). When the expected count was less than 5, the Fisher chi-square test was used instead of the chi-square test. Spearman correlation analyses were used. The association of clinical and biological characteristics was evaluated through linear and multivariable regression analyses. A two-tailed
Results
PARTICIPANT DEMOGRAPHIC DATA:
The study population included 60 AD patients with an average age of 69.77±10.18 (female: 40, male: 20) and 28 NCs with an average age of 70.00±8.07 (female: 18, male: 10). There were no significant differences in age, sex, education, smoking, alcohol use, blood pressure or lipids (including CHO, HDL, LDL and TGs) between the 2 groups (shown in Table 1). Neuropsychological assessment scales (including the MMSE, MoCA and Activities of daily living [ADL]), plasma α-syn level and AD7c-NTP levels were different between the 2 groups.
RELATIONSHIP BETWEEN PLASMA α-SYN AND URINARY AD7C-NTP LEVELS:
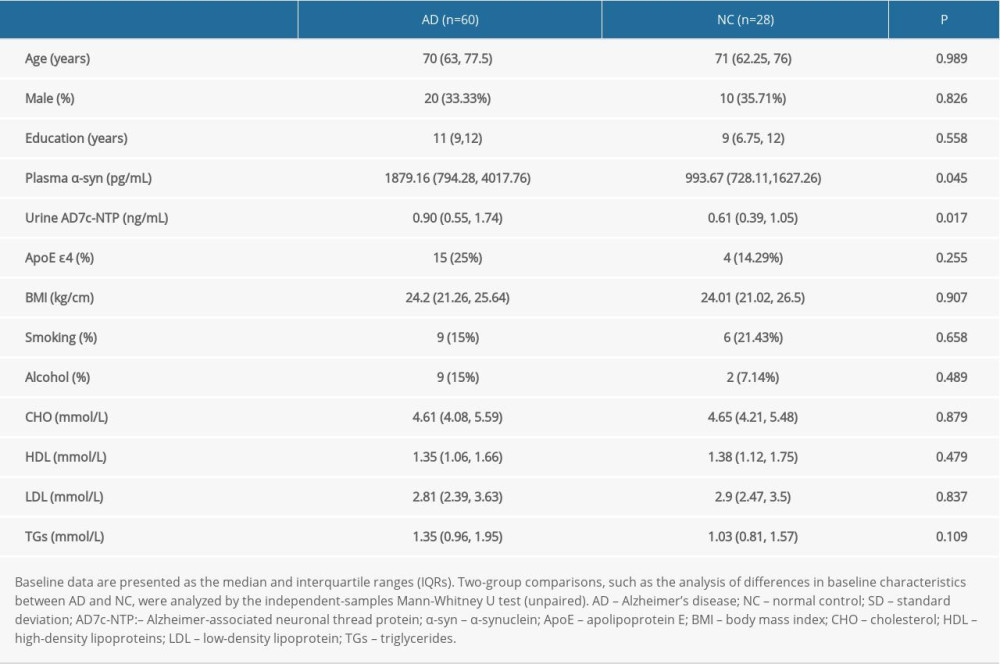
The concentration of plasma α-syn in the AD group was higher than that in the NC group (Z=-2.007, p=0.045). Spearman correlation analysis showed that plasma α-syn levels were negatively associated with urinary AD7c-NTP levels (r=0.231, P=0.03) (shown in Figure 1). However, no relationships were found between the plasma α-syn levels and age (r=0.146, P=0.176), sex (Z=−1.03, P=0.303), education (r=0.044, P=0. 683), body mass index (BMI) (r=0.145, P=0.179), smoking (Z=−0.1, P=0.92) and alcohol use (Z=−0.7, P=0.484).
The concentration of urinary AD7c-NTP in the AD group was higher than that in the NC group (Z=−2.383, P=0.017) (shown in Figure 1). However, no relationships were found between the urinary AD7c-NTP levels and age (r=−0.065, P=0.548), sex (Z=−1.056, P=0.291), education (r=−0.095, P=0.378), BMI (r=−0.024, P=0.827), smoking (Z=−1.215, P=0.224) or alcohol use (Z=−0.24, P=0.811).
RELATIONSHIP BETWEEN PLASMA α-SYN LEVELS AND NEUROPSYCHOLOGICAL ASSESSMENT SCALE SCORES:
Spearman correlation analysis revealed that plasma α-syn levels were negatively associated with MMSE scores (r=−0.283,
RELATIONSHIP BETWEEN URINARY AD7C-NTP LEVELS AND NEUROPSYCHOLOGICAL ASSESSMENT SCALE SCORES:
Spearman correlation analysis revealed that urinary AD7c-NTP levels were negatively associated with MMSE scores (r=−0.355,
RELATIONSHIPS BETWEEN PLASMA α-SYN AND URINARY AD7C-NTP LEVELS AND APOE ɛ4:
Two independent-samples Mann-Whitney U tests (unpaired) revealed that plasma α-syn levels (Z=−0.147,
RELATIONSHIPS BETWEEN PLASMA α-SYN AND URINARY AD7C-NTP LEVELS AND LIPIDS:
Spearman correlation analysis revealed no relationship between plasma α-syn levels and levels of CHO (r=0.085, P=0.432), HDL (r=0.076, P=0.484), LDL (r=0.037, P=0.733) or TGs (r=0.123, P=0.253) (shown in Figure 2).
Spearman correlation analysis revealed no relationship between urinary AD7c-NTP levels and levels of CHO (r=−0.018, P=0.867), HDL (r=0.163, P=0.13), LDL (r=−0.061, P=0.57), or TGs (r=−0.045, P=0.678) (shown in Figure 3).
Discussion
We found increased α-Syn protein levels in patients with AD compared with NCs, which is unusual because this is a biomarker for Parkinson’s disease (PD) [28]. At the beginning of the study, we first excluded patients with other neurological diseases, especially PD. All patients underwent MRI to rule out other brain diseases leading to cognitive decline. The subjects were independently recruited by 3 experienced doctors to ensure the correct diagnosis. While tau is a biomarker of AD and α-syn is a pathological feature in PD and dementia with Lewy bodies (DLB), the presence of copathologies is important and very common in the above diseases [29]. α-Syn is a 140-amino-acid protein that is encoded by the alpha-synuclein gene (SNCA) and expressed at high levels in neuronal presynaptic terminals [30]. Nuclear magnetic resonance (NMR) spectroscopy experiments have shown that monomeric α-syn can directly interact with tau variants through its highly negatively charged C-terminus, promoting amyloid fibrillation and accumulation in vitro [31]. Electron microscopy confirmed the immunopositivity of tubulin polymerization promoting protein (TPPP/p25) in postmortem AD brain tissue. TPPP/p25 was suggested to be a new marker of α-syn [32]. These pathological features appear not only in the human brain but also in peripheral fluids. CSF α-syn levels are higher in patients with mild cognitive impairment (MCI) and AD than in healthy controls [33,34]. Sylvie Slaets’s study showed that CSF α-syn levels were significantly higher in AD than in synucleinopathies [33]. Vergallo’s study showed that CSF α-syn had positive correlations with both CSF t-tau and p-tau181 concentrations [35]. Filippo’s study [6] showed that compared with NCs, AD patients had lower concentrations of α-syn and its heterocomplexes, such as α-syn/Aβ and α-syn/tau, in red blood cells. To the best of our knowledge, no study has compared α-syn levels in human plasma between AD patients and NCs. In our study, AD patients had higher plasma α-syn concentrations than NCs [medians and IQRs: 1879.16 (794.28, 4017.76) vs 993.67 (728.11,1627.26), respectively,
AD7c-NTP is a transmembrane phosphate protein, and its molecular weight is approximately 41 kDa [36]. AD7c-NTP has high sensitivity and specificity in both urine and CSF. It was one of the potential biomarkers of early AD [37]. AD7c-NTP immunoreactivity was found to co-localize with neurofibrillary tangles and dystrophic neurites [38]. Li’s results showed that urinary AD7c-NTP levels in the subjective cognitive decline group (0.7561±0.5657 ng/mL) were not different than levels in either depressive state (0.7527±0.5607 ng/mL) or normal control (0.7214±0.5077 ng/mL) groups [27]. However, in Zhang’s study, urinary AD7c-NTP levels were significantly higher in the lower cognitive function group [0.48 (0.21–1.00) ng/mL] than in the NC group [0.25 (0.04–0.44) ng/ml;
Another study of 329 cognitively normal right-handed Han Chinese subjects showed that urinary AD7c-NTP levels had a positive relationship with ApoE grade in the cognitively normal population and that urinary AD7c-NTP levels were significantly higher in subjects with ApoE ɛ4 [0.6074 (0.6541) ng/mL] than in subjects without ApoE ɛ4 [0.4368 (0.3392) ng/mL] [22]. ApoE is a cholesterol/lipid transporter in the central nervous system and is also known to be the most influential genetic risk factor for AD [42]. A study in mice showed that ApoE ɛ4, but not ApoE ɛ2 or ApoE ɛ3, increased α-syn pathology and astrogliosis. ApoE ɛ4 also worsened neuronal and synaptic loss and impaired behavioral performance at 9 months of age [43]. However, in our study, we did not find any association with ApoE ɛ4. It may be that the sample size in our study was too small.
α-Syn can interact with lipoproteins in human plasma [44]. A study showed that hyperlipidaemia had a significant direct impact on tau protein deposition in the brain and damaged the blood-brain barrier [45]. Dyslipidaemia was shown to be a risk factor for AD [46]. Cédric Eichmann’s study showed that the family of synucleins in humans, including mutants of the α-syn family, can form HDL-like particles with different morphologies [47]. An 11-amino acid sequence from the ApoB protein LDL binding peptide can transport α-syn, which is targeted by small interfering ribonucleic acid (siRNA), across the blood-brain barrier [48]. Higher low-density lipoprotein cholesterol (LDL-C) levels were associated with a higher risk of early-onset AD [49]. A study showed that urinary AD7c-NTP levels were also associated with human blood lipids [21]. Thus, we supported the notion that α-syn had a relationship with AD through lipoproteins. However, in our study, we did not find any association with lipids, including CHO, HDL, LDL, and TGs. Therefore, the levels of AD7c-NTP and plasma α-syn are not consistent with the levels of ApoE or lipids.
We first analyzed the relationship between α-syn, AD7c-NTP, and ApoE. Further analysis was performed to determine whether they were associated with lipids in AD patients. This is a new clinical perspective in the diagnosis of AD. Currently, few biomarkers are used by neurologists in the clinic, and few studies have explored the relationship between them. All of the 3 biomarkers are related to Aβ and tau, which are the hallmarks of AD. Moreover, we found that α-syn levels are positively correlated with AD7c-NTP levels. Above, we speculate that α-syn and AD7c-NTP, together, may be implicated in Aβ and tau pathologies.
However, how α-syn and AD7c-NTP are involved in AD pathogenesis and the interplay between them are still unclear. Large sample size studies, multicentre studies, and longitudinal studies should be performed for confirmation. In addition, a more elaborate case history of patients should be provided. The one limitation in the current study is that we quantified the protein levels of α-syn and AD7c-NTP, but not ribonucleic acid (RNA) levels. Thus, whether α-syn and AD7c-NTP’s protein and RNA enrichment show consistency is still unknown, and this is an interesting question for future investigation. All the patients received a criterion standard biomarker-based diagnosis of AD and were matched for age and sex with the NC group in our study. Future studies may further investigate the interactions between α-syn and AD7c-NTP.
Conclusions
This study showed that the levels of plasma α-syn protein and urinary AD7c-NTP were significantly increased in patients with AD compared with normal age-matched individuals, but not with ApoE alleles or serum lipid levels. Thus, plasma α-syn levels may play an important role in the pathology of AD and this progression was not associated with lipids.
Figures
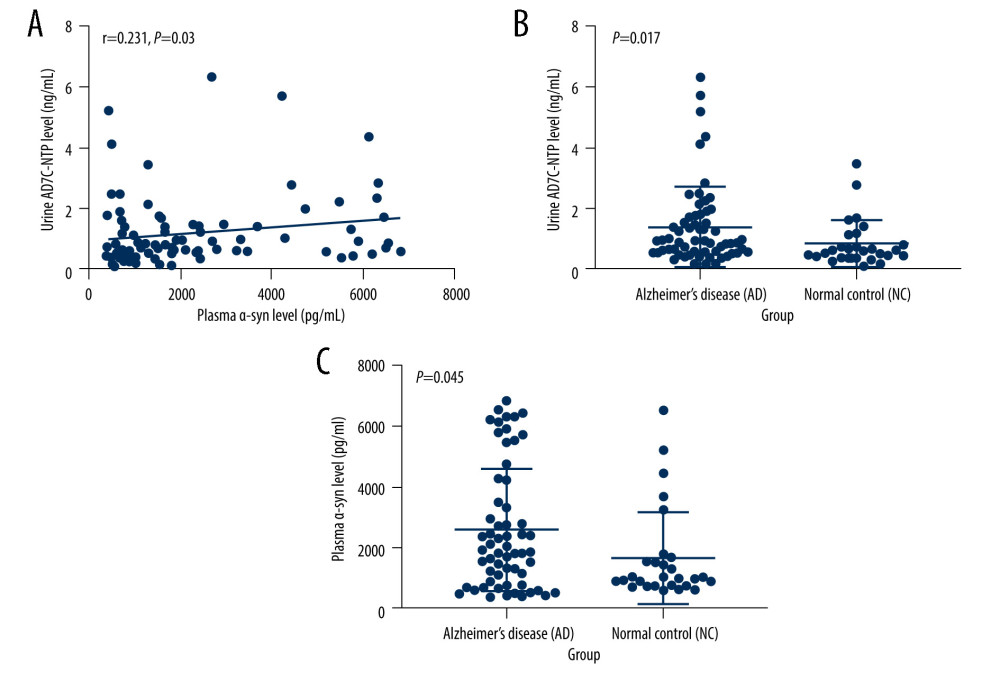 Figure 1. (A–C) Relationship between plasma α-syn and urinary AD7c-NTP levels. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). AD7c-NTP – Alzheimer-associated neuronal thread protein; α-syn – α-synuclein.
Figure 1. (A–C) Relationship between plasma α-syn and urinary AD7c-NTP levels. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). AD7c-NTP – Alzheimer-associated neuronal thread protein; α-syn – α-synuclein. 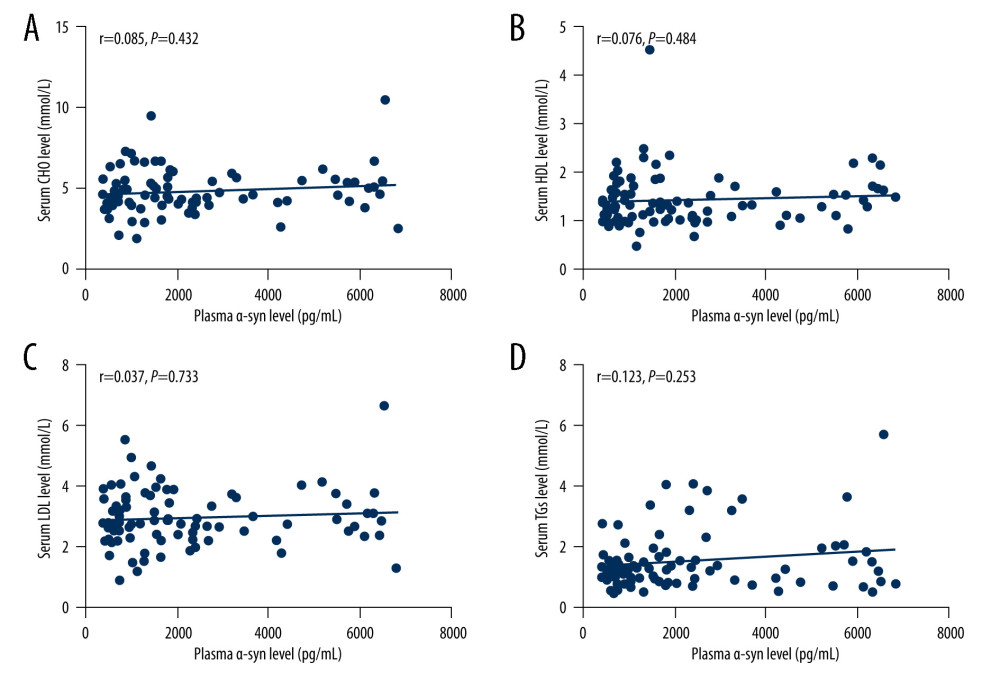 Figure 2. (A–D) Relationships between plasma α-syn levels and lipids. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). α-syn – α-synuclein; CHO – cholesterol; HDL – high-density lipoproteins; LDL – low-density lipoprotein; TGs – triglycerides.
Figure 2. (A–D) Relationships between plasma α-syn levels and lipids. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). α-syn – α-synuclein; CHO – cholesterol; HDL – high-density lipoproteins; LDL – low-density lipoprotein; TGs – triglycerides.  Figure 3. (A–D) Relationships between urinary AD7c-NTP levels and lipids. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). AD7c-NTP – Alzheimer-associated neuronal thread protein; CHO – cholesterol; HDL – high-density lipoproteins; LDL – low-density lipoprotein; TGs – triglycerides.
Figure 3. (A–D) Relationships between urinary AD7c-NTP levels and lipids. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). AD7c-NTP – Alzheimer-associated neuronal thread protein; CHO – cholesterol; HDL – high-density lipoproteins; LDL – low-density lipoprotein; TGs – triglycerides. References
1. Lyketsos CG, Carrillo MC, Ryan JM, Neuropsychiatric symptoms in Alzheimer’s disease: Alzheimers Dement, 2011; 7(5); 532-39
2. Scheltens P, Blennow K, Breteler MM, Alzheimer’s disease: Lancet, 2016; 388(10043); 505-17
3. Sabayan B, Sorond F, Reducing risk of dementia in older age: JAMA, 2017; 317(19); 2028
4. , The need for early detection and treatment in Alzheimer’s disease [editorial]: EBioMedicine, 2016; 9; 1-2
5. Lloret A, Esteve D, Lloret MA, When does Alzheimer’s disease really start? The role of biomarkers: Int J Mol Sci, 2019; 20(22); 5536
6. Baldacci F, Daniele S, Piccarducci R, Potential diagnostic value of red blood cells α-synuclein heteroaggregates in Alzheimer’s disease: Mol Neurobiol, 2019; 56(9); 6451-59
7. Wang J, Zheng B, Yang S, Differential circulating levels of naturally occurring antibody to α-synuclein in Parkinson’s disease dementia, Alzheimer’s disease, and vascular dementia: Front Aging Neurosci, 2020; 12; 571437
8. Bassil F, Brown HJ, Pattabhiraman S, Amyloid-beta (Aβ) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of Lewy body disorders with Aβ pathology: Neuron, 2020; 105(2); 260-275.e6
9. Bassil F, Meymand ES, Brown HJ, α-Synuclein modulates tau spreading in mouse brains: J Exp Med, 2021; 218(1); e20192193
10. Masliah E, Rockenstein E, Veinbergs I, beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease: Proc Natl Acad Sci USA, 2001; 98(21); 12245-50
11. Mandal PK, Pettegrew JW, Masliah E, Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease: Neurochem Res, 2006; 31(9); 1153-62
12. Oláh J, Vincze O, Virók D, Interactions of pathological hallmark proteins: tubulin polymerization promoting protein/p25, beta-amyloid, and alpha-synuclein: J Biol Chem, 2011; 286(39); 34088-100
13. de la Monte SM, Xu YY, Wands JR, Modulation of neuronal thread protein expression with neuritic sprouting: Relevance to Alzheimer’s disease: J Neurol Sci, 1996; 138(1–2); 26-35
14. Monte SM, Ghanbari K, Frey WH, Characterization of the AD7C-NTP cDNA expression in Alzheimer’s disease and measurement of a 41-kD protein in cerebrospinal fluid: J Clin Invest, 1997; 100(12); 3093-104
15. Jin H, Wang R, Alzheimer-associated neuronal thread protein: Research course and prospects for the future: J Alzheimers Dis, 2021; 80(3); 963-71
16. Alzheimer’s Association, 2016 Alzheimer’s disease facts and figures: Alzheimers Dement, 2016; 12(4); 459-509
17. Vos SJ, Xiong C, Visser PJ, Preclinical Alzheimer’s disease and its outcome: A longitudinal cohort study: Lancet Neurol, 2013; 12(10); 957-65
18. Corder EH, Saunders AM, Strittmatter WJ, Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families: Science, 1993; 261(5123); 921-23
19. Varkey J, Isas JM, Mizuno N, Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins: J Biol Chem, 2010; 285(42); 32486-93
20. Paslawski W, Zareba-Paslawska J, Zhang X, α-synuclein-lipoprotein interactions and elevated ApoE level in cerebrospinal fluid from Parkinson’s disease patients: Proc Natl Acad Sci USA, 2019; 116(30); 15226-35
21. Li Y, Guan S, Jin H, The relationship between urinary Alzheimer-associated neuronal thread protein and blood biochemical indicators in the general population: Aging (Albany NY), 2020; 12(15); 15260-80
22. Li Y, Kang M, Sheng C, Relationship between urinary Alzheimer-associated neuronal thread protein and apolipoprotein Epsilon 4 allele in the cognitively normal population: Neural Plast, 2020; 2020; 9742138
23. Zhao N, Liu CC, Qiao W, Bu G, Apolipoprotein E, receptors, and modulation of Alzheimer’s disease: Biol Psychiatry, 2018; 83(4); 347-57
24. Kaur U, Lee JC, Unroofing site-specific α-synuclein-lipid interactions at the plasma membrane: Proc Natl Acad Sci USA, 2020; 117(32); 18977-83
25. Cummings J, The National Institute on Aging-Alzheimer’s Association Framework on Alzheimer’s disease: Application to clinical trials: Alzheimers Dement, 2019; 15(1); 172-78
26. Sperling RA, Aisen PS, Beckett LA, Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease: Alzheimers Dement, 2011; 7(3); 280-92
27. Li Y, Kang M, Wang H, Urinary Alzheimer-associated neuronal thread protein is not elevated in patients with subjective cognitive decline and patients with depressive state: J Alzheimers Dis, 2019; 71(4); 1115-23
28. Bieri G, Brahic M, Bousset L, LRRK2 modifies α-syn pathology and spread in mouse models and human neurons: Acta Neuropathol, 2019; 137(6); 961-80
29. Rodriguez-Vieitez E, Nielsen HM, Associations between APOE variants, Tau and α-synuclein: Adv Exp Med Biol, 2019; 1184; 177-86
30. Hsu LJ, Mallory M, Xia Y, Expression pattern of synucleins (non-Abeta component of Alzheimer’s disease amyloid precursor protein/alpha-synuclein) during murine brain development: J Neurochem, 1998; 71(1); 338-44
31. Lu J, Zhang S, Ma X, Structural basis of the interplay between α-synuclein and Tau in regulating pathological amyloid aggregation: J Biol Chem, 2020; 295(21); 7470-80
32. Kovács GG, László L, Kovács J, Natively unfolded tubulin polymerization promoting protein TPPP/p25 is a common marker of alpha-synucleinopathies: Neurobiol Dis, 2004; 17(2); 155-62
33. Slaets S, Vanmechelen E, Le Bastard N, Increased CSF α-synuclein levels in Alzheimer’s disease: Correlation with tau levels: Alzheimers Dement, 2014; 10(5 Suppl); S290-98
34. Shi M, Tang L, Toledo JB, Cerebrospinal fluid α-synuclein contributes to the differential diagnosis of Alzheimer’s disease: Alzheimers Dement, 2018; 14(8); 1052-62
35. Vergallo A, Bun RS, Toschi N, Association of cerebrospinal fluid α-synuclein with total and phospho-tau181protein concentrations and brain amyloid load in cognitively normal subjective memory complainers stratified by Alzheimer’s disease biomarkers: Alzheimers Dement, 2018; 14(12); 1623-31
36. De La Monte SM, Wands JR, The AD7c-NTP neuronal thread protein biomarker for detecting Alzheimer’s disease: J Alzheimers Dis, 2001; 3(3); 345-53
37. Jin H, Guan S, Wang R, The Distribution of urinary Alzheimer-associated neuronal thread protein and its association with common chronic diseases in the general population: J Alzheimers Dis, 2018; 65(2); 433-42
38. Li P, Quan W, Wang Z, AD7c-NTP impairs adult striatal neurogenesis by affecting the biological function of MeCP2 in APP/PSl transgenic mouse model of Alzheimer’s disease: Front Aging Neurosci, 2021; 12; 616614
39. Zhang Y, Li Y, Wang R, Elevated urinary AD7c-NTP levels in older adults with hypertension and cognitive impairment: J Alzheimers Dis, 2020; 74(1); 237-44
40. Ma L, Chen J, Wang R, The level of Alzheimer-associated neuronal thread protein in urine may be an important biomarker of mild cognitive impairment: J Clin Neurosci, 2015; 22(4); 649-52
41. Kahle PJ, Jakowec M, Teipel SJ, Combined assessment of tau and neuronal thread protein in Alzheimer’s disease CSF: Neurology, 2000; 54(7); 1498-504
42. Chen Y, Strickland MR, Soranno A, Holtzman DM, Apolipoprotein E: Structural insights and links to Alzheimer disease pathogenesis: Neuron, 2021; 109(2); 205-21
43. Zhao N, Attrebi ON, Ren Y, APOE4 exacerbates α-synuclein pathology and related toxicity independent of amyloid: Sci Transl Med, 2020; 12(529); eaay1809
44. Emamzadeh FN, Allsop D, α-Synuclein interacts with lipoproteins in plasma: J Mol Neurosci, 2017; 63(2); 165-72
45. Vemuri P, Lesnick TG, Przybelski SA, Age, vascular health, and Alzheimer disease biomarkers in an elderly sample: Ann Neurol, 2017; 82(5); 706-18
46. Stukas S, Robert J, Wellington CL, High-density lipoproteins and cerebrovascular integrity in Alzheimer’s disease: Cell Metab, 2014; 19(4); 574-91
47. Eichmann C, Kumari P, Riek R, High-density lipoprotein-like particle formation of Synuclein variants: FEBS Lett, 2017; 591(2); 304-11
48. Spencer B, Trinh I, Rockenstein E, Systemic peptide mediated delivery of an siRNA targeting α-syn in the CNS ameliorates the neurodegenerative process in a transgenic model of Lewy body disease: Neurobiol Dis, 2019; 127; 163-77
49. Wingo TS, Cutler DJ, Wingo AP, Association of early-onset Alzheimer disease with elevated low-density lipoprotein cholesterol levels and rare genetic coding variants of APOB: JAMA Neurol, 2019; 76(7); 809-17
Figures
 Figure 1. (A–C) Relationship between plasma α-syn and urinary AD7c-NTP levels. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). AD7c-NTP – Alzheimer-associated neuronal thread protein; α-syn – α-synuclein.
Figure 1. (A–C) Relationship between plasma α-syn and urinary AD7c-NTP levels. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). AD7c-NTP – Alzheimer-associated neuronal thread protein; α-syn – α-synuclein. Figure 2. (A–D) Relationships between plasma α-syn levels and lipids. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). α-syn – α-synuclein; CHO – cholesterol; HDL – high-density lipoproteins; LDL – low-density lipoprotein; TGs – triglycerides.
Figure 2. (A–D) Relationships between plasma α-syn levels and lipids. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). α-syn – α-synuclein; CHO – cholesterol; HDL – high-density lipoproteins; LDL – low-density lipoprotein; TGs – triglycerides. Figure 3. (A–D) Relationships between urinary AD7c-NTP levels and lipids. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). AD7c-NTP – Alzheimer-associated neuronal thread protein; CHO – cholesterol; HDL – high-density lipoproteins; LDL – low-density lipoprotein; TGs – triglycerides.
Figure 3. (A–D) Relationships between urinary AD7c-NTP levels and lipids. The figures were made using GraphPad Prism (version 8.0.2, GraphPad Software). AD7c-NTP – Alzheimer-associated neuronal thread protein; CHO – cholesterol; HDL – high-density lipoproteins; LDL – low-density lipoprotein; TGs – triglycerides. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952